Abstract
Although there is long association of medical hyperthermia and immune stimulation, the relative lack of a quantifiable and reproducible effect has limited the utility and advancement of this relationship in preclinical/clinical cancer and non-cancer settings. Recent cancer-based immune findings (immune checkpoint modulators etc.) including improved mechanistic understanding and biological tools now make it possible to modify and exploit the immune system to benefit conventional cancer treatments such as radiation and hyperthermia. Based on the prior experience of our research group including; cancer-based heat therapy, magnetic nanoparticle (mNP) hyperthermia, radiation biology, cancer immunology and Cowpea Mosaic Virus that has been engineered to over express antigenic proteins without RNA or DNA (eCPMV/VLP). This research was designed to determine if and how the intra-tumoral delivery of mNP hyperthermia and VLP can work together to improve local and systemic tumor treatment efficacy. Using the C3H mouse/MTG-B mammary adenocarcinoma cell model and the C57-B6 mouse/B-16-F10 melanoma cancer cell model, our data suggests the appropriate combination of intra-tumoral mNP heat (e.g. 43°C/30–60 minutes) and VLP (100 μg/200 mm3 tumor) not only result in significant primary tumor regression but the creation a systemic immune reaction that has the potential to retard secondary tumor growth (abscopal effect) and resist tumor rechallenge. Molecular data from these experiments suggest treatment based cell damage and immune signals such as Heat Shock Protein (HSP) 70/90, calreticulin, MTA1 and CD47 are potential targets that can be exploited to enhance the local and systemic (abscopal effect) immune potential of hyperthermia cancer treatment.
Keywords: magnetic nanoparticle, hyperthermia, immunotherapy, viral-like nanoparticle (VLP), cancer therapy, abscopal effect
INTRODUCTION
Hyperthermia cancer treatment
Most cancer patients are currently treated with multimodality therapy including various combinations of the following agents: chemotherapy, surgery, hyperthermia, radiation, molecular targeting, and immunotherapy. How these modalities are optimally combined to achieve the best outcome is a central question in translational cancer research. Hyperthermia therapy has historically been used to treat various types of cancer and has used many types of applications/applicators. At many levels, hyperthermia has been effective, however its lack of an inherent positive therapeutic ratio (tumor-normal tissue toxicity), especially at cytolytic doses, has limited utility and efficacy in many settings. Therefore, hyperthermia is often used in combination to enhance the efficacy of more established cancer treatment modalities, such as chemotherapy and radiation therapy. In the studies demonstrated here we have used an alternating magnetic field and a nontoxic iron oxide nanoparticle (mNP) to produce therapeutic heat (described below). Although this technique is capable of generating tissue ablative temperatures and thermal doses, we have explored lower, non-ablative doses, in order to determine if such treatment can be used effectively with an immunostimulatory agent (VLP) to create an immune based local and/or systemic anti-cancer effect (abscopal or anti-metastatic effect).
The ability of local cancer treatments to induce a reproducible and effective systemic/metastatic cancer response has been extremely elusive. Such a situation is known as the “abscopal effect”. The abscopal effect of radiation therapy is a rare but well documented event. Although the mechanism is unclear most believe the abscopal effect to be immunologically based. A hyperthermia based abscopal effect is even less well documented. Maybe this is because so few patients have been treated with hyperthermia and/or hyperthermia treatment techniques are so variable, as compared to radiation. It is however known that hyperthermia cancer treatment, and maybe especially mNP hyperthermia cancer treatment, stimulates a cell damage response that includes immune-related stimulatory molecules. This situation provides the potential for effectively using mNP hyperthermia, or other types of hyperthermia, with radiation and/or other types of immunomodulatory agent, such as VLP, to stimulate a systemic anti-tumor immune effect and an overall improved prognosis. This approach is attractive because the cell stress and cell death caused by the heat and/or radiation is likely to support stronger antitumor immune response at the tumor microenvironment level. [1–9]
The abscopal effect has recently been well reviewed in multiple places (Grass, 2016). The main concepts are: that the effect is immune based and is due to the tendency of radiation therapy (RT) damage of tumors to create a more immunogenic tumor microenvironment than the immune suppressive environment that characterizes untreated tumors. Clearly, RT by itself is generally not sufficient to create an effective and reliable antitumor immunity (Siva, 2015). Studies report that the damage of RT alone recruits M2 type tissue repair macrophages that suppress adaptive immunity (Crittenden, 2014). The crucial aspect appears to be “immunogenic cell death” (ICD) that occurs when cells die in a manner that stimulates the immune system. ICD is characterized by a grouping of the following danger associated molecular signals (DAMPs): calreticulin expression on the cell surface, release of ATP, release of HMGB1 protein, and expression of type one interferons (IFN alpha and beta) (Grass, 2016). When the tumor environment is sufficiently immunogenic, tumor associated antigens (normal proteins expressed at the wrong level, wrong tissue or wrong time during development) and neoantigens (mutant proteins expressed by tumors due to accumulated mutations) are taken up by antigen presenting cells that go to the lymph nodes, present them to T cells and stimulate an adaptive immune response against tumor cells. That adaptive immune response not only impacts tumors near that lymph node but also can become a systemic response against the same tumor wherever it may occur. [10–27]
In one recent paper (Wang et al, 2014) the abscopal effect it was demonstrated in a Walker-256 carcinoma using magnetic seed-mediated hyperthermia at a higher temperatures (42°C – 46°C for 30 minutes and 50°C – 55°C for 10 minutes). The abscopal endpoint effect was regression of a non-heated tumor (contralateral) following heat treatment of one tumor in rat with bilateral tumor growth. It was also noted that the levels of CD4+ and CD8+ lymphocytes and IFN-γ and IL-2 cytokines were significantly increased in the blood of hyperthermia groups compared with those of the three control groups; the increase in CD8+ cells was higher than that of the CD4+ cells. Our group also demonstrated this situation, showing that a mild hyperthermia treatment, of an established tumor, can generate systemic resistance to rechallenge (Toraya-Brown, 2014).
