Abstract
Objective:
To determine outcomes among patients with migraine in the emergency department (ED) who receive IV hydromorphone vs IV prochlorperazine + diphenhydramine.
Methods:
This study was conducted in 2 EDs in New York City. Patients who met international criteria for migraine were eligible for participation if they had not used an opioid within the previous month. Clinicians, participants, investigators, and research personnel were blinded to treatment. Patients were randomized in blocks of 4. Participants received hydromorphone 1 mg or prochlorperazine 10 mg + diphenhydramine 25 mg. Diphenhydramine was administered to prevent akathisia, a common side effect of IV prochlorperazine. The primary outcome was sustained headache relief, defined as achieving a headache level of mild or none within 2 hours of medication administration and maintaining that level for 48 hours without the requirement of rescue medication. A planned interim analysis was conducted once 48-hour data were available for 120 patients.
Results:
The trial was halted by the data monitoring committee after 127 patients had been enrolled. The primary outcome was achieved in the prochlorperazine arm by 37 of 62 (60%) participants and in the hydromorphone arm by 20 of 64 (31%) participants (difference 28%, 95% confidence interval 12–45, number needed to treat 4, 95% confidence interval 2–9).
Conclusions:
IV hydromorphone is substantially less effective than IV prochlorperazine for the treatment of acute migraine in the ED and should not be used as first-line therapy.
ClinicalTrials.gov identifier:
Classification of evidence:
This study provides Class I evidence that for patients in the ED with migraine, IV prochlorperazine + diphenhydramine is superior to IV hydromorphone.
Migraine patients visit US emergency departments (EDs) 1.2 million times annually.1 Parenteral opioids are used to treat migraine in >50% of all ED visits.1 Hydromorphone, the parenteral opioid used most commonly, is administered in 25% of all migraine visits.1
Experts caution against the use of opioids for migraine, although scant high-quality data exist.2,3 A recent guideline statement from the American Headache Society identified no randomized studies of hydromorphone for acute migraine.4 Only lower-quality evidence exists for any parenteral opioid.4
On the basis of correlative data, some have linked ED use of parenteral opioids for migraine with an increased frequency of return visits to an ED5 and refractoriness to standard migraine medication during subsequent medical encounters.6 Outpatient data indicate that use of oral opioids for migraine may be associated with progression of episodic migraine to chronic migraine.7 These associations have never been demonstrated in randomized studies.
Given the disconnect between expert opinion and clinical emergency practice, the dearth of high-quality data, and the very large number of patients with migraine who present to EDs annually, we conducted a randomized trial in which we compared IV hydromorphone to IV prochlorperazine, a guideline-endorsed antimigraine therapeutic. Our primary hypothesis was that use of prochlorperazine would result in a greater rate of sustained headache relief than hydromorphone. Secondary hypotheses related to downstream migraine outcomes: that patients receiving prochlorperazine would visit EDs less frequently over the subsequent month and that 3-month migraine functional disability scores would be better in the prochlorperazine arm.
METHODS
Study design.
This was a randomized, double-blind, ED-based study. Outcomes were assessed for up to 4 hours in the ED. A 48-hour phone call determined intensity of headache since ED discharge and the patient's overall satisfaction with the investigational medication. Phone calls at 1 and 3 months determined the patient's subsequent migraine course, including the number of headache days, return visits to the ED, and effect of migraine on the patient’s daily life. Our primary research question was as follows: Among patients who present to an ED with migraine, would IV prochlorperazine + diphenhydramine cause greater rates of sustained headache relief than IV hydromorphone (Class I evidence)?
Standard protocol approvals, registrations, and patient consents.
The Albert Einstein College of Medicine Institutional Review Board reviewed and approved this study. It was registered at http://clinicaltrials.gov (NCT02389829). We obtained written consent from all study participants.
Study setting.
We conducted this study in 2 EDs of Montefiore Medical Center, an urban teaching medical center located in the Bronx, NY. Salaried research associates staffed the EDs 24 h/d, 7 d/wk during the accrual period. These research associates each underwent individualized training on how to apply headache classification criteria and to ascertain outcomes. The training included mock patient interviews.
Population of interest.
Eligible patients were adults ≥21 years of age who presented to the EDs for treatment of migraine (International Classification of Headache Disorders 3 beta)8 rated as moderate or severe in intensity. Patients were excluded from participation if the treating physician had suspicion for a disease process other than migraine, including those patients who required emergent brain imaging, those with a temperature ≥100.4°F, or patients with objective neurologic findings. Patients were also excluded for allergy or contraindications to any of the investigational medications, including pregnancy or breastfeeding, patient-reported use of opioids within the previous month, history of addiction to prescription or illicit opioids, or prior use of methadone.
