Abstract
Objective:
To estimate the prevalence of elevated brain amyloid and reduced cortical thickness (as a marker for neurodegeneration) in a defined population.
Methods:
Mayo Clinic Study of Aging participants underwent MRI to assess a composite Alzheimer disease (AD) signature cortical thickness measure and PET to assess brain amyloid accumulation. Participants were characterized as having elevated amyloid (A+/A−), reduced cortical thickness (N+/N−), and A+N+, A+N−, A−N+, or A−N−. The prevalence of AD biomarkers was derived by adjusting for nonparticipation and standardizing to the Olmsted County, Minnesota, population.
Results:
Among 1,646 participants without dementia (mean age 70.8 years; 53.2% men), the prevalence (95% confidence interval) of amyloidosis was 21.1% (19.1%–23.2%): women, 24.3%; men, 17.5%. The prevalence of reduced cortical thickness was 28.9% (26.4%–31.5%): women, 27.9%; men, 30.2%. The prevalence estimates of biomarker categories were as follows: A−N−: 61.4%; A+N−: 9.7%; A−N+: 17.4%; and A+N+: 11.5%, and varied by sex and by APOE ε4 carrier status. In men, prevalence estimates were as follows: A−N−: 62.6%; A+N−: 7.3%; A−N+: 19.9%; and A+N+: 10.2%. In women, prevalence estimates were as follows: A−N−: 60.4%; A+N−: 11.7%; A−N+: 15.3%; and A+N+: 12.6%. In ε4 carriers, prevalence estimates were as follows: A−N−: 54.6%; A+N−: 16.6%; A−N+: 12.4%; and A+N+: 16.4%. In non-ε4 carriers, prevalence estimates were as follows: A−N−: 63.3%; A+N−: 6.9%; A−N+: 19.9%; and A+N+: 10.0%.
Conclusions:
These prevalence estimates are important for understanding age-related trends in amyloid positivity and AD signature cortical thickness in the population, and for potentially projecting the future burden of biomarkers in elderly persons.
To determine the effect of interventions for reducing the burden of the clinical dementia phenotype prior to widespread initiation of interventions, it is necessary to understand the prevalence of Alzheimer disease (AD) biomarkers (i.e., elevated brain amyloid [A+] or neurodegeneration [N+]) in the population without dementia. A problem, however, is that estimates of prevalence (defined as the proportion of individuals in a defined population with a given condition or characteristic) of AD biomarkers in the population without dementia are lacking because few studies have the ability to estimate prevalence.
Staging of AD-related pathology is determined using MRI measures of atrophy, PET measures of amyloid PET and brain metabolism, and CSF amyloid β42,1,2 from which participants are characterized as A−N−, A+N−, A−N+, or A+N+.1,3,4 The frequency of AD biomarkers has been reported in persons attending memory clinics and convenience samples such as the Alzheimer's Dementia Neuroimaging Initiative (ADNI).5 These frequencies do not translate to population-based estimates needed for public health planning.
Frequencies of A/N biomarkers have been published for cognitively normal Mayo Clinic Study of Aging (MCSA) participants based on amyloidosis (amyloid PET) and neurodegeneration (MRI hippocampal volume or FDG brain metabolism),6 and using different definitions of neurodegeneration.7 Unlike hippocampal volume, which varies by sex and is highly correlated with head size, and FDG PET, which is not entirely specific for AD, AD signature cortical thickness does not vary by sex or head size and predicts memory decline. Thus, in the present study, AD signature cortical thickness was used as a marker for neurodegeneration (N+).7,8 The objective of this present study was to estimate the prevalence of the A/N categories of AD pathology in the population without dementia (cognitively normal and mild cognitive impairment [MCI]) by weighting the frequencies to account for potential nonparticipation bias in the MCSA and standardizing to the Olmsted County, Minnesota, population.
METHODS
Sampling frame and recruitment of participants.
To establish the MCSA, Olmsted County residents aged 70–89 years on October 1, 2004, were enumerated using the medical records linkage system of the Rochester Epidemiology Project (REP).9–11 From this enumeration, potential participants were selected using a random sampling strategy stratified by age and sex to ensure equal allocation by age and sex. Exclusion criteria were dementia, being in hospice, or being terminally ill. Using the same study protocols, we began continuous recruitment of participants in 2008; in 2012 and 2015, we began to recruit participants aged 50–69 years and persons with dementia, respectively. The demographic and clinical characteristics of all randomly selected participants were abstracted from the medical records using the REP in order to assess potential nonparticipation bias. Persons who agreed to a telephone-only component but declined face-to-face participation were considered nonparticipants.12 Data were abstracted for 4,552 (89% of 5,110) nonparticipants who provided authorization for use of their medical records in research.