MATERIALS and METHODS
MTG-B/C3H and B6/B16 Mouse Model
This research utilized two mouse models: MTG-B mammary adenocarcinoma in a sygeneic C3H mouse and a B16-F10 melanoma studied in syngeneic C57-BL6 mice. We grow the tumors intradermally because it is an orthotopic site and because we can then access the tumor readily to inject reagents for immune studies and because it provides ease of measurement these studies typically utilized tumor regrowth, tumor rechallenge and metastatic endpoints.
Iron Oxide Nanoparticle (IONP)/Alternating Magnetic Field (AMF) Technology and Treatment
In these studies, we used BNF iron oxide nanoparticles (product number 10-00-801 from Micromod Partikeltechnologie GmBH, Rostock, Germany). These iron oxide nanoparticles (mNP) are composed of a hematite core surrounded with crystals of average diameter of approximately 20 nm (total core diameter approximately 40 nm) and a hydroxyethyl starch or dextran shell to a final average hydrodynamic diameter of 117 nm. The mNP were concentrated to a final particle concentration of 44 mg/ml and iron concentration of 28 mg/ml. A cooled helical coil with an inner diameter of 4 cm and is composed of hollow copper tubing was used to generate AMF. The AMF coil (Fluxtrol Inc) was powered at variable 10 KW generator (Huttinger Elektronik GmbH, Freiburg, Germany) at a field of 150 kHz and 400 Oe. The AMF coil and generator were cooled by a chiller (Tek-Temp Instruments, Croydon PA.) operating at 20°C and four gallons per minute.
Figure 1 demonstrates the AMF treatment facility. Figure 2 demonstrates intratumoral injection of mNP into a flank MTG-B mammary tumor. Figure 3 demonstrates mNP biodistribution in an injected (tumor cut section) 10 minutes following injection. Figure 4 demonstrates a mouse positioned in the AMF coil.
Figure 1.
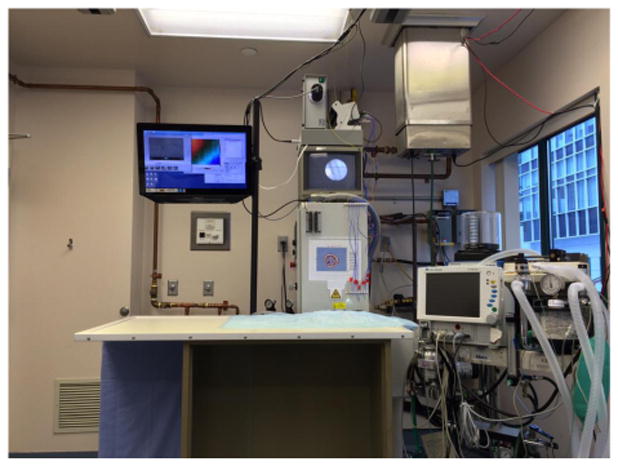
Figure 2.
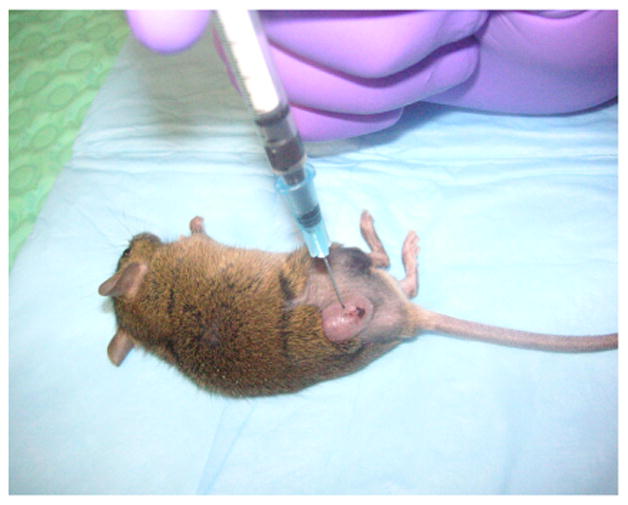
Figure 3.
