Abstract
There are many examples where the use of chemicals have had profound unintended consequences, such as fertilizers reducing crop yields (paradox of enrichment) and insecticides increasing insect pests (by reducing natural biocontrol). Recently, the application of agrochemicals, such as agricultural disinfectants and fungicides, has been explored as an approach to curb the pathogenic fungus, Batrachochytrium dendrobatidis (Bd), which is associated with worldwide amphibian declines. However, the long-term, net effects of early-life exposure to these chemicals on amphibian disease risk have not been thoroughly investigated. Using a combination of laboratory experiments and analysis of data from the literature, we explored the effects of fungicide exposure on Bd infections in two frog species. Extremely low concentrations of the fungicides azoxystrobin, chlorothalonil, and mancozeb were directly toxic to Bd in culture. However, estimated environmental concentrations of the fungicides did not reduce Bd on Cuban tree frog (Osteopilus septentrionalis) tadpoles exposed simultaneously to any of these fungicides and Bd, and fungicide exposure actually increased Bd-induced mortality. Additionally, exposure to any of these fungicides as tadpoles resulted in higher Bd abundance and greater Bd-induced mortality when challenged with Bd post-metamorphosis, an average of 71 days after their last fungicide exposure. Analysis of data from the literature revealed that previous exposure to the fungicide itraconazole, which is commonly used to clear Bd infections, made the critically endangered booroolong frog (Litoria booroolongensis) more susceptible to Bd. Finally, a field survey revealed that Bd prevalence was positively associated with concentrations of fungicides in ponds. Although fungicides show promise for controlling Bd, these results suggest that, if fungicides do not completely eliminate Bd or if Bd re-colonizes, exposure to fungicides has the potential to do more harm than good. To ensure that fungicide applications have the intended consequence of curbing amphibian declines, researchers must identify which fungicides do not compromise the pathogen resistance mechanisms of amphibians.
Keywords: Batrachochytrium salamandrivorans, agrochemicals, pesticides, biocontrol, parasite, chytrid fungus
Introduction
Synthetic chemicals have had enormous value to society, such as by treating and curing diseases and revolutionizing agricultural production. However, they can also have unanticipated consequences (Pimentel et al. 1973). In fact, there are several examples where applications of chemicals to the environment have had exactly the opposite effects than were intended. For example, the application of fertilizers can destabilize crop herbivore dynamics resulting in larger herbivore outbreaks that, in some years, can result in zero crop yields, a phenomenon called the paradox of enrichment (Rosenzweig 1971). The overuse of antibiotics in intensified livestock operations and medicine has resulted in antimicrobial resistance and perhaps an even more challenging public health crisis to manage (Young et al. 2016). Similarly, the application of insecticides has, in some cases, actually increased pests by having more adverse effects on arthropod predators of the pests than on the pests themselves, thus adversely affecting natural biocontrol (Desneux et al. 2007, Douglas et al. 2015). Additionally, there are numerous examples where chemicals can have substantial non-target effects that can disrupt rather than enhance ecosystem functions and services (McMahon et al. 2012, Halstead et al. 2014, Staley et al. 2014). These examples highlight the need to comprehensively understand the complex effects that chemicals can have on ecosystems to avoid inadvertent and undesirable ramifications.
Recently, several researchers have explored the effects of pesticides on the chytrid fungus, Batrachochytrium dendrobatidis (Bd), a pathogen associated with worldwide amphibian declines (Kilpatrick et al. 2010). For example, studies have explored how Bd is affected by herbicides (Gahl et al. 2011, McMahon et al. 2013, Rohr et al. 2013, Buck et al. 2015, Jones et al. 2016), insecticides (Davidson et al. 2007, Gaietto et al. 2014, Buck et al. 2015, Jones et al. 2016), fungicides (Johnson et al. 2003, Woodhams et al. 2012, McMahon et al. 2013, Gaietto et al. 2014, Hudson et al. 2016), and agricultural disinfectants (Johnson et al. 2003, Bosch et al. 2015). Additionally, many researchers are exploring biocides as management tools to control Bd (Woodhams et al. 2011). For example, Hanlon et al. (Hanlon et al. 2012) showed that the common agricultural fungicide thiophanate-methyl cleared frogs of Bd and had positive effects on their health, and Woodhams et al. (Woodhams et al. 2011) highlighted several studies where fungicides and disinfectants were being explored as management tools. More specifically, Bosch et al. (2015) successfully eradicated Bd from a field site using a fungicide and an agricultural disinfectant, and Hudson et al. (2016) temporarily reduced fungal loads on amphibians in the wild using in situ exposure to a fungicide. Thus, chemicals, such as fungicides and disinfectants with fungicidal properties, show exciting promise for managing amphibian chytridiomycosis. However, the long-term, net effects of early-life exposure to these chemicals on amphibian disease risk have not been thoroughly investigated.
In addition to intentional exposure to products with fungicidal properties to manage Bd, amphibians are also regularly and unintentionally exposed to fungicides from direct overspray, drift, and run-off (Maltby et al. 2009, McMahon et al. 2011). Fungicides have experienced a greater increase in use in the last two decades than herbicides and insecticides. For example, in the US from 2004-2005, only 2% of all corn, soybean, and wheat fields were sprayed with fungicides, but this number increased to nearly 30% by 2009 (Belden et al. 2010). The soybean industry estimates that 60 to 70% of soybean seed planted in 2014 had a fungicide seed treatment, compared to 30% in 2008 and 8% in 1996 (http://unitedsoybean.org/article/six-things-farmers-should-know-about-seed-treatments/). Additionally, in the last 15 years in the US, an average of two new fungicides were registered for use each year, and in 2011 alone, the US Environmental Protection Agency reviewed the registration of 14 fungicides for 49 new uses, and all of the 14 fungicides are in use today (Battaglin et al. 2011).
Despite the widespread and increasing use of fungicides, there are few studies of the direct and indirect effects of fungicides on aquatic ecosystems (but see McMahon et al. 2011, McMahon et al. 2012) compared to the copious research on insecticides and herbicides (e.g. Relyea 2005, Rohr and Crumrine 2005, Rohr and McCoy 2010). For instance, a review of the indirect effects of pesticides on aquatic food webs found that only two out of the 150 research articles written from 1970–2002 addressed the use of a fungicide (Fleeger et al. 2003). Fungicides also are often very broad spectrum, affecting common and vital physiological processes, such as cellular respiration and immunity (Maltby et al. 2009). Additionally, modeling and monitoring suggest that fungicides might accumulate in freshwater habitats to levels above those considered safe for chronic exposure of some aquatic organisms (Deb et al. 2010).
The effects of pesticides on host-parasite interactions in particular can be extremely complex, having both positive and negative effects. As mentioned, several studies have shown that fungicides can be directly toxic to Bd (Hanlon and Parris 2012, McMahon et al. 2013). Chemical contaminants can also have negative effects on hosts by altering host immunity or parasite virulence (Relyea and Hoverman 2006, Rohr et al. 2006a, Jayawardena et al. 2016). Indeed, many pesticides are known to be immunomodulators (Voccia et al. 1999, Rohr et al. 2008b, Rohr and McCoy 2010, Rohr et al. 2015) and thus might have unfavorable effects on amphibian disease risk. Given the complexity of these direct and indirect effects, it is not surprising that studies exploring the effects of pesticides on Bd infections have produced mixed results (Davidson et al. 2007, Hanlon et al. 2012, McMahon et al. 2013, Buck et al. 2015, Hanlon et al. 2015, Jones et al. 2016).
Ultimately, what must be quantified is the sum of the positive and negative effects of contaminants to assess an overall or net effect (Rohr et al. 2008a). Additionally, net effects must be considered across life stages because exposure to chemicals early in life can have effects that persist into adulthood (e.g. Rohr and Palmer 2005, Rohr et al. 2006b). As an example, although the pesticide atrazine was directly toxic to Bd in culture (McMahon et al. 2013), the net effect of amphibian early-life exposure to this pesticide was an increase in Bd-induced mortality because it had adverse effects on frog tolerance of infections that occurred later in life (Rohr et al. 2013).
Here, we use a combination of experiments and analysis of data from the literature to explore the net effects of exposure to four common fungicides on Bd infections in two frog species. To test for direct effects, we exposed Bd in culture to three commonly used fungicides each at five ecologically relevant concentrations. To test for indirect and persistent effects of fungicides, we conducted a laboratory experiment where tadpoles were exposed to these three fungicides or a control and challenged with Bd during the fungicide exposure period and/or after metamorphosis. To compare our results on these three fungicides used commonly in agriculture to itraconazole, the fungicide commonly used to clear amphibians of Bd (Garner et al. 2009, Berger et al. 2010), we analyzed data from the literature on the effects of itraconazole on susceptibility to Bd. Finally, to evaluate the relevance of our results to natural settings, we tested whether fungicide levels in waterbodies and frog tissues were correlated with the prevalence of Bd infections.