Standard protocol approvals, registrations, and patient consents.
All study protocols were approved by the institutional review boards of the Mayo Clinic and the Olmsted Medical Center. Written informed consent was obtained from all participants.
Clinical evaluations and diagnostic assessment.
Participants were evaluated by 3 study personnel. A study coordinator assessed memory (using questions from the Blessed Test), medical conditions, and sociodemographic information, and interviewed a study partner to assess functioning of the participant. A physician assessed memory and medical conditions and performed a neurologic evaluation, and each participant completed a neuropsychological testing battery that consisted of 9 tests to assess performance in 4 cognitive domains: memory (Wechsler Memory Scale–Revised Logical Memory II [delayed recall], Wechsler Memory Scale–Revised Visual Reproduction II [delayed recall], and the Auditory Verbal Learning Test [delayed recall]), attention-executive function (Trail Making Test Part B and Digit Symbol Substitution from the Wechsler Adult Intelligent Scale–Revised), language (Boston Naming Test and category fluency), and visuospatial skills (Block Design and Picture Completion tests from the Wechsler Adult Intelligent Scale–Revised). For diagnostic purposes, the raw scores from each cognitive domain were transformed into age-adjusted scores, averaged, and scaled to allow comparisons across domains.13 The raw test scores were also averaged and scaled to create a z score for each of the 4 cognitive domains, and a global cognitive z score was calculated by averaging and scaling the 4 domain z scores. The coordinator and physician, and a neuropsychologist, reviewed all information for a participant and assigned a diagnosis of MCI by consensus: (1) cognitive concern by the individual, informant, or physician; (2) impairment in one or more cognitive domains; (3) essentially normal functional activities; and (4) absence of dementia (DSM-IV) according to published data.14 Participants were considered cognitively normal (CN) if they did not meet criteria for MCI or dementia. Only 22 participants with dementia had imaging data and were therefore excluded.
Covariates.
The coordinator assessed depressive symptoms (Beck Depression Inventory) and measured weight and height to estimate body mass index (BMI). APOE ε4 genotyping was performed. Research nurses abstracted information on clinical comorbidities (e.g., type 2 diabetes, hypertension) from participant medical records using REP resources.
Assessment of imaging biomarkers.
Beginning in 2005, participants were invited to undergo MRI to assess structural brain changes and in 2008 they were invited to undergo Pittsburgh compound B (PiB) PET imaging to assess amyloid accumulation. For this study, we considered a baseline visit as the first visit on or after August 1, 2008, at which both MRI and PiB PET imaging were performed.
Neuroimaging protocols.
MRI was performed at 3T and a marker for neurodegeneration was derived from FreeSurfer (v5.3) as an AD-signature meta–region of interest (ROI) composed of the surface-area weighted average of the mean cortical thickness in the following individual ROIs: entorhinal, inferior temporal, middle temporal, and fusiform from both hemispheres as described.15 An abnormal (reduced) AD signature cortical thickness (N+) was defined as <2.67 mm to capture the earliest stages of abnormality.15
Amyloid PET imaging was performed using 11C-PiB PET. A CT was obtained for attenuation correction. Amyloid PET imaging consisted of four 5-minute dynamic frames acquired 40–60 minutes after injection of 11C-PiB; images were analyzed with a fully automated image-processing pipeline.16 PiB PET ROIs were derived from automatically labeled ROIs from an MRI template.17 An amyloid PET standardized uptake value ratio (SUVR) was calculated from the voxel number weighted average of the median uptake in the parietal, temporal, prefrontal, orbitofrontal, precuneus, anterior and posterior cingulate, and precuneus ROIs referenced to the cerebellar gray crus region. An abnormal (elevated) amyloid (A+) was defined as PiB SUVR >1.42, based on a single cutpoint using the reliable worsening cutpoint method.15 PET values were gray matter and white matter sharpened, but were not partial volume corrected.
Statistical analyses.
Population prevalence was estimated from the frequencies of A/N categories for 5-year, 20-year, and the entire 50–89 year age ranges. First, to account for potential bias from nonparticipation in the face-to-face MCSA, the frequencies were adjusted by the probability of participating in the MCSA using inverse probability weighting (IPW) where weights were a function of age, sex, and education18–20 such that MCSA participants who were more like nonparticipants in the MCSA in regard to age, sex, and education were assigned a greater weight (figure 1; IPW adjustment 1). Second, to account for potential bias from nonparticipation in imaging studies among MCSA participants, the frequencies were adjusted by the probability of being imaged using IPW where weights were a function of age, sex, education, and MCI status (figure 1; IPW adjustment 2). For both sets of IPWs, we derived the individual weights using logistic regression models; there were no differences when the clinical covariates were considered in deriving IPWs, thus they were not included in the final IPW estimates. Finally, the adjusted frequency estimates were directly standardized by age, and age and sex, where applicable, to the 2010 Olmsted County population aged 50–89 years, using the sampling fractions from our population-based sampling.21–23
Figure 1. Conceptual design of estimating prevalence.
