Abstract
Background
Systemic lupus erythematosus (SLE) is frequently characterized by type I interferon (IFN) pathway activation. We previously found a missense SNP in the purine nucleoside phosphorylase (PNP) gene was associated with high IFN in SLE (rs1049564, P=1.24 ×10−7). PNP is a key enzyme in purine metabolism. In this study, we perform functional follow-up of this polymorphism in human cells.
Methods
Type I IFN was measured in patient sera using a reporter cell assay. Structural modeling of the PNP variant was performed using Pymol software. PNP mRNA, protein levels and type I IFN-induced gene expression were measured in lymphoblastoid cell lines with known PNP rs1049564 genotypes. Cell cycle was assayed using flow cytometry.
Results
Structural modeling indicated no major disruption in folding related to rs1049564. We found that homozygous rs1049564 TT lymphoblastoid cells had decreased PNP mRNA and protein levels, and TT cells had reduced PNP enzymatic activity even when the amount of PNP was controlled. TT cells had a 2-fold increase in S-phase block as compared to homozygous CC cells. The S-phase block could be pharmacologically reversed with hypoxanthine and adenosine, supporting relative PNP deficiency as the cause of the S-phase block. Type I IFN-induced transcripts were increased in a dose-response fashion related to the rs1049564 T allele both at baseline and after type I IFN stimulation.
Conclusions
The rs1049564 PNP T allele is a loss-of-function variant, inducing S-phase block and IFN pathway activation in lymphocytes. The S-phase block can be rescued in our in vitro experiments, suggesting a potential for personalized therapeutics.
INTRODUCTION
SLE is a severe multi-system autoimmune disorder, caused by a combination of genetic predisposition and environmental factors that result in an irreversible break in immunologic self-tolerance.(1) Interferon alpha (IFN-α) is a type I interferon which plays an important role in setting thresholds for self-reactivity and autoimmunity. Circulating serum IFN-α levels are high in SLE patients,(2) and this IFN pathway activation is heritable within SLE families,(3) supporting a role for IFN-α as a primary causal factor in human SLE. Case-control genetic studies in SLE have demonstrated a remarkable over-representation of genes involved in type I IFN signaling, production and response.(4) Previous work from our group demonstrated that many of these SLE-risk genetic polymorphisms contribute to high IFN levels in SLE patients.(5, 6) These data support the idea that gain-of-function polymorphisms in the IFN pathway are a common pathogenic mechanism in SLE, and high IFN-α represents a stable, heritable, molecular subphenotype important to SLE etiology.
We have subsequently shown that some of the established SLE-risk loci are characterized by strong sub-phenotype effects, which are much greater than the overall case-control effect size.(7) For example, >70% of the risk of SLE related to the IRF5 gene is found in a specific subset of patients defined by an autoantibody profile.(7) IRF5 is one of the strongest overall risk factors for SLE, suggesting that heterogeneity in genetic association is a common phenomenon in SLE. To directly address biological heterogeneity in SLE and identify additional novel genetic factors associated with IFN pathway dysregulation, we performed a genome-wide association study (GWAS) comparing high IFN SLE patients to low IFN SLE patients.(8) This case-case sub-phenotype mapping strategy allowed us to detect genetic influences on serum IFN-α in SLE, thus identifying important pathogenic SLE-associated genes which may not be detectable with standard case-control analyses. Using this approach we were able to identify a novel association between a missense SNP in the PNP gene with high serum IFN [rs1049564; Odds Ratio=2.08 95% CI (1.34 – 3.21); PMeta-analysis=1.24 ×10−7) in European-American (EA) SLE patients.(8) This locus has not been previously identified in case-control SLE genetics studies, and this study supports a role for PNP in the dysregulation of IFN-α observed in SLE.
The PNP gene located at chromosome 14q13.1 encodes purine nucleotide phosphorylase, an enzyme involved in purine metabolism. PNP is found in most tissues and is expressed at particularly high levels in lymphoid tissues.(9) This enzyme reversibly catalyzes the phosphorolysis of purine nucleosides and deoxynucleosides (guanosine, deoxyguanosine (dGuo), inosine and deoxyinosine) to their respective purine bases and ribose 1-phosphate. PNP together with adenosine deaminase (ADA), serve a key role in purine catabolism (salvage pathway) (Supplementary Figure 1). In normal conditions, the rate of phosphorolysis of dGuo markedly exceeds the rate of phosphorylation.(10) PNP deficiency results in increased phosphorylation of dGuo and subsequent accumulation of deoxy-GTP (dGTP), a potent feedback inhibitor of human ribonucleotide reductase. This causes depletion of intracellular dCTP levels and subsequent inhibition of DNA synthesis and cell division (Supplementary Figure 1). Rare autosomal recessive deficiency of the PNP gene in humans results in a metabolic disorder characterized by defective T-cell and immunity and variable B-cell immunity (9–12). PNP-deficient subjects present with 7–11% of their circulating lymphocytes blocked in S phase.(13) Several rare coding-change mutations in the PNP gene have been reported that produce variable degrees of enzymatic activity correlated with nucleoside accumulation and clinical severity.(14–17) In addition to immunodeficiency, PNP deficient subjects have developed autoimmune disorders, such as SLE, autoimmune hemolytic anemia, and idiopathic thrombocytopenic purpura.(9) The SNP we identified in our IFN-α GWAS (rs1049564) is a common coding-change variant (SER to GLY at amino acid position 51) which did not cause complete enzyme deficiency as seen with other polymorphisms (18), although quantitative impacts upon enzyme function are not ruled out by these earlier studies. Given its relevance in lymphocyte purine metabolism and previous association with autoimmunity, PNP is a fascinating genetic association with SLE. To functionally confirm our genetic association, in this study we delineate the cellular and molecular disturbances associated with the PNP allele, and relate these molecular changes back to SLE immuno-phenotype.
METHODS
Reagents
Human recombinant PNP was purchased from Calbiochem, EMD Millipore (Billerica, MA). Xanthine oxidase, obtained from bovine milk as an ammonium sulfate suspension, and Inosine, HEPES, INT, Triton X-100, 2′-Deoxyguanosine, hypoxanthine, adenine, and 2′-deoxycytidine were purchased from Sigma-Aldrich (St. Louis, MO). Potassium phosphate monobasic, Potassium phosphate dibasic and 1-Step™ Ultra TMB-ELISA Substrate Solution were obtained from ThermoFischer Scientific (Waltham, MA). Affinity purified rabbit anti-PNP monoclonal antibody (# A304-240A), goat anti-rabbit IgG-heavy and light chain, highly cross adsorbed HRP conjugated antibody, and Mouse Reference Serum were purchased from Bethyl labs (Montgomery, TX). Mouse anti-human PNP (aa68-289) monoclonal antibody was ordered from LifeSpan BioSciences (Seattle, WA). Horse serum and RPMI 1640 medium were purchased from ThermoFisher Scientific.
