Abstract
Craniospinal irradiation is standard radiotherapy (RT) for localized intracranial nongerminoma germ cell tumors (NGGCT). Given its toxicity, there is interest in using smaller fields. We examined outcomes of NGGCT patients receiving reduced-volume RT at a single institution. Records of 16 patients who received reduced-volume RT as part of definitive treatment between 1996 and 2016 were reviewed. Median age at presentation was 10.8 years (range, 4.6-41.0 years). Ten patients had pineal tumors and 6 had suprasellar tumors. All received chemotherapy and 9 patients received second-look surgery thereafter. RT volume was tumor-only to a median of 54 Gy (range, 50.4-54 Gy) in 3 patients and whole-ventricle irradiation to a median of 30.6 Gy (range, 30.6-36 Gy) with a boost to 54 Gy in 13 patients. Median follow-up was 4.1 years (range, 1.9-19.3 years). Three patients recurred locally at a median 9.9 months (range, 9.6-10.6 months) after diagnosis, and one of these developed leptomeningeal relapse after 30 months. One patient expired from disease 2.6 years post-diagnosis and another due to stroke 19.3 years post-diagnosis. Fourteen patients are alive with no evidence of disease. Kaplan-Meier estimates of the 4-year overall survival (OS) and failure-free survival (FFS) are 92% (95% confidence interval [CI], 57%-99%) and 81% (95% CI, 53%-94%), respectively. Excellent disease control was observed in these patients with no initial relapses outside of these RT fields. The results of ACNS1123 may better delineate patterns of failure and identify subgroups likely to benefit from this approach.
Keywords: radiation therapy, germ cell tumors, chemotherapy, CNS tumors, tumor markers, surgery
Introduction
Primary intracranial germ cell tumors (GCTs) are rare and heterogeneous neoplasms most commonly arising from the regions of the pineal gland and/or the suprasellar cistern. [1] While pure germinomas are highly radiosensitive and have been shown to have survival rates exceeding 90% when treated with RT alone [2,3], nongerminoma germ cell tumors (NGGCTs) treated with RT alone have lower 5-year survival rates of 20-50%. [4-8] In light of the relative radioresistance of NGGCT, management generally consists of multimodality treatment with chemotherapy, second-look surgery (SLS) in cases of incomplete response to induction chemotherapy, and RT. The current standard of care for M0 NGGCT is craniospinal irradiation (CSI) to 30-36 Gy with a primary site boost to 54-60 Gy. However, late toxicities of CSI in pediatric patients are a major concern, such as neurocognitive, growth, auditory, and endocrine effects, as well as risk of stroke and secondary neoplasms. [9]
Though RT is an essential component in the management of NGGCT, the optimal volume is unclear. [10] Limited trials have reported encouraging preliminary results with chemotherapy followed by whole ventricular irradiation (WVI) or local field irradiation for NGGCT, as is used in the treatment of germinoma. [11] The Children's Oncology Group (COG) trials examining outcomes of patients receiving limited radiation fields are ongoing but results have not yet been published. Therefore, we report our institutional experience with reduced-volume RT in patients with intracranial M0 NGGCT.
Methods and Materials
Patient population
This is a single-institution cohort of patients treated with RT to tumor only or to ventricles after being diagnosed with localized primary intracranial NGGCTs between 1994 and 2016. After approval by our internal Institutional Review Board, we identified 16 consecutive patients who were treated with limited RT fields as part of their definitive treatment. All patients in the study time period with unequivocal radiographic (MRI brain and spine) and cytological absence of neuraxial dissemination on lumbar puncture were treated with reduced-volume RT Patients were excluded from consideration for reduced-volume RT in cases of uncertain tumor extension or spread on MRI, tumor in locations other than suprasellar or pineal regions, or mature teratoma with normal tumor markers. Patients were also excluded if CSF cytology was not obtained before treatment or if ventricular fluid rather than lumbar puncture was used for cytologic examination. RT field was selected independent of chemotherapy regimen.
The basic characteristics of the 16 patients are shown in Table 1. On presentation, the median age was 10.8 years (range, 4.6-41.0 years) with 15 of these ≤ 20 years of age. Eleven patients were male and 5 were female. Ten patients had pineal tumors and 6 had suprasellar tumors; no patients with presented with multiple tumor foci. All 16 patients had negative spine MRI on presentation and lumbar puncture with negative cytology. At diagnosis, patients had serum and CSF serum β-human chorionic gonadotropin (β-hCG) and α-fetoprotein (AFP) levels measured. Six patients had biopsy without resection prior to initiation of any therapy: 1 with mixed components, 1 choriocarcinoma, 2 mature teratoma, 1 immature teratoma, and 1 germinoma. Both patients found to have mature teratoma on biopsy had elevated tumor markers consistent with NGGCT. The patient diagnosed with germinoma on biopsy demonstrated an elevated, rising AFP level and thus was deemed to have NGGCT. The remaining patients were diagnosed based on radiographic findings and elevated tumor markers.
Table 1. Summary of patient population characteristics.
| Characteristic | n (%) |
|---|---|
| Total patients | 16 |
| Age at diagnosis (y) | |
| Median | 10.8 |
| Range | 4.6-41.0 |
| Sex | |
| Male | 11 (69%) |
| Female | 5 (31%) |
| Primary site | |
| Pineal | 10 (63%) |
| Suprasellar | 6 (38%) |
| Tumor size (largest dimension) | |
| Median | 3.2 cm |
| Range | 1.2-5.7 cm |
| Follow-up time (y) | |
| Median | 4.1 |
| Range | 1.9-19.3 |
| Management of primary tumor | |
| Surgery | 10 (63%) |
| Chemotherapy regimen | |
| As per MSKCC 94-125 | 2 (13%) |
| As per COG ACNS 0122 | 9 (56%) |
| As per COG ACNS 1123 | 5 (31%) |
| RT field used | |
| Tumor only | 3 (19%) |
| Whole ventricle | 13 (81%) |
Disease management
Two patients were treated as per, but not enrolled on, a modified schedule of institutional protocol MSKCC 94-125 [12], consisting of 2 cycles of Regimen A (cisplatin, etoposide, cyclophosphamide, and bleomycin), 2 cycles of Regimen B (carboplatin, etoposide, and bleomycin), followed by 1 additional cycle of Regimens A and B in cases of complete remission (CR) or by second-look surgery (SLS) ± WVI to 54 Gy in cases of residual disease. One of these patients achieved CR after chemotherapy alone but developed progression 5 months later in the same location as the primary tumor and was treated with 2 cycles of high-dose thiotepa, stem-cell rescue, and tumor-only RT to 50.4 Gy. Nine patients were treated as per, but not enrolled on, COG protocol ACNS0122 [13] and received 6 cycles of induction chemotherapy. Of these, 3 patients achieved complete response (CR) and then received reduced-volume RT, instead of CSI per protocol, and 6 patients demonstrated residual radiological abnormalities and were treated with SLS and reduced-volume RT, instead of CSI per protocol. Of the 9 patients treated as per ACNS0122, 2 patients received tumor-only RT to 50.4 Gy and 7 patients received WVI to 30.6-36 Gy with a boost to 54 Gy. Five patients were treated as per the NGGCT arm of COG protocol ACNS1123 [14], consisting of 6 cycles of induction chemotherapy ± SLS followed by WVI to 30.6 Gy with a boost to 54 Gy.
Four patients received four cycles of chemotherapy and 12 patients received six cycles. Of 10 patients receiving surgery as part of definitive treatment, 1 had subtotal resection before initiation of chemotherapy, 3 patients had surgery after initiation but before completion of chemotherapy regimen, 6 had surgery after completion of chemotherapy. One patient had surgery upon developing local progression 5 months after achieving CR to chemotherapy and thereafter received tumor-only RT.
All patients had normalized serum β-hCG and AFP levels prior to the start of RT. Of the 16 total patients, 3 patients were treated with 3D conformal radiotherapy (3D-CRT), 9 with dose painting intensity modulated radiotherapy (DP-IMRT), and 4 with proton beam therapy (PBT).
Statistical analysis
Statistical analysis was performed with Stata Version 13.0 (StataCorp, College Station, TX, USA). Failure-free survival (FFS) was calculated from the time of diagnosis of NGGCT to first relapse at any site. The Kaplan-Meier method was used to assess FFS and overall survival (OS).
Results
Detailed patient-level information is displayed in Table 2. Median follow-up duration was 4.1 years (range, 1.9-19.3 years). Tumor size information was available for 13 patients (81%); the median largest dimension was 3.2 cm (range, 1.2-5.7cm). All 16 patients had one or more elevated serum or CSF tumor markers. In patients with elevated markers, median serum β-hCG was 806 IU/L (range, 78-3193 IU/L), median serum AFP was 125 IU/L (range, 11-26346 IU/L), median CSF β-hCG was 636 IU/L (range, 61-2477 IU/L), and median AFP was 29 IU/L (range, 21-1305 IU/L). Histopathologic analysis information is available for 10 patients receiving surgery as part of definitive treatment: 7 mature teratoma, 2 immature teratoma, and 1 with components of yolk sac tumor and immature teratoma.
Table 2. Characteristics of M0 NGGCT patients receiving reduced-volume RT.
| Age/Sex | Primary site | Pretherapy biopsy? | Serum β-hCG | Serum AFP | CSF β-hCG | CSF AFP | Chemotherapy regimen (as per) | Surgery | RT field | Type of initial relapse | Disease status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 41/M | Suprasellar | Yolk sac + immature teratoma | Elevated | Elevated | 0 | 5309 | MSKCC 94-125 | Upfront | WVI + boost | None | DOC |
| 8/M | Suprasellar | None† | 78 | 4.6 | 508 | 0 | MSKCC 94-125 | No (CR) | IFRT (salvage) | Local | NED |
| 7/M | Pineal | Choriocarcinoma | 517 | <2 | 2477 | 0 | COG ACNS 0122 | SLS | IFRT | None | NED |
| 5/F | Suprasellar | Mature teratoma | 1517 | 305 | 991 | 21 | COG ACNS 0122 | SLS | IFRT | None | NED |
| 8/F | Suprasellar | None† | 1012 | 358 | 304 | 25 | COG ACNS 0122 | SLS | WVI + boost | None | NED |
| 10/F | Suprasellar | None† | 202 | 60 | 636 | 29 | COG ACNS 0122 | No (CR) | WVI + boost | None | NED |
| 10/M | Pineal | Immature teratoma | 11 | 125 | 1136 | 28 | COG ACNS 0122 | SLS | WVI + boost | None | NED |
| 14/M | Pineal | None† | 1149 | 2160 | 9.0 | 1305 | COG ACNS 0122 | SLS | WVI + boost | Local | DOD |
| 12/M | Pineal | None† | <2 | 26346 | ** | ** | COG ACNS 0122 | No (CR) | WVI + boost | Local | NED |
| 12/F | Pineal | Germinoma* | 2.0 | 11 | 8 | <2 | COG ACNS 0122 | No (CR) | WVI + boost | None | NED |
| 15/M | Pineal | None† | 36 | 486 | 20 | 135 | COG ACNS 0122 | SLS | WVI + boost | None | NED |
| 20/M | Pineal | None† | <2 | 49 | <2 | 32 | COG ACNS 1123 | SLS | WVI + boost | None | NED |
| 11/M | Pineal | None† | 600 | 76 | 601 | 25 | COG ACNS 1123 | SLS | WVI + boost | None | NED |
| 6/F | Suprasellar | None† | 3193 | 5.4 | 2124 | <2 | COG ACNS 1123 | No (CR) | WVI + boost | None | NED |
| 12/M | Pineal | Mature teratoma | 24 | 6.0 | 61 | <2 | COG ACNS 1123 | No (unsafe) | WVI + boost | None | NED |
| 8/M | Pineal | Mixed‡ | <2 | 72 | <2 | <2 | COG ACNS 1123 | SLS | WVI + boost | None | NED |
Patient was diagnosed with NGGCT due to presence of elevated AFP.
Patients were diagnosed with serum markers.
Histology was comprised primarily of mature teratoma and yolk sac elements.
= Tumor marker information was not available.
NED = No evidence of disease, IFRT = involved field radiation therapy, WVI + boost = whole ventricle radiation with primary site boost, SLS = second-look surgery, CR = complete remission, DOD = Died of disease, DOC = Died of other causes
For 3 patients receiving tumor-only RT, the median dose was 54 Gy (range, 50.4-54 Gy). For 13 patients receiving WVI + boost, the median whole ventricle dose was 30.6 Gy (range, 30.6-36) and all received a boost to the primary tumor to 54 Gy.
A total of 3 patients developed recurrences at the site of the primary tumor at a median of 9.9 months (range, 9.6-10.6 months) after initial diagnosis, all of whom were diagnosed according to MRI and elevated serum tumor markers.
One patient who achieved CR after completing definitive treatment with 2 cycles cisplatin, etoposide, cyclophosphamide, and bleomycin and 2 cycles of carboplatin, etoposide, and bleomycin without upfront RT (MSKCC 94-125) developed a local progression 5 months later and was treated with 2 cycles of high-dose thiotepa, stem-cell rescue, and tumor-only RT to 50.4 Gy; this patient showed no evidence of disease at last follow-up, 17.5 years after initial diagnosis. After upfront treatment with 6 cycles of chemotherapy as per ACNS0122, second-look surgery revealing mature teratoma, and WVI to 36 Gy and tumor boost to 54 Gy, one patient developed local recurrence and was treated with 3 cycles of viscristine/cisplatin/cyclophosphamide and 2 cycles of cyclophosphamide/thiotepa, and subsequently developed multiple local recurrences treated with chemotherapy and stereotactic radiosurgery. This patient went on to develop leptomeningeal relapse 30 months after initial diagnosis and died 2 weeks later, a total of 2.6 years after initial diagnosis. After 6 cycles of chemotherapy as per ACNS0122 with complete biochemical and radiographic response followed by WVI to 30.6 Gy and tumor boost to 54 Gy, another patient developed local recurrence and was treated with 2 cycles of gemcitabine/paclitaxel/oxalplatin, 2 cycles of high-dose carboplatin/thiotepa stem-cell rescue, followed by 6 cycles of oral etoposide; this patient showed no evidence of disease at last follow-up, 4.2 years after initial diagnosis. No patients developed initial relapse outside of the limited RT fields. One patient expired 19.3 years after diagnosis due to stroke without evidence of relapse.
Overall survival and failure-free survival Kaplan-Meier curves are shown in Figure 1. Kaplan-Meier estimates of the 4-year overall survival (OS) and failure-free survival (FFS) are 92% (95% confidence interval [CI], 57%-99%) and 81% (95% CI, 53%-94%), respectively.
Figure 1.
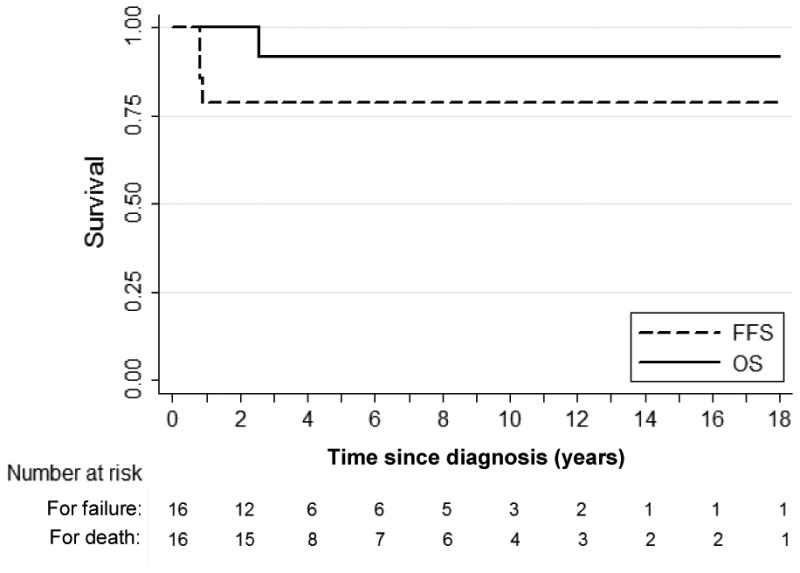
Kaplan-Meier overall survival and failure-free survival curves for all patients included in study. Median follow-up duration was 4.1 years (range, 1.9-19.3 years). OS = Overall survival, FFS = failure-free survival.
Discussion
The Children's Oncology Group (COG) trials for children with ependymoma (ACNS0121 and ACNS0831), glioma (ACNS0221), standard-risk medulloblastoma (ACNS0331), and NGGCT (ACNS1123) have tested the hypothesis that reducing of the target volume of RT is associated with a decrease in side effects without compromise of local control rates. [15] The NGGCT arm of ACNS1123 is examining survival in patients receiving WVI + boost in patients with M0 NGGCT who achieve a CR with induction chemotherapy and those who show a partial response (PR) to induction chemotherapy with SLS revealing mature teratoma. The NGGCT arm of ACNS1123 has been permanently closed as of November 1st, 2016; the number of relapses in patients receiving reduced dose/volume of irradiation after CR/PR to induction chemotherapy exceeded the threshold set in the design of the protocol. However, full results have yet to be published and may shed light on patterns of failure and specific subgroups that preferentially benefited from reduced-volume RT; analysis of specific patient histologies and of patients who experienced CR after induction chemotherapy may contribute to a better understanding of the future of risk-stratified therapy.
While data from ACNS1123 have not yet been reported, several reasons may account for a potential discrepancy between the trial and the present study. The confidence intervals for OS and FFS reported in our study are broad, reflecting the small sample size; the true survival may be on the lower end of the estimate intervals. Additionally, 6 of 13 patients treated with WVI received doses in excess of the protocol-stipulated 30.6 Gy, which may have contributed to greater local control. The patient cohort reported in our study may have represented a lower risk group than that studied in ACNS1123. The SIOP CNS GCT 96 phase II trial defined patients with serum AFP >1000 ng/mL or age <6 years to be “high-risk”, but only 4 patients in the present study met these criteria. [16] None of the patients in the current study had multifocal disease, which was included in ACNS1123 and may portend a worse prognosis. [17] While ACNS1123 included patients with unifocal parenchymal extension, none of the patients in our study had obvious parenchymal extension. There may also be unrecorded discrepancies, such as differences in histological, immunohistochemical, and molecular profiles. Despite potential differences, forthcoming data from ACNS1123 are needed to assess if and how our results compare.
In our series of 16 patients, 3 patients developed local recurrences, all approximately 10 months after initial diagnosis. The patient who developed recurrence after CR to chemotherapy without upfront RT was diagnosed in the late 1990s. However, the current consensus for treatment is that patients with multimodal therapy involving chemotherapy and radiation have superior outcomes [13,18], and this patient's relapse is consistent with the idea that chemotherapy alone is suboptimal.
Kaplan-Meier estimates of the 4-year overall survival (OS) and failure-free survival (FFS) are 92% and 81%, respectively. These estimates are comparable to existing literature on NGGCTs treated with induction chemotherapy followed by RT; one multi-institutional study from Robertson, et al. estimated 4-year OS and FFS rates of 74% and 67%, respectively, [18] another study by the German Cooperative Group estimated 5-year OS and FFS rates of 67% and 57% [19], respectively, and the COG ACNS0122 study estimated 5-year OS and FFS rates of 93% and 84%, respectively. [13] An overview of key studies examining the use of reduced-volume RT in conjunction with chemotherapy for NGGCT is shown in Table 3. Much of the existing literature examining reduced-field RT focuses on tumor-only RT, as opposed to WVI, making comparison of our results with existing literature challenging. In addition, studies have examined a variety of approaches to chemotherapy and surgery over the last 30 years. Optimal chemotherapy itself has not been conclusively defined to date. Studies have reported FFS and OS rates at 2-4 years ranging between 60-80% [5,11,16,18,20,21], which are consistent with the 4-year OS and FFS 95% confidence intervals of 57-99% and 53-94%, respectively, in the present study. Given the significant heterogeneity in treatment in the literature, the forthcoming results of ACNS1123, which treated patients with WVI, will likely serve as the best comparator for our results.
Table 3. Selected recent studies examining reduced-volume RT used in conjunction with chemotherapy for M0 intracranial NGGCT.
| Lead author (Year) [Ref number] | # of patientsa | Treatment(s) | Relevant outcome measures | Conclusions regarding reduced-field RT |
|---|---|---|---|---|
| Matsutani (1997)[5] | 19 | Chemo→RT→Chemo Pre- and post-RT chemo: Cisplatin/VP-16 or carboplatin/VP-16 RT: 50-60 Gy |
Mdn f/u: 8.1 yrs (entire cohort) FFS at 2 yrs: 56% 24% of failures outside of primary site |
Histologies conferring a poor prognosis (e.g. choriocarcinoma, yolk sac tumor, embryonal carcinoma) may benefit from more aggressive therapy |
| Robertson (1997)[18] | 11 | Surgery→Chemo→RT→Chemo Pre-RT chemo: VP-16+cisplatin x 3-4 Local RT: mdn 50.4 Gy (range, 20-55 Gy) Post-RT chemo: Vinblastine +bleomycin + VP-16 + carboplatin x 4 |
Mdn f/u: 2.7 yrs Relapses: 3 distant (at 1.1, 2.5, 7.3 yrs) 2 local (at 0.1, 2 yrs) 4-yr OS: 74%, 4-yr FFS: 67% |
The observed spinal relapses suggest a potential role for CSI in patients not achieving CR to chemotherapy |
| Buckner (1999)[11] | 7 | Chemo±surgery→RT Chemo: VP-16 + cisplatin x 4 Local RT: mdn 54 Gy (range, 30.6-59.4 Gy) |
Mdn f/u: 3.5 yrs Relapses: None |
Chemotherapy followed by reduced-dose tumor-only RT is safe and associated with low morbidity |
| Patte (2002)[20]b | 33 | Chemo±surgery→RT Chemo: Carboplatin / VP-16 + VP-16 / ifosfamide x 3-4 Local RT: 50-55 Gy |
Mdn f/u: 5.1 yrs Relapses: Exact # unknown (“mainly spinal” at mdn 11 months) EFS: 67±14%, OS: 77±8% |
The observed spinal relapses reflect the possibility that CSI may be needed in higher-risk patients |
| Calaminus (2012)[16]b | 146 | Chemo→RT Chemo: Cisplatin + VP-16 + ifosfamide Local RT: 54 Gy |
Mdn f/u: 4.4 yrs Relapses: 23 local, 5 distant, 8 combined FFS at 4.4 yrs=69±4%, OS at 3.4 yrs=78±4% 12/22 patients with AFP > 1000 ng/mL relapsed |
Local RT is sufficient for local disease control but CSI may be needed to control microscopic disease |
| Robertson (2014)[21] | 16 | Chemo±surgery±chemo→RT Chemo: Cisplatin + VP-16 + ifosfamide x 4 Post-surgery chemo: Carboplatin + cyclophosphamide x 2 RT: WVI to 36 Gy + tumor boost (54 Gy) |
Mdn f/u 7.1 yrs Relapses: 1 local, 3 spine, 1leptomeninges, 1 combined |
CR before RT was not a prognostic factor; given the high rate of relapse in WVI patients, CSI should be recommended even to patients achieving CR to chemotherapy |
| Current study (2017) | 16 | Chemo±surgery→RT Chemo: COG protocols, as described RT: WVI to mdn 30.6 Gy (range, 30.6-36 Gy) + tumor-boost (54 Gy) or tumor-only to mdn 54 Gy (range 50.4-54 Gy) |
Mdn f/u: 4.1 yrs Relapses: 3 local (at 0.8, 0.8, and 0.9 yrs), with 1 also relapsing in leptomeninges (2.5 yrs) 4-yr OS: 92%, 4-yr FFS: 81% |
Reduced-volume RT may be feasible in low-risk subgroups, but further data and longer-term follow-up are needed |
Only includes NGGCT patients receiving chemotherapy and reduced-volume RT.
Data is available in abstract-form only.
CR=complete response, CSI=craniospinal irradiation, Gy = gray, f/u = follow-up, FFS=failure-free survival, EFS=event-free survival, IC=intracranial, NGGCT=non-germinoma germ cell tumor, M0 = non-metastatic, mdn=median, OS=overall survival, PR=partial response, VP-16 = etoposide, WVI = whole ventricular irradiation, yrs=years.
Several of the studies from large French, German, and Japanese cooperative groups have found high rates of distant relapse, including spinal and/or leptomeningeal failure [5,16,18,20,21], as compared to studies in which patients have received CSI. [12,13,19] The 2014 study by Robertson et al. [21] used two stages of chemotherapy to give patients several chances to achieve CR before whole ventricle RT. However, there was no benefit noted with this approach, and patients still experienced a high rate of spinal relapse. The study authors point out the inadequacy of current staging, measures of dissemination, and possibly even conventional- and high-dose chemotherapy as upfront treatments for NGGCT in some patients; future diagnostic techniques and small molecule therapies may assist with more reliably selecting patients suited for reduced-field RT. [21]
Patient subgroup analysis in our study is limited by sample size. The SIOP CNS GCT 96 phase II trial studied 154 patients with intracranial M0 NGGCT and showed that the magnitude of AFP elevation may be a poor prognostic factor in children with NGGCT. Patients with AFP levels above 1000 μg/L at diagnosis exhibited a progression-free survival (PFS) of 33%, leading the study authors to advocate for intensified chemotherapy for these patients in a subsequent trial. [16] In our study, of 2 patients with serum AFP > 1000 μg/L, one died of disease and one is alive with no evidence of disease at last follow-up. Histology indicating pure endodermal sinus, choriocarcinoma, embryonal carcinoma, or predominantly malignant mixed GCT has been shown to have a 5-year OS rate of 9%. [5] Among 3 biopsy-confirmed patients in our sample with one of these histologies, one patient died of stroke 19.3 years post-diagnosis but no failures or deaths due to disease have yet been recorded.
Previous studies have documented that the majority of NGGCT relapses occur within 18 months of diagnosis. [1,5,22,23] However, relapses have been documented as late as 68 months, reinforcing the importance of continued surveillance.
The role of RT technique in the treatment of intracranial M0 NGGCT is an area of continued investigation. One study compared dose-volume histograms of patients receiving 3D-CRT and IMRT for WVI and showed that IMRT reduced the irradiated volume receiving doses of 20 Gy, 30 Gy, and 40 Gy by 8%, 12%, and 9%, respectively, when compared with 3D-CRT. In addition, IMRT provided statistically significant reductions of median irradiated volumes at all dose levels. However, RT dose to peripheral areas of the body was 1.9 times higher with IMRT than with 3D-CRT. [24] Our institution previously compared DP-IMRT to standard IMRT and found that DP-IMRT decreased mean dose to whole brain, temporal lobes, hippocampi, cochleae, and optic nerves. [25] Whole ventricular PBT is similarly associated with reduced volume of cerebral cortex receiving dose as compared to 3D-CRT and IMRT. [26]
Limitations of our study include the small sample size, retrospective design, heterogeneous patient population and histopathologies represented, and probable confounders within the analysis that are unable to be accounted for. Additionally, patients were carefully selected for treatment with limited RT fields, which likely represents a source of selection bias. In light of the recent closure of the nongerminoma stratum of ACNS1123, we reaffirm the latest Children's Oncology Group recommendation that newly diagnosed NGGCT patients receive 6 cycles of induction chemotherapy ± SLS in cases of partial response according to the published results of ACNS0122. [13] This should be followed with CSI to 36 Gy with boost to primary sites for a total dose of 54 Gy. However, the data presented in the current study raise interest for continued inquiry into patient subgroups of intracranial M0 NGGCT that are likely to benefit from reduced-volume RT, particularly as advances in treatment further enable long-term survival.
Compliance with Ethical Standards
Ira Dunkel reports personal fees from Bayer Health Care Pharmaceuticals, Bristol-Myers Squibb, Ipsen, Eisai, and Pfizer, and grants from Genentech and Parexel (GSK, Novartis), all outside the submitted work. Mark Souweidane reports personal fees from Aesculap outside the submitted work. All other authors declare no conflicts. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Acknowledgments
Funding was provided by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748). This work is also supported by a gift from Jack and Susan Rudin.
References
- 1.Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. Journal of neurosurgery. 1985;63(2):155–167. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 2.Kamoshima Y, Sawamura Y. Update on current standard treatments in central nervous system germ cell tumors. Current opinion in neurology. 2010;23(6):571–575. doi: 10.1097/WCO.0b013e32833ff522. [DOI] [PubMed] [Google Scholar]
- 3.Echevarria ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. The oncologist. 2008;13(6):690–699. doi: 10.1634/theoncologist.2008-0037. [DOI] [PubMed] [Google Scholar]
- 4.Schild SE, Haddock MG, Scheithauer BW, Marks LB, Norman MG, Burger PC, Wong WW, Lyons MK, Schomberg PJ. Nongerminomatous germ cell tumors of the brain. International journal of radiation oncology, biology, physics. 1996;36(3):557–563. doi: 10.1016/s0360-3016(96)00354-9. [DOI] [PubMed] [Google Scholar]
- 5.Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, Seto T. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. Journal of neurosurgery. 1997;86(3):446–455. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 6.Drummond KJ, Rosenfeld JV. Pineal region tumours in childhood. A 30-year experience. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1999;15(2-3):119–126. doi: 10.1007/s003810050347. discussion 127. [DOI] [PubMed] [Google Scholar]
- 7.Jaing TH, Wang HS, Hung IJ, Tseng CK, Yang CP, Hung PC, Lui TN. Intracranial germ cell tumors: a retrospective study of 44 children. Pediatric neurology. 2002;26(5):369–373. doi: 10.1016/s0887-8994(01)00419-2. [DOI] [PubMed] [Google Scholar]
- 8.Packer RJ, Cohen BH, Cooney K. Intracranial germ cell tumors. The oncologist. 2000;5(4):312–320. [PubMed] [Google Scholar]
- 9.Fossati P, Ricardi U, Orecchia R. Pediatric medulloblastoma: toxicity of current treatment and potential role of protontherapy. Cancer treatment reviews. 2009;35(1):79–96. doi: 10.1016/j.ctrv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Calaminus G, Bamberg M, Baranzelli MC, Benoit Y, di Montezemolo LC, Fossati-Bellani F, Jurgens H, Kuhl HJ, Lenard HG, Curto ML, et al. Intracranial germ cell tumors: a comprehensive update of the European data. Neuropediatrics. 1994;25(1):26–32. doi: 10.1055/s-2008-1071577. [DOI] [PubMed] [Google Scholar]
- 11.Buckner JC, Peethambaram PP, Smithson WA, Groover RV, Schomberg PJ, Kimmel DW, Raffel C, O'Fallon JR, Neglia J, Shaw EG. Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(3):933–940. doi: 10.1200/JCO.1999.17.3.933. [DOI] [PubMed] [Google Scholar]
- 12.Kellie SJ, Boyce H, Dunkel IJ, Diez B, Rosenblum M, Brualdi L, Finlay JL. Primary Chemotherapy for Intracranial Nongerminomatous Germ Cell Tumors: Results of the Second International CNS Germ Cell Study Group Protocol. Journal of Clinical Oncology. 2004;22(5):846–853. doi: 10.1200/JCO.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Goldman S, Bouffet E, Fisher PG, Allen JC, Robertson PL, Chuba PJ, Donahue B, Kretschmar CS, Zhou T, Buxton AB, Pollack IF. Phase II Trial Assessing the Ability of Neoadjuvant Chemotherapy With or Without Second-Look Surgery to Eliminate Measurable Disease for Nongerminomatous Germ Cell Tumors: A Children's Oncology Group Study. Journal of Clinical Oncology. 2015;33(22):2464–2471. doi: 10.1200/JCO.2014.59.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chemotherapy Followed by Radiation Therapy in Treating Younger Patients With Newly Diagnosed Localized Central Nervous System Germ Cell Tumors. https://ClinicalTrials.gov/show/NCT01602666.
- 15.Merchant TE, Hodgson D, Laack NN, Wolden S, Indelicato DJ, Kalapurakal JA. Children's Oncology Group's 2013 blueprint for research: radiation oncology. Pediatric blood & cancer. 2013;60(6):1037–1043. doi: 10.1002/pbc.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calaminus G, Frappaz D, Kortmann RD, Alapetite C, Garre ML, Ricardi U, Saran FH, N J. GC-11. RISK ADAPTED IRRADIATION IS FEASIBLE IN INTRACRANIAL NONGERMINOMATOUS GERM CELL TUMOURS (NGGCT): FINAL RESULTS OF SIOP CNS GCT 96. Neuro-Oncology. 2012;14(Suppl 1):i49–55. doi: 10.1093/neuonc/nos101. [DOI] [Google Scholar]
- 17.Aizer AA, Sethi RV, Hedley-Whyte ET, Ebb D, Tarbell NJ, Yock TI, MacDonald SM. Bifocal intracranial tumors of nongerminomatous germ cell etiology: diagnostic and therapeutic implications. Neuro-Oncology. 2013;15(7):955–960. doi: 10.1093/neuonc/not050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. Journal of neuro-oncology. 1997;32(1):71–80. doi: 10.1023/a:1005732105727. [DOI] [PubMed] [Google Scholar]
- 19.Calaminus G, Bamberg M, Harms D, Jürgens H, Kortmann RD, Sörensen N, Wiestler OD, Göbel U. AFP/β-HCG Secreting CNS Germ Cell Tumors: Long-Term Outcome with Respect to Initial Symptoms and Primary Tumor Resection. Results of the Cooperative Trial MAKEI 89. Neuropediatrics. 2005;36(02):71–77. doi: 10.1055/s-2005-837582. [DOI] [PubMed] [Google Scholar]
- 20.Patte C, Frappaz D, Raquin MA, Bouffet E, Kalifa C, Baranzelli MC. Treatment of Primary Intracranial Germ Cell Tumours with Carboplatin-based Chemotherapy and Focal Irradiation. In: Harnden P, Joffe JK, Jones WG, editors. Germ Cell Tumours V; The Proceedings of the Fifth Germ Cell Tumour Conference Devonshire Hall, University of Leeds; 13th-15th September, 2001; Springer London, London: 2002. pp. 131–131. [DOI] [Google Scholar]
- 21.Robertson PL, Jakacki R, Hukin J, Siffert J, Allen JC. Multimodality therapy for CNS mixed malignant germ cell tumors (MMGCT): results of a phase II multi-institutional study. Journal of neuro-oncology. 2014;118(1):93–100. doi: 10.1007/s11060-013-1306-0. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman HJ, Otsubo H, Hendrick EB, Humphreys RP, Drake JM, Becker LE, Greenberg M, Jenkin D. Intracranial germ-cell tumors in children. Journal of neurosurgery. 1991;74(4):545–551. doi: 10.3171/jns.1991.74.4.0545. [DOI] [PubMed] [Google Scholar]
- 23.Dearnaley DP, A'Hern RP, Whittaker S, Bloom HJ. Pineal and CNS germ cell tumors: Royal Marsden Hospital experience 1962-1987. International journal of radiation oncology, biology, physics. 1990;18(4):773–781. doi: 10.1016/0360-3016(90)90396-2. [DOI] [PubMed] [Google Scholar]
- 24.Chen MJ, Santos Ada S, Sakuraba RK, Lopes CP, Goncalves VD, Weltman E, Ferrigno R, Cruz JC. Intensity-modulated and 3D-conformal radiotherapy for whole-ventricular irradiation as compared with conventional whole-brain irradiation in the management of localized central nervous system germ cell tumors. International journal of radiation oncology, biology, physics. 2010;76(2):608–614. doi: 10.1016/j.ijrobp.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Yang JC, Terezakis SA, Dunkel IJ, Gilheeney SW, Wolden SL. Intensity-Modulated Radiation Therapy With Dose Painting: A Brain-Sparing Technique for Intracranial Germ Cell Tumors. Pediatric blood & cancer. 2016;63(4):646–651. doi: 10.1002/pbc.25867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JY, Park J. Understanding the Treatment Strategies of Intracranial Germ Cell Tumors: Focusing on Radiotherapy. Journal of Korean Neurosurgical Society. 2015;57(5):315–322. doi: 10.3340/jkns.2015.57.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]