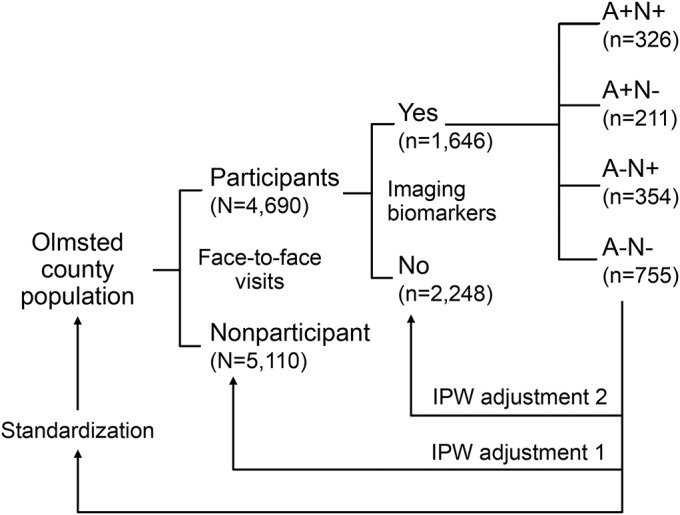
Inverse probability weighting (IPW) adjustment 1 adjusts for nonparticipation in the Mayo Clinic Study of Aging (MCSA); IPW adjustment 2 adjusts for participation in MCSA, but not in imaging studies; standardization involves direct standardization to the Olmsted County 2010 population by age and or sex, where applicable. Of the 5,110 nonparticipants in the MCSA, 1,822 declined the face-to-face evaluation and participated by telephone only; 3,288 refused to participate. A total of 796 people who participated in the face-to-face evaluation were lost to the study prior to August 1, 2008, or were not eligible for imaging.
RESULTS
A total of 1,646 MCSA participants without dementia completed both MRI and PET scanning at the same evaluation between August 1, 2008, and October 20, 2016. The mean (SD) age at imaging was 70.8 (9.8) years, 53.2% were men, 97.1% had ≥12 years of education, 28.0% had an APOE ε4 allele, and 13.6% had prevalent MCI (table 1). Compared to women, men were older (p = 0.02); had a higher frequency of hypertension (p = 0.005), diabetes (p < 0.001), dyslipidemia (p = 0.01), and coronary artery disease (p < 0.001); had a lower global cognitive z score (p < 0.001); and had a higher frequency of an abnormal AD signature cortical thickness (N+) (p = 0.001).
Table 1.
Characteristics of participants with MRI and PiB PET by sex and nonimaged participants at first visit on or after August 1, 2008
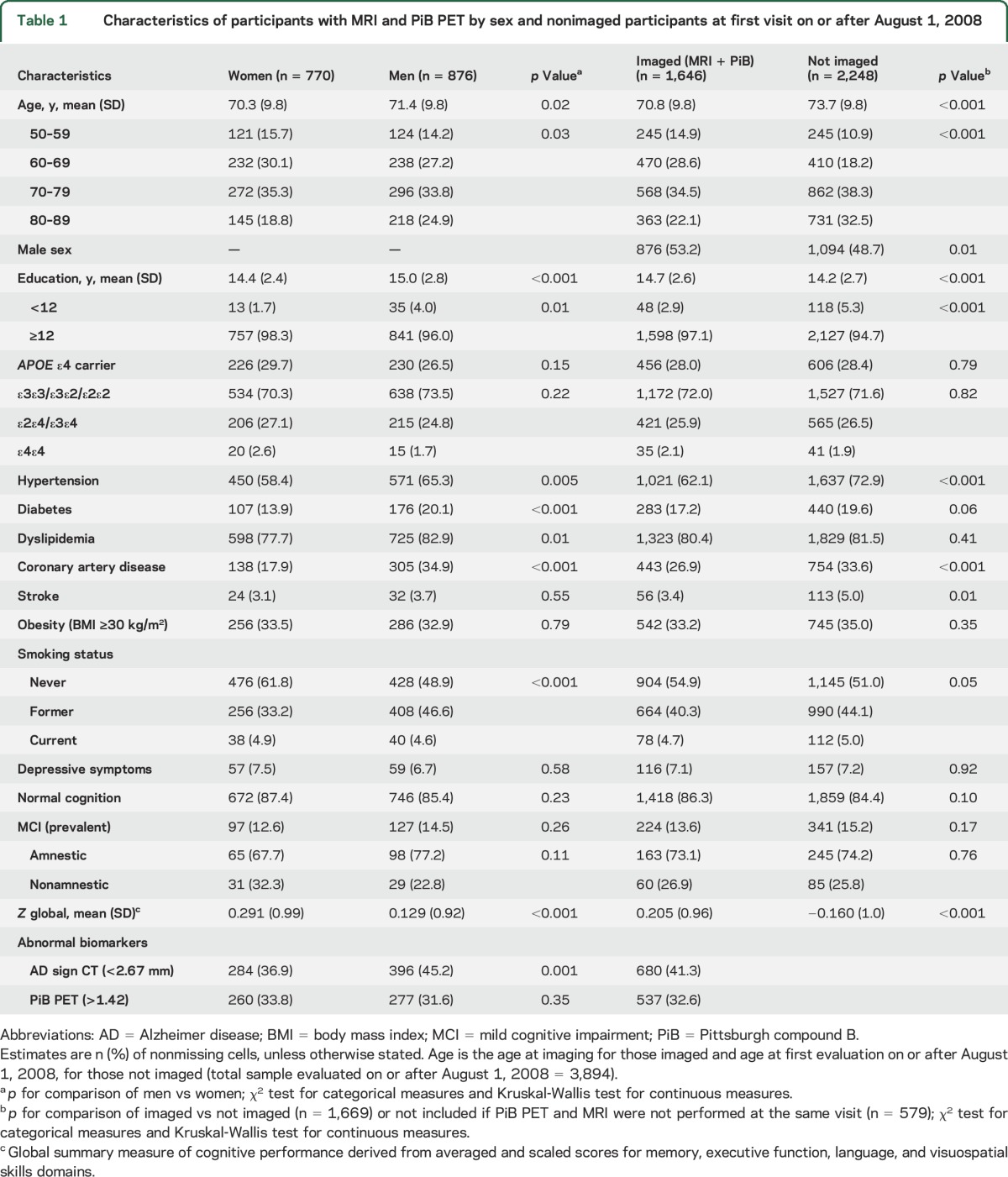