Investigational medications.
Medications in each study arm were prochlorperazine 10 mg IV administered over 5 minutes + diphenhydramine 25 mg, which was coadministered to prevent akathisia, and hydromorphone 1 mg IV administered over 5 minutes + normal saline placebo.
A second dose of the same medication was offered to each participant 1 hour after the initial medication infusion was begun. Patients randomized to prochlorperazine did not receive a second dose of diphenhydramine.
When choosing doses of medications for this study, we were limited by the absence of dose-finding studies and so opted for the highest commonly used and well-tolerated dose. For IV prochlorperazine, this was 10 mg. Diphenhydramine was coadministered with the first dose of prochlorperazine to prevent akathisia, an evidence-based strategy.9 With regard to hydromorphone, a 1 mg + 1 mg dosing strategy has been shown to be highly effective and safe for the management of acute severe pain in the ED setting and thus was incorporated into this study.10
Participants were stratified by presenting level of pain (moderate or severe) and study site. Assignment was concealed. The research pharmacist determined assignment on the basis of a random number sequence generated online. Randomization occurred in blocks of 4. Research participants, clinicians, and research personnel were blinded with the following mechanism. The research pharmacist sent 2 vials containing clear solution to the ED. Patients randomized to the prochlorperazine arm received 1 vial with prochlorperazine and a second vial of diphenhydramine. Those randomized to the hydromorphone arm received 1 vial with hydromorphone and a second vial with normal saline. An equal amount of clear solution was in both vials, which were used sequentially by the clinical nurse. The nurse withdrew the blinded investigational solutions from the vials, inserted the solutions into a 50-mL bag of normal saline, and administered the 50 mL normal saline + investigational medications as a rapid IV infusion over 5 minutes. A vial containing the second dose of investigational medication was linked by alphanumeric code to the first dose. This second dose, if required, was administered with the same blinding methodology.
Any further treatment, including outpatient prescriptions, was provided at the discretion of the attending emergency physician.
Measures and outcomes.
The primary assessment used the 4-item descriptive scale recommended for use in migraine clinical trials. With this scale, headache is described as severe, moderate, mild, or none.11 This assessment was performed every hour in the ED until the patient was discharged or until 4 hours had elapsed. Functional impairment was assessed with a recommended 4-item scale: severely impaired (cannot get out of bed), moderately impaired (great deal of difficulty doing what I usually do), mildly impaired (some difficulty doing what I usually do), or not impaired.11 Preference for a specific medication is a highly patient-centered outcome in which individuals determine for themselves the benefit of a particular drug vs the adverse effects experienced. With this in mind, we included in this study a simple query that has been used in multiple ED-based trials: “The next time you come to the ER [emergency room] for treatment of migraine, do you want to receive the same medication again?”12 Adverse events were assessed with an open-ended format. At the 48-hour follow-up, participants were asked to recall 2 specific adverse events: restlessness and drowsiness. They rated their restless and drowsiness after investigational medication administration using the phrases “a lot,” “a little,” or “none.” Longer-term outcomes were assessed with 2 instruments validated for use in a general migraine population. The 6-item Headache Impact Test (HIT6) rates the severity of the underlying migraine disorder on a scale that ranges from 36 to 78, with higher scores indicating greater severity.13 The HIT6 asks patients with migraine to recall the effects of their headaches over the previous 4 weeks. Similar to the HIT6, the 5-item Migraine Disability Assessment Scale (MIDAS) allows patients with migraine to rate the influence of their headaches on daily life over the previous 3 months. Zero indicates no headache-related functional impairment; scores >20 indicate severe headache-related impairment.14
The primary outcome for this study was sustained headache relief. This outcome required a participant to experience a reduction in headache level to mild or none within 2 hours of medication administration, to not require rescue analgesics, and to not relapse to worse than mild for 48 hours.
Secondary endpoints were need for a second dose of the per-protocol medication, need for off-protocol rescue medication, ED length of stay, frequency of adverse events in the ED, functional scale at the time of ED discharge, frequency of return visits to the ED for headache, number of headache days during the month after study enrollment, HIT6 score at 1 month, and MIDAS score at 3 months.
Analysis.