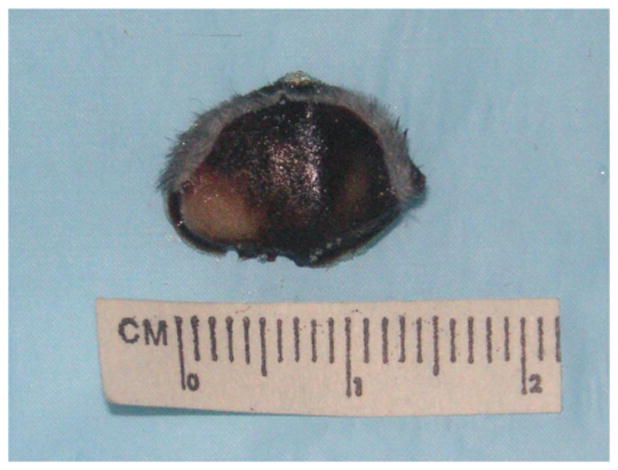
Figure 4.
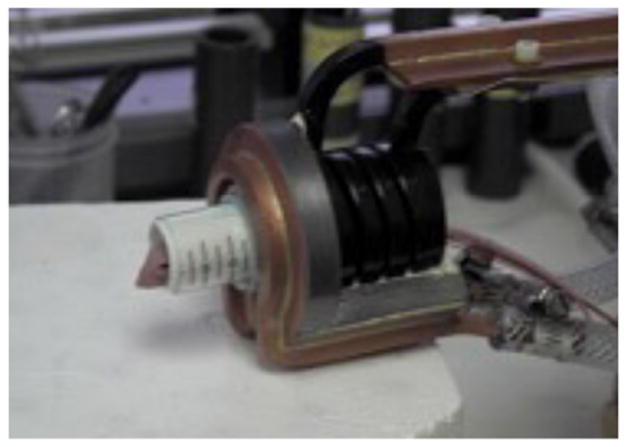
IONP Tumor cell uptake and migration
In an example below (Giustini et al., figure 5) most cancer cells are programmed to endocytose specific sized material, such as mNPs, at a relatively high and rapid level. The results show dextran-coated mNP are 90% intracellular at four (4) hours following direct mNP injection into a mouse mammary adenocarcinoma (MTGB). In spite of this situation, at least two significant mNP cancer therapy challenges remain: 1) ensuring that the mNP distribute uniformly throughout the tumor (local and systemic delivery) and 2) improving the percentage of mNP that reach the tumor following systemic injection. However the fluidity and migratory capability of mNP-induced hyperthermia have the potential to create a pseudo-systemic/regional therapy that is not possible with most other therapeutic modalities. In Figure 2 when demonstrate a lymph node with a very significant level of iron oxide nanoparticles. This situation occurred 90 minutes following the direct injection of mNPs into a mouse flank tumor (MTG-B mammary adenocarcinoma). Figure 7 is a higher magnification photomicrograph showing the majority of the mNPs located in lymph node sinus region. This is to be expected since fluid or material draining from the tumor to this regional node would enter the sinus before filtering to the lymph node parenchyma. The phenomena is, however, most significant because this is also the route cancer cells take when migrating tumor to the lymph node. Because most cancer cells readily take up mNP, the simultaneous presence of migrating cancer cells and mNP affords a unique, highly specific opportunity.
Figure 5.
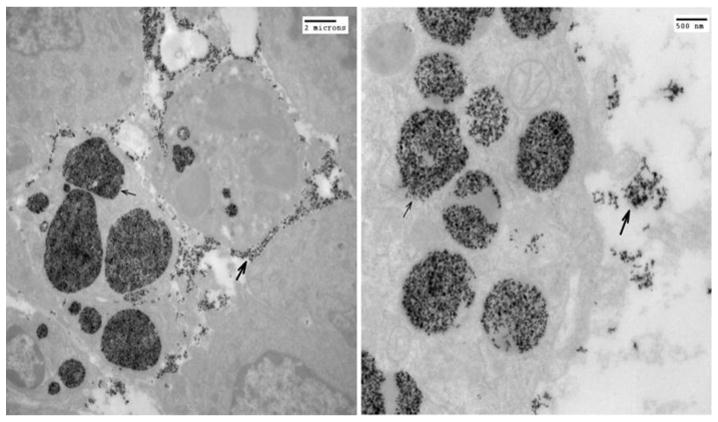
Figure 7.
In vivo mNP hyperthermia treatment
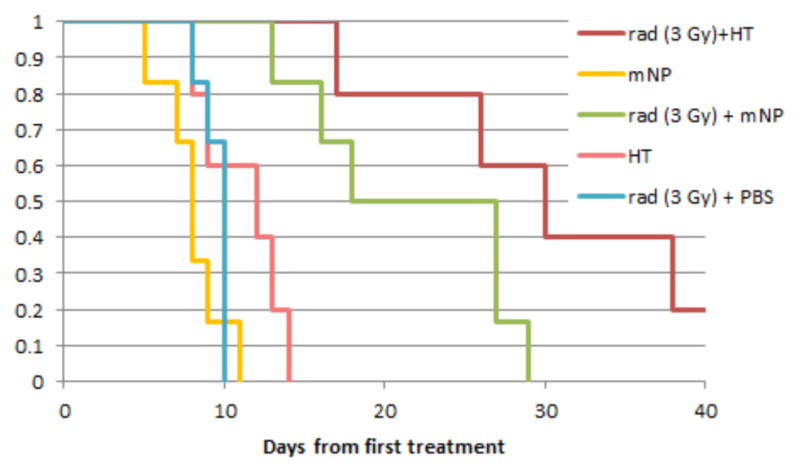
Mice in the study demonstrated in figure 8 received radiation alone (3 x 5 Gy), mNPs alone, mNP + AMF (thermal doses of 43°C/30 min x 2 x CEM 30) or a combination. This study shows radiation and hyperthermia combined have the greatest benefit. While two small thermal doses demonstrate little tumor cytotoxicity, it is clear that when combined with radiation its effect is notable. It is this type of sub-lethal damage/effect that makes low dose hyperthermia attractive for combination with immunotherapy. Ongoing studies are examining the relationship and effect of hyperthermia – immunotherapy combination.