Compared to participants with imaging, MCSA participants who were seen on or after August 1, 2008, but not included (n = 2,248), were older (mean age 73.7 vs 70.8 years; p < 0.001), and fewer were men (48.7% vs 53.2%; p = 0.01), and had a higher frequency of hypertension (p < 0.001), coronary artery disease (p < 0.001), and stroke (p = 0.01), and a lower education (p < 0.001) and global cognitive z score (p < 0.001) (table 1). Similar patterns were observed for imaged MCSA participants compared with the total MCSA participants not included in the study (table e-1 at Neurology.org).
Across A/N biomarker categories (unadjusted for age), the A+N+ group had the highest mean age (79.3 years), the frequency of men was highest in A−N+ (60.7%) or A+N+ (55.5%), and frequency of women was highest in A+N− (54.5%); the frequency of an APOE ε4 allele was highest in the A+N− (48.6%) and A+N+ (41.1%) groups (table e-2). The frequencies of hypertension, diabetes, dyslipidemia, and coronary artery disease were highest in A+N+ followed by A−N+ groups and lowest in the A−N− group. The frequency of prevalent MCI was highest in the A+N+ group (30.1%), followed by the A+N− group (15.2%).
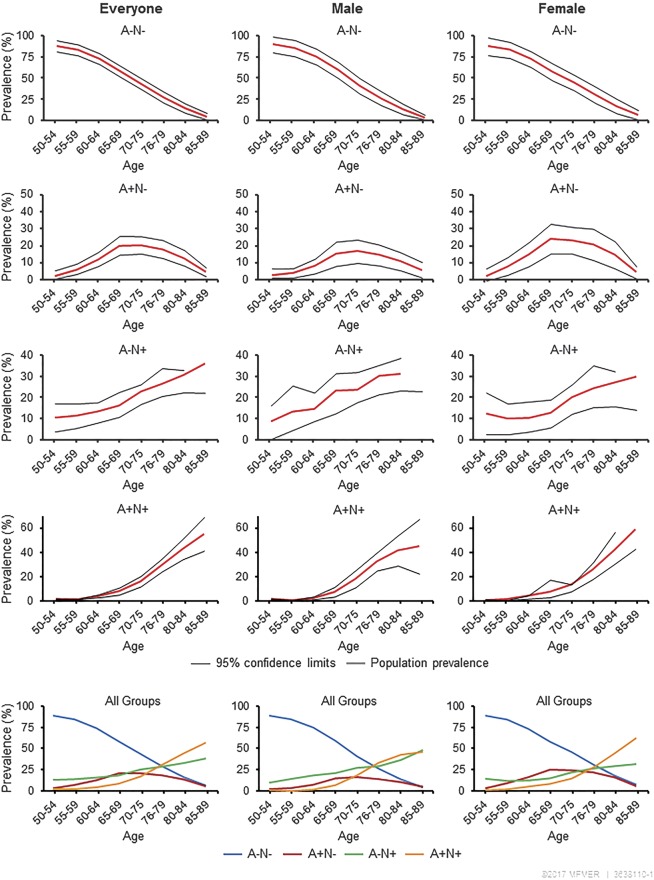
Amyloid levels increased with age in both men and women. AD signature cortical thickness decreased with age and was similar in men and women in the youngest and oldest ages, but lower in men than women in the intervening age groups (figure e-1). The overall prevalence of amyloidosis was 21.1% (95% confidence interval 19.1%–23.2%). The prevalence was higher in women, 24.3% (21.1%–27.5%), than men, 17.5% (15.0%–20.0%). The prevalence of reduced AD signature cortical thickness was 28.9% (26.4%–31.5%), similar in men, 30.2% (26.4%–33.9%), and women, 27.9% (24.4%–31.4%). Both A+ and N+ increased with age, N+ more steeply than A+ after 69 years; A+ plateaued after age 79 in men, but continued to increase in women (figure e-2).