The primary analyses addressed the following question: “How effective in relieving acute migraine is a single dose of prochlorperazine vs hydromorphone?” For the purpose of this primary analysis, any study participant who requested a second dose of medication was counted as an outcome failure. A secondary analysis allowed for 1 additional dose of the same medication if requested. This second component of the analysis addressed the question, “How effective in relieving acute migraine is 1 or 2 doses of each of these migraine medications?” For the purpose of this latter analysis, any study participant who requested an off-protocol medication to treat the migraine was counted as an outcome failure. For the primary analysis, we compared the frequency of sustained headache relief between those randomized to prochlorperazine and those randomized to hydromorphone. We report point estimates, difference (absolute risk reduction), and number needed to treat (NNT), all bounded by 95% confidence intervals (CIs).
Sample size calculation.
In a randomized study of prochlorperazine 10 mg + diphenhydramine 25 mg IV for acute migraine, 65% (95% CI 48–79) of patients experienced sustained headache relief.15 In an open-label study of morphine 8 mg IV (an equi-analgesic dose to hydromorphone 1 mg IV), 24% (95% CI 11–45) of patients experienced sustained headache relief.16 A sample size calculation based on the point estimate of one of these estimates and the proximal boundary of the 95% CI of the other (65% vs 45%) required 94 patients in each group with a 2-sided α of 0.05 and a β of 0.20. Adding to this 10% for lost to follow-up and protocol violations, we determined the need for 208 patients.
A planned interim analysis was conducted after analyzable 48 hours data were available for 120 participants. The purpose of this analysis was to identify overwhelming superiority of one of the arms of the study on the primary outcome.
RESULTS
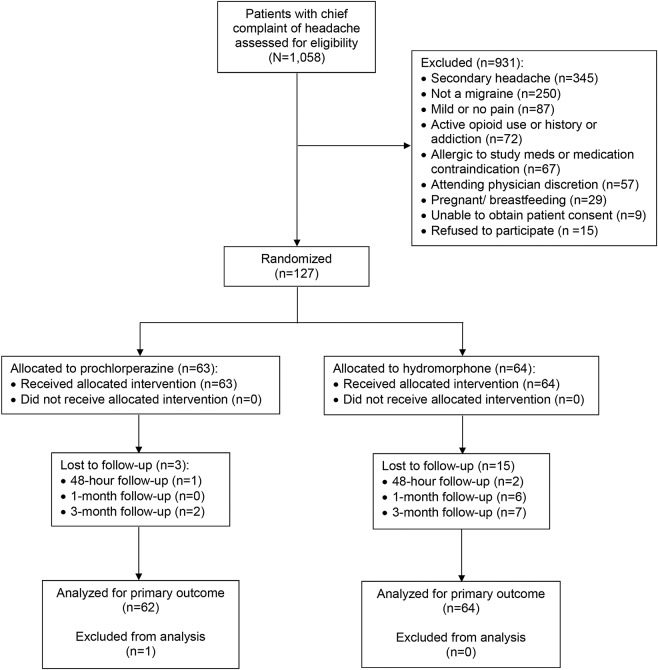
Enrollment in the study began in March 2015. The study was halted by the data monitoring committee in June 2016 after 127 patients had been enrolled because of overwhelming superiority of one of the treatment arms. Primary outcome data were available for 126 of 127 (99%) participants (figure).
Figure. CONSORT flow diagram.
Although 2 patients in the hydromorphone arm were lost to follow-up at 48 hours, we were able to include these patients in the primary outcome analysis because they received rescue medication in the emergency department, thus failing the primary outcome. CONSORT = Consolidated Standards of Reporting Trials.