Figure 8.
Immunogenic Viral – Like Nanoparticles
The viral-like nanoparticle platform we have used in these treatments is a modified version of the Cowpea Mosaic Virus (CPMV). The RNA has been removed from the VLP rendering it non-infectious and the protein coat engineered to stimulate a greater immune response. This is done through parallel expression of a viral protease (Pro) and the VP60 precursor protein encoding large and small coat proteins. While similar in protein organization, the eCPMV (empty) platform nanotechnology offers key advantages over the naturally occurring Cowpea mosaic virus such as it is not infectious and the empty interior cavity can be engineered to carry medical payload.
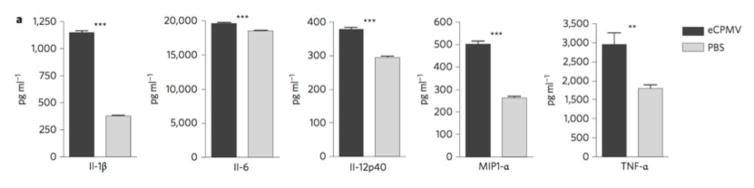
Figure 9 is a computer rendition of a CPMV/VLP (approximate 30nm diameter). Transmission electron micrographs (taken by the Hoopes laboratory group) in figures 10 and 11 demonstrate the association of 30 nm viral-like nanoparticles VLP and B16 F10 murine melanoma cells. The cells were harvested and processed for TEM six hours following the addition of the VLP to cells/cell culture media. Figure 10 demonstrates VLP aggregate adjacent (possibly attached) to the outer plasma membrane of a B16 melanoma cell. Figure 11 demonstrates an intracellular vesicle containing VLP. Figure 12 demonstrates the inherent immunogenicity of eCPMV/VLP: a) bone marrow derived dendritic cells (BMDC) exposed to eCPMV produce elevated levels of pro-inflammatory cytokines in vitro. Cells (n=6/group) were cultured for 24 hours with 20 μg eCPMV (dark grey bars) and cytokine levels were analyzed using multiplexed luminex array. Data for the bar graph using an unpaired student T-test: **p<0.05, **p<0.01, ***p<0.001 (Lizotte PH, Steinmetz NF, Fiering SN et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol. 2016; 11(3): 295–303.
Figure 9.
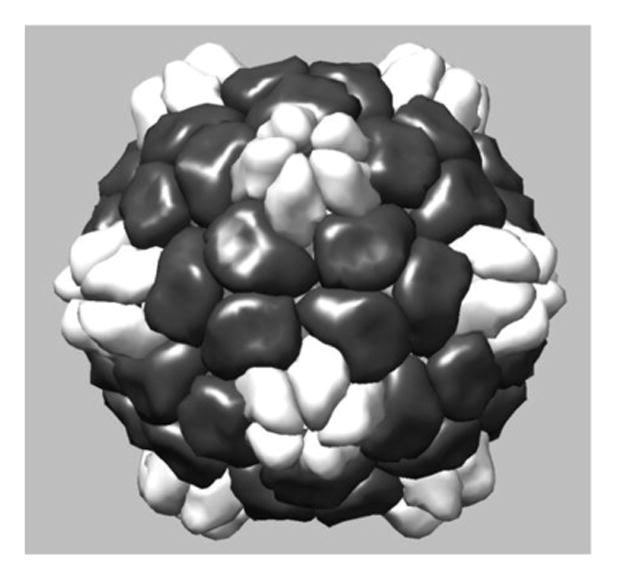
Figure 10.
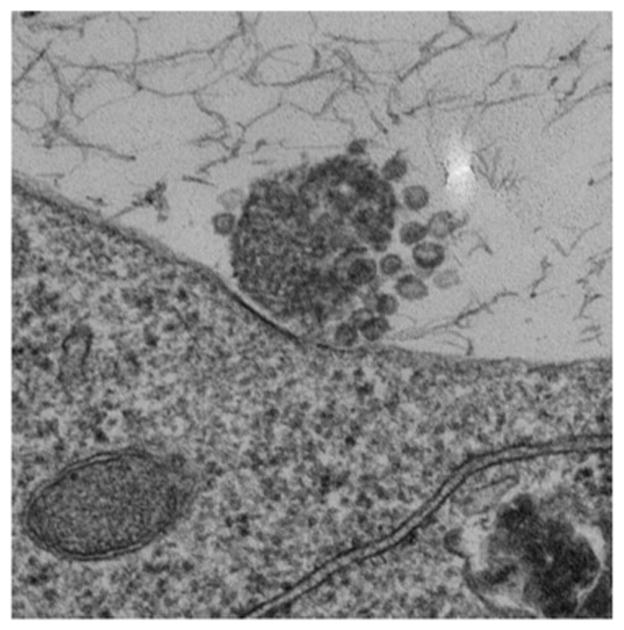
Figure 11.