In men and women combined, the prevalence of A/N biomarker categories for 50–89 years combined was A−N−: 61.4%; A+N−: 9.7%; A−N+: 17.4%; and A+N+: 11.5% (table 2). The prevalence of A−N− declined with age; A+N− was <3% before age 60, increased with age until the 70s, and declined thereafter; A−N+ was <13% before age 65 and increased with age; A+N+ was 0% below age 60 years and increased to 53.8% in 85- to 89-year-olds. Age-specific frequencies did not differ much from age-specific estimates of prevalence; however, larger differences were observed between frequencies and summary prevalence estimates (IPW-adjusted, pooled across age, and age and sex categories, and standardized to the population). Prevalence of A/N biomarker categories varied by sex and APOE ε4 carrier status.
Table 2.
Prevalence of abnormal imaging biomarker categories in men and women

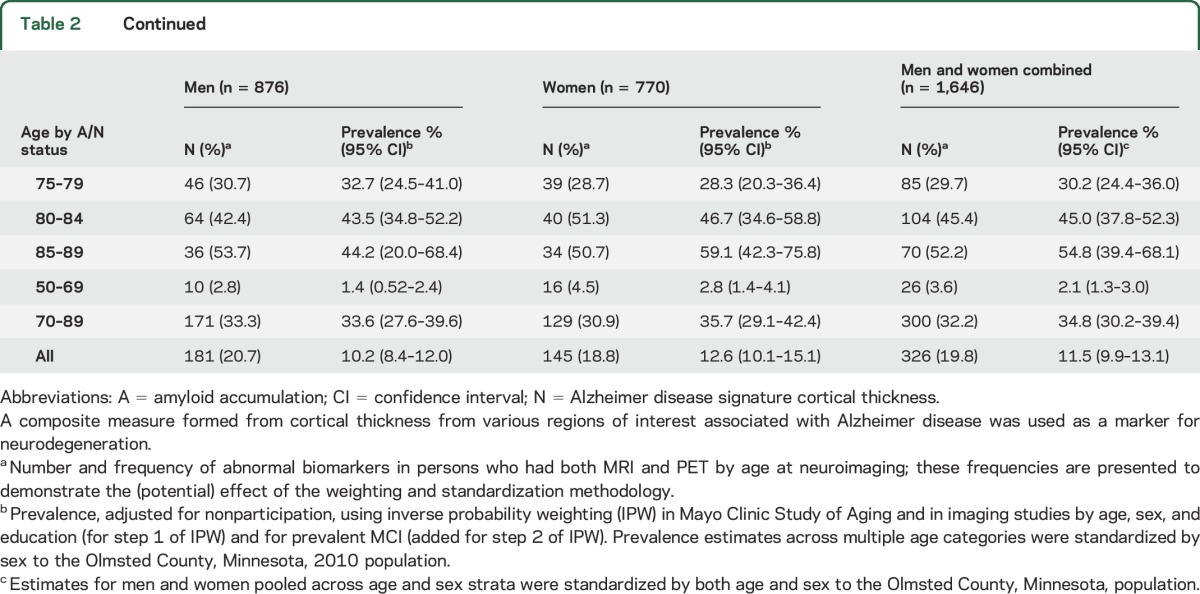
In men, the prevalence of biomarkers (50–89 years) was A−N−: 62.6%; A+N−: 7.3%; A−N+: 19.9%; and A+N+: 10.2% (table 2). In women, estimates were A−N−: 60.4%; A+N−: 11.7%; A−N+: 15.3%; and A+N+: 12.6%. Thus, A+N+ prevalence was comparable in men and women, A+N− was more prevalent in women, A−N+ was more prevalent in men, and A−N− was similar in men and women. Across 20-year age groups, A+N+ prevalence was similar in both age groups (but slightly higher in women); A+N− prevalence was higher in women in both the 50–69 and 70–89 age groups. Conversely, the prevalence of A−N+ was higher in men than in women in both age groups. Figure 2 graphically presents these age-specific and summary estimates (all biomarker groups) of prevalence.