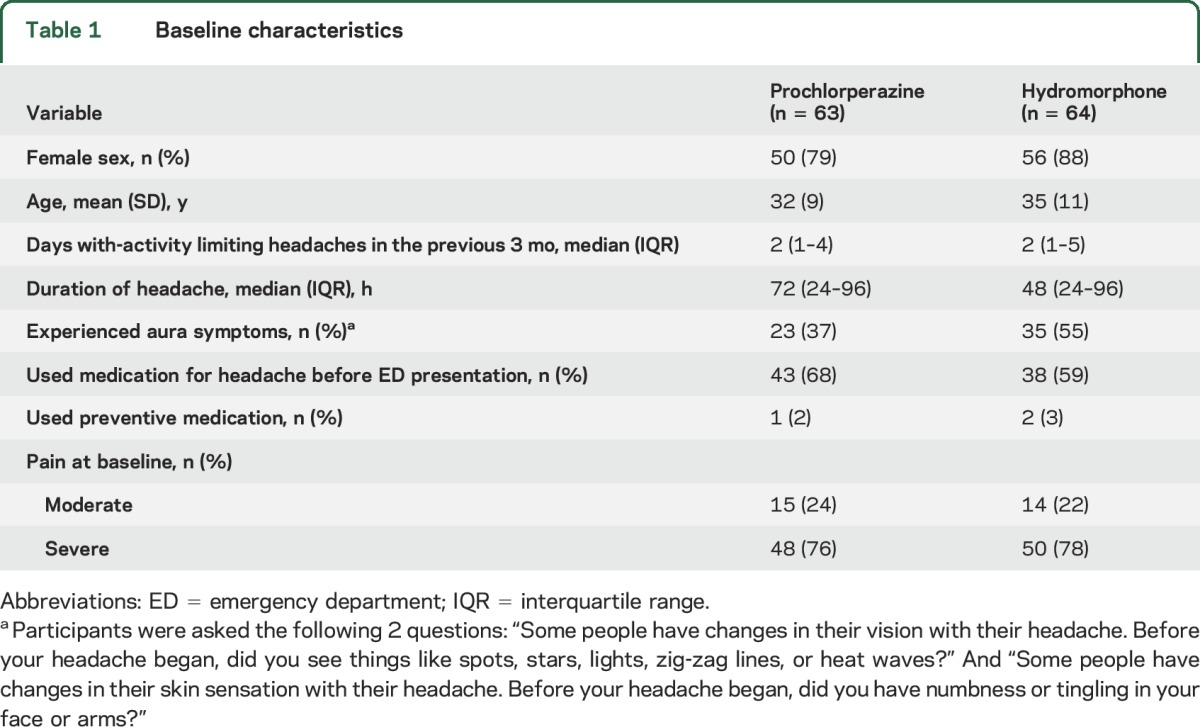
Baseline characteristics were similar between the groups (table 1). As is common in ED-based headache studies, a large percentage of study participants were women, and many study participants did not self-medicate before ED presentation.
Table 1.
Baseline characteristics
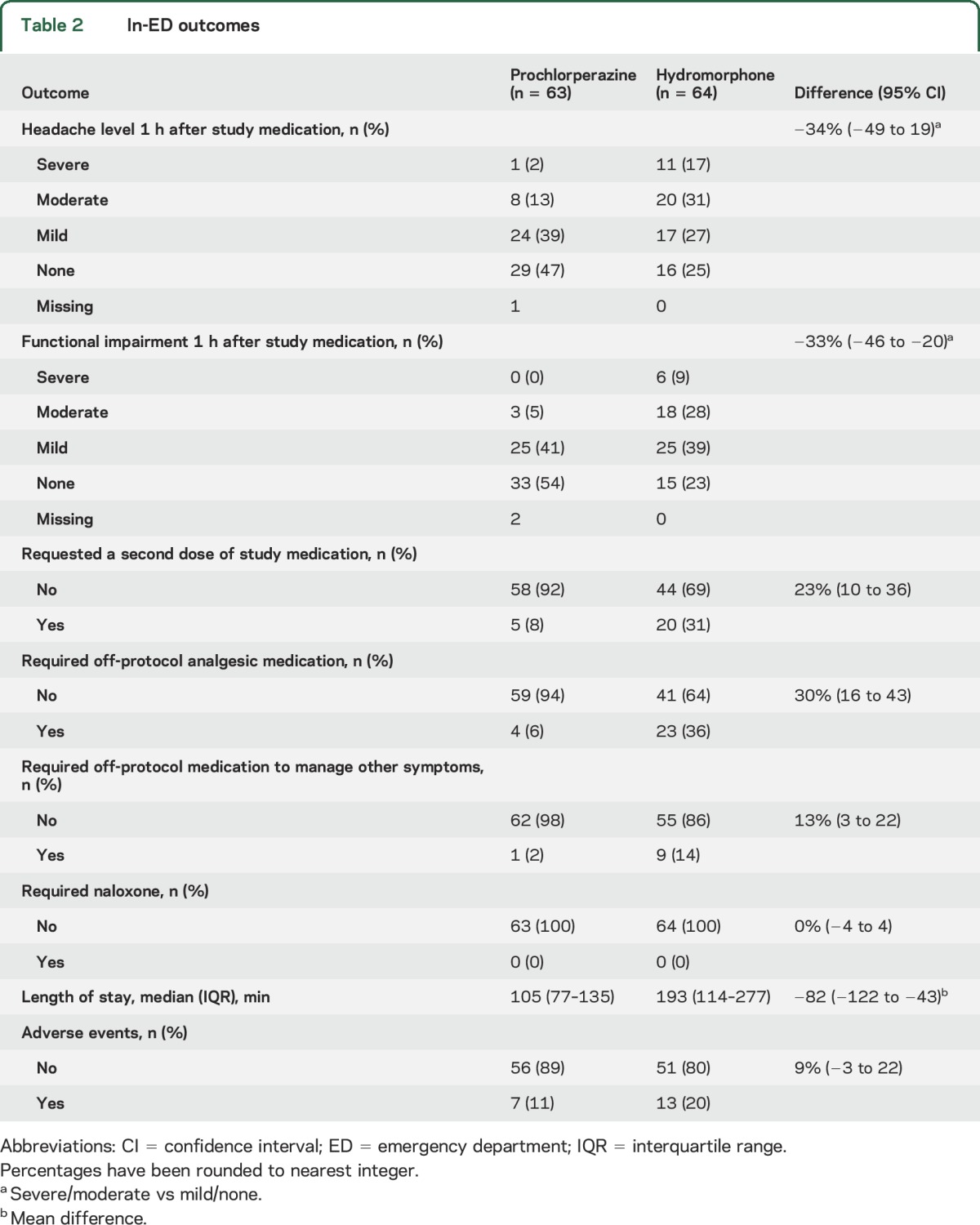
The primary outcome, sustained headache relief for 48 hours after 1 dose of investigational medication, was achieved in the prochlorperazine arms by 37 of 62 (60%) participants and in the hydromorphone arm by 20 of 64 (31%) participants (difference 28%, 95% CI 12–45, NNT 4, 95% CI 2–9). The secondary outcome of sustained headache relief after 1 or 2 doses of medication was achieved in the prochlorperazine arm by 37 of 62 (60%) participants and in the hydromorphone arm by 26 of 64 (41%) participants (difference 19%, 95% CI 2–36, NNT 6, 95% CI 3–52). Other in-ED outcomes are reported in table 2. There were large between-group discrepancies in functional impairment, need for additional medication, and ED length of stay. The most common