We predicted that each fungicide would be directly toxic to Bd. Given that most of these fungicides are documented to suppress immune responses of vertebrates (Colosio et al. 1996, Corsini et al. 2006, McMahon et al. 2011), we also expected that exposure to each fungicide would alter amphibian-Bd interactions (indirect effect). The direction of the net effect of fungicide exposure, however, was challenging to predict a priori. Additionally, we predicted that if any fungicide delayed metamorphosis, it would increase Bd-loads on frogs by increasing the amount of time tadpoles were exposed to this predominantly aquatic fungus.
Background on pathogen and fungicides
Bd is a pathogenic chytrid fungus that causes chytridiomycosis in many amphibians. It is a major contributor to global amphibian declines and has been found on six continents (Kilpatrick et al. 2010). Bd infects amphibians by colonizing keratin-containing body regions, infecting the mouthparts of anuran tadpoles and later spreading to the skin as the skin becomes keratinized during metamorphosis (McMahon and Rohr 2015). Infection does not usually cause tadpole mortality, but post-metamorphic anurans can be extremely susceptible to Bd (McMahon et al. 2013, Gervasi et al. 2017) because the infection disrupts osmotic and electrolyte balance that is controlled by their skin, which can eventually lead to cardiac arrest (Voyles et al. 2009). Cellular immunity is an important defense against Bd and despite Bd being immunosuppressive, amphibians can acquire immunological resistance to Bd that overcomes this immunosuppression (McMahon et al. 2014).
Four fungicides were studied here: azoxystrobin, chlorothalonil, mancozeb, and itraconazole. The first three fungicides rank among the top five in the United States based on usage (Grube et al. 2011). Azoxystrobin has experienced a recent increase in usage in the US in the last couple of decades to combat the emergence of soybean rust and is also used commonly on grain crops (Battaglin et al. 2011). Chlorothalonil is used on a wide variety of crop species, as well as residential and golf course turf (Caux et al. 1996), and is the fungicide most commonly used in Latin America, a place where Bd effects on amphibians have been severe (Ghose et al. 2014). Mancozeb is primarily applied to potatoes to reduce fungal pathogens and is also sprayed on other food crops (Maltby et al. 2009, Grube et al. 2011). Azoxystrobin and chlorothalonil inhibit cellular respiration, and mancozeb disrupts lipid metabolism (Caux et al. 1996, Maltby et al. 2009, Battaglin et al. 2011), and thus all three chemicals have modes of action that affect vital physiological processes of many organisms, from bacteria to vertebrate animals. The half-lives and estimated peak environmental concentrations (based on US EPA EXAMS-PRZM software) of azoxystrobin, chlorothalonil, and mancozeb are 11-17 d, 1 to 48 h, and 1-2 d and ~2 ppb, ~164 ppb, and ~58 ppb, respectively.
Itraconazole is not heavily used in agriculture but is used in the medical and veterinary fields and is probably the most commonly used fungicide to clear Bd infections of amphibians (KuKanich 2008, Garner et al. 2009, Berger et al. 2010). Its mode of action is to inhibit fungal-mediated synthesis of ergosterol, and it can also inhibit cytochrome P450, which is important in metabolizing potentially toxic compounds (KuKanich 2008).
Materials and Methods
Preparation of Bd inoculum
Bd inoculum was prepared by adding 1 mL of Bd stock (isolate SRS 812 isolated from a Rana catesbeiana in 2006 captured near the Savannah River Ecology Lab, SC, USA, passed through culture ~12 times) cultured in 1% tryptone broth, to a 1% tryptone agar plate. The plates were maintained at 23°C (estimate optimal temperature for growth for this culture) for approximately 1 week to allow Bd proliferation. Plates were inspected microscopically to verify that the zoospores were viable (swimming) and then they were then flooded with ultrapure water to suspend the zoospores. Water from the multiple agar plates was homogenized to create the Bd positive (Bd+) solutions. Zoospore density was standardized among replicates and concentrations above the target concentration were diluted with ultrapure water. The Bd negative (Bd−) solution was created using the same method, except that no Bd was added to the 1% tryptone agar plates.
Effects of fungicides on Bd in culture
To test for direct effects of the fungicides on Bd in culture, we used methods developed by McMahon et al. (2013). In a sterile, laminar flow hood, 10-mL glass test tubes were filled with Bd+ solution (total concentration: 3.8×104 zoospores/mL), 1% tryptone broth, and one of four fungicide stocks (0.001×, 0.01×, 0.1×, 1.0×, and 10×estimate environmental concentration [EEC] or less for each of the three fungicides) or two control treatments (water and acetone solvent controls). Each treatment (concentration of each fungicide) was replicated 5 times for a total of 25 experimental units per fungicide, plus 10 control replicates. Test tubes were maintained at 23°C for 10 d after which Bd quantities were quantified by counting a 10-µL aliquot of Bd with a hemocytometer.
Effects of fungicides on Bd growth on frogs in the laboratory
The objectives of this experiment were to test for (1) the direct effects of fungicides on amphibians, (2) indirect effects of fungicides mediated by Bd, and (3) persistent effects of fungicide exposure on amphibian susceptibility to Bd. To accomplish these objectives, we collected Cuban tree frog tadpoles (Osteopilus septentrionalis) from a pond near the University of South Florida Botanical Gardens during the month of September 2012. We chose this frog species because it is common locally but is susceptible to chytrid infections (Rohr et al. 2013). In summary, we conducted a 4 × 2 × 2 study with exposure to one of four fungicide treatments during larval development (azoxystrobin, chlorothalonil, mancozeb, solvent control) crossed with Bd exposure or not during larval development crossed with Bd exposure or not after metamorphosis (see below; Table S1, Fig. S1).
Each individual tadpole was weighed and staged (Gosner 1960) before the start of the experiment; stages and weights were equally distributed across all treatments. Individual tadpoles were exposed to treatments in a 500-mL glass jar filled with 300 mL of artificial spring water (ASW, Cohen et al. 1980). Throughout the experiment, animals were fed fish flakes and Sera Micron ad libitum.
The four fungicide treatments were an acetone control or the EEC of azoxystrobin (2.06 µg/L), chlorothalonil (164 µg/L, we used the concentration of 30.0 µg/L because 164 µg/L will kill most of the frogs; McMahon et al. 2011, 2012), or mancozeb (57.6 µg/L). The treatments were applied to the water in each jar at the start of the experiment. These treatments were re-applied with every water change, which occurred weekly until metamorphosis or up to 12 weeks.
The Bd exposures occurred during the tadpole stage (simultaneous exposure with the fungicide treatments), after metamorphosis (delayed exposure; after fungicide treatments), both during the tadpole stage and after metamorphosis (double exposure; during and after fungicide treatments), or not at all (sham-exposed during both life stages). For the tadpole exposures, a 1-mL Bd inoculum (2.88 × 106 – 4.25×106 zoospores/mL) was added to the appropriate jar during weeks 1 and 3 of the experiment. All animals that were randomly assigned not to receive Bd during a given exposure period received a sham Bd exposure (see Preparation of Bd stocks).
The tadpoles were checked daily for metamorphosis or mortality. All animals that metamorphosed were swabbed (snout to vent and down each leg 5 times each), weighed, and maintained individually in 1-L plastic deli cups at 23°C. Each post-metamorphic juvenile frog received vitamin- and mineral-dusted crickets ad libitum and weekly changes of wet papers towel. All animals that died were swabbed, weighed, and preserved. After 12 weeks, any tadpoles that had not metamorphosed were swabbed (mouthparts only; see McMahon and Rohr 2015) and euthanized using 0.5% MS-222. Animals were then weighed, staged, and snout-vent length (SVL) was measured. The mouthparts were removed and stored in 95% ethanol to later measure Bd loads.
At week 22, all metamorphic frogs were exposed to either 1mL of Bd+ inoculum (1.69×106 zoospores/mL) or 1mL of the Bd− inoculum. For animals that were exposed only to a fungicide or control treatment as tadpoles, this was their first exposure to Bd; for the simultaneous Bd-fungicide treatment animals, this was the second Bd exposure. Five weeks after post-metamorphic exposure to Bd or the sham inoculum, the right hind limb (15 times hip to toe) of all frogs were swabbed. To optimize Bd growth, all frogs were moved to 17°C at week 32; all frogs were swabbed (15 times hip to toe) and then euthanized during week 38 (Fig. 2b).