Figure 2. Prevalence of biomarker categories overall, by sex and age group (years).
Summary figures (bottom) show all 4 biomarker categories (A−N−, A+N−, A−N+, and A+N+) on the same plot for both sexes combined and for men and women, separately, where N represents a composite Alzheimer disease signature cortical thickness as a marker for neurodegeneration. By permission of Mayo Foundation for Medical Education and Research. All rights reserved.
In APOE ε4 carriers, the prevalence of biomarker categories for 50- to 89-year-olds was A−N−: 54.6%; A+N−: 16.6%; A−N+: 12.4%; and A+N+: 16.4%. In non-ε4 carriers, the prevalence was A−N−: 63.3%; A+N−: 6.9%; A−N+: 19.9%; and A+N+: 10.0%. By 20-year age groups, A−N− was most prevalent in 50- to 69-year-old non-ε4 carriers; A+N− was most prevalent in 70- to 89-year-old APOE ε4 carriers; A−N+ was most prevalent in 70- to 89-year-old non-ε4 carriers; and A+N+ was most prevalent in 70- to 89-year-old APOE ε4 carriers (figure 3).
Figure 3. Prevalence of biomarker categories by APOE ε4 carrier status.

Figure shows prevalence of A/N biomarker categories, by 20-year age groups, and APOE ε4 allele status, where N represents a composite Alzheimer disease signature cortical thickness as a marker for neurodegeneration. By permission of Mayo Foundation for Medical Education and Research. All rights reserved.
DISCUSSION
Our findings demonstrate an increasing prevalence of abnormal AD biomarkers with age that varied with biomarker groups. Specifically, the prevalence of A+N+ and A−N+ increased with age whereas A+N− increased to the early 70s, then declined. These estimates of prevalence also varied by sex and by APOE ε4 allele. There were small differences between age-specific observed frequencies and estimated prevalence, but there were larger differences when frequencies across age, and age and sex categories were adjusted for nonparticipation and standardized to the Olmsted County population (prevalence).
Our findings are consistent with those of others. The overall prevalence of amyloidosis of 21% among 50- to 89-year-olds in the present study is similar to 24% reported for 50- to 90-year-olds in a meta-analysis of amyloid positivity.24 Similarly, a study that combined data from several clinic- and population-based studies showed an increasing trend in amyloid positivity with age from <20% at or below age 40 years to 50% by age 100 years.25 Other studies, including the Harvard Brain Aging Study,26 participants with MCI in the ADNI, and in the European Consortium,27,28 have reported estimates of A−N+ frequency that are higher in men vs women. In contrast, one study among CN participants reported a lower frequency of A−N+ in men vs women based on CSF β-amyloid and tau.29 The prevalence estimates for A/N categories are also consistent with our unadjusted estimates of frequencies previously reported among a smaller sample of CN MCSA participants.6
Despite the cross-sectional design, the low prevalence of positive biomarkers in 50- to 69-year-olds is consistent with the strong age dependence of declines in cortical thickness and elevations in brain amyloid levels.30 The high prevalence of A+N+ among persons 70 and older is consistent with increasing incidence of MCI and AD dementia with increasing age. The higher prevalence of A+N− in women than in men (1.8-fold higher in 50- to 69-year-olds and 1.4-fold higher in 70- to 89-year-olds) raises questions about potential differences in the mechanisms that underlie the development of A+ in women vs men. Understanding these mechanisms will inform the development of strategies to reduce the burden of amyloid positivity in women, some of which may also benefit men.
In contrast to A+N−, the prevalence of A−N+ was higher in men than in women in all age groups except age 50–54. This further underscores the importance of understanding how and why the prevalence of these markers varies by sex. The higher prevalence of A−N+ in men vs women may partly explain the earlier occurrence and higher prevalence of MCI in men compared to women in our previous studies31,32 and is consistent with studies suggesting that neurodegeneration, rather than amyloid, triggers symptomatic cognitive impairment.33 The higher prevalence of A−N+ in older women (11% in 50- to 69-year-olds, 25% in 70- to 89-year-olds) may have implications for later onset of clinical symptoms in women. In particular, since the prevalence of A−N+ continues to increase with age, this together with the higher prevalence of A+N+ in women than in men may contribute to a higher prevalence of AD dementia in women at advanced ages. We also observed a higher prevalence of A+ categories in APOE ε4 carriers vs non-ε4 carriers that is consistent with the known association of the APOE ε4 allele with amyloidosis.