adverse symptom reported in open-ended question format by patients receiving prochlorperazine was anxiety or restlessness, which was reported by 3 (5%) patients. For hydromorphone, the most common adverse event was dizziness or weakness, reported by 9 (14%) patients. No more than 2 study participants reported any other side effect in this open-ended format.
Table 2.
In-ED outcomes
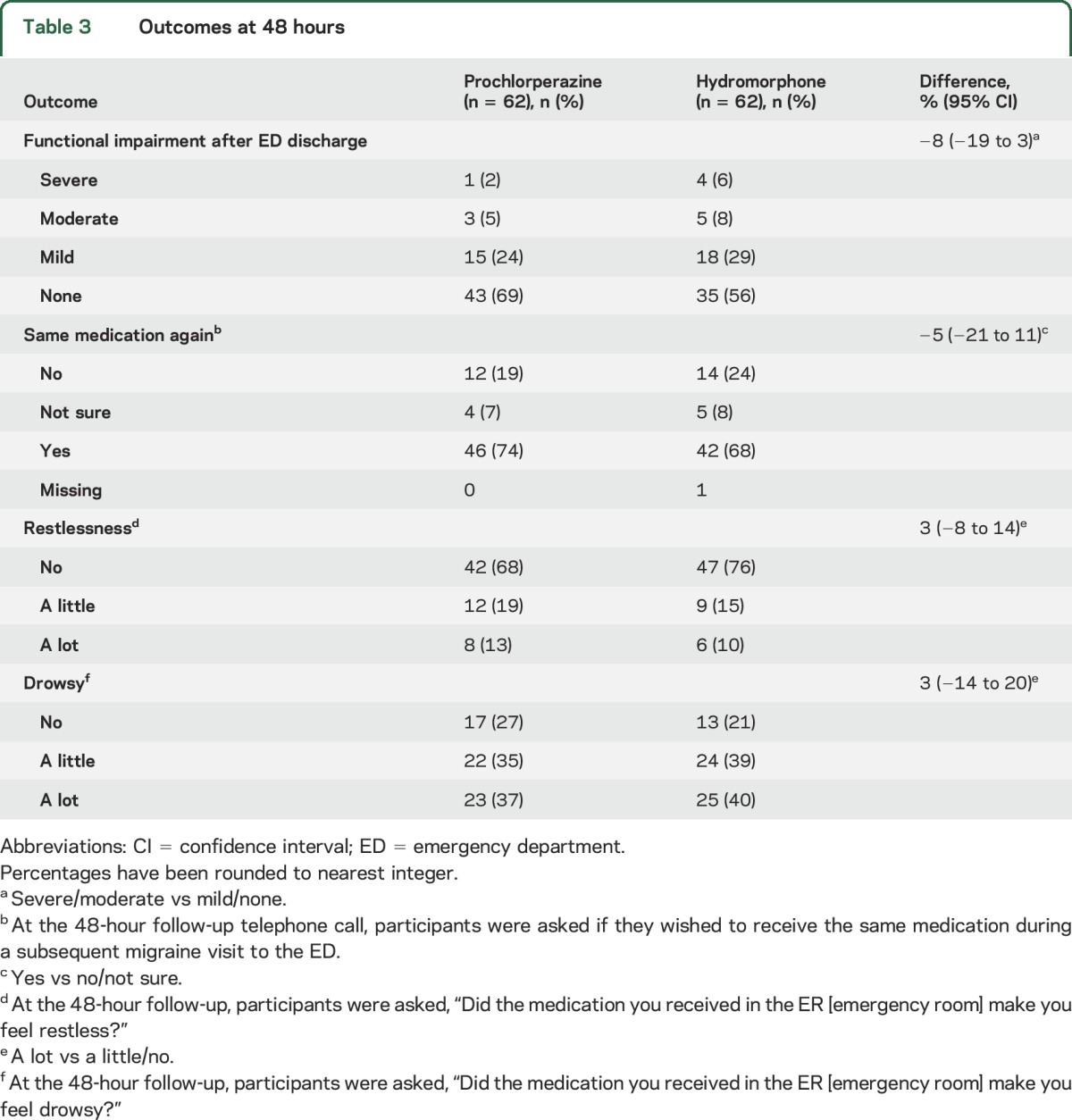
Outcomes during the 48 hours after discharge are reported in table 3. Among patients who improved after receiving the investigational medications, there was a similar frequency of headache relapse. Of the 52 patients who received only prochlorperazine + diphenhydramine in the ED and improved to pain levels of mild or none before discharge, 15 (29%) reported a moderate or severe headache within 48 hours of ED discharge vs 13 (33%) of the 39 patients who improved to mild or none after receiving only hydromorphone (95% CI for difference of 4% −15 to 23). As depicted in table 3, the frequency of functional impairment after ED discharge was similar between the groups. Restlessness was not reported more frequently in either arm. Similarly, drowsiness was equally distributed between the groups. Desire to receive the same medication again during a subsequent ED visit was reported by nearly three-fourths of the prochlorperazine group and two-thirds of the hydromorphone group.
Table 3.
Outcomes at 48 hours
One- and 3-month follow-up data are reported in the supplemental data at Neurology.org. There were no between-group differences in these longer-term outcomes, including a comparable number of headache days, return visits to EDs, and functional disability scores.
DISCUSSION
In this randomized, comparative effectiveness study, IV hydromorphone was substantially less effective than IV prochlorperazine for the treatment of acute migraine. Twice as many patients who received single-dose prochlorperazine achieved sustained headache relief compared with those who received single-dose hydromorphone. Hydromorphone was also associated with longer ED throughput times and an increased requirement of additional medication to treat headache and related symptoms.
