Abstract
Morphine has been shown to increase the expression of brain-derived neurotrophic factor (BDNF) in the brain. However, little is known about the effect of morphine withdrawal on BDNF and its precursor protein, or proBDNF, which induces neuronal apoptosis. In this work, we examined whether BDNF and proBDNF levels change in rats chronically injected with escalating doses of morphine and those who undergo spontaneous withdrawal for 60 hr. We observed, in the frontal cortex and striatum, that the ratio of BDNF to proBDNF changed depending upon the experimental paradigm. Morphine treatment and morphine withdrawal increased both BDNF and proBDNF levels. However, the increase in proBDNF immunoreactivity in withdrawal rats was more robust than that observed in morphine-treated rats. proBDNF is processed either intracellularly by furin or extracellularly by the tissue plasminogen activator (tPA)/plasminogen system or matrix metalloproteases (MMPs). To examine the mechanisms whereby chronic morphine treatment and morphine withdrawal differentially affects BDNF/proBDNF, the levels MMP-3 and -7, furin, and tPA were analyzed. We found that morphine increases tPA levels whereas withdrawal causes a decrease. To confirm the involvement of tPA in the morphine-mediated effect on BDNF/proBDNF, we exposed cortical neurons to morphine in the presence of the tPA inhibitor PAI-1. This inhibitor reversed the morphine-mediated decrease in proBDNF, supporting the hypothesis that morphine increases the availability of BDNF by promoting the extracellular processing of proBDNF by tPA. Because proBDNF could negatively influence synaptic repair, preventing withdrawal is crucial for reducing neurotoxic mechanisms associated with opioid abuse.
Keywords: BDNF, proBDNF, furin, opioid abuse, tissue plasminogen activator
Introduction
Brain-derived neurotrophic factor (BDNF) is a neurotrophin that prevents the injury of different neuronal populations (Han and Holtzman 2000; Hyman et al. 1991; Mamounas et al. 1995) and plays a pivotal role in brain plasticity-related processes such as learning and memory (Park and Poo 2013). BDNF may also have a role in the molecular and cellular modifications underlying opioid abuse because higher blood levels of BDNF are associated with increased craving for heroin (Heberlein et al. 2011). In addition, a polymorphism (Val66Met) in the BDNF gene, which regulates the release of BDNF (Egan et al. 2003), has been linked to heroin-seeking behavior (Greenwald et al. 2013). In animals, morphine administration increases BDNF expression in selected brain areas (Hatami et al. 2007; Koo et al. 2012; Mashayekhi et al. 2012; Numan et al. 1998; Vargas-Perez et al. 2009). These and other findings (Akbarian et al. 2002) have indicated that BDNF mediates behavioral adaptations underlying opioid addiction (Bolanos and Nestler 2004). Thus, BDNF may be a tool for diagnosis and a target for a therapeutic intervention in opiate abusers.
BDNF is synthesized as a larger glycosylated precursor, proBDNF (Mowla et al. 2001), which exhibits biological effects opposite of those of mature BDNF (mBDNF). In fact, when proBDNF, through sortilin, binds to the p75 neurotrophin receptor (p75NTR), it promotes neuronal apoptosis (Teng et al. 2005), presynaptic terminal retraction (Yang et al. 2009), long-term depression (Nagappan et al. 2009; Woo et al. 2005), and proinflammatory responses (Luo et al. 2016). When released extracellularly, proBDNF is immediately processed to mBDNF by plasmin or metalloproteases (Lee et al. 2001; Pang et al. 2004). Intriguingly, the levels and activity of tissue plasminogen activator (tPA), a serine protease that converts plasminogen into plasmin, are up-regulated by morphine (Nagai et al. 2004; Yamada et al. 2005). Because tPA promotes neurite outgrowth (Jacovina et al. 2001; Seeds et al. 1999) and long-term potentiation (Centonze et al. 2002), two neurotrophic properties that are similar to that of BDNF, one could suggest that the tPA-mediated processing of proBDNF to mBDNF may underlie morphine-induced synaptic plasticity.
One of the most profound side effects of opiates is dependence that if not maintained, leads to withdrawal. The common physical symptoms of opioid withdrawal include nausea, diarrhea, tachycardia, and the development of hyperalgesia among others. Incidentally, several psychological symptoms of withdrawal persist well past the physical symptoms. These include irritability, anxiety, and drug cravings that can continue months to years after last drug use, leading to relapse. The hypothalamic-pituitary-adrenal axis is affected by withdrawal, leading to maladaptive changes marked by enhanced sensitivity to stressors (Valentino and Van Bockstaele 2015), which produce heightened anxiety-like states and dysphoria that can increase susceptibility to relapse (Schluger et al. 2003). Additionally, physical symptoms may be accompanied by neurodegeneration. In fact, in animal models, morphine withdrawal negatively affects synaptic plasticity and neuronal survival (Bandaru et al. 2011; Campbell et al. 2015), which might be attributed to the neurotoxic effects of proBDNF (Teng et al. 2005).