The characteristics of participants by sex and biomarker group also generate hypotheses about etiologic factors for prevalence of AD biomarkers. The high frequency of vascular risk factors in men may contribute to the higher prevalence of A−N+ in men than in women, potentially due to cerebrovascular disease.34 The high frequency of vascular risk factors in the A+N+ and A−N+ groups suggests that lifestyle and related vascular risk factors may have additive effects on the development of AD-related neurodegeneration.35 The high frequency of low education in A+N+ and A−N+ groups raises the question that sociodemographic and lifestyle factors may be linked to greater vascular pathology leading to neurodegeneration.
The A−N− group, a large component of the study, was much healthier than the groups with pathology. They were younger (69% aged <70 years; table e-2), had the lowest frequencies of diabetes, dyslipidemia, hypertension, stroke, and coronary artery disease, and had the highest level of education compared to remaining groups. This suggests that an increasing burden of chronic conditions with aging may contribute to brain pathology; prevention of these conditions may offer opportunities for intervention.
Limitations of our study include possible nonparticipation bias, but adjustment for potential nonparticipation bias had minimal effects on the unadjusted age-specific frequencies. Yet, potential residual bias from factors that differed in participants and nonparticipants could contribute to underestimation of prevalence estimates. Tau imaging was not considered in AD biomarkers since relatively few MCSA participants had undergone tau imaging at the onset of the present study. Since this is a prevalence study, we cannot make reliable inferences about etiology or causation. Contrary to amyloid positivity, hippocampal volume, and FDG hypometabolism that have been associated with clinical progression, data on the predictive role of AD signature cortical thickness for progression are limited.7 The predominant race/ethnicity of the MCSA is 96% white; therefore estimates of prevalence need to be explored in nonwhite populations.
The strengths of our article include the large sample size that enhances the internal validity of the study, the ability to characterize AD pathology in participants using amyloid and neurodegeneration biomarkers, and the population-based study design. More importantly, conducting the study in a defined population, combined with our ability to adjust for potential nonparticipation bias using information from participant medical records, enabled us to estimate prevalence in the population without dementia.
These estimates of prevalence may have important implications for projections of the future population burden of age-related amyloid positivity and reduced AD signature cortical thickness. The role of AD signature cortical thickness as a predictor of progression and the public health implications remain to be determined prospectively.
ACKNOWLEDGMENT
The authors thank the Mayo Clinic Study of Aging staff and participants for their involvement; Mary J. Dugdale, RN, Connie J. Fortner, RN, and Julie A. Gingras, RN, for the abstraction of medical record data; and Sondra L. Buehler for administrative assistance.
GLOSSARY
- A+
elevated brain amyloid
- AD
Alzheimer disease
- ADNI
Alzheimer's Dementia Neuroimaging Initiative
- CN
cognitively normal
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- IPW
inverse probability weighting
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- N+
elevated brain neurodegeneration
- PiB
Pittsburgh compound B
- REP
Rochester Epidemiology Project
- ROI
region of interest
- SUVR
standardized uptake value ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Rosebud Roberts: generated the first draft and completed the final draft, study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision, obtained grant funding. David Knopman: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Jeremy Syrjanen: analysis of data, critical revision of the manuscript for important intellectual content. Jeremiah Aakre: analysis of data, critical revision of the manuscript for important intellectual content. Maria Vassilaki: critical revision of the manuscript for important intellectual content. Walter Kremers: analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Michelle Mielke: study concept and design, critical revision of the manuscript for important intellectual content. Mary Machulda: acquisition of data, critical revision of the manuscript for important intellectual content. Jonathan Graff-Radford: critical revision of the manuscript for important intellectual input. Yonas Geda: critical revision of the manuscript for important intellectual input. Prashanthi Vemuri: critical revision of the manuscript for important intellectual input, obtaining grant funding. Val Lowe: study concept and design, acquisition of data, analysis and interpretation, study supervision, critical revision of the manuscript for important intellectual content, obtaining grant funding. Clifford Jack: study concept and design, acquisition of data, analysis and interpretation; study supervision, critical revision of the manuscript for important intellectual content, obtaining grant funding. Ronald Petersen: study concept and design, acquisition of data, study supervision, analysis and interpretation, critical revision of the manuscript for important intellectual content, obtaining grant funding.