These results are similar to those of an earlier nonexperimental study of hydromorphone vs metoclopramide in which hydromorphone was associated with less relief of headache, more frequent use of rescue medication, and longer ED throughput times.17 Meperidine, the parenteral opioid studied most commonly, affords less relief than dihydroergotamine-based protocols but is comparable in efficacy to antidopaminergics and parenteral ketorolac.18 One other ED-based study of opioids for migraine followed up patients systematically after the ED visit. That randomized study of meperidine vs dihydroergotamine demonstrated comparable rates of headache recurrence after initial successful treatment and comparable rates of functional impairment after ED discharge.19
IV prochlorperazine is a guideline-endorsed, first-line acute migraine therapeutic.4 It is more efficacious than sumatriptan and, compared to placebo, has an NNT of 3 with regard to relief of headache in the ED.20 In our study, 86% of patients reported headache levels of mild or none 1 hour after prochlorperazine administration, and 96% of patients reported mild or no functional impairment at 1 hour. These very successful results are typical of IV prochlorperazine and other IV antidopaminergics, which have emerged as a first-line treatment of acute migraine in the ED.4
Post-ED outcomes of patients in this study were typical. More than one-fourth of patients who experienced initial relief of headache reported a moderate or severe headache within 48 hours of discharge.21 Follow-up of patients 1 and 3 months later revealed persistent suffering. MIDAS scores >5, indicating persistent headache-related functional impairment, were reported by nearly 50% of the study population. MIDAS scores >20, indicating persistent severe headache related disability, were reported by 20% of the study population. Although beyond the scope of typical ED care, the high frequency of persistent functionally impairing headaches should be understood so that patients can be directed to appropriate outpatient care.
While this study demonstrates the overwhelming superiority of prochlorperazine over hydromorphone for initial management of acute migraine, these data do not provide a rationale to avoid hydromorphone for patients with a history of nonresponse to antidopaminergics, nor do they suggest that treatment with a parenteral opioid leads to long-term sequelae. It is widely believed that treatment in the ED with parenteral opioids leads to addiction in some patients by initiating a cycle of return visits and increasingly frequent treatment with parenteral opioids. In our data, we found no evidence of this cycle among patients randomized to hydromorphone.
In nonrandomized studies, use of opioids has been linked to detrimental post-ED sequelae, including increased frequency of ED headache visits5 and subsequent less responsiveness to standard migraine medication.6,22 The data from this study do not support an association between administration of opioids and 1- or 3-month outcomes. The most plausible explanation for this discrepancy is that the nonrandomized studies did not control for potential confounders such as severity of the underlying headache disorder, concomitant medication-overuse headache, and use of opioids at baseline. Mechanistically, it seems unlikely that 1 or 2 doses of opioids would lead to any sequelae beyond the 48-hour time frame, although theoretically, if the opioid caused the patient to experience euphoria, the patient may be more likely to visit subsequently. This would initiate a cycle in which the patient would present to the ED with increasing frequency. However, our data do not indicate that patients receiving opioids were more likely to revisit the ED. Entry criteria for this study required patients not to have used opioids during the previous month and to have no personal history of addiction to prescription or illicit opioids. Therefore, patients included in this study consisted of a population of migraineurs at lower risk for subsequent problems with opioid use. These data cannot be generalized to an unselected ED population. Similarly, we enrolled patients from 2 distinct neighborhoods in the Bronx, NY. These data are therefore most appropriately generalizable to urban US EDs.
Other limitations of this work are that we did not ascertain whether study participants had previously been exposed to hydromorphone or prochlorperazine. Prior exposure to one of these medications may have unblinded the study participant. We did not exclude patients who also met analgesic overuse criteria. In addition, we did not account for the use of preventives or migraine medication in the time period after ED discharge. Our failure to account for this may have biased the 1- and 3-month outcomes toward the null hypothesis.
In this randomized, double-blind study, we found that IV hydromorphone is substantially less effective than IV prochlorperazine + diphenhydramine for the treatment of acute migraine.