SLE Patients
SLE patients were recruited after providing full informed consent as approved by the institutional review board. RBC samples were obtained for 24 patients and all were genotyped at the PNP rs1048564 polymorphism (CC=9, CT=10, TT=5). Human RBCs were chosen as source of PNP enzyme to study PNP enzyme activity in SLE patients because they are deficient in de novo synthesis of purines, and thus dependent on purine salvage pathway and are a rich source of PNP (15, 19). For the primary B cell experiments, we recalled 10 subjects back in for a fresh sample (CC=4, CT=4, TT=2).
Molecular Modelling
The crystal structure of the PNP trimeric complex was obtained from the structural Protein Data Bank (PDB code 3PHB) and was analyzed for subunit contacts, distances and conformation using the molecular graphics program PyMol V1.8.04.
B-lymphoblastoid Cell Lines
Epstein-Barr virus-transformed B-lymphoblastoid cell lines derived from European ancestry populations were obtained from Coriell Cell repositories (Camden, NJ). A total of 24 B-lymphoblastoid cell lines that were homozygous (CC or TT) and heterozygous (CT) at the rs1049564 SNP (at least n = 6 for each genotype category) were studied. Cells were grown in RPMI 1640 medium (GIBCO) supplemented with 1X GlutaMax (GIBCO), 0.01M HEPES buffer (GIBCO), Gentamicin (40 μg/ml), 10% horse serum (reported to have very low amounts of PNP activity) at 37°C under 5% CO2.
Isolation of primary B cells from SLE patients
Heparinized peripheral blood was diluted threefold with wash buffer (PBS + 10 mM HEPES/0.1% BSA/2 mM EDTA/20 μg/mL Gentamicin), layered onto Ficoll-paque (GE Healthcare, Pittsburgh, PA), and centrifuged at 1400 rpm for 30 min. Buffy coats were collected and washed twice by repeated centrifugation at 1200 rpm for 10 min and resuspension in wash buffer. B cells were isolated from PBMCs by negative selection using the Human Pan B cell isolation kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s protocol. Flow cytometric analysis was used to confirm that B cell purity was >95%.
Detection of PNP mRNA, protein level, and enzymatic activity in B-lymphoblastoid cell lines and primary cells from SLE patients
PNP mRNA detection
Total mRNA was purified from the B-lymphoblastoid cell lysates and SLE patient B cells using the Qiagen Turbocapture 96-well RNA purification kit (Qiagen, Valencia, CA, USA) as per manufacturer protocol. cDNA was made from the mRNAs, and was quantified using real-time PCR. Forward (ACCATGGAGAACGGATACACC) and reverse (GGCACTGTACTTCGGGGAAA) primers for the PNP gene were designed using primer designing tool from NCBI: Primer BLAST. Each sample and control was run in duplicate.
PNP ELISA
PNP protein was quantified in cell lysates and SLE patient RBCs using an ELISA method. 5% packed RBC samples were prepared from whole blood by repeated suspension in 0.9% cold isotonic saline and centrifugation. ELISA plates (Nunc, Maxisorp) were coated with 0.5 μg/ml of PNP mouse anti-human monoclonal antibody (Ab) (LS bio), washed with 0.5% Tween-20 in PBS (wash buffer) and treated with 0.25% blocking solution (BioRad) for 2 hrs at room temperature. Wells were then washed and standards and cell and RBC lysates were added in duplicate and plates placed at 4°C overnight. Wells were washed and overlain with anti-PNP rabbit polyclonal Ab (1 μg/ml; Bethyl labs) for 1 hour. Bound rabbit Ab was detected using a HRP conjugated goat anti-rabbit IgG Ab (Bethyl). The wells were washed, and 0.1 ml 1-Step Ultra TMB-ELISA (Thermo Fischer) was added. The reaction was stopped after 15 min using 2M sulphuric acid and absorbance was measured at 450nm using a microplate reader.
Colorimetric enzyme assay to measure PNP activity
Cell lysates were prepared from 4 × 107 B-lymphoblastoid cells of each genotype. RBC lysates were prepared from 5% packed RBCs as detailed above. This assay measures the hypoxanthine product of the PNP reaction, formazan, which is produced when the PNP catalyzes phosphorolysis of Inosine (Ino) in presence of inorganic orthophosphate (Pi) to release hypoxanthine (Hx) (20–23). Assay mixtures consisting of 100 mM HEPES (pH 7.0), 1 mM inosine, 50 mM potassium phosphate (pH 7.0), 0.075% Triton X-100, 1 mM 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride, xanthine oxidase (20 munits), and 0–2.2 munits of PNP or cell and RBC lysates were incubated at 25 °C. Recombinant human PNP (Calbiochem) was used as a standard. PNP activity was measured by monitoring highly colored formazan product for 10 min at 490nm for each sample in duplicate. Linearity of the PNP reaction was maintained provided the absorbance at 490nm did not exceed 2.0 absorbance units. PNP enzyme activity was reported as the amount of PNP activity per nanogram of PNP protein standardize for the amount of enzyme present.
Cell cycle analysis experiments
Exponentially growing cells (2 × 106 cells/ml) were cultured in flat bottom 24 well microtiter plates (BD Falcon) in culture medium with increasing concentrations of 2′-Deoxyguanosine (No 2′-dGuo, 200 μM, 350 μM, 500 μM, 650 μM, 800 μM) for 24 hours at 37°C under 5% CO2. For rescue experiments, cells were incubated with an experimentally determined optimal dose of 2′-dGuo ± hypoxanthine, deoxycytidine, and adenine. Cell cycle phase was determined using the Alexa 488 Click-It Plus Edu Flow Cytometry Assay kit (Life technologies) as per the kit instructions. Briefly, after a 18 hour culture period, cells were pulsed with the thymidine analogue, EdU (5-ethynyl-2′-deoxyuridine) for 2 hours. Incorporated EdU into DNA during active DNA synthesis was detected post-culture by specific conjugation to Alexa fluor 488. Total DNA content was measured by using the FxCycle violet stain (Life technologies). Cells were stained using LIVE/DEAD® Far Red dye and analyzed on a BD LSR II flow cytometer. Each experiment was performed in duplicate.