STUDY FUNDING
The study was funded by the NIH (P50 AG016574, U01 AG006786, R01 AG041851, R01 NS097495, R01 AG011378), the Elsie and Marvin Dekelboum Family Foundation, the GHR Foundation, and the Mayo Foundation for Medical Education and Research, and was made possible by the Rochester Epidemiology Project (R01 AG034676).
DISCLOSURE
R. Roberts receives research funding from the NIH/NIA and Roche. D. Knopman serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by Biogen, TauRX Pharmaceuticals, Lilly Pharmaceuticals, and the Alzheimer's Disease Cooperative Study; and receives research support from the NIH. J. Syrjanen and J. Aakre report no disclosures relevant to the manuscript. M. Vassilaki receives research funding from Roche. W. Kremers receives research funding from NIH and Roche. M. Mielke consults for Eli Lilly and Lysosomal Therapeutics, Inc., receives unrestricted research grants from Biogen and Roche, and receives research funding from the NIH/NIA and the Department of Defense. M. Machulda receives research support from the NIH/NIA & NIDCD. J. Graff-Radford reports no disclosures relevant to the manuscript. Y. Geda receives funding from NIH and Roche. P. Vemuri receives NIH funding. V. Lowe serves on scientific advisory boards for Bayer Schering Pharma, Piramal Life Sciences, and Merck Research, and receives research support from GE Healthcare, Siemens Molecular Imaging, Avid Radiopharmaceuticals, and the NIH (NIA, NCI). C. Jack serves on a scientific advisory board for Eli Lilly & Company; receives research support from the NIH/NIA and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation; and holds stock in Johnson & Johnson. R. Petersen is a consultant for Roche, Inc., Biogen, Inc., Merck, Inc., Eli Lilly and Company, and Genentech, Inc.; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging–Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol 2012;71:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 2014;13:614–629. [DOI] [PubMed] [Google Scholar]
- 5.Mueller SG, Weiner MW, Thal LJ, et al. The Alzheimer's Disease Neuroimaging Initiative. Neuroimaging Clin N Am 2005;15:869–877, xi–xii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack CR Jr, Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral b-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol 2014;13:997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR Jr, Wiste HJ, Weigand SD, et al. Different definitions of neurodegeneration produce similar frequencies of amyloid and neurodegeneration biomarker groups by age among cognitively non-impaired individuals. Brain 2015;138:3747–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz CG, Gunter JL, Wiste HJ, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. Neuroimage Clin 2016;11:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St. Sauver JL, Grossardt BR, Melton LJ III. History of the Rochester Epidemiology Project: half a century of medical records linkage in a United States population. Mayo Clin Proc 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knopman DS, Roberts RO, Geda YE, et al. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology 2010;34:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's older Americans normative studies: WAIS-R norms for ages 56 to 97. Clin Neuropsychol 1992;6:1–104. [Google Scholar]
- 14.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 15.Jack CR Jr, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement 2017;13:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR Jr, Wiste HJ, Knopman DS, et al. Rates of beta-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology 2014;82:1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR Jr, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016;87:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler RC, Little RJ, Groves RM. Advances in strategies for minimizing and adjusting for survey nonresponse. Epidemiol Rev 1995;17:192–204. [DOI] [PubMed] [Google Scholar]
- 19.D'Agostino RB Jr, Rubin DB. Estimating and using propensity scores with partially missing data. J Am Stat Assoc 2000;95:749–759. [Google Scholar]
- 20.Little RJA. Survey nonresponse adjustments for estimates of means. Int Stat Rev (Netherlands) 1986;54:139–157. [Google Scholar]
- 21.Ahmad OB, Boschi-Pinto CB, Lopez AD, Murray CJL, Lozano R, Inoue M. Age Standardization of Rates: A New WHO Standard. Geneva: World Health Organization; 2001. [Google Scholar]
- 22.Kahn HA, Sempos CT. Statistical Methods in Epidemiology. New York: Oxford Universtiy Press; 1989. [Google Scholar]
- 23.Cochran WG. Sampling Techniques, 3rd ed. New York: John Wiley & Sons; 2007. [Google Scholar]
- 24.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA 2015;313:1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015;313:1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol 2014;71:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen RC, Aisen P, Boeve BF, et al. Mild cognitive impairment due to Alzheimer disease in the community. Ann Neurol 2013;74:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caroli A, Prestia A, Galluzzi S, et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): prediction of progression. Neurology 2015;84:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol 2013;12:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knopman DS, Jack CR Jr, Wiste HJ, et al. Age and neurodegeneration imaging biomarkers in persons with Alzheimer disease dementia. Neurology 2016;87:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic Study of Aging. Neurology 2010;75:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology 2012;78:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer's disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis. Alzheimers Dement 2017;7:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 2015;138:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]