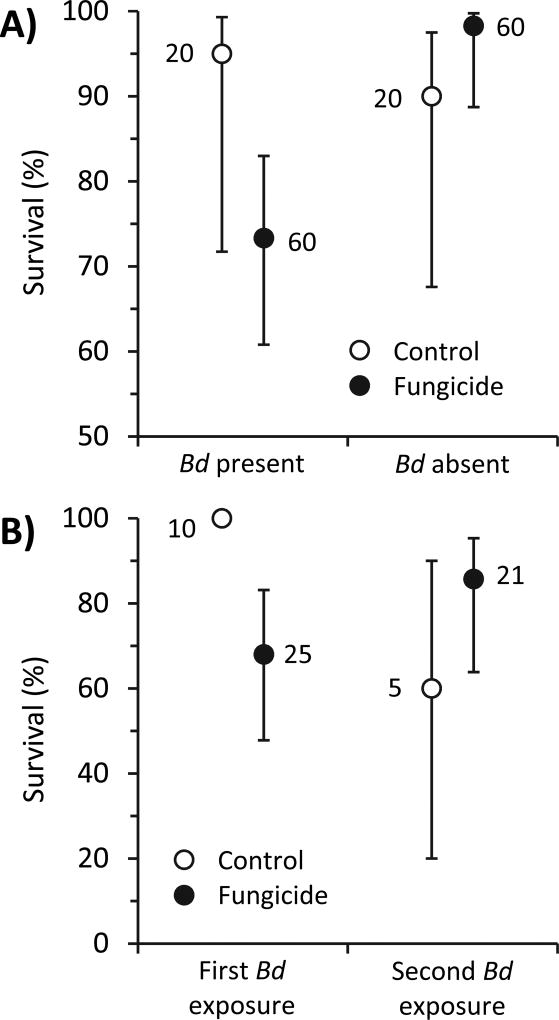
Fig. 2.
Mean (± 95% CI) survival of Cuban tree frogs exposed A) to fungicide and Batrachochytrium dendrobatidis (Bd) treatments simultaneously as tadpoles (Fungicide × Bd: Χ2 = 6.47, P=0.011) or B) to fungicides treatments as tadpoles and then to Bd for the first or second time as a post-metamorphic frog (a mean of 71 days after their last exposure to fungicide; Fungicide × number of Bd exposures: Χ2 = 6.89, P=0.009). There were no statistically significant differences among the three tested fungicides (azoxystrobin, chlorothalonil, mancozeb) and thus they were pooled for subsequent analyses to increase statistical power and facilitate visualization. Numbers next to data points are associated sample sizes (which vary because of mortality and re-assignment of frogs to appropriate treatments if they died before receiving their assigned Bd exposure; see text for details).
Both swabs and tissues from the mouthparts of tadpoles provided DNA to quantify Bd abundance. Bd DNA was amplified through qPCR to calculate Bd infection abundance. All qPCR was performed according to Hyatt et al. (2007), using StepOne™ Real-Time PCR System (qPCRStepOne™).
We initially had 10 replicate frogs for each of these 16 treatments (160 frogs total). However, if a frog was assigned to receive a Bd exposure after metamorphosis and it died before this exposure, the animal was re-assigned to the appropriate treatment. For example, if a frog was assigned to receive Bd exposure both before and after metamorphosis but died before receiving the second Bd exposure after metamorphosis, it was shifted from the double Bd exposure treatment to the Bd before metamorphosis-only treatment. Likewise, if a frog was assigned to only receive Bd after metamorphosis and it died before receiving this treatment, it was reassigned to the no Bd before or after metamorphosis treatments. Hence, this had the effect of increasing sample sizes in some groups while decreasing the sample size in others. Sample sizes ended up as follows: simultaneous exposure n=53; delayed exposure n = 35; double exposure n=25; sham-exposure n=42.
Effects of the fungicide itraconazole on Bd growth on frogs in the laboratory
Itraconazole is probably the most commonly used fungicide to clear frogs of Bd because of the existence of established amphibian application protocols (Garner et al. 2009, Berger et al. 2010) and thus is extremely relevant to the amphibian-Bd system. To evaluate whether itraconazole had similar effects as azoxystrobin, chlorothalonil, and mancozeb and to provide a test of the effects of fungicides on Bd infections by a completely independent laboratory (adding to the weight of evidence), we searched the literature for studies that exposed amphibians to itraconazole and then challenged them with Bd. Cashins et al. (2013) conducted a study on the critically endangered booroolong frogs (Litoria booroolongensis) that satisfied this search criteria. However, they did not test for the effects of itraconazole on Bd prevalence in their study (see Data analysis for analyses).
Effects of the fungicides on Bd prevalence in the field
To investigate for an association between fungicide exposure and Bd infections, we collected water, bed sediment, Bd, and frog tissue samples concurrently from 21 sites in seven states (CA, CO, GA, ID, LA, ME, and OR) in 2009 and 2010 and quantified Bd and fungicide concentrations. Details on the site locations, characteristics, and sampling design are provided in detail in Battaglin et al. (2016) and Smalling et al. (2015) and thus are only briefly covered here. Approximately 1 L of water and a stainless steel scoop of bed sediment were collected from each site to quantify pesticide or pesticide degradates. One to 15 adult frogs were collected at each site (see Results for details on frogs species collected) by hand or net and swabbed for Bd (Hyatt et al. 2007). The frogs were euthanized, wrapped in aluminum foil, and then placed in a freezer for later whole-body analysis of pesticides and pesticide degradates (Battaglin et al. 2016).
Filtered water samples were analyzed for a suite of pesticides and pesticide degradates by extracting onto a solid phase extraction cartridge, spiking the samples with a recovery surrogate, eluting the cartridge with ethyl acetate, adding a deuterated internal standard, and analyzing the extracts on an Agilent (Santa Clara, CA) 7890 gas chromatograph coupled to an Agilent 5975 (Folsom, CA) mass spectrometer. Data for all pesticides were collected in selective ion monitoring mode with each compound having one quantifier ion and one to two qualifier ions. Wet sediments (10 g) were analyzed similarly to the water samples with the following exceptions. The sediment was homogenized with sodium sulfate, extracted using pressurized liquid extraction, dried over sodium sulfate, reduced, and sulfur was removed by gel permeation chromatography. Samples were subjected to a clean-up method with 6% deactivated Florisil. Frog tissue samples were analyzed similarly to the water samples with the following exceptions. Individual whole frogs (3–5 g) were thawed, homogenized with sodium sulfate (Na2SO4) using a mortar and pestle, extracted three times with dichloromethane using pressurized liquid extraction, dried over Na2SO4, and reduced to 1 mL. Ten percent by volume of each raw extract was allowed to evaporate to a constant weight in a fume hood for gravimetric lipid determination to the nearest 0.001 g using a microbalance. A majority of the lipid was removed using gel permeation chromatography followed by 6% deactivated Florisil previously activated at 550 °C for 16 h (Battaglin et al. 2016).
Data analysis
All statistical analyses were conducted using R statistical software. For the study that examined Bd responses to three fungicides, we conducted analyses on each of the three fungicides separately. We tested for a relationship between fungicide concentration and Bd abundance (rounded zoospore equivalents) using a negative binomial distribution (function: glmmadmb, package: glmmadmb). We tested for differences among the concentrations using a sequential Bonferroni adjustment.
For the laboratory study on frogs, we used a generalized linear model with a binomial error distribution to determine whether the fungicide treatments, Bd treatments, and their interaction significantly affected whether a frog lived or died during the experiment, and used a factorial ANOVA to evaluate how treatments affected log mass at and time to metamorphosis. Additionally, we conducted two analyses on Bd abundance. First, we compared the loads of frogs exposed to Bd for the first time across fungicide treatments to see if simultaneous exposure to Bd and fungicides resulted in different fungal loads than early-life exposure to fungicides and later-life exposure to Bd. For frogs exposed simultaneously to Bd and fungicide treatments, the response variable was Bd load at metamorphosis or Bd load at death if they did not reach metamorphosis. For frogs exposed to Bd after they were exposed to fungicide treatments (i.e. after metamorphosis), we used the mid-survey (experimental day 155) Bd load or Bd load at death if they did not reach the mid-survey swabbing. Second, we compared the Bd load of post-metamorphic frogs receiving their first and second exposures to Bd using the mid-survey swabs. In all analyses treating Bd abundance on the frogs as a response, we included fungicide treatments, timing of Bd exposures, their interaction, and initial mass (unless it was not significant) as fixed effects. We analyzed Bd abundance using a zero-inflated negative binomial model (it was a better fit [AIC=1009] than the negative binomial model [AIC=1038]; function: zeroinfl, package: pscl).
To test whether the fungicide itraconazole was immunosuppressive to booroolong frogs in the Cashins et al. (2013) study, we applied a Chi square analysis to their data to compare Bd prevalence of frogs previously exposed to itraconazole or not.