IFN-α stimulation of B-lymphoblastoid cell lines and primary B cells from SLE patients
Type I IFN activity in B-lymphoblastoid cells and primary B cells was measured using a functional reporter assay which has been described in detail previously. (24–26) In this modified assay, B-lymphoblastoid cells and primary B cells were incubated with increasing doses of IFN-α (0 Units/mL, 25 Units/mL and 100 Units/mL) for 6 hours. Then real-time PCR using SYBR green assays was used to quantify three canonical IFN-α-induced transcripts (IFIT1, MX1 and PKR) in the B-lymphoblastoid cell and SLE B cell lysates. The relative expression of these three genes was summed to generate a score reflecting the degree of IFN-induced gene expression in the cells.
Statistical Analysis
For mRNA expression levels, PNP protein levels, and enzyme activities, samples were assayed in duplicate and the average of the duplicates used for data analysis. All normally distributed data are expressed as the mean ± SD of these averages while non-normally distributed data are expressed as the median ± interquartile range. Data from the different genotype groups were compared by Student’s t-test for normally distributed data and by Mann-Whitney U or Kruskal-Wallis test for non-normal data. Non-linear regression analysis was used to identify the best fit model for analyzing relationship between rs1049564 genotypes with relative PNP mRNA expression levels and IFN induced mRNA expression in B-lymphoblastoid cells and SLE B cells treated with increasing doses of IFN. Extra sum-of-squares F test was used to compare different regression models for the same response (IFN induction) in 3 different genotype groups and to determine statistically significant differences in slope for each data set (3 genotype groups) for the given best fit model.
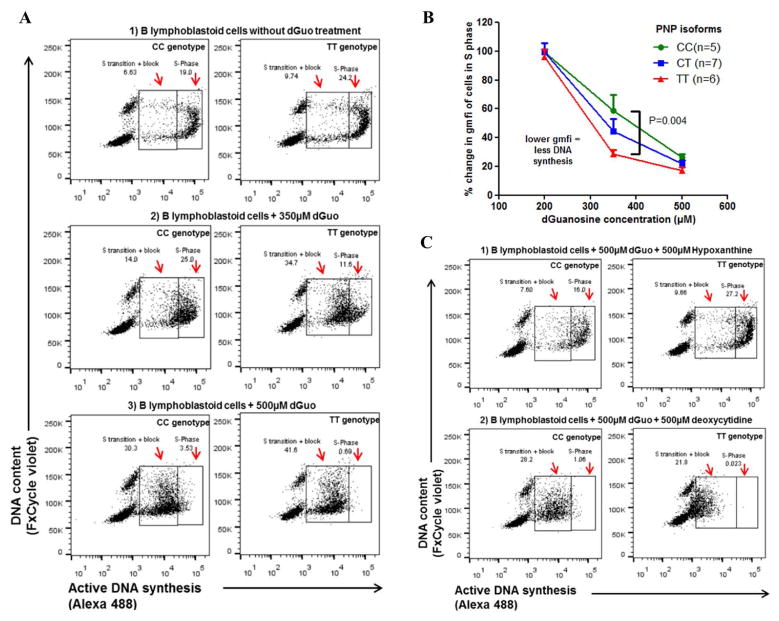
Flow analysis was carried out using FlowJo_V10. Doublets (SSC-W vs. SSC-H and FSC-W vs. FSC-H), dead cells (SSC-A vs. Live/Dead dye FarRed-A) and debris (SSC-A vs. FSC-A) were excluded. FxCycle single cells were selected for further analysis (FxCycle-W vs. FxCycle-H. Cells in different phases of cell cycle were identified based on their DNA content and on their level of incorporated EDU (FxCycle violet-W vs. Alexa 488-A). Cells that show increased levels of both labels are in S-phase of the cell cycle. Figure 3A shows scatter plots of B-lymphoblastoid cells analyzed for cell cycle phase with gates drawn around cells in S phase and around cells that have recently entered S phase or that are blocked in S phase (S transition + block). The alexa fluor 488 emission (geometric mean fluorescence intensity; GMFI) was used as a measure of DNA synthesis. Statistical analysis was performed using Graph Prism (version 4.0 for Windows; CA, USA). p <0.05 was considered significant.
Figure 3. B lymphoblastoid cell lines with the rs1049564 variant show increased susceptibly to dGuo-induced S-phase block and toxicity as determined by cytometric cell cycle analysis.
(3A) Scatter plots of B cell lines with TT or CC genotype with DNA content shown on the y-axis and incorporated alexa fluor 488 Edu shown on the x-axis. Gates drawn show cells in S phase (S-Phase) and cells entering S phase or in block (S transition + block). Panel 1 to 3 shows different treatments received by the cells that have the normal (left) and variant (right) PNP isoform. (3B) Graph showing exogenous dGuo-induced S-phase block of cell lines treated with increasing dGuo doses displayed on the x-axis. B cell lines with the PNP variant are blocked at lower dGuo doses. Y-axis shows % GMFI of treated cells compared to cells grown in medium alone; each point is the average of 5 to 7 different cell lines with the same PNP isoforms. Samples were assayed in duplicate. Kruskal-Wallis test with Dunn’s post-test used to compare the three genotypes. (3C) Hypoxanthine reverses the dGuo-induced S-phase block in the PNP variant cell lines. Cells were treated with dGuo ± different rescue agents as shown.