The aim of the present study was to establish whether morphine withdrawal affects proBDNF processing. Our findings demonstrate that chronic morphine and withdrawal differentially alter proBDNF and mBDNF levels. Moreover, we have established a correlation between morphine withdrawal, tPA levels and activity, and proBDNF processing.
Materials and Methods
Reagents
Morphine sulfate was received from the National Institutes of Drug Abuse, (NIH, Division of Neuroscience & Behavioral Research, Research Triangle Park, NC). Morphine was diluted in isotonic and filtered sterilized saline. All other chemicals were commercially obtained, reagent grade.
Animals
All studies were carried out following the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health and approved by Georgetown University Animal Care and Use Committee.
Three-month-old male Sprague-Dawley rats (Charles River, Germantown, MD) were acclimated for a minimum of one week prior to conducting experiments. Animals were housed under standard conditions, two per cage, with food and water available ad libitum, and were maintained on a 12-h light/dark cycle for the duration of the treatment protocols. Rats received either a single injection of morphine (10 mg/kg), subcutaneously (s.c.) or saline, or were treated with escalating doses of morphine from 10 mg/kg to 30 mg/kg twice at day over the course of five days to cause dependence as previously described (Campbell et al. 2013). To cause morphine withdrawal, animals were treated with increasing dosages of morphine for five days as described above, after which, morphine treatment was ceased. Withdrawal symptoms were observed for 60 hr and included diarrhea, weight loss, piloerection, and irritability, compared to control animals (Campbell et al. 2013). After the treatments, animals were anesthetized with a mixture of ketamine/xylazine (80 mg/kg and 20 mg/kg, respectively) and intracardially perfused with ice-cold phosphate buffered saline. Whole brains were quickly removed and frontal cortex and striatum were dissected on ice for biochemical analysis.
Cortical neurons
Cortical neurons were prepared from the cortex of embryonic (E17–18) Sprague Dawley rats (Charles River, MA) following an established protocol (Avdoshina et al. 2010). Cells were seeded onto poly-L-lysine pre-coated plates in Neurobasal Medium containing 2% B27 supplement, 25 nM glutamate, 0.5 mM L-glutamine, and 1% antibiotic-antimycotic solution (Invitrogen, Carlsbad, CA). Cultures were grown at 37°C in 5% CO2/95% air for 8 days on glass coverslips. The presence of neurons and non-neuronal cells was verified by staining coverslips with the following antibodies: class III β-tubulin (1:1000; Covance, Emeryville, CA), glial fibrillary acidic protein (1:250, Abcam, Cambridge, MA), ionized calcium binding adaptor molecule 1 (1:500; Wako Chemicals USA, Inc., Richmond, VA) to visualize neurons, astrocytes and microglia, respectively. At the time of the experiments, cultures contained ~5% non-neuronal cells.
Western blot analysis
Brain areas were homogenized in RIPA buffer (EMD Millipore, Billerica, MA) containing protease-phosphatase inhibitors (Roche Applied Science, Indianapolis, IN) by sonication and protein elution at 4°C. For the detection of mBDNF and proBDNF, lysates were subjected to immunoprecipitation, as previously described (Bachis et al. 2012). In brief, protein levels in the lysates were measured by the Bradford Coomassie blue colorimetric assay (Bio-Rad Laboratories Inc, Hercules, CA). Equal amount of proteins were precleared using precipHen (Aves Labs, Tigar, OR) or protein A-Sepharose beads (Sigma Aldrich, St Louis, MO), and the supernatant was then incubated for 18 hr at 4°C with an anti-BDNF antibody (5 μg/ml, EMD Millipore), as previously described (Lim et al. 2011). Immunoprecipitates were collected by centrifugation for 5 min at 5000 rpm. Beads were washed in lysis buffer and immune complexes were resolved by SDS-PAGE. Proteins were transferred onto a PVDF membrane and blocked with TBS-T (25 mM Tris and 1% Tween) containing 5% milk powder. Blots were then incubated overnight with the anti-BDNF antibody (1:1000, Promega Corp, Madison, WI).
For matrix metalloproteinases (MMPs), tissue lysates were loaded on a PVDF membrane and analyzed by an anti-MMP-3 (1:500, EMD Millipore) and anti-MMP-7 (1:200, Abcam) antibodies. Blots were washed with TBS-T, and incubated with correspondent peroxidase-conjugated secondary antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hr at room temperature. To assure for equal protein loading membranes were stripped with Restore Western Blot Stripping Buffer (Invitrogen) for 30 min at 37°C and re-probed with a mouse monoclonal β-actin (1:5000, Sigma- Aldrich) antibody.