Finally, to evaluate how fungicides affected Bd infections in the field, we used multiple regression with a binomial error distribution (function: glm, package stats) to test how the concentration of fungicides in frog tissues, sediment, and water affected Bd prevalence and quantify the fit of this model using the Nagelkerke Index (Nagelkerke 1991). We did not analyze Bd abundance data because we did not know the time course of infection for each frog, and thus we conservatively focused on prevalence. However, we did evaluate whether any differences among frog species in their fungicides or Bd loads could account for any detected patterns between fungicides and Bd prevalence. All p-values were calculated using log-likelihood ratio tests (function: Anova, package: car).
Results
Effects of fungicides on Bd in culture
Relative to the acetone control, all tested concentrations of azoxystrobin and chlorothalonil (Concentration: X2=19.96, p<0.001 and X2=15.22, p<0.001, respectively; Table 1) and all tested concentrations of mancozeb except the lowest concentration (Concentration: X2=6.81, p=0.009; Table 1) significantly reduced Bd abundance in culture relative to the controls.
Table 1.
Effects of various concentrations of the fungicides azoxystrobin, chlorothalonil, and mancozeb on the abundance on Batrachochytrium dendrobatidis zoospores cultured in welled plates.
| Concentration (µg/L) | Mean log10 zoospores/mLa |
Standard error |
Sequential Bonferronib |
|
|---|---|---|---|---|
| Azoxystrobin | ||||
| 0.000 | 5.65 | 0.15 | a | |
| 0.002 | 1.03 | 1.03 | b | |
| 0.020 | 0.00 | 0.00 | b | |
| 0.206 | 2.27 | 0.94 | b | |
| 2.060 | 0.00 | 0.00 | b | |
| 20.600 | 0.00 | 0.00 | b | |
| Chlorothalonil | ||||
| 0.000 | 5.65 | 0.15 | a | |
| 0.030 | 0.85 | 0.85 | b | |
| 0.300 | 1.04 | 1.04 | b | |
| 3.000 | 1.53 | 0.95 | b | |
| 30.000 | 1.75 | 1.08 | b | |
| 300.000 | 0.00 | 0.00 | b | |
| Mancozeb | ||||
| 0.000 | 5.65 | 0.15 | a | |
| 0.057 | 4.81 | 0.45 | a,b | |
| 0.570 | 1.04 | 1.04 | b | |
| 5.700 | 3.70 | 0.99 | b | |
| 57.600 | 0.00 | 0.00 | c | |
| 576.000 | 0.68 | 0.68 | c | |
These means are from five independent samples per concentration.
Concentrations that do not share a letter are significantly different from one another based on post hoc tests among concentrations within a fungicide.
Effects of fungicides on Bd growth on frogs in the laboratory
There were no significant effects of the fungicides, Bd treatments, or their interactions on time to metamorphosis or mass at metamorphosis (Tables S1 & S2). For the Bd analyses, we first tested for an effect of fungicide treatments on Bd abundance on frogs that were exposed to Bd for the first time, which included frogs exposed to Bd and the fungicide treatments simultaneously and frogs exposed to fungicide treatments as tadpoles but to Bd an average of 71 days after metamorphosis. In these analyses, frogs were not all swabbed at the same time because they metamorphosed or died at different times. Duration of time that Bd grew on the frogs was never a significant predictor in the models (X2=2.97, p>0.225). However, whether a frog was swabbed at death was always a significant predictor (p<0.05) because frogs that died from infection had, on average, more Bd than frogs swabbed while alive. Therefore, we included whether a frog was swabbed at death as a categorical factor rather than time for Bd growth in each model. Initial mass was included in most models because it was generally a negative predictor of Bd intensity.
In these analyses, mean Bd abundance on frogs never differed significantly among the three fungicide treatments, regardless of whether Bd exposure occurred simultaneous with the fungicide exposure (X2=2.65, p=0.618) or later in life (X2=0.61, p=0.961; Table S3). In addition, exposure to any of the three fungicides resulted in significantly more Bd on frogs compared to frogs exposed to the solvent control (Fungicide main effect: p<0.05; Table S3). Hence, we pooled the fungicides together for ease of interpretation and to increase statistical power. There was no significant effect of the fungicides on Bd abundance when the fungicide and Bd exposures occurred simultaneously as tadpoles (Fig. 1A); however, when the Bd exposure occurred after metamorphosis, an average of 71 days since the previous exposure to fungicides, the previous fungicide exposure caused a nearly 3.5-fold increase Bd abundance on frogs relative to the solvent controls (Fungicide×timing of Bd exposure: X2=25.98, p<0.001; Fig. 1A).
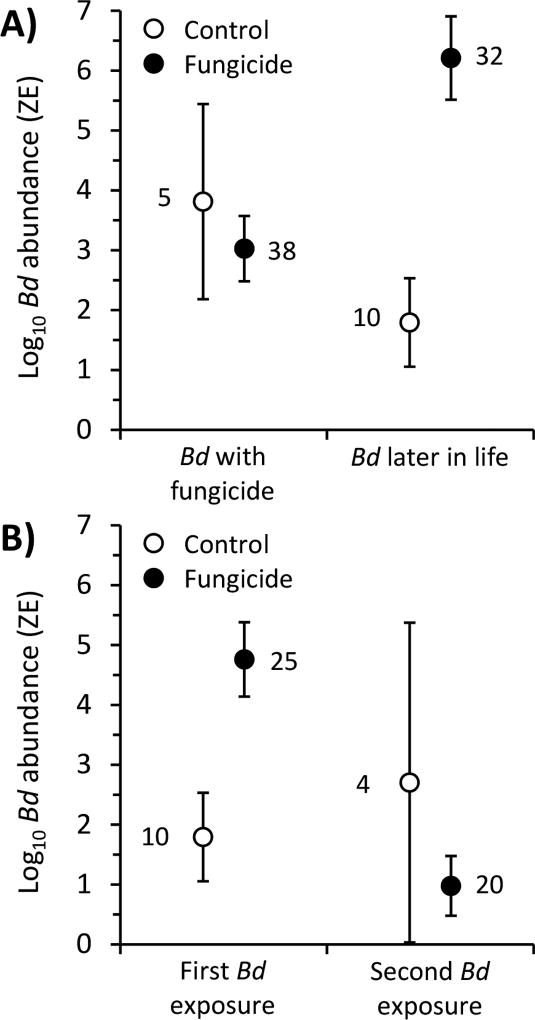
Fig. 1.
Effects of fungicide treatments on mean (± 1 SE) log10 Batrachochytrium dendrobatidis (Bd) abundance on Cuban tree frogs A) exposed to Bd for the first time as tadpoles simultaneous with the fungicide treatments versus for the first time as post-metamorphic frogs, 71 days after the previous exposure to fungicide treatments (Fungicide × timing of Bd exposure: X2=25.98, p<0.001), and B) exposed to Bd for the first versus second time as a post-metamorphic frog (Fungicide × number of Bd exposures: X2=15.47, p<0.001). There were no statistically significant differences among the three tested fungicides (azoxystrobin, chlorothalonil, mancozeb) and thus they were pooled for subsequent analyses to increase statistical power and facilitate visualization. Numbers next to data points are associated sample sizes (which vary because of mortality and re-assignment of frogs to appropriate treatments if they died before receiving their assigned Bd exposure; see text for details).
We also compared the Bd load of post-metamorphic frogs receiving their first and second exposures to Bd. These analyses revealed a significant two-way interaction between the fungicide treatment and number of Bd exposures (X2=15.47, p<0.001). Frogs exposed to fungicide and Bd for the first time had higher Bd loads than frogs exposed to solvent and Bd for the first time (X2=12.85, p=0.002; Fig. 1B, Table S4). However, the opposite trend occurred on the second exposure. Frogs exposed to fungicide and Bd for the second time had lower Bd loads than frogs exposed to the solvent and Bd for the second time, although not significantly so because of low sample size and high variability in the control (X2=2.95, p=0.229; Fig. 1B, Table S4).
For the analyses on survival, we conducted binomial survival analyses that assessed how treatments affected the probabilities of surviving the length of the experiment. In these analyses, mean mortality of frogs exposed to the three fungicides never differed, regardless of whether Bd exposure occurred simultaneous with the fungicide exposure (X2=1.33, p=0.515), later in life (delayed) (X2=0.23, p=0.892), or both (double exposure; X2=2.35, p=0.309). However, for the simultaneous and delayed exposure treatments, each fungicide caused significantly greater mortality than the solvent control (p<0.04). Hence, we again pooled the fungicides for ease of interpretation and to increase statistical power.
We compared Bd-induced mortality in the fungicide versus control treatments for frogs exposed to Bd for the first time. Despite not having significantly more Bd than controls, tadpoles exposed simultaneously to fungicide and Bd had greater mortality than tadpoles exposed to solvent, fungicides alone, and Bd alone, resulting in a significant interaction between fungicide and Bd treatments (Fungicide*Bd: X2=6.47, p=0.011; Fig. 2A). A similar but more pronounced pattern was observed when the Bd exposure occurred after metamorphosis, there was significantly greater Bd-induced mortality if frogs were previously exposed to fungicide than solvent control (Fungicide*Bd: X2=6.28, p=0.012; Fig. 2B).