RESULTS
Structural analysis of the Ser-to-Gly substitution at position 51 in PNP
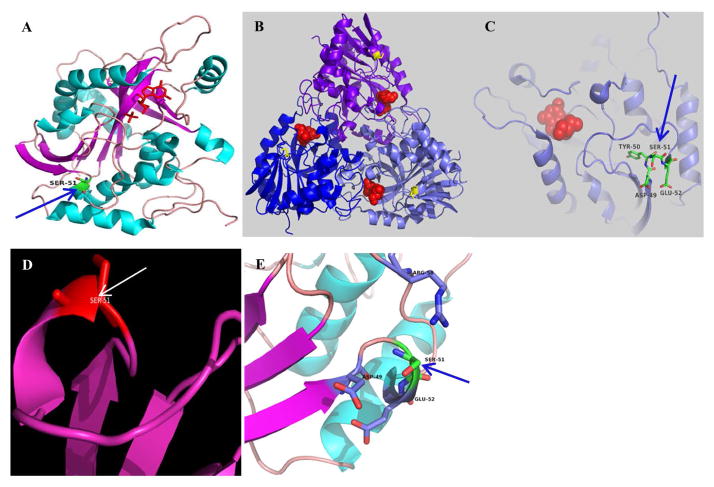
PNP exists as a trimer, in which subunits are related by a crystallographic 3-fold axis. Each subunit contains an eight-stranded mixed beta-sheet and a five-stranded mixed beta-sheet which join to form a distorted beta-barrel structure. This core beta-structure is flanked by seven alpha-helices in a manner that generates a novel folding pattern (Figure 1A). The active site, which was characterized from binding of the substrate analogs 8-iodoguanine and 5′-iodoformycin B, is located near the subunit-subunit boundary within the trimer (Figure 1B) and involves seven different segments from one subunit and an additional short segment from an adjacent subunit. In the crystal, the phosphate-binding site is probably occupied by a sulfate ion. The specificity of purine nucleoside phosphorylase for guanine, hypoxanthine, and their analogs can be explained on the basis of the arrangement of hydrogen bond donors and acceptors in the active site. The amino acid position 51 is at the beginning of a helical turn in one of the connecting loops between two beta-pleated strands of the central β-sheet forming the structure (Figure 1C, 1D). The location is not directly interacting with the substrate-binding site and is at a distance from the trimer interface (Figure 1B, 1C). Ser-to-Gly substitution at position 51 will likely affect the local conformation of the α-helical segment (Ser a helical former, Gly a helical breaker when on the surface) and introduce more flexible local conformation. Also the loss of the hydroxyl group may affect the local water network formed by the charged residues located around (Figure 1E). These results are consonant with the previous functional findings that the Ser-Gly substitution at position 51 does not result in complete loss of enzymatic function.(18)
Figure 1. 3-D ribbon diagram of the structure of the PNP trimeric complex (PDB code 3PHB) containing the position of the G51S polymorphism.
(A) Helical regular segments are represented in cyan and beta strands in magenta with the Ser51 position in green. (B) The trimeric PNP structure with the ligand (in red) and Ser51 (in yellow). (C) The location of the Ser51 containing loop with respect to the binding site. (D) The conformation of Ser51 in detail. (E) The distribution of the local charged residues. Diagrams created using program PyMOL (The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC.).
PNP rs1049564 variant shows lower enzyme activity and decreased PNP mRNA expression levels in B-lymphoblastoid cell lines and primary cells from SLE patients
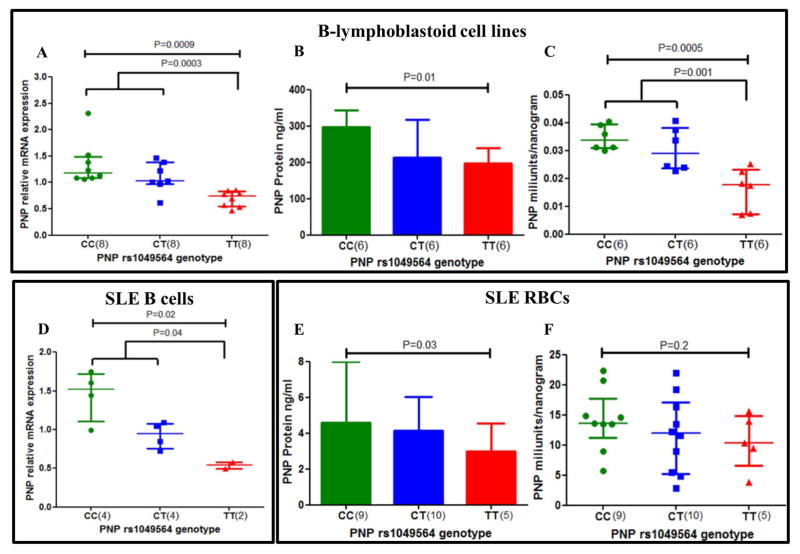
We next measured PNP mRNA expression and protein expression levels in cell lysates of B-lymphoblastoid cells and primary cells from SLE patients of different PNP genotypes to see if levels vary with presence of the variant allele. Interestingly, PNP mRNA expression levels were significantly decreased in cell lines with the homozygous TT variant of rs1049564 (Figure 2A). We observed similar results for PNP mRNA expression levels in freshly isolated primary B cells from SLE patients (Figure 2D). This is not necessarily expected of a coding-change polymorphism, but protein levels were also reduced in B lymphoblastoid cells (Figure 2B) as well as SLE patient RBCs (Figure 2E), supporting the idea that PNP is decreased quantitatively in rs1049564 TT homozygotes. To measure PNP enzymatic activity, we used a colorimetric assay that specifically detects the hypoxanthine product of the PNP reaction via the formation of formazan. The specificity of the reaction has previously been established in the absence of inosine and using a PNP inhibitor,(27) and also has been used in assays to test the activity of mutated PNP constructs. (28) PNP enzyme activity was calculated as per unit mass for B-lymphoblastoid cells and for SLE RBCs with various PNP genotypes. Figure 2C and 2F demonstrates the PNP activity of lymphoblastoid cells and SLE RBCs with different genotypes respectively. Cell lines with the TT genotype had significantly lower enzyme activity as compared to cell lines that were heterozygous CT and homozygous for the normal variant CC (2C). We observed similar trend for SLE patients (2F). These graphs presents enzymatic activity corrected for total enzyme mass, and thus the decreased enzyme activity cannot be attributed to decreased protein amount in the TT cells. These results indicate that the decreased PNP enzyme function is due to a combination of structural protein changes and decreased mRNA and protein expression levels. In addition, evidence from the publically available eQTL data base GTEx portal (https://www.gtexportal.org/home/) shows that the rs1049564 variant allele is associated with low levels of PNP expression in whole blood.
Figure 2. The PNP rs1049564 variant has lower enzyme activity and decreased mRNA expression levels in B lymphoblastoid cells and primary cells from SLE patients.