GLOSSARY
- CI
confidence interval
- ED
emergency department
- HIT6
Headache Impact Test
- MIDAS
Migraine Disability Assessment Scale
- NNT
number needed to treat
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
B.W.F. conceived and designed the trial, managed the data, including quality control, analyzed the data, and drafted the manuscript. E.I. supervised the conduct of the trial and data collection, managed the data, including quality control, and drafted the manuscript. C.S. designed the trial, supervised the conduct of the trial and data collection, and contributed substantially to revisions of the manuscript. A.L. supervised the conduct of the trial and data collection, analyzed the data, and contributed substantially to revisions of the manuscript. K.R. and E.Z. supervised the conduct of the trial and data collection and contributed substantially to revisions of the manuscript. D.R.V. analyzed the data and contributed substantially to revisions of the manuscript. P.E.B. and E.J.G. conceived and designed the trial and contributed substantially to revisions of the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Friedman BW, West J, Vinson DR, Minen MT, Restivo A, Gallagher EJ. Current management of migraine in US emergency departments: an analysis of the National Hospital Ambulatory Medical Care Survey. Cephalalgia 2015;35:301–309. [DOI] [PubMed] [Google Scholar]
- 2.Langer-Gould AM, Anderson WE, Armstrong MJ, et al. The American Academy of Neurology's top five choosing wisely recommendations. Neurology 2013;81:1004–1011. [DOI] [PubMed] [Google Scholar]
- 3.Loder E, Weizenbaum E, Frishberg B, Silberstein S; American Headache Society Choosing Wisely Task Force. Choosing wisely in headache medicine: the American Headache Society's list of five things physicians and patients should question. Headache 2013;53:1651–1659. [DOI] [PubMed] [Google Scholar]
- 4.Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016;56:911–940. [DOI] [PubMed] [Google Scholar]
- 5.Colman I, Rothney A, Wright SC, Zilkalns B, Rowe BH. Use of narcotic analgesics in the emergency department treatment of migraine headache. Neurology 2004;62:1695–1700. [DOI] [PubMed] [Google Scholar]
- 6.Jakubowski M, Levy D, Goor-Aryeh I, Collins B, Bajwa Z, Burstein R. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache 2005;45:850–861. [DOI] [PubMed] [Google Scholar]
- 7.Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache 2008;48:1157–1168. [DOI] [PubMed] [Google Scholar]
- 8.Olesen J, Bendtsen L, Dodick D, et al. ; Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 9.Vinson DR, Drotts DL. Diphenhydramine for the prevention of akathisia induced by prochlorperazine: a randomized, controlled trial. Ann Emerg Med 2001;37:125–131. [DOI] [PubMed] [Google Scholar]
- 10.Chang AK, Bijur PE, Campbell CM, Murphy MK, Gallagher EJ. Safety and efficacy of rapid titration using 1 mg doses of intravenous hydromorphone in emergency department patients with acute severe pain: the “1+1” protocol. Ann Emerg Med 2009;54:221–225. [DOI] [PubMed] [Google Scholar]
- 11.Tfelt-Hansen P, Pascual J, Ramadan N, et al. ; International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: third edition: a guide for investigators. Cephalalgia 2012;32:6–38. [DOI] [PubMed] [Google Scholar]
- 12.Friedman BW, Bijur PE, Lipton RB. Standardizing emergency department-based migraine research: an analysis of commonly used clinical trial outcome measures. Acad Emerg Med 2010;17:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res 2003;12:963–974. [DOI] [PubMed] [Google Scholar]
- 14.Stewart WF, Lipton RB, Whyte J, et al. An international study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology 1999;53:988–994. [DOI] [PubMed] [Google Scholar]
- 15.Friedman BW, Esses D, Solorzano C, et al. A randomized controlled trial of prochlorperazine versus metoclopramide for treatment of acute migraine. Ann Emerg Med 2008;52:399–406. [DOI] [PubMed] [Google Scholar]
- 16.Friedman BW. The efficacy of intravenous morphine for acute migraine. Acad Emerg Med 2013;20(suppl 1):S95. [Google Scholar]
- 17.Griffith JD, Mycyk MB, Kyriacou DN. Metoclopramide versus hydromorphone for the emergency department treatment of migraine headache. J Pain 2008;9:88–94. [DOI] [PubMed] [Google Scholar]
- 18.Friedman BW, Kapoor A, Friedman MS, Hochberg ML, Rowe BH. The relative efficacy of meperidine for the treatment of acute migraine: a meta-analysis of randomized controlled trials. Ann Emerg Med 2008;52:705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carleton SC, Shesser RF, Pietrzak MP, et al. Double-blind, multicenter trial to compare the efficacy of intramuscular dihydroergotamine plus hydroxyzine versus intramuscular meperidine plus hydroxyzine for the emergency department treatment of acute migraine headache. Ann Emerg Med 1998;32:129–138. [DOI] [PubMed] [Google Scholar]
- 20.Jones J, Sklar D, Dougherty J, White W. Randomized double-blind trial of intravenous prochlorperazine for the treatment of acute headache. JAMA 1989;261:1174–1176. [PubMed] [Google Scholar]
- 21.Friedman BW, Hochberg ML, Esses D, et al. Recurrence of primary headache disorders after emergency department discharge: frequency and predictors of poor pain and functional outcomes. Ann Emerg Med 2008;52:696–704. [DOI] [PubMed] [Google Scholar]
- 22.Ho TW, Rodgers A, Bigal ME. Impact of recent prior opioid use on rizatriptan efficacy: a post hoc pooled analysis. Headache 2009;49:395–403. [DOI] [PubMed] [Google Scholar]