We also compared the Bd-induced mortality of post-metamorphic frogs receiving their first and second exposures to Bd. If metamorphs were exposed to Bd for the first time, there was greater Bd-induced mortality among frogs previously exposed to fungicide than solvent control (X2=6.28, p=0.012, Fig. 2B). However, similar to our Bd abundance results, the opposite trend was observed when metamorphs were exposed to Bd for the second time; there was greater Bd-induced mortality among frogs previously exposed to solvent control than fungicides, although not significantly so (X2=1.51, p=0.220; Fig. 2B). This resulted in a significant interaction between fungicide treatment and number of Bd exposures (X2=6.90, p=0.009) because there was a significant effect during the first exposure and not during the second exposure.
Effects of the fungicide itraconazole on Bd growth on frogs in the laboratory
Booroolong frogs previously exposed to itraconazole in the Cashins et al. (2013) study had significantly higher Bd prevalence when exposed to Bd later in life (91%, 10/11 frogs) than frogs not previously exposed to itraconazole (50%, 14/28 frogs; X2=5.58, p=0.018).
Effects of the fungicides on Bd growth on frogs in the field
Twelve different fungicides were detected in the 21 wetlands that were sampled (Table S5). The three most common fungicides detected in sediment were pyraclostrobin (52%), chlorothalonil (33%), and tebuconazole (24%), and the only fungicide detected from water samples at multiple sites was azoxystrobin (19%, Table S5). Given the infrequency in which individual fungicides were detected, we combined fungicide concentrations for the analyses.
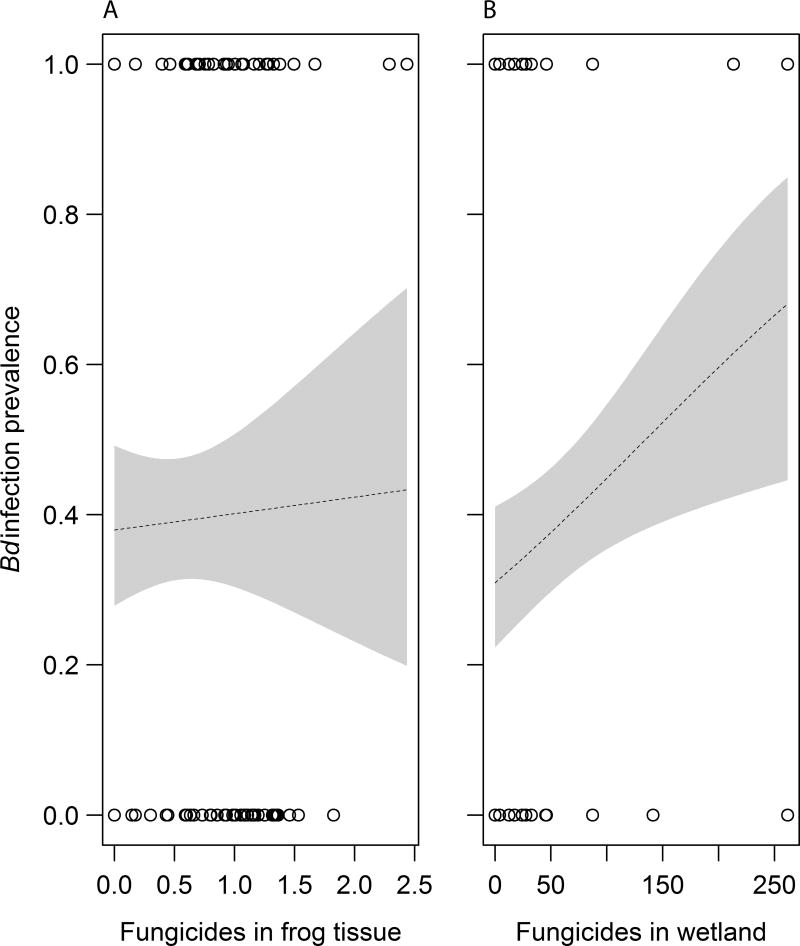
A total of 138 frogs were swabbed for Bd and also had fungicides quantified from their tissues. Of these frogs, 54 were positive for Bd and 72 had quantifiable levels of fungicides in their tissues. Only four wetlands had detectable levels of fungicides in the water column, whereas all but three had detectable levels in sediment (Table S5). Given how few wetlands had detectable fungicide in the water column, we chose to simply add the water column and sediment concentrations to reflect overall environmental exposure, but admit that much of this exposure appears to be through sediment. Bd prevalence on frogs was not significantly related to the concentration of fungicides in frog tissues (X2=0.03, p=0.858, Nagelkerke Index=0.001, Fig. 3A), but was positively related to the concentration of fungicides (sediment plus water) in waterbodies (X2=7.10, p=0.008, Nagelkerke Index=0.069, Fig. 3B). Two genera of frogs were captured, Pseudacris (65%) and Rana (35%; Fig. S2). Five species were captured but 65% were just two species, Pseudacris regilla and P. triseriata (Fig. S2). Loads of Bd were higher for genus Pseudacris than Rana, but Pseudacris was less likely to be found at sites with high concentrations of fungicides (Fig. S2). Thus, differences in Bd loads across genera do not seem capable of accounting for the detected positive relationship between fungicides and Bd (Fig. 3).
Fig. 3.
Relationship between the prevalence of Batrachochytrium dendrobatidis (Bd) infections of 138 frogs and the concentration of fungicides in frog tissues (log10(µg/kg+1)-transformed, X2=0.03, p=0.858, A) and in wetlands (sediment [µg/kg] plus water [µg/L], X2=7.10, p=0.008, B). Shown are logistic regression plots and associated 95% confidence bands (shaded).
Discussion
When tadpoles were exposed to both a fungicide and Bd simultaneously in the laboratory, none of the three fungicides affected Bd loads on the frogs compared to the no-fungicide control. In contrast, frogs exposed to Bd as metamorphs, an average of 71 days since any fungicide exposure, had significantly greater Bd abundance and greater Bd-induced mortality than frogs exposed to the solvent control. The type of applied fungicide did not matter with all samples exhibiting the same reaction to the three fungicides, azoxystrobin, chlorothalonil, and mancozeb. Research from a completely independent laboratory on the critically endangered booroolong frog found exactly the same results for itraconazole (Cashins et al. 2013), the most commonly used fungicide to clear frogs of Bd (Garner et al. 2009, Berger et al. 2010). Booroolong frogs previously exposed to itraconazole had significantly greater Bd prevalence when challenged with Bd later in life than frogs not previously exposed to itraconazole. Hence, despite four commonly used fungicides being directly toxic to Bd (see Table 1), all paradoxically increased Bd infections by having persistent adverse effects on frog resistance to this fungal pathogen.
Interestingly, the increase in Bd abundance and Bd-induced mortality associated with fungicide exposure in our laboratory study was greater if we exposed the frogs to Bd an average of 71 days after the fungicide exposure than if we exposed the frogs to Bd and fungicide simultaneously. This result is probably a product of two factors. First, the culture experiment demonstrated that all three tested fungicides are directly toxic to Bd and thus almost certainly reduced the abundance of Bd on frogs when the two occurred simultaneously. However, any direct toxicity to Bd was clearly not as strong as the adverse effect of the fungicides on the fungal defenses of the frogs. Second, Bd is believed to consume keratin, which is only found on the mouthparts of tadpoles but is throughout the skin of metamorphs (McMahon and Rohr 2015). Hence, Bd might also be able to proliferate more rapidly after metamorphosis, amplifying the effect of fungicide exposure more so after than before metamorphosis. Given that the fungicides did not affect timing of or size at metamorphosis, these traits seem unlikely to explain any observed effects.
The observed persistent effects of early-life exposure to fungicides on infectious disease risk are consistent with several previous toxicological studies. Several pesticides have been shown to cause changes in host-parasite dynamics (Relyea and Hoverman 2006, Rohr et al. 2006a, Rohr and McCoy 2010) and have delayed effects on host behavior, growth, physiology, and survival (e.g. Rohr and Palmer 2005, Rohr et al. 2006b, Jones et al. 2009, Rohr et al. 2013). Similar to our findings, other studies have shown that fungicides can be directly toxic to Bd (Hanlon and Parris 2012, McMahon et al. 2013). In contrast to our work, some of these previous studies revealed that fungicide exposures can actually reduce Bd growth rates on frogs, but these previous studies did not test for the effects of sequential fungicide and Bd exposures and did not test for persistent adverse effects of early-life exposure to fungicides (Hanlon et al. 2012, Hanlon and Parris 2012, McMahon et al. 2013, Hanlon et al. 2015).