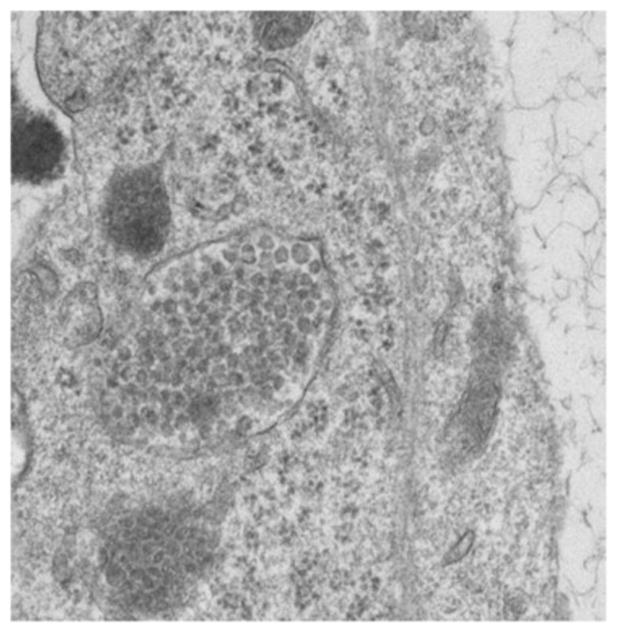
Figure 12.
Hyperthermia and VLP Effects
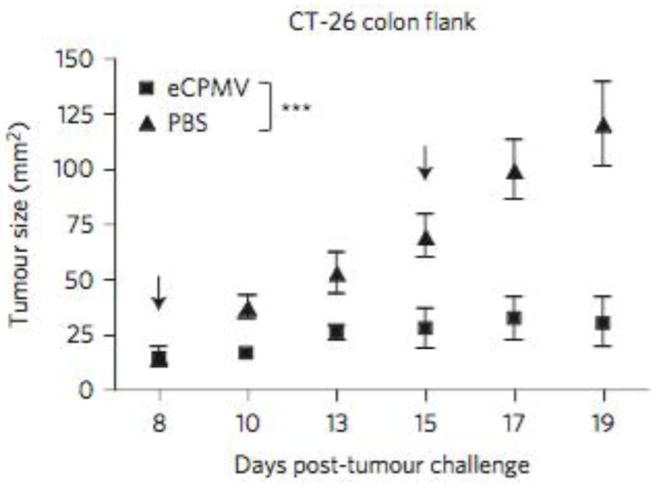
In study demonstrated in figure 13 (below) C57BL/6 mice (6–8 weeks old) were injected intradermally (ID) on 1.25 × 105 B16F10 cells, to establish dermal tumors of about 5 mm × 6 mm on both flanks of the mice. The left side tumor was injected with approximately 300 μg mNP (140 ug Fe). The right side tumor received no mNP (no heat). Using the previously described AMF exposure, tumor were heated to 43°C for 30 min. In support of the abscopal effect the heated tumors on the left flank disappeared completely in 5 days while the non-heated right-flank tumors grew significantly slower than in the unheated group (Toraya-Brown S, Hoopes P.J., Fiering S.N. et al. “Local hyperthermia treatment of tumors induces CD8 (+) T cell-mediated resistance against distal and secondary tumors” Nanomedicine: nanotechnology, biology, and medicine. 2014; 10 (6): 1273–85). In figure 13 Lizotte, Fiering, Steinmetz et al demonstrated the very significant treatment efficacy of eCPMV when used in the CT-26 colon cancer mouse flank model. The flank tumors were injected once with eCPMV on day eight as shown in figure 14. Lizotte PH, Steinmetz NF, Fiering SN et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016; 11 (3): 295–303.
Figure 13.
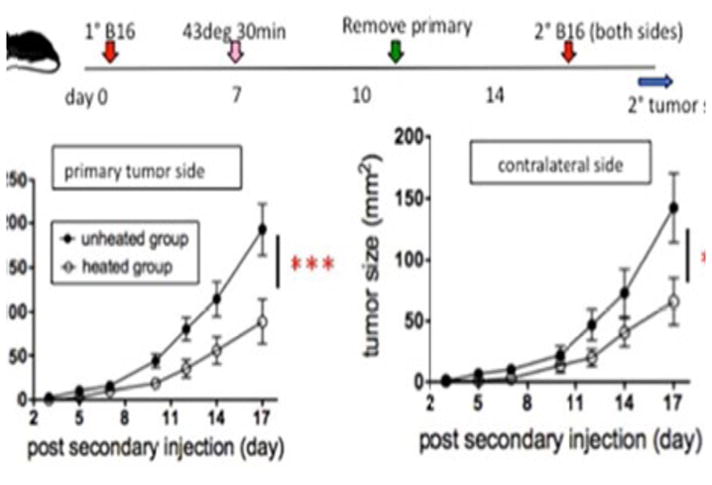
Figure 14.