Immunoreactivity was detected by enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA). The intensity of immunoreactive bands was quantified using ImageJ (National Institute of Health, Bethesda, MD).
Enzyme-Linked Immunosorbent Assay (ELISA)
Protein levels in the lysates were measured by the Bradford Coomassie blue colorimetric assay (Bio-Rad). Fifty μg of proteins were then used to measure the levels of mBDNF and proBDNF by ELISA immunoassay kits from Aviscera Bioscience (Yoshida et al. 2012) or Promega Corp., respectively, according to the manufacturer’s instructions, as previously described (Bachis et al. 2012). For the proBDNF ELISA, recombinant mBDNF did not demonstrate measurable immunoreactivity below 5ng/ml as determined by running in parallel a standard curve with proBDNF. Above this concentration, recombinant mBDNF demonstrated an approximately 14% cross reactivity with the ELISA kit (supplementary figure).
For the measurement of mBDNF and proBDNF in neuronal culture media, medium from neurons was collected on ice, centrifuged at 10,000 rpm for 10 min and concentrated using Amicon® ultracentrifugal filters (EMD Millipore), following the manufacturer’s instructions.
Furin levels were determined using an ELISA from R&D System (Minneapolis, MN) as previously described (Bachis et al. 2012). Total and functionally active tPA levels were determined using ELISA from Innovative Research Inc. (Novi, MI), according to the manufacturer instructions.
Statistical Analysis
Statistical analysis was calculated by ANOVA and post-hoc Tukey test for multiple comparisons using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA). P values of <0.05 indicate statistical significance.
Results
Morphine and morphine withdrawal change the ratio mBDNF/proBDNF in vivo
Morphine dependence and withdrawal differentially affect the expression of pro-inflammatory cytokines in the frontal cortex and striatum but not in the hippocampus or cerebellum (Campbell et al. 2013). To examine whether the same treatments alter mBDNF and proBDNF levels, rats were injected subcutaneously for 5 days with saline or escalating doses of morphine that induce dependence, as described in Materials and Methods. Rats were euthanized 2 hr after the last injection. For comparison, rats also received an acute treatment of saline or an acute dose of morphine and were sacrificed two hours after the injection. To determine the effect of withdrawal, rats received an escalating dose of morphine for five days and were allowed to undergo spontaneous withdrawal for 60 hr prior to euthanasia.
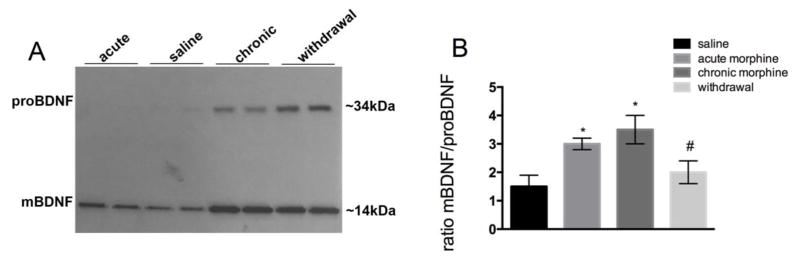
The levels of mBDNF and proBDNF were then first determined in the frontal cortex by Western blot analysis. Tissue lysates were immunoprecipitated with a BDNF antibody that recognizes both mBDNF and proBDNF immunoreactivity and probed with a different BDNF antibody, as previously described (Bachis et al. 2012). Two immunoreactive bands migrating at an apparent molecular weight of ~14 kDa and ~34 kDa corresponding to mBDNF and proBDNF, respectively, were detected (Fig. 1A). All morphine paradigms increased mBDNF, although the intensity of the 14kDa band was stronger in the chronic morphine treated and withdrawal rats than acute morphine group (Fig. 1A). Likewise, the 34kDa proBDNF band was increased in chronic morphine and withdrawal groups when compared to either saline or acute morphine (Fig. 1A). Moreover, densitometric analysis of the 14kDa and 34kDa bands revealed that morphine withdrawal significantly decreased the ratio mBDNF/proBDNF when compared to chronic morphine treatment (Fig. 1B).
Figure 1. Chronic morphine treatment and morphine withdrawal change the ratio proBDNF/mBDNF in the frontal cortex.
Male rats were treated s.c. with saline or escalating doses of morphine for five days and were euthanized 2 hr later. Another group of rats received an acute dose of morphine 2 hr prior to euthanasia. A third group of animals received chronic morphine and was allowed to undergo spontaneous withdrawal for 60 hr. The levels of mBDNF and proBDNF were then measured by Western blot in tissue lysates from the frontal cortex following immunoprecipitation. A: Example of Western blot analysis. Lysates were immunoprecipitated with an antibody against BDNF. The blot was then analyzed with a BDNF antibody that recognizes both the mature (14kDa) and pro form of BDNF (34 kDa). B. Densitometric analysis of BDNF-like immunoreactivity expressed as the ratio mBDNF/proBDNF immunoreactivity. Data are the mean ± SEM of 4 rats per group. *p<0.01 vs saline, #p<0.05 vs chronic morphine.
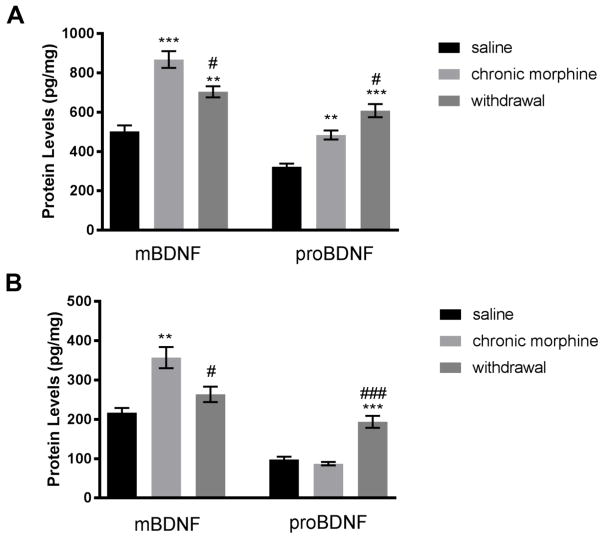
To confirm the effect of morphine and morphine withdrawal, we analyzed mBDNF and proBDNF levels in the frontal cortex by ELISA. Both chronic morphine administration and withdrawal increased mBDNF compared to saline controls (Fig. 2A). Furthermore, the increase in proBDNF by withdrawal was significantly higher than that obtained after chronic morphine administrations (Fig. 2A), supporting the data obtained by Western blot analysis. Thus, we suggest that chronic morphine treatment and withdrawal differentially affect proBDNF levels.
Figure 2. Morphine withdrawal increases proBDNF.
Male rats were treated s.c. with saline or escalating doses of morphine for five days. A group of animals was allowed to undergo spontaneous withdrawal for 60 hr. The levels of mBDNF and proBDNF were then measured by ELISA in the frontal cortex (A) and striatum (B). Data, expressed as pg/mg of protein, are the mean ± SEM of five animals per group. **p<0.01, ***p<0.001 vs saline; #p<0.05, ##p<0.01 and ###p<0.001 vs chronic morphine.
BDNF is produced in neurons of the cerebral cortex and is delivered by anterograde transport to striatal neurons (Altar et al. 1997). Therefore, we examined whether the effects of morphine and withdrawal seen in the frontal cortex, could be reproducible in the striatum using the same animal groups. We observed that chronic morphine treatment increased the levels of mBDNF without a significant increase in proBDNF (Fig. 2B). In animals undergoing withdrawal, there was a significant increase in proBDNF while mBDNF did not change compared to saline controls (Fig. 2B). Thus, withdrawal decreases the ratio mBDNF/proBDNF in this brain area as well.
Chronic morphine and processing enzymes
proBDNF is cleaved intracellularly by the enzyme furin, and extracellularly by MMPs, in particular MMP-3 and -7, and plasmin (Pang et al. 2004). Morphine may change the activity of one or more of these processing enzymes. Indeed, morphine has been shown to increase the levels and activity of tPA (Nagai et al. 2004; Yamada et al. 2005), a serine protease that is released in an activity dependent manner (Bruno and Cuello 2006) and plays a role in proBDNF processing by converting plasminogen into plasmin (Pang et al. 2004). Therefore, in an attempt to reveal the molecular mechanisms through which chronic morphine treatment and withdrawal could be affecting the levels of proBDNF, we determined the levels of these enzymes in the frontal cortex of chronic morphine and withdrawal animals.
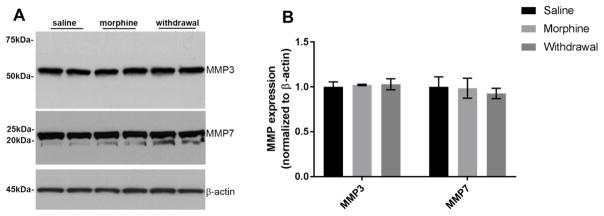
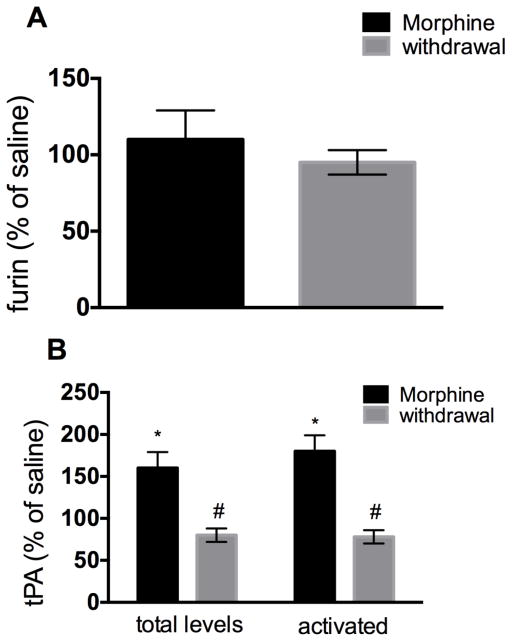
Western blot analysis of MMP-3 and -7 immunoreactivity revealed that neither chronic morphine nor withdrawal changed their levels when compared to saline (Fig. 3). Similarly, using a sensitive ELISA (Bachis et al. 2012) we found no changes in furin levels (Fig. 4A) in any treatment groups, overall suggesting that the levels of these enzymes are not affected by morphine or withdrawal. However, chronic morphine and withdrawal differentially affected tPA. In fact, while chronic morphine increased tPA levels, withdrawal decreased it when compare to saline control (Fig. 4B).
Figure 3. Morphine does not change the levels of MMPs.
Cortical lysates were obtained from animals treated with saline, chronic morphine or undergoing withdrawal. A. Example of a Western blot of lysates from 2 rats each group analyzed for MMP-3 and MMP-7 immunoreactivity. MMP-3 and MMP-7 bands migrate with an apparent molecular weight of 53kDa and 22kDa, respectively. The blot was washed and reprobed with a β actin antibody for loading control. B. Relative levels of MMPs were calculated by optical density and normalized by β-actin immunoreactivity. Data, expressed as arbitrary units, are the mean ± SEM of four separate samples each group.
Figure 4. Morphine and morphine withdrawal differentially change the levels of tPA.
Male rats were treated s.c. with saline or escalating doses of morphine. A group of animals was allowed to undergo spontaneous withdrawal for 60 hr. Levels of furin (A), total tPA and activated tPA (B) were then measured in the frontal cortex by ELISA. Data, expressed as % of saline control, are the mean ± SEM of five animals per group. *p<0.01 vs. saline control, #p<0.05 vs. saline control.
Increased levels of tPA alone may not reveal whether this enzyme is actively promoting the processing of proBDNF. Therefore, we determined the levels of functionally active tPA by ELISA. We found that while chronic morphine treatment increased activated tPA levels when compared to saline controls, withdrawal decreased them (Fig. 4B). Collectively, these data suggest that the different ratio mBDNF/proBDNF evoked by chronic morphine administration and morphine withdrawal is due to processing of proBDNF.
Chronic morphine affects the release of proBDNF and mBDNF in vitro
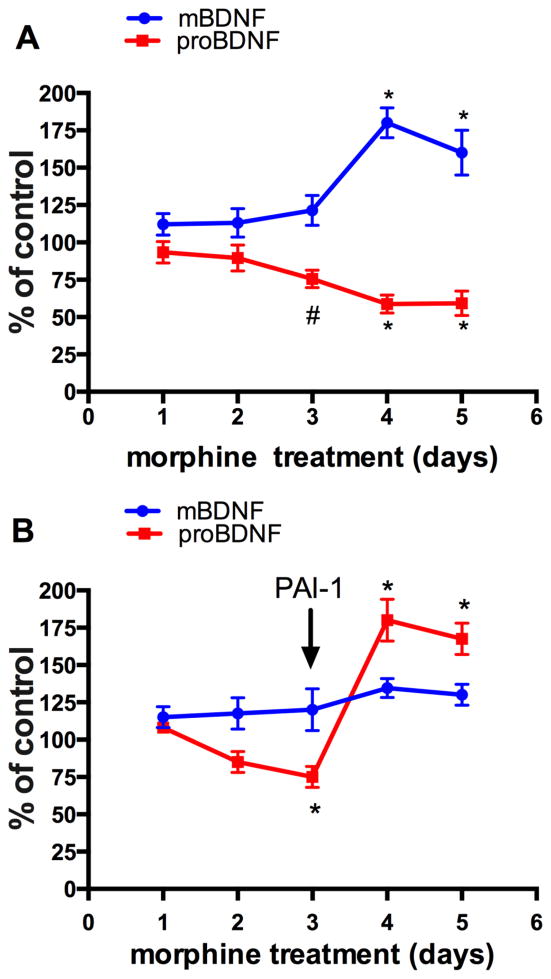
proBDNF is neurotoxic after it has been released into the synaptic cleft. However, measuring proBDNF release in vivo is challenging. Therefore, we used primary rat cortical cells in culture. Cortical neurons were exposed to 1μM morphine every 24 hr for 5 days. The amount of mBDNF and proBDNF released by the cells was determined in the concentrated medium by ELISA using an antibody specific for mBDNF or proBDNF, as previously described (Bachis et al. 2012). The time course analysis shows that morphine elicits a temporal increase in mBDNF in the medium on days 4 and 5 (Fig. 5A) concomitantly to a reduction of proBDNF by day 3 of treatment (Fig. 5A).
Figure 5. Morphine increases the release of mBDNF and proBDNF in vitro.
Rat cortical neurons were exposed to 1 μM, morphine or saline control each day for five days. (A) Medium was collected at the indicated time points, and levels of BDNF and proBDNF were measured by ELISA in the concentrated media. Data are expressed as % of control because the basal levels of neurotrophins fluctuated throughout the experiments. (B) Cortical neurons were exposed to morphine or saline control for 3 days. Starting at day 3, PAI-1 (100ng/ml) was added concomitantly with morphine. Data, expressed as % of control, are the mean ± SEM of three separate experiments in duplicate. *p<0.01, #p<0.05 vs control.
To establish whether there is a correlation between morphine, tPA, and proBDNF, cortical neurons were exposed to morphine for different time points, alone or in combination with plasminogen activator inhibitor-1 (PAI-1), a tPA inhibitor (Cale and Lawrence 2007). PAI-1 was added to neurons concomitantly with morphine, starting at day 3. Low concentrations of PAI-1 (100ng/ml) were used due to its toxicity (Flavin et al. 2000). We observed that PAI-1 abrogated both the morphine-mediated decrease of proBDNF release and increase in mBDNF release (Fig. 5B), further suggesting a central role of tPA in regulating morphine-induced changes in the mBDNF/proBDNF ratio.
Discussion
We have previously shown that morphine withdrawal promotes neuronal apoptosis by increasing pro-inflammatory cytokines and microglia (Campbell et al. 2015). The present work aims to provide more insights into the neurotoxic mechanisms of morphine withdrawal, by looking at its effect on proBDNF. proBDNF is a secreted protein that binds to p75NTR and induces neurotoxic events such as apoptosis (Teng et al. 2005), growth cone collapse (Sun et al. 2012), presynaptic terminal retraction (Je et al. 2013), and axonal degeneration (Bachis et al. 2012). Our study shows that mBDNF is increased by both chronic morphine treatment and withdrawal but withdrawal augments proBDNF so that it significantly decreases the ratio mBDNF/proBDNF. Moreover, we have shown that morphine dependence and morphine withdrawal differentially alter the levels of tPA, a protease that is implicated in proBDNF cleavage. Overall, our findings suggest that chronic morphine and morphine withdrawal alters the ratio mBDNF/proBDNF by modifying proBDNF processing.
proBDNF is packaged in dense core vesicles (Dieni et al. 2012), transported as a complex with Huntingtin associated protein-1 and sortilin (Chen et al. 2005; Yang et al. 2011) and released into the synaptic cleft where it can be cleaved by MMPs and the serine protease plasmin (Pang et al. 2004). Plasmin, which is initially produced as inactive plasminogen, requires cleavage by tPA (Plow et al. 1995) which is released upon neuronal activation (Bruno and Cuello 2006). Decreased tPA release has been shown to correlate with the ability of proBDNF to induce neuronal apoptosis (Head et al. 2009), supporting the notion that neuronal activity coordinates the simultaneous release of proneurotrophins and processing enzymes (Bruno and Cuello 2006). Our findings show that neither furin nor MMP levels are changed by chronic morphine or withdrawal but tPA is activated by morphine and reduced by withdrawal. In addition, the tPA inhibitor PAI-1 reversed the effect of chronic morphine on mBDNF/proBDNF in cortical neurons, suggesting that the tPA/plasminogen system most likely mediates morphine-induced processing of proBDNF to mBDNF. Our data support previous studies showing that repeated doses of morphine increase tPA expression (Berta et al. 2013). Our data join a growing number of studies that have shown the importance of tPA in proBDNF processing and neuronal plasticity. Indeed, tPA knockout mice, which exhibit increased levels of proBDNF in the hippocampus (Pang et al. 2004), have impaired synaptic plasticity (Matys et al. 2004; Pawlak et al. 2003) and reduced long term potentiation (Calabresi et al. 2000). Importantly, this scenario is similar to that described in the brain of rodents undergoing morphine withdrawal. In fact, opiate withdrawal has been shown to decreases the number and complexity of dendritic spines in brain reward circuitry neurons (Robinson et al. 2002) including the ventral tegmental area (Russo et al. 2009; Spiga et al. 2003). Although more studies are needed to examine in more details the neuroanatomical induction of proBDNF during withdrawal, our data suggest that proBDNF might be responsible for some of the morphological changes induced by morphine withdrawal.
The data presented here indicate that morphine withdrawal increases proBDNF by reducing its extracellular processing. Unprocessed extracellular proBDNF can lead to activation of p75NTR, which results in damage to neuronal processes and remodeling (Teng et al., 2005), as well as reduced long-term potentiation. Moreover, when proBDNF is not cleaved, the availability of mBDNF is drastically reduced. Lack of or reduced mBDNF has been suggested to be one of the causes of the neuropathology observed in various neurodegenerative diseases, including Parkinson’s (Howells et al. 2000; Toda et al. 2003) and Huntington’s disease (Ciammola et al. 2007), and human immunodeficiency virus (HIV)-associated dementia (HAND) (Bachis et al. 2012). While transcriptional mechanisms mediate the decrease in mBDNF in Parkinson’s and Huntington’s disease (Zuccato and Cattaneo 2009), in HAND it appears that impaired processing of proBDNF is the main cause of low levels of mBDNF. In fact, the basal ganglia of HAND exhibit reduced furin and tPA levels, concomitantly to higher levels of proBDNF, than HIV subjects without dementia (Bachis et al. 2012). This scenario is similar to that characterized here, supporting a suggestion that opioid withdrawal, not abuse, may accelerate the progression of neurological impairment such as that seen in HAND subjects (Bandaru et al. 2011).
In this study, we present evidence that morphine withdrawal increases the pro-apoptotic proBDNF. However, proBDNF is not the only neurotrophin that is neurotoxic by binding to p75NTR. In fact, pro-nerve growth factor binds to p75NTR and promotes neurotoxicity of various cell types (Beattie et al. 2002; Lebrun-Julien et al. 2010; Nykjaer et al. 2004). Moreover, the intracellular or extracellular processing of proBDNF, in addition to mBDNF, generates a BDNF prodomain, the amino-terminal portion of proBDNF (Anastasia et al. 2013). The prodomain interacts with sortilin and promotes growth cone retraction, similar to proBDNF (Anastasia et al. 2013). In our study, we did not examine whether withdrawal affects these two ligands, which are also released from neurons (Anastasia et al. 2013; Harrington et al. 2004); moreover, the antibody against proBDNF could detect the BDNF prodomain as well. Therefore, we cannot rule out that other pro-apoptotic neurotrophins could be released during morphine withdrawal and be involved in its neurotoxic effects. In addition, withdrawal from morphine can positively affect the production of pro-inflammatory cytokines such as interleukin-1β and tumor necrosis factor-α, and negatively affect the production of anti-inflammatory cytokines such as CCL5 (Campbell et al. 2013; Desjardins et al. 2008; Eisenstein et al. 2006; Hutchinson et al. 2011). Likewise, withdrawal can increase susceptibility to outside pathogens (Eisenstein et al. 2006). For example, opioid withdrawal can reduce the levels of circulating lymphocytes in morphine-dependent macaques (Weed and Steward 2005), or exacerbate the neurotoxic effect of the HIV protein gp120 (Campbell et al. 2015). Moreover, an in vitro study of HIV-infected lymphocytes has revealed enhanced HIV replication in both abrupt (cessation of treatment) and precipitated (by naloxone) opioid withdrawal (Desjardins et al. 2008). Thus, one could suggest that opioid withdrawal in viral infectious diseases may increase neuronal susceptibility to the toxic effects of the virus.
Drug abuse is a complex disease. In humans, it often involves cycles of intoxication followed by withdrawal either during recovery from opioid abuse or during intermittent periods where addicts “seek out” more abusive substances. In the future, it will be important to establish whether drug abusers with a single nucleotide polymorphism in the BDNF gene, such as Val66Met, which impairs the release of mBDNF (Egan et al. 2003), are more at risk than non-polymorphic subjects to develop neurological alterations.
Supplementary Material
A. Representative proBDNF standard curve obtained using the ELISA kit, as described in Materials and Methods. B. Cross reactivity of different concentrations (ng/ml) of BDNF as detected by the proBDNF ELISA.
Acknowledgments
This work was supported by HHS grants 1R01 NS079172 and 1R21 NS102121 to I.M., 1F31DA032282 to L.A.C., T32 NS041218 to E.W. and Georgetown University Music for the Mind award to A.B.
References
- Akbarian S, Rios M, Liu RJ, Gold SJ, Fong HF, Zeiler S, Coppola V, Tessarollo L, Jones KR, Nestler EJ, Aghajanian GK, Jaenisch R. Brain-derived neurotrophic factor is essential for opiate-induced plasticity of noradrenergic neurons. J Neurosci. 2002;22:4153–4162. doi: 10.1523/JNEUROSCI.22-10-04153.2002. 20026381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K, Lee FS, Hempstead BL, Bracken C. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat Commun. 2013;4:2490. doi: 10.1038/ncomms3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia. 2010;58:1630–1639. doi: 10.1002/glia.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci. 2012;32:9477–9484. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Patel N, Ewaleifoh O, Haughey NJ. A failure to normalize biochemical and metabolic insults during morphine withdrawal disrupts synaptic repair in mice transgenic for HIV-gp120. J Neuroimmune Pharmacol. 2011;6:640–649. doi: 10.1007/s11481-011-9289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta T, Liu YC, Xu ZZ, Ji RR. Tissue plasminogen activator contributes to morphine tolerance and induces mechanical allodynia via astrocytic IL-1beta and ERK signaling in the spinal cord of mice. Neuroscience. 2013;247:376–385. doi: 10.1016/j.neuroscience.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci U S A. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Napolitano M, Centonze D, Marfia GA, Gubellini P, Teule MA, Berretta N, Bernardi G, Frati L, Tolu M, Gulino A. Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur J Neurosci. 2000;12:1002–1012. doi: 10.1046/j.1460-9568.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- Cale JM, Lawrence DA. Structure-function relationships of plasminogen activator inhibitor-1 and its potential as a therapeutic agent. Curr Drug Targets. 2007;8:971–981. doi: 10.2174/138945007781662337. [DOI] [PubMed] [Google Scholar]
- Campbell LA, Avdoshina V, Day C, Lim ST, Mocchetti I. Pharmacological induction of CCL5 in vivo prevents gp120-mediated neuronal injury. Neuropharmacology. 2015;92:98–107. doi: 10.1016/j.neuropharm.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LA, Avdoshina V, Rozzi S, Mocchetti I. CCL5 and cytokine expression in the rat brain: differential modulation by chronic morphine and morphine withdrawal. Brain Behav Immun. 2013;34:130–140. doi: 10.1016/j.bbi.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Napolitano M, Saulle E, Gubellini P, Picconi B, Martorana A, Pisani A, Gulino A, Bernardi G, Calabresi P. Tissue plasminogen activator is required for corticostriatal long-term potentiation. Eur J Neurosci. 2002;16:713–721. doi: 10.1046/j.1460-9568.2002.02106.x. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. 25/26/6156 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciammola A, Sassone J, Cannella M, Calza S, Poletti B, Frati L, Squitieri F, Silani V. Low brain-derived neurotrophic factor (BDNF) levels in serum of Huntington’s disease patients. Am J Med Genet B Neuropsychiatr Genet. 2007;144:574–577. doi: 10.1002/ajmg.b.30501. [DOI] [PubMed] [Google Scholar]
- Desjardins S, Belkai E, Crete D, Cordonnier L, Scherrmann JM, Noble F, Marie-Claire C. Effects of chronic morphine and morphine withdrawal on gene expression in rat peripheral blood mononuclear cells. Neuropharmacology. 2008;55:1347–1354. doi: 10.1016/j.neuropharm.2008.08.027. [DOI] [PubMed] [Google Scholar]
- Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, Gundelfinger ED, Kojima M, Nestel S, Frotscher M, Barde YA. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196:775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eisenstein TK, Rahim RT, Feng P, Thingalaya NK, Meissler JJ. Effects of opioid tolerance and withdrawal on the immune system. J Neuroimmune Pharmacol. 2006;1:237–249. doi: 10.1007/s11481-006-9019-1. [DOI] [PubMed] [Google Scholar]
- Flavin MP, Zhao G, Ho LT. Microglial tissue plasminogen activator (tPA) triggers neuronal apoptosis in vitro. Glia. 2000;29:347–354. [PubMed] [Google Scholar]
- Greenwald MK, Steinmiller CL, Sliwerska E, Lundahl L, Burmeister M. BDNF Val(66)Met genotype is associated with drug-seeking phenotypes in heroin-dependent individuals: a pilot study. Addict Biol. 2013;18:836–845. doi: 10.1111/j.1369-1600.2011.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000;20:5775–5781. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci U S A. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatami H, Oryan S, Semnanian S, Kazemi B, Bandepour M, Ahmadiani A. Alterations of BDNF and NT-3 genes expression in the nucleus paragigantocellularis during morphine dependency and withdrawal. Neuropeptides. 2007;41:321–328. doi: 10.1016/j.npep.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–825. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein A, Dursteler-MacFarland KM, Lenz B, Frieling H, Grosch M, Bonsch D, Kornhuber J, Wiesbeck GA, Bleich S, Hillemacher T. Serum levels of BDNF are associated with craving in opiate-dependent patients. J Psychopharmacol. 2011;25:1480–1484. doi: 10.1177/0269881111411332. [DOI] [PubMed] [Google Scholar]
- Howells DW, Porritt MJ, Wong JYF, Batchelor PE, Kalnins R, Hughes AJ, Donnan GA. Reduced BDNF mRNA expression in the