There are several potential mechanisms by which early-life exposure to chemicals can have persistent effects on infectious disease risk. Pesticide exposure early in life can induce stress responses, elevating cortisol, corticosterone, or other stress-related hormones; chronic levels of these hormones have been associated with persistent immunomodulation (Martin et al. 2010, McMahon et al. 2011, McMahon et al. 2017). Chemical contaminants have also been shown to disrupt the microbiome of hosts. The gut microbiome has been linked to immune development in vertebrates (Hooper et al. 2012) and the skin microbiome in amphibians has been shown to inhibit the growth of Bd (Bletz et al. 2013). Two recent studies revealed that tadpole exposure to chemical contaminants reduced their gut and skin microbiota and reductions in gut microbiota were associated with reduced resistance to skin-penetrating gut nematodes and Bd later in life (Knutie et al. in review, Knutie et al. in press). Understanding the mechanisms by which pesticides cause long-term impacts on host defenses will be necessary to improve the design of pesticides.
Interestingly, the pattern of higher Bd abundance in fungicide- than solvent-exposed frogs was not apparent upon a second exposure to Bd. Frogs exposed to fungicides and to Bd for the second time had similar Bd loads as control frogs and had lower Bd loads than fungicide-exposed frogs exposed to Bd for the first time when exposed at the same life stage (Fig. 1B). This pattern was most likely caused by Bd-induced mortality. Frogs exposed to fungicide and Bd for the first time had significantly higher mortality than frogs exposed to solvent and Bd for the first time. In fact, Bd only caused significant mortality when frogs were exposed to fungicides. Hence, the most Bd-susceptible individuals were not available to be exposed to Bd for a second time in the fungicide treatments but were available for exposure to Bd a second time in the control treatment. Consequently, selection is a resonable explanation for the change in Bd abundance and mortality patterns across fungicide treatments between the first and second Bd exposures (Rohr et al. 2008a).
Despite the broad spectrum nature of many fungicides (Maltby et al. 2009), we did not detect strong direct effects of the tested fungicides on Cuban tree frogs in our laboratory experiments. The fungicides alone did not affect survival or timing of or size at metamorphosis. Rather, most of the adverse or beneficial effects of fungicides were only apparent in the presence of Bd. The fungicides reduced Bd growth on frogs when the exposures occurred simultaneously. However, they tended to increase Bd-induced mortality regardless of the timing of exposures. These findings emphasize the importance of considering the effects of contaminants within a community context (Relyea and Hoverman 2006, Rohr et al. 2006a), quantifying the net effects of contaminants (the sum of the beneficial and adverse effects) (Rohr et al. 2008a), and testing for delayed or persistent effects of chemicals (Rohr and Palmer 2005, Rohr et al. 2006b, Jones et al. 2009, Rohr and Palmer 2013).
The patterns we observed in nature were consistent with our laboratory findings because fungicides were generally associated with greater rather than less Bd. In our field survey, we revealed that the greater the concentration of fungicides in a given wetland, the greater the prevalence of chytrid fungal infections in frogs (Fig. 3B). In contrast, fungicide concentrations at the level of individual frogs were less correlated with Bd prevalence (Fig. 3A), perhaps because of individual-level variation in susceptibility, exposure, and timing and duration of infections, variation that is reduced at the level of the wetland. In addition to reflecting current exposure, detectable levels of fungicides in wetlands must also reflect some level of previous fungicide exposure, which, according to our laboratory experiments, can persistently compromise host resistance to Bd. Importantly, given that Bd can be quite persistent in the presence of abundant hosts and that many fungicides often degrade rapidly in the environment, exposure to fungicide followed by exposure to Bd is almost certainly more frequent than simultaneous exposure to the two factors, suggesting that persistent adverse effects of previous fungicide exposure might be common. Additionally, our field patterns revealed that fungicides were rarely detected in the water column but were regularly detected in sediments, suggesting that adsorption might be important for many fungicides and that benthos-dwelling tadpole species might have higher fungicide exposure than species found more commonly in other microhabitats. Additional field data and field manipulations would be invaluable in determining the absolute magnitude of these effects and the specific fungicides that are driving these patterns.
Recently, there have been some exciting findings that suggest applications of fungicides or disinfectants with fungicidal properties might be an effective tool for curbing amphibian declines associated with Bd (Bosch et al. 2015, Hudson et al. 2016). Bosch et al. (2015) used fungicides and agricultural disinfectants to successfully eradicate Bd from a field site and Hudson et al. (2016) temporarily reduced fungal loads on amphibians in the wild using in situ exposure to fungicides. However, our results show that many fungicides can also have adverse effects, such as persistently compromising amphibian defenses against pathogens. Thus, fungicides might work well if they completely eliminate Bd from the environment and if Bd is unlikely to return soon after. However, if a fungicide application does not eradicate Bd, if it does eradicate Bd but Bd re-colonizes, or if fungicide-induced suppression of host defenses affects resistance to other virulent pathogens in the environment (bacteria, viruses, macroparasites, protozoa, etc.), our results suggest that fungicide applications could cause more harm than good. Indeed, if fungicides and disinfectants do not clear both the frog and environment of Bd, our results suggest that they can eventually elevate Bd prevalence. Additionally, many broad spectrum pesticides have widespread non-target effects (Jones et al. 2009, Halstead et al. 2014), but the consequences of fungicide exposure on non-target organisms are not fully understood. Although fungicides show promise for controlling Bd, the recently discovered chytrid of salamanders Batrachochytrium salamandrivorans (Martel et al. 2013), and perhaps even other emerging fungal diseases, such as those of bats, bees, corals, and snakes (Allender et al. 2011, Cameron et al. 2011, Warnecke et al. 2012), for all of the reasons just provided, we encourage greater research on and caution in using fungicides for managing infectious diseases of wildlife. In particular, research is needed to more concretely identify which fungicides can control fungal pathogens without compromising host resistance and tolerance of pathogens.
Although synthetic chemicals provide an enormous value to society, they also have had many unintended consequences (Pimentel et al. 1973). Examples include the paradox of enrichment, where fertilizers reduce crop yields (Rosenzweig 1971) and cases where insecticides cause greater pest outbreaks by reducing natural biocontrol (Desneux et al. 2007, Douglas et al. 2015). Here, we provided yet another example to this growing list. Fungicides paradoxically increased fungal loads and fungal-induced mortality of amphibians. These findings highlight the importance of understanding the role of multiple simultaneous and sequential stressors in biodiversity declines and disease emergences and the need to comprehensively understand the complex effects that chemicals can have on ecosystems to avoid inadvertent and undesirable ramifications.
Supplementary Material
Acknowledgments
We thank D. L. Calhoun, S. Paschke, and K. Smalling for feedback on this manuscript. This research was supported by grants from the National Science Foundation (EF-1241889), National Institutes of Health (R01GM109499, R01TW010286), US Department of Agriculture (NRI 2006-01370, 2009-35102-0543), and US Environmental Protection Agency (CAREER 83518801) to J.R.R., and The University of Tampa’s Dana Faculty Development Grant to T.A.M. The field research was supported by the US Geological Survey’s Amphibian Research and Monitoring Initiative. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. W.A.B. did not materially contribute to the model application described in this publication. J.R.R. and J.B. are equal first authors.