Pathology and Immunopathology
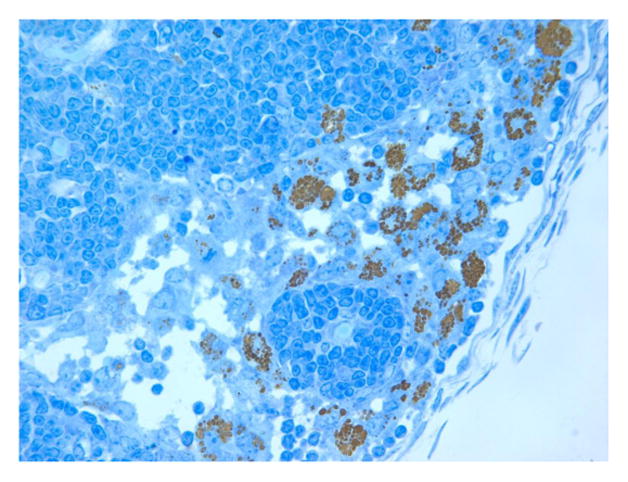
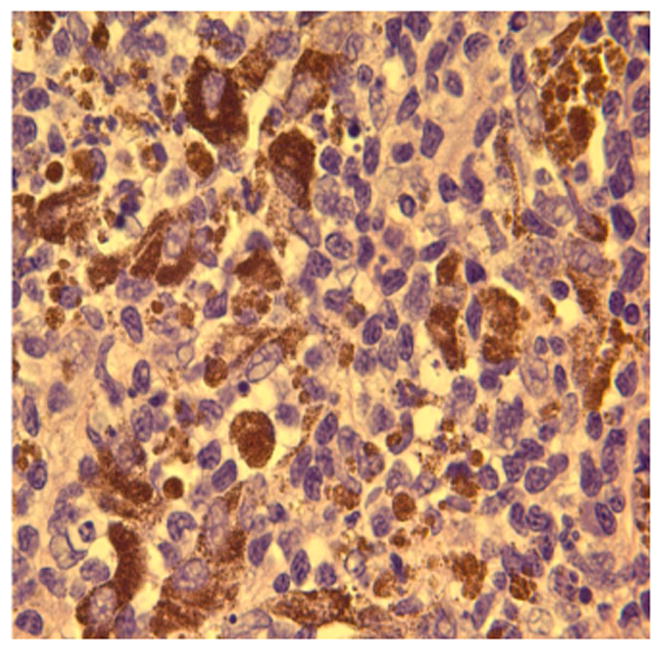
We have used a variety of anatomic (H&E) and immunohistochemical pathology stains and markers to identify the inflammatory and immune cells that infiltrate the tumor and prei-tumor region post treatment. Below are examples such tissues following treatment. Figures 10 represents a highly inflamed/immune cell infiltrated melanoma 21 days post treatment. The tissue is primarily populated by dying melanoma cells, macrophages and lymphocytes, which according to CD11b and CD11c are probably B cells. Figure 16 represents pro-apoptotic cleaved caspase marker in a murine tumor post mNP hyperthermia and VLP. Figures 17 and 18 are high magnification H&E and IHC (CD11c) photomicrographs, from tissue post VLP treatment.
Figure 16.
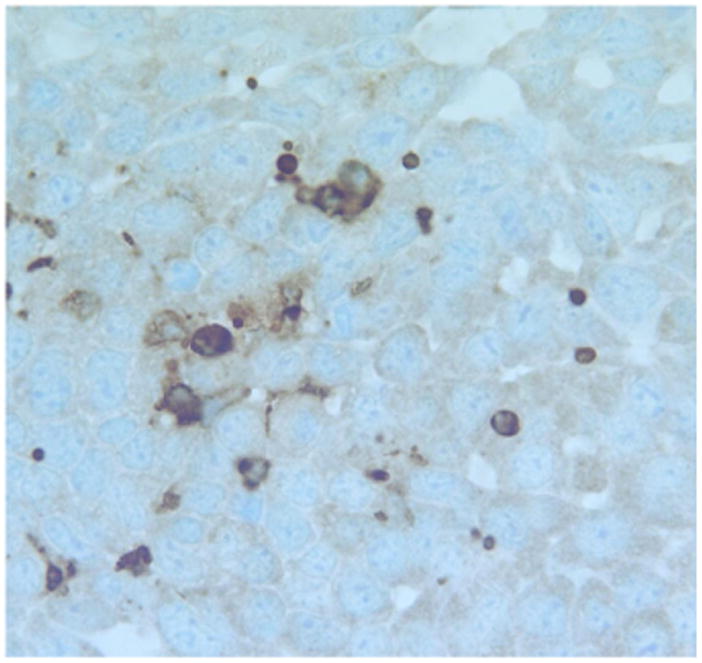
Figure 17.
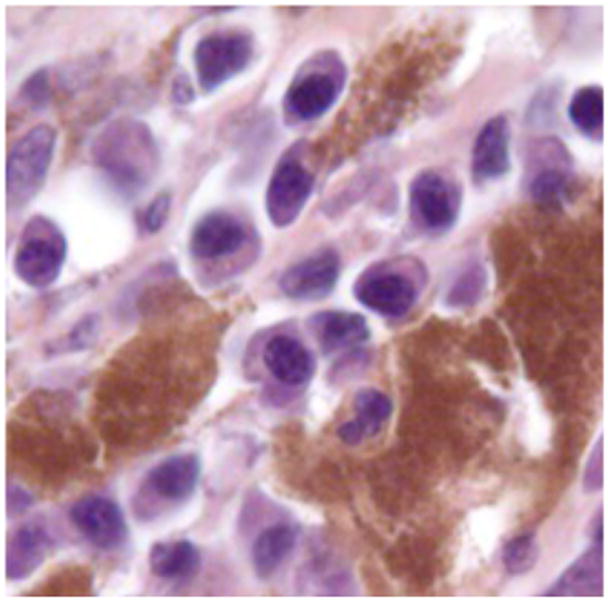
Figure 18.

Assessment of Pathobiological and Therapeutic Markers
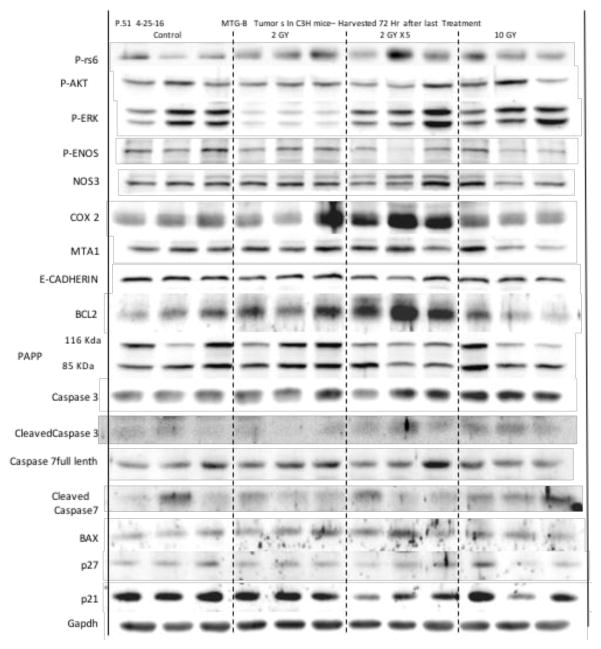
In addition to the qualitative and quantitative histopathological and immunohistochemistry assessment described above, western blot assessments of tumor and peri-tumor tissue, following VLP or mNP hyperthermia are used to identify and semi-quantify potentially important therapuetic and immune proteins. Preliminary results, in figures 19 and 20 below, suggests apoptosis markers (BAX, Caspase-3), heat shock proteins, calreticulin, CD47 and MTA1 are potentially important proteins in the radiation- hyperthermia- abscopal/immune-pathway.
Figure 19.
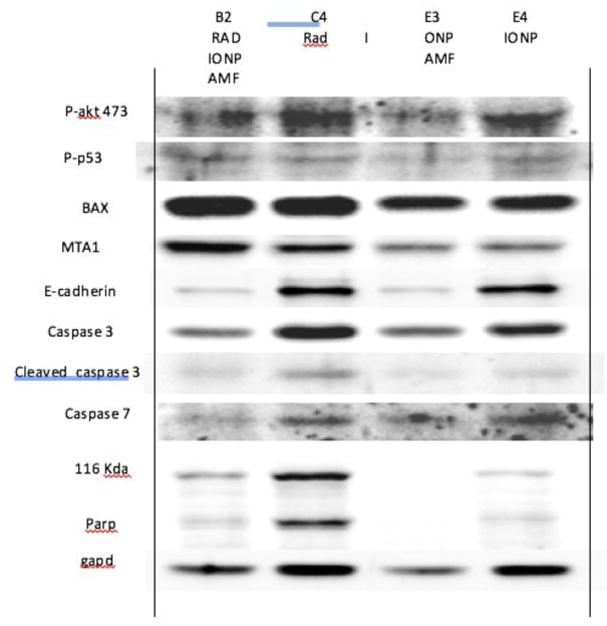
Figure 20.
RESULTS
These studies demonstrate that magnetic hyperthermia and VLP not only provide basic treatment benefit alone but also create anti-tumor immune based activity that enhances the overall therapeutic effect.
Modest local hyperthermia activates the anti-cancer immune system and has the potential create or assist the abscopal effect. mNP heating may have give an advantage over other types of heating (mechanism unknown).
Combining heat with an immunostimulatory agent such as eCPMV or other immune therapeutics (such as anti-PD1 or STING agonist/stimulator of interferon genes) has promise.
CONCLUSIONS
In these preliminary studies we have shown that magnetic nanoparticle hyperthermia and VLP treated murine tumors have the ability to not only be clinically effective but to stimulate immunological activity. This study is too limited in size, scope, and statistics to make conclusive determinations regarding the treatment efficacy and abscopal effect contributions of each modality. However, it can be clearly stated that these cancer treatment modalities all have significant independent therapeutic responses and include immune components.
Figure 6.
Figure 15.
Acknowledgments
The authors would like to acknowledge James D. Petryk, Hugo, MN. and Alicia A. Petryk, PhD, Univesity of Bridgeport, Bridgeport, CT for their assistance with mNP heating activity.
References
- 1.Gilchrist RK, et al. Selective inductive heating of lymph nodes. Annals of surgery. 1957;146(4):596–606. doi: 10.1097/00000658-195710000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan A, et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. Journal of Neuro-Oncology. 2006;78(1):7–14. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- 3.Cassim SM, Hoopes PJ, et al. Iron oxide nanoparticle hyperthermia and radiation cancer treatment. Proc SPIE. 2009;7181:71810O. doi: 10.1117/12.810035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maier-Hauff K, et al. Intracranial Thermotherapy using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. Journal of Neuro-Oncology. 2006;81(1):53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 5.Johannsen M, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. International Journal of Hyperthermia. 2007;23(3):315–323. doi: 10.1080/02656730601175479. [DOI] [PubMed] [Google Scholar]
- 6.Giustini AJ, Hoopes PJ, et al. Magnetic Nanoparticle Hyperthermia in Cancer Treatment. Nano LIFE. 2010;1(1–2):17–32. doi: 10.1142/S1793984410000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang G, Liao Y, Baker I. Surface engineering of core/shell iron/iron oxide nanoparticles from microemulsions for hyperthermia. Materials Science and Engineering: C. 2010;30(1):92–97. doi: 10.1016/j.msec.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennis CL, et al. Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia. Nanotechnology. 2009;20:395103. doi: 10.1088/0957-4484/20/39/395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. International journal of radiation oncology, biology, physics. 1984;10(6):787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 10.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5(6):404–7. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. Int J Radiat Oncol Biol Phys. 2014;88(5):986–975. doi: 10.1016/j.ijrobp.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varn FS, Andrews EH, Mullins DW, Cheng C. Integrative analysis of breast cancer reveals prognostic haematopoietic activity and patient-specific immune response profiles. Nat Commun. 2016;7:10248. doi: 10.1038/ncomms10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer. 2016;40(1):10–24. doi: 10.1016/j.currproblcancer.2015.10.003. [DOI] [PubMed] [Google Scholar]; Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356(1):82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Crittenden MR, Savage T, Cottam B, Baird J, Rodriguez PC, Newell P, et al. Expression of arginase I in myeloid cells limits control of residual disease after radiation therapy of tumors in mice. Radiat Res. 2014;182(2):182–90. doi: 10.1667/RR13493.1. [DOI] [PubMed] [Google Scholar]
- 15.Toraya-Brown S, Sheen MR, Baird JR, Demidenko E, Hoopes PJ, Fiering SN, et al. Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors. Nanomedicine: nanotechnology, biology, and medicine. 2014;10(6):1273–85. doi: 10.1016/j.nano.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–10. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-Dependent Cytosolic DNA SensingPromotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41(5):843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baird JR, Friedman D, Cottam B, Dubensky TW, Jr, Kanne DB, Bambina S, et al. Radiotherapy Combined with Novel STING-Targeting Oligonucleotides Results in Regression of Established Tumors. Cancer Res. 2016;76(1):50–61. doi: 10.1158/0008-5472.CAN-14-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2014;30(8):531–9. doi: 10.3109/02656736.2014.968640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toraya-Brown S, Sheen MR, Baird JR, Barry S, Demidenko E, Turk MJ, et al. Phagocytes mediate targeting of iron oxide nanoparticles to tumors for cancer therapy. Integrative biology: quantitative biosciences from nano to macro. 2013;5(1):159–71. doi: 10.1039/c2ib20180a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, et al. Abscopal antitumor immune effects of magnet-mediated hyperthermia at a high therapeutic temperature on Walker-256 carcinosarcomas in rats. Oncol Lett. 2014 Mar;7(3):764–770. doi: 10.3892/ol.2014.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol. 2016;11(3):295–303. doi: 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varn FS, Andrews EH, Mullins DW, Cheng C. Integrative analysis of breast cancer reveals prognostic haematopoietic activity and patient-specific immune response profiles. Nat Commun. 2016;7:10248. doi: 10.1038/ncomms10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiteside TL. Disarming suppressor cells to improve immunotherapy. Cancer Immunother. 2012;61(2):283–8. doi: 10.1007/s00262-011-1171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammerich L, Binder A, Brody JD. In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Mol Oncol. 2015;9(10):1966–81. doi: 10.1016/j.molonc.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KL, Twyman RM, Fiering S, Steinmetz NF. Virus-based nanoparticles as platform technologies for modern vaccines. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8(4):554–78. doi: 10.1002/wnan.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebel ME, Chartrand K, Tarrab E, Savard P, Leclerc D, Lamarre A. Potentiating Cancer Immunotherapy Using Papaya Mosaic Virus-Derived Nanoparticles. Nano Lett. 2016;16(3):1826–32. doi: 10.1021/acs.nanolett.5b04877. [DOI] [PubMed] [Google Scholar]