PNP mRNA levels are shown for (2A) B cell lines and for (2D) primary B cells from SLE patients of different genotypes. PNP protein levels are shown for (2B) B cell lines and for (2E) RBC lysates from SLE patients of different genotypes. PNP enzyme activity is shown for (2C) B cell lines and for (2F) RBC lysates of SLE patients of different genotypes. Y axis shows the PNP enzyme activity per unit mass for the cells studied. Enzyme activity was determined by colorimetric assay. The median and interquartile ranges are shown for all scatter plots and box plots. Mann-Whitney U was used to test significant differences for mRNA and protein levels between genotype groups. Unpaired t-test was used to compare enzyme activity between genotypes. For B lymphoblastoid cell lines, n = 6 to 8 for each genotype studied as indicated. For primary B cell and RBC samples, n = 2 to 10 as indicated.
PNP rs1049564 T allele results in S phase block in a dose-response fashion, which can be pharmacologically corrected with hypoxanthine and adenine
To determine if the rs1049564 T allele which reduces PNP enzyme function impacts cell biology in lymphocytes, we studied cell cycle in the B-lymphoblastoid cell lines of various genotypes that were exposed to a dose curve of increasing amounts of 2′-dGuo. The 2′-dGuo is expected to antagonize potential S-phase block induced by nucleotide imbalance related to deficiency of PNP. Figure 3A shows the cell cycle diagrams of cells with TT or CC genotypes, documenting a greater degree of S-phase block in cells with the TT genotype. Figure 3B illustrates dGuo dose curve and resultant proportional S-phase block in cells of each genotype, establishing a dose response effect of the T allele at the 350 μM dGuo concentration. At this concentration of dGuo, cell lines with TT genotype had a ~2 fold increase (P=0.004) in S-phase block as compared to cells lines with the CC genotype (Figure 3). Higher concentrations of dGuo led to a more complete block in all genotypes, and less differentiation between genotypes. DGuo-induced S-phase block in B cells can be reversed by treatment of cells with hypoxanthine or adenine, while this block in T cells can be reversed by exogenous addition of deoxycytidine (dCyd).(29) We observed that exogenously added hypoxanthine and adenine rescued the B-lymphoblastoid cells from dGuo dependent toxicity while exogenously added dCyd had no impact on cell cycle phase (Figure 3C). Exogenously added hypoxanthine mitigates dGuo induced S-phase block by competing for HGPRT (Supplementary figure1). B-lymphoblastoid cells are derived from primary B cells, and therefore our findings are consistent with rescue of B cells from dGuo-induced S-phase block caused by a decrease in PNP enzyme function.(29) Taken together, these results demonstrate that the rs1049564 T allele in PNP causes a dose-dependent increase in S-phase block which can be reversed pharmacologically.
The rs1049564 variant is associated with higher IFN induced mRNA expression in B-lymphoblastoid cell lines and B cells from SLE patients
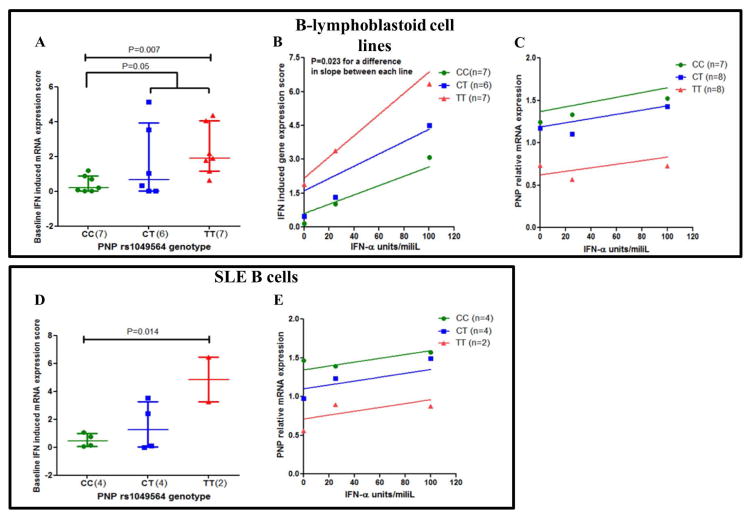
Based on the observation that the PNP rs1049564 variant was associated with high serum type I IFN activity in SLE patients, we evaluated IFN-induced gene expression in B-lymphoblastoid cells and B cells from SLE patients with different PNP genotypes at baseline, and after treatment with increasing doses of IFN-α. In baseline unstimulated cells, we observed an additive effect of the T allele on IFN-induced gene expression (Figure 4A and 4D). When cells were treated with increasing doses of IFN-α there was a dose dependent increase in the IFN induced mRNA expression score in all 3 genotypes in B lymphoblastoid cells (Figure 4B). It was evident that cell lines with the homozygous variant allele (TT) had significantly higher induction of IFN-induced gene expression as compared to cells lines that were heterozygous CT and homozygous for the normal allele CC at baseline and also at each increasing dose of IFN-α. The slopes of the lines of each genotype dose curve were significantly different by sum-of-squares F test (p<0.023 for each). For SLE B cells treated with increasing doses of IFN-α there was a dose dependent increase in the IFN induced mRNA expression score in all 3 genotypes. The IFN dose response curves for B cells in all 3 genotypes were similar. We also checked to see if IFN-α exposure changed the expression of PNP mRNA. As shown in Figure 4C and 4E, while the rs1049564 genotype exerted a large influence on PNP expression, there was no appreciable induction of PNP mRNA with increasing doses of IFN-α, in both B lymphoblastoid cells as well as SLE B cells and thus PNP was not IFN-induced.
Figure 4. The rs1049564 variant associates with higher IFN induced mRNA expression score at baseline and after IFN induction in B lymphoblastoid cell lines (A–C) and in SLE B cells (D–E).
Scatter dot plots show mRNA expression levels for IFN induced genes in cells at baseline for (A) B-lymphoblastoid cells and for (D) SLE B cells with different genotypes. (B) Figure shows the association of rs1049564 genotypes with IFN induced mRNA expression levels in B lymphoblastoid cells treated with increasing doses of IFN. Y-axis shows IFN induced gene expression score (calculated from genes MX1, IFIT1, PKR) as outlined in methods section. (C and E) shows non-linear regression plot of rs1049564 genotypes with relative PNP mRNA expression levels in cells treated with increasing doses of IFN. PNP mRNA expression levels for (C) B-lymphoblastoid cells and for (E) SLE B cells with different PNP genotypes treated with increasing doses of IFN-α. Experiments carried out in duplicates. Average values are shown for 4A and 4D. For B lymphoblastoid cell lines, n= 6 to 8 for each genotype studied as indicated. For primary B cell and RBC samples, n=2 to 4 as indicated.
DISCUSSION
SLE is a heritable polygenic condition that results from the synergistic effect of different genetic loci which impact various immune system phenotypes in concert. A major frontier is in understanding the molecular and immunologic significance of the genetic variations that are associated with SLE, as this knowledge will allow us to better understand disease etiology and personalize treatment. Our previous GWAS data implicate the PNP gene polymorphism in SLE pathogenesis through high circulating IFN-α levels (30), and in this study here we performed functional studies to understand how this causal variant alters cell biology. PNP rs1049564 is a common coding-change variant which does not cause complete enzymatic deficiency, and in silico bioinformatic analysis using PolyPhen2 and SIFT predict this SNP as non-damaging (8). In structural analysis, we found that the Gly-to-Ser substitution at position 51 is not part of the substrate-binding site. In the functional studies presented here we show that the cells carrying the rs1049564 PNP polymorphism have lower enzyme activity; decreased PNP mRNA and protein expression, altered DNA synthesis and associated cell cycle block, and higher expression of IFN induced genes at baseline and upon IFN stimulation.
The coding-change SNP rs1049564 is a part of a haplotype that spans the PNP gene region including approximately 5 kb upstream and downstream of the gene. ENCODE data shows that SNP rs1049564 is located in a DNAase hypersensitivity cluster very close to 4 different transcription factor binding sites. Additionally, rs1049564 is in linkage with the 3′ region which also contains transcription factor binding sites. Our data supports the concept that both coding change and regulatory elements in this haplotype contribute to the overall genetic effects we observe. We find evidence for a difference in mRNA and protein levels in association with the variant, which should not arise from the coding change SNP and would be more likely due to regulatory elements in LD. When we control for the amount of protein, there is still a decreased activity of the enzyme, supporting the idea that the coding change polymorphism is also impacting enzyme function. Overall, our results indicate that cells carrying this variant in PNP have decreased enzyme function, dysfunction in lymphocyte cell cycle, and an increased type I IFN response.
Besides antiviral and antimicrobial effects, type I IFNs also exert pleiotropic cellular effects such as altered cell cycle regulation, and inhibition of cell proliferation and differentiation (31). The antiproliferative effect of IFN occurs through inhibition of DNA replication and modulation of genes that regulate the cell cycle (32). Few studies have examined the effect of cell cycle stage and progression on IFN production or on IFN-induced gene expression. In a study using Daudi cells (B lymphoblast cells), both constitutive and IFN-α-induced expression of IFN-inducible genes varied at different points in the cell cycle (33). This study reported that cells released from G1/S block had increased expression levels of IFN-inducible proteins (100- and 46-kDa 2–5(A)- synthetases (OAS), IFN-inducible mRNAs, and expression of IFN stimulatory gene factor (ISGF)-3 complex in the absence of exogenous IFN-α (33). The authors found that Daudi cells were treated with IFN-α during G1/S phase showed increased tyrosine phosphorylation of the kinases JAK-1 and Tyk-2 and of the polypeptide p91 by IFN-α as compared with non-synchronized cells. Overall, Daudi cells showed the greatest sensitivity to IFN signaling in late S-phase and a decreased response to IFN-α-signaling in several IFN inducible genes during transition from G1/S to G2/M. The increased sensitivity to IFN signaling in Daudi cells during or after cell cycle block may be influenced by the prolonged stay in one phase of the cell cycle since cells treated with cell cycle blockers appeared to be more sensitive to IFN signaling than cells not treated with blockers. Other studies using human melanoma cells in G1/S phase and mouse embryo fibroblast in late S phase show that production of IFN-α and IFN-β is higher as cells progress from G1 to late S phase and that OAS activity correlates with type I IFN levels in these cultures (34–36) In an additional study, chick embryo fibroblast cell cultures were synchronized for different cell cycle phases and virally induced to produce type I IFN at various phases of the cell cycle. Cultures infected at the early S phase had comparatively higher levels of IFN in the medium as compared to those infected at early G2 and late G2 phases of the cell cycle (37). Taken together, these studies show that production of type I IFNs and induction of IFN inducible genes differ based on cell cycle phase, with highest levels occurring at or around S-phase. These studies help to explain why we find increased response to IFN-α and expression of IFN-induced genes in cells that carry the PNP variant and link the S-phase block we observe with the IFN pathway.
We used B lymphoblastoid cells and primary B cells from patients for our study, which are not a major IFN-α secreting cell type. Hence it would be interesting to carry out a similar study using different immune cell types, including plasmacytoid dendritic cells (pDCs) which are major IFN-α producing cells (38), to see if the PNP variant impacts type I IFN production. Cell cycle-related proteins, such as the polo-like kinases (PLKs) and NIMA-related kinases, appear to play important roles in antiviral and type I IFN responses, in non-dividing cells such as plasmacytoid dendritic cells (DCs).(39–42) A recent study demonstrated that PLKs are essential for activation of IFN-inducing pathways in conventional and plasmacytoid DCs (42). Similarly, polymorphisms in PNP, a cell cycle-related enzyme, may directly impact IFN production or response by plasmacytoid DCs independent of the cell cycle phase. However our data showing cell-cycle changes in lymphocytes carrying the PNP polymorphism would suggest that the variant alters immune function in multiple cell types via numerous regulatory effects.
In summary, our results implicate a polymorphism in the PNP gene in SLE pathogenesis that alters enzyme function and causes cell cycle dysfunction in lymphocytes, which is associated with increased type I IFN responses. We also find that the cell cycle abnormality is “druggable” in cells in vitro; suggesting that targeting the cell cycle in SLE with agents that normalize cell cycle phase could provide a novel strategy for the treatment of SLE. Future work in various human cell types could enable directed targeting, as different cell types preferentially respond to different agents to reverse the S phase block. This could provide a path from genetic heterogeneity to molecular and clinical heterogeneity, and thereby a novel and personalized therapeutic approach.
Supplementary Material
PNP regulates deoxynucleotide (dNTP) levels indirectly by maintaining pools of dNTP precursors and thus PNP enzyme activity impacts cell cycle and proliferation of T and B cells. PNP deficiency leads to accumulation of dGTP/GTP, and inhibition of ribonucleotide reductase (RNR) and consequently DNA synthesis and cell apoptosis. dGMP: deoxyguanosine monophosphate; dGDP: deoxyguanosine diphosphate; dGTP: deoxyguanosine triphosphate; GMP: guanosine monophosphate; GDP: guanosine diphosphate; GTP: guanosine triphosphate; NT: nucleotide; AMP: adenosine monophosphate; IMP: inosine monophosphate; 5′ NT: 5′ nucleotidase; ADA: Adenosine deaminase AK: Adenosine kinase; dCK: deoxycytidine kinase; GDA: Guanine deaminase; HGPRT: hypoxanthine-guanine-phosphoribosyltransferase; PNP: Purine nucleotide phosphorylase; XO: Xanthine oxidase; Enzymes: 5′ NT: 5′ nucleotidase; ADA: Adenosine deaminase; ADK: Adenosine kinase; AMPDA: AMP deaminase; APRT: Adenine phosphoribosyl transferase; dCK: deoxycytidine kinase; GDA: Guanine deaminase; HGPRT: hypoxanthine-guanine-phosphoribosyltransferase; IMPD: IMP dehydrogenase; PNP: Purine nucleotide phosphorylase; PP-ribose-P: 5-phosphoribosyl-1 pyrophosphate; RNR: Ribonucleotide reductase; XO: Xanthine oxidase
Acknowledgments
Funding Sources: MA Jensen - Mayo Clinic Career Development Award in Rheumatoid Arthritis Research; TB Niewold – Colton Center for Autoimmunity, NIH (AR060861, AR057781, AR065964, AI071651), Rheumatology Research Foundation, CureJM Foundation, the Mayo Clinic Foundation, and the Lupus Foundation of Minnesota.
Footnotes
Financial Disclosures and Conflict of Interest: The authors report no financial conflict of interest.
References
- 1.Harley JB, Kelly JA, Kaufman KM. Unraveling the genetics of systemic lupus erythematosus. Springer Semin Immunopathol. 2006;28(2):119–30. doi: 10.1007/s00281-006-0040-5. [DOI] [PubMed] [Google Scholar]
- 2.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301(1):5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 3.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8(6):492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghodke-Puranik Y, Niewold TB. Genetics of the type I interferon pathway in systemic lupus erythematosus. International Journal of Clinical Rheumatology. 2013;8(6):657–69. doi: 10.2217/ijr.13.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kariuki SN, Kirou KA, MacDermott EJ, Barillas-Arias L, Crow MK, Niewold TB. Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. J Immunol. 2009;182(1):34–8. doi: 10.4049/jimmunol.182.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58(8):2481–7. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niewold TB, Kelly JA, Kariuki SN, Franek BS, Kumar AA, Kaufman KM, et al. IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Ann Rheum Dis. 2012;71(3):463–8. doi: 10.1136/annrheumdis-2011-200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kariuki SN, Ghodke-Puranik Y, Dorschner JM, Chrabot BS, Kelly JA, Tsao BP, et al. Genetic analysis of the pathogenic molecular sub-phenotype interferon-alpha identifies multiple novel loci involved in systemic lupus erythematosus. Genes Immun. 2014 doi: 10.1038/gene.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markert ML. Purine nucleoside phosphorylase deficiency. Immunodefic Rev. 1991;3(1):45–81. [PubMed] [Google Scholar]
- 10.Hershfield MS, Mitchell BS. Immunodeficiency diseases caused by adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency. In: Scriver CR, WSSly ALB, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 1995. pp. 1725–68. [Google Scholar]
- 11.Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2(9):764–6. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki Y, Iseki M, Yamaguchi S, Kurosawa Y, Yamamoto T, Moriwaki Y, et al. Direct evidence of autosomal recessive inheritance of Arg24 to termination codon in purine nucleoside phosphorylase gene in a family with a severe combined immunodeficiency patient. Hum Genet. 1998;103(1):81–5. doi: 10.1007/s004390050787. [DOI] [PubMed] [Google Scholar]
- 13.Rijkers GT, Zegers BJ, Spaapen LJ, Rutgers DH, Kuis W, Roord JJ, et al. Mononuclear cells in S-phase in a patient with purine nucleoside phosphorylase deficiency. Adv Exp Med Biol. 1984;165(Pt B):171–4. doi: 10.1007/978-1-4757-0390-0_34. [DOI] [PubMed] [Google Scholar]
- 14.Al-Saud B, Alsmadi O, Al-Muhsen S, Al-Ghonaium A, Al-Dhekri H, Arnaout R, et al. A novel mutation in purine nucleoside phosphorylase in a child with normal uric acid levels. Clin Biochem. 2009;42(16–17):1725–7. doi: 10.1016/j.clinbiochem.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Bzowska A, Kulikowska E, Shugar D. Purine nucleoside phosphorylases: properties, functions, and clinical aspects. Pharmacol Ther. 2000;88(3):349–425. doi: 10.1016/s0163-7258(00)00097-8. [DOI] [PubMed] [Google Scholar]
- 16.Grunebaum E, Zhang J, Roifman CM. Novel mutations and hot-spots in patients with purine nucleoside phosphorylase deficiency. Nucleosides Nucleotides Nucleic Acids. 2004;23(8–9):1411–5. doi: 10.1081/NCN-200027647. [DOI] [PubMed] [Google Scholar]
- 17.Osborne WR, Scott CR. Purine nucleoside phosphorylase deficiency. Measurement of variant protein in four families with enzyme-deficient members by an enzyme-linked immunosorbent assay. Am J Hum Genet. 1980;32(6):927–33. [PMC free article] [PubMed] [Google Scholar]
- 18.Aust MR, Andrews LG, Barrett MJ, Norby-Slycord CJ, Markert ML. Molecular analysis of mutations in a patient with purine nucleoside phosphorylase deficiency. Am J Hum Genet. 1992;51(4):763–72. [PMC free article] [PubMed] [Google Scholar]
- 19.Kim BK, Cha S, Parks RE., Jr Purine nucleoside phosphorylase from human erythrocytes. I. Purification and properties. J Biol Chem. 1968;243(8):1763–70. [PubMed] [Google Scholar]
- 20.Kalckar HM. Differential spectrophotometry of purine compounds by means of specific enzymes; determination of hydroxypurine compounds. J Biol Chem. 1947;167(2):429–43. [PubMed] [Google Scholar]
- 21.Chu SY, Cashion P, Jiang M. A new colorimetric assay for purine nucleoside phosphorylase. Clin Biochem. 1989;22(5):357–62. doi: 10.1016/s0009-9120(89)80032-3. [DOI] [PubMed] [Google Scholar]
- 22.Halfpenny AP, Brown PR. Optimized assay for purine nucleoside phosphorylase by reversed-phase high-performance liquid chromatography. J Chromatogr. 1980;199:275–82. doi: 10.1016/s0021-9673(01)91379-2. [DOI] [PubMed] [Google Scholar]
- 23.Uitendaal MP, De Bruyn CH, Oei TL, Hosli P, Griscelli C. A new ultramicrochemical assay for purine nucleoside phosphorylase. Anal Biochem. 1978;84(1):147–53. doi: 10.1016/0003-2697(78)90493-1. [DOI] [PubMed] [Google Scholar]
- 24.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54(6):1906–16. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 25.Niewold TB, Adler JE, Glenn SB, Lehman TJ, Harley JB, Crow MK. Age- and sex-related patterns of serum interferon-alpha activity in lupus families. Arthritis Rheum. 2008;58(7):2113–9. doi: 10.1002/art.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niewold TB, Rivera TL, Buyon JP, Crow MK. Serum type I interferon activity is dependent on maternal diagnosis in anti-SSA/Ro-positive mothers of children with neonatal lupus. Arthritis Rheum. 2008;58(2):541–6. doi: 10.1002/art.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frederiks WM, Bosch KS, Van Gulik T. A quantitative histochemical procedure for the demonstration of purine nucleoside phosphorylase activity in rat and human liver using Tetranitro BT and xanthine oxidase as auxiliary enzyme. Histochem J. 1993;25(1):86–91. doi: 10.1007/BF00161048. [DOI] [PubMed] [Google Scholar]
- 28.Erion MD, Takabayashi K, Smith HB, Kessi J, Wagner S, Honger S, et al. Purine nucleoside phosphorylase. 1. Structure-function studies. Biochemistry. 1997;36(39):11725–34. doi: 10.1021/bi961969w. [DOI] [PubMed] [Google Scholar]
- 29.Sidi Y, Mitchell BS. 2′-deoxyguanosine toxicity for B and mature T lymphoid cell lines is mediated by guanine ribonucleotide accumulation. J Clin Invest. 1984;74(5):1640–8. doi: 10.1172/JCI111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kariuki SN, Ghodke-Puranik Y, Dorschner JM, Chrabot BS, Kelly JA, Tsao BP, et al. Genetic analysis of the pathogenic molecular sub-phenotype interferon-alpha identifies multiple novel loci involved in systemic lupus erythematosus. Genes Immun. 2015;16(1):15–23. doi: 10.1038/gene.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36(2):166–74. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada H, Ochi K, Nakada S, Nemoto T, Horiguchi-Yamada J. Changes of cell cycle-regulating genes in interferon-treated Daudi cells. Mol Cell Biochem. 1994;136(2):117–23. doi: 10.1007/BF00926071. [DOI] [PubMed] [Google Scholar]
- 33.Kumar R, Korutla L, Zhang K. Cell cycle-dependent modulation of alpha-interferon-inducible gene expression and activation of signaling components in Daudi cells. J Biol Chem. 1994;269(41):25437–41. [PubMed] [Google Scholar]
- 34.Creasey AA, Eppstein DA, Marsh YV, Khan Z, Merigan TC. Growth regulation of melanoma cells by interferon and (2′-5′)oligoadenylate synthetase. Mol Cell Biol. 1983;3(5):780–6. doi: 10.1128/mcb.3.5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells V, Mallucci L. Expression of the 2–5A system during the cell cycle. Exp Cell Res. 1985;159(1):27–36. doi: 10.1016/s0014-4827(85)80034-3. [DOI] [PubMed] [Google Scholar]
- 36.Wells V, Mallucci L. Cell cycle regulation (G1) by autocrine interferon and dissociation between autocrine interferon and 2′,5′-oligoadenylate synthetase expression. J Interferon Res. 1988;8(6):793–802. doi: 10.1089/jir.1988.8.793. [DOI] [PubMed] [Google Scholar]
- 37.Lee SH, Rozee KR. Variation of interferon production during the cell cycle. Appl Microbiol. 1970;20(1):11–5. doi: 10.1128/am.20.1.11-15.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzgerald-Bocarsly P, Dai J, Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008;19(1):3–19. doi: 10.1016/j.cytogfr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amit I, Garber M, Chevrier N, Leite AP, Donner Y, Eisenhaure T, et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science. 2009;326(5950):257–63. doi: 10.1126/science.1179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10(4):265–75. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- 41.Seeburg DP, Pak D, Sheng M. Polo-like kinases in the nervous system. Oncogene. 2005;24(2):292–8. doi: 10.1038/sj.onc.1208277. [DOI] [PubMed] [Google Scholar]
- 42.Chevrier N, Mertins P, Artyomov MN, Shalek AK, Iannacone M, Ciaccio MF, et al. Systematic discovery of TLR signaling components delineates viral-sensing circuits. Cell. 2011;147(4):853–67. doi: 10.1016/j.cell.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PNP regulates deoxynucleotide (dNTP) levels indirectly by maintaining pools of dNTP precursors and thus PNP enzyme activity impacts cell cycle and proliferation of T and B cells. PNP deficiency leads to accumulation of dGTP/GTP, and inhibition of ribonucleotide reductase (RNR) and consequently DNA synthesis and cell apoptosis. dGMP: deoxyguanosine monophosphate; dGDP: deoxyguanosine diphosphate; dGTP: deoxyguanosine triphosphate; GMP: guanosine monophosphate; GDP: guanosine diphosphate; GTP: guanosine triphosphate; NT: nucleotide; AMP: adenosine monophosphate; IMP: inosine monophosphate; 5′ NT: 5′ nucleotidase; ADA: Adenosine deaminase AK: Adenosine kinase; dCK: deoxycytidine kinase; GDA: Guanine deaminase; HGPRT: hypoxanthine-guanine-phosphoribosyltransferase; PNP: Purine nucleotide phosphorylase; XO: Xanthine oxidase; Enzymes: 5′ NT: 5′ nucleotidase; ADA: Adenosine deaminase; ADK: Adenosine kinase; AMPDA: AMP deaminase; APRT: Adenine phosphoribosyl transferase; dCK: deoxycytidine kinase; GDA: Guanine deaminase; HGPRT: hypoxanthine-guanine-phosphoribosyltransferase; IMPD: IMP dehydrogenase; PNP: Purine nucleotide phosphorylase; PP-ribose-P: 5-phosphoribosyl-1 pyrophosphate; RNR: Ribonucleotide reductase; XO: Xanthine oxidase