Footnotes
Additional supporting information may be found in the online version of this article at http://onlinelibrary.wiley.com/doi/10.1002/eap.xxxx/suppinfo
Data Availability
Data available from Figshare: https://doi.org/10.6084/m9.figshare.5197378.v1
Literature Cited
- Allender MC, Dreslik M, Wylie S, Phillips C, Wylie DB, Maddox C, Delaney MA, Kinsel MJ. Chrysosporium sp. infection in eastern massasauga rattlesnakes. Emerging Infectious Diseases. 2011;17:2383–2384. doi: 10.3201/eid1712.110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglin W, Smalling K, Anderson C, Calhoun D, Chestnut T, Muths E. Potential interactions among disease, pesticides, water quality and adjacent land cover in amphibian habitats in the United States. Science of the Total Environment. 2016;566:320–332. doi: 10.1016/j.scitotenv.2016.05.062. [DOI] [PubMed] [Google Scholar]
- Battaglin WA, Sandstrom MW, Kuivila KM, Kolpin DW, Meyer MT. Occurrence of azoxystrobin, propiconazole, and selected other fungicides in US streams, 2005–2006. Water, Air, & Soil Pollution. 2011;218:307–322. [Google Scholar]
- Belden J, McMurry S, Smith L, Reilley P. Acute toxicity of fungicide formulations to amphibians at environmentally relevant concentrations. Environmental Toxicology and Chemistry. 2010;29:2477–2480. doi: 10.1002/etc.297. [DOI] [PubMed] [Google Scholar]
- Berger L, Speare R, Pessier A, Voyles J, Skerratt LF. Treatment of chytridiomycosis requires urgent clinical trials. Diseases of Aquatic Organisms. 2010;92:165–174. doi: 10.3354/dao02238. [DOI] [PubMed] [Google Scholar]
- Bletz MC, Loudon AH, Becker MH, Bell SC, Woodhams DC, Minbiole KP, Harris RN. Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecology Letters. 2013;16:807–820. doi: 10.1111/ele.12099. [DOI] [PubMed] [Google Scholar]
- Bosch J, Sanchez-Tome E, Fernandez-Loras A, Oliver JA, Fisher MC, Garner TWJ. Successful elimination of a lethal wildlife infectious disease in nature. Biology Letters. 2015;11 doi: 10.1098/rsbl.2015.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck JC, Hua J, Brogan WR, III, Dang TD, Urbina J, Bendis RJ, Stoler AB, Blaustein AR, Relyea RA. Effects of pesticide mixtures on host-pathogen dynamics of the amphibian chytrid fungus. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL. Patterns of widespread decline in North American bumble bees. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:662–667. doi: 10.1073/pnas.1014743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashins SD, Grogan LF, McFadden M, Hunter D, Harlow PS, Berger L, Skerratt LF. Prior infection does not improve survival against the amphibian disease chytridiomycosis. PLoS One. 2013;8:e56747. doi: 10.1371/journal.pone.0056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux PY, Kent RA, Fan GT, Stephenson GL. Environmental fate and effects of chlorothalonil: a Canadian perspective. Critical Reviews in Environmental Science and Technology. 1996;26:45–93. [Google Scholar]
- Cohen LM, Neimark H, Eveland LK. Schistomsoma mansoni: Response of cercariae to a thermal gradient. Journal of Parasitology. 1980;66:362–364. [PubMed] [Google Scholar]
- Colosio C, Barcellini W, Maroni M, Alcini D, Bersani M, Cavallo D, Galli A, Meroni P, Pastorelli R, Rizzardi GP, Soleo L, Foa V. Immunomodulatory effects of occupational exposure to mancozeb. Archives of Environmental Health. 1996;51:445–451. doi: 10.1080/00039896.1996.9936044. [DOI] [PubMed] [Google Scholar]
- Corsini E, Viviani B, Birindelli S, Gilardi F, Torri A, Codeca I, Lucchi L, Bartesaghi S, Galli CL, Marinovich M, Colosio C. Molecular mechanisms underlying mancozeb-induced inhibition of TNF-alpha production. Toxicology and Applied Pharmacology. 2006;212:89–98. doi: 10.1016/j.taap.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Davidson C, Benard MF, Shaffer HB, Parker JM, O'Leary C, Conlon JM, Rollins-Smith LA. Effects of chytrid and carbaryl exposure on survival, growth, and skin peptide defenses in foothill yellow-legged frogs. Environmental Science & Technology. 2007;41:1771–1776. doi: 10.1021/es0611947. [DOI] [PubMed] [Google Scholar]
- Deb D, Engel BA, Harbor J, Hahn L, Lim KJ, Zhai T. Investigating potential water quality impacts of fungicides used to combat soybean rust in Indiana. Water Air and Soil Pollution. 2010;207:273–288. [Google Scholar]
- Desneux N, Decourtye A, Delpuech J-M. The sublethal effects of pesticides on beneficial arthropods. Pages 81–106 Annual Review of Entomology. 2007 doi: 10.1146/annurev.ento.52.110405.091440. [DOI] [PubMed] [Google Scholar]
- Douglas MR, Rohr JR, Tooker JF. Neonicotinoid insecticide travels through a soil food chain, disrupting biological control of non-target pests and decreasing soya bean yield. Journal of Applied Ecology. 2015;52:250–260. [Google Scholar]
- Fleeger JW, Carman KR, Nisbet RM. Indirect effects of contaminants in aquatic ecosystems. Science of the Total Environment. 2003;317:207–233. doi: 10.1016/S0048-9697(03)00141-4. [DOI] [PubMed] [Google Scholar]
- Gahl MK, Pauli BD, Houlahan JE. Effects of chytrid fungus and a glyphosate-based herbicide on survival and growth of wood frogs (Lithobates sylvaticus) Ecological Applications. 2011;21:2521–2529. doi: 10.1890/10-2319.1. [DOI] [PubMed] [Google Scholar]
- Gaietto KM, Rumschlag SL, Boone MD. Effects of pesticide exposure and the amphibian chytrid fungus on gray treefrog (Hyla chrysoscelis) metamorphosis. Environmental Toxicology and Chemistry. 2014;33:2358–2362. doi: 10.1002/etc.2689. [DOI] [PubMed] [Google Scholar]
- Garner TWJ, Garcia G, Carroll B, Fisher MC. Using itraconazole to clear Batrachochytrium dendrobatidis infection, and subsequent depigmentation of Alytes muletensis tadpoles. Diseases of Aquatic Organisms. 2009;83:257–260. doi: 10.3354/dao02008. [DOI] [PubMed] [Google Scholar]
- Gervasi SS, Stephens PR, Hua J, Searle CL, Xie G, Urbina J, Olson D, Bancroft BA, Weis V, Hammond JI, Relyea RA, Blaustein AR. Linking ecology and epidemiology to understand predictors of multi-host responses to an emerging pathogen, the amphibian chytrid fungus. PLoS One. 2017;12:e0167882. doi: 10.1371/journal.pone.0167882. doi:0167810.0161371/journal.pone.0167882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose SL, Donnelly MA, Kerby J, Whitfield SM. Acute toxicity tests and meta-analysis identify gaps in tropical ecotoxicology for amphibians. Environmental Toxicology and Chemistry. 2014;33:2114–2119. doi: 10.1002/etc.2665. [DOI] [PubMed] [Google Scholar]
- Gosner N. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- Grube A, Donaldson D, Kiely T, Wu L. Pesticide industry sales and usage: 2006 and 2007 market estimates. U.S. Environmental Protection Agency; Washington, D.C.: 2011. [Google Scholar]
- Halstead NT, McMahon TA, Johnson SA, Raffel TR, Romansic JM, Crumrine PW, Rohr JR. Community ecology theory predicts the effects of agrochemical mixtures on aquatic biodiversity and ecosystem properties. Ecology Letters. 2014;17:932–941. doi: 10.1111/ele.12295. [DOI] [PubMed] [Google Scholar]
- Hanlon SM, Kerby JL, Parris MJ. Unlikely remedy: Fungicide clears infection from pathogenic fungus in larval southern leopard frogs (Lithobates sphenocephalus) PLoS One. 2012;7 doi: 10.1371/journal.pone.0043573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon SM, Lynch KJ, Kerby JL, Parris MJ. The effects of a fungicide and chytrid fungus on anuran larvae in aquatic mesocosms. Environmental Science and Pollution Research. 2015;22:12929–12940. doi: 10.1007/s11356-015-4566-8. [DOI] [PubMed] [Google Scholar]
- Hanlon SM, Parris MJ. The impact of pesticides on the pathogen Batrachochytrium dendrobatidis independent of potential hosts. Archives of Environmental Contamination and Toxicology. 2012;63:137–143. doi: 10.1007/s00244-011-9744-1. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson MA, Young RP, Lopez J, Martin L, Fenton C, McCrea R, Griffiths RA, Adams S, Gray G, Garcia G, Cunningham AA. In-situ itraconazole treatment improves survival rate during an amphibian chytridiomycosis epidemic. Biological Conservation. 2016;195:37–45. [Google Scholar]
- Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, Dalton A, Kriger K, Hero M, Hines H, Phillott R, Campbell R, Marantelli G, Gleason F, Colling A. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms. 2007;73:175–192. doi: 10.3354/dao073175. [DOI] [PubMed] [Google Scholar]
- Jayawardena UA, Rohr JR, Navaratne AN, Amerasinghe PH, Rajakaruna RS. Combined effects of pesticides and trematode infections on hourglass tree frog Polypedates cruciger. EcoHealth. 2016;13:111–122. doi: 10.1007/s10393-016-1103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Berger L, Philips L, Speare R. Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms. 2003;57:255–260. doi: 10.3354/dao057255. [DOI] [PubMed] [Google Scholar]
- Jones DK, Dang TD, Urbina J, Bendis RJ, Buck JC, Cothran RD, Blaustein AR, Relyea RA. Effect of simultaneous amphibian exposure to pesticides and an emerging fungal pathogen, Batrachochytrium dendrobatidis. Environmental Science & Technology. 2016 doi: 10.1021/acs.est.6b06055. [DOI] [PubMed] [Google Scholar]
- Jones DK, Hammond JI, Relyea RA. Very highly toxic effects of endosulfan across nine species of tadpoles: Lag effects and family-level sensitivity. Environmental Toxicology and Chemistry. 2009;28:1939–1945. doi: 10.1897/09-033.1. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Briggs CJ, Daszak P. The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends in Ecology & Evolution. 2010;25:109–118. doi: 10.1016/j.tree.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Knutie SA, Gabor CR, Kohl KD, Rohr JR. Do host-associated gut microbiota mediate the effect of an herbicide on disease risk in frogs? Journal of Animal Ecology. doi: 10.1111/1365-2656.12769. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutie SA, Wilkinson CL, Kohl KD, Rohr JR. Early-life disruption of host microbiota decreases later-life resistance to infections. Nature Communications. doi: 10.1038/s41467-017-00119-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukanich B. A review of selected systemic antifungal drugs for use in dogs and cats. Veterinary Medicine. 2008;103:41–50. [Google Scholar]
- Maltby L, Brock TCM, van den Brink PJ. Fungicide risk assessment for aquatic ecosystems: Importance of interspecific variation, toxic mode of action, and exposure regime. Environmental Science & Technology. 2009;43:7556–7563. doi: 10.1021/es901461c. [DOI] [PubMed] [Google Scholar]
- Martel A, Spitzen-van der Sluijs A, Blooi M, Bert W, Ducatelle R, Fisher MC, Woeltjes A, Bosman W, Chiers K, Bossuyt F. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proceedings of the National Academy of Sciences. 2013;110:15325–15329. doi: 10.1073/pnas.1307356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Hopkins WA, Mydlarz LD, Rohr JR. The effects of anthropogenic global changes on immune functions and disease resistance. Pages 129–148 Year in Ecology and Conservation Biology 2010. 2010 doi: 10.1111/j.1749-6632.2010.05454.x. [DOI] [PubMed] [Google Scholar]
- McMahon TA, Boughton RK, Martin LB, Rohr JR. Exposure to the herbicide atrazine nonlinearly affects tadpole corticosterone levels. Journal of Herpetology. 2017;52:270–273. [Google Scholar]
- McMahon TA, Halstead NT, Johnson S, Raffel TR, Romansic JM, Crumrine PW, Boughton RK, Martin LB, Rohr JR. The fungicide chlorothalonil is nonlinearly associated with corticosterone levels, immunity, and mortality in amphibians. Environmental Health Perspectives. 2011;119:1098–1103. doi: 10.1289/ehp.1002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon TA, Halstead NT, Johnson S, Raffel TR, Romansic JM, Crumrine PW, Rohr JR. Fungicide-induced declines of freshwater biodiversity modify ecosystem functions and services. Ecology Letters. 2012;15:714–722. doi: 10.1111/j.1461-0248.2012.01790.x. [DOI] [PubMed] [Google Scholar]
- McMahon TA, Rohr JR. Transition of chytrid fungus infection from mouthparts to hind limbs during amphibian metamorphosis. EcoHealth. 2015;12:188–193. doi: 10.1007/s10393-014-0989-9. [DOI] [PubMed] [Google Scholar]
- McMahon TA, Romansic JM, Rohr JR. Nonmonotonic and monotonic effects of pesticides on the pathogenic fungus Batrachochytrium dendrobatidis in culture and on tadpoles. Environmental Science & Technology. 2013;47:7958–7964. doi: 10.1021/es401725s. [DOI] [PubMed] [Google Scholar]
- McMahon TA, Sears BF, Venesky MD, Bessler SM, Brown JM, Deutsch K, Halstead NT, Lentz G, Tenouri N, Young S, Civitello DJ, Ortega N, Fites JS, Reinert LK, Rollins-Smith LA, Raffel TR, Rohr JR. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature. 2014;511:224–227. doi: 10.1038/nature13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerke NJ. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. [Google Scholar]
- Pimentel D, Hurd LE, Bellotti AC, Forster MJ, Oka IN, Sholes OD, Whitman RJ. Food production and energy crisis. Science. 1973;182:443–449. doi: 10.1126/science.182.4111.443. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. URL http://www.R-project.org/ [Google Scholar]
- Relyea R, Hoverman J. Assessing the ecology in ecotoxicology: a review and synthesis in freshwater systems. Ecology Letters. 2006;9:1157–1171. doi: 10.1111/j.1461-0248.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- Relyea RA. The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecological Applications. 2005;15:618–627. doi: 10.1890/1051-0761(2006)016[2022:tioiah]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Civitello DJ, Crumrine PW, Halstead NT, Miller AD, Schotthoefer AM, Stenoien C, Johnson LB, Beasley VR. Predator diversity, intraguild predation, and indirect effects drive parasite transmission. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:3008–3013. doi: 10.1073/pnas.1415971112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Crumrine PW. Effects of an herbicide and an insecticide on pond community structure and processes. Ecological Applications. 2005;15:1135–1147. [Google Scholar]
- Rohr JR, Kerby JL, Sih A. Community ecology as a framework for predicting contaminant effects. Trends in Ecology & Evolution. 2006a;21:606–613. doi: 10.1016/j.tree.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Rohr JR, McCoy KA. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environmental Health Perspectives. 2010;18:20–32. doi: 10.1289/ehp.0901164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Palmer BD. Aquatic herbicide exposure increases salamander desiccation risk eight months later in a terrestrial environment. Environmental Toxicology and Chemistry. 2005;24:1253–1258. doi: 10.1897/04-448r.1. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Palmer BD. Climate change, multiple stressors, and the decline of ectotherms. Conservation Biology. 2013;27:741–751. doi: 10.1111/cobi.12086. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Halstead NT, McMahon TA, Johnson SA, Boughton RK, Martin LB. Early-life exposure to a herbicide has enduring effects on pathogen-induced mortality. Proceedings of the Royal Society B-Biological Sciences. 2013;280:20131502. doi: 10.1098/rspb.2013.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Sessions SK, Hudson PJ. Understanding the net effects of pesticides on amphibian trematode infections. Ecological Applications. 2008a;18:1743–1753. doi: 10.1890/07-1429.1. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Sager T, Sesterhenn TM, Palmer BD. Exposure, postexposure, and density-mediated effects of atrazine on amphibians: Breaking down net effects into their parts. Environmental Health Perspectives. 2006b;114:46–50. doi: 10.1289/ehp.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N, Hoverman JT, Johnson CM, Johnson LB, Lieske C, Piwoni MD, Schoff PK, Beasley VR. Agrochemicals increase trematode infections in a declining amphibian species. Nature. 2008b;455:1235–1239. doi: 10.1038/nature07281. [DOI] [PubMed] [Google Scholar]
- Rosenzweig Ml. Paradox of enrichment: Destabilization of exploitation ecosystems in ecological time. Science. 1971;171:385–387. doi: 10.1126/science.171.3969.385. [DOI] [PubMed] [Google Scholar]
- Smalling KL, Reeves R, Muths E, Vandever M, Battaglin WA, Hladik ML, Pierce CL. Pesticide concentrations in frog tissue and wetland habitats in a landscape dominated by agriculture. Science of the Total Environment. 2015;502:80–90. doi: 10.1016/j.scitotenv.2014.08.114. [DOI] [PubMed] [Google Scholar]
- Staley ZR, Rohr JR, Senkbeil JK, Harwood VJ. Agrochemicals indirectly increase survival of E. coli O157:H7 and indicator bacteria by reducing ecosystem services. Ecological Applications. 2014;24:1945–1953. doi: 10.1890/13-1242.1. [DOI] [PubMed] [Google Scholar]
- Voccia I, Blakley B, Brousseau P, Fournier M. Immunotoxicity of pesticides: a review. Toxicology and Industrial Health. 1999;15:119–132. doi: 10.1177/074823379901500110. [DOI] [PubMed] [Google Scholar]
- Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, Cook D, Webb R, Alford RA, Skerratt LF, Speare R. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 2009;326:582–585. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- Warnecke L, Turner JM, Bollinger TK, Lorch JM, Misra V, Cryan PM, Wibbelt G, Blehert DS, Willis CKR. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6999–7003. doi: 10.1073/pnas.1200374109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams DC, Bosch J, Briggs CJ, Cashins S, Davis LR, Lauer A, Muths E, Puschendorf R, Schmidt BR, Sheafor B, Voyles J. Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Frontiers in Zoology. 2011;8 doi: 10.1186/1742-9994-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams DC, Geiger CC, Reinert LK, Rollins-Smith LA, Lam B, Harris RN, Briggs CJ, Vredenburg VT, Voyles J. Treatment of amphibians infected with chytrid fungus: learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy. Diseases of Aquatic Organisms. 2012;98:11–25. doi: 10.3354/dao02429. [DOI] [PubMed] [Google Scholar]
- Young S, Nayak B, Sun S, Badgley BD, Rohr JR, Harwood VJ. Vancomycin-resistant enterococci and bacterial community structure following a sewage spill into an aquatic environment. Applied and Environmental Microbiology. 2016;82:5653–5660. doi: 10.1128/AEM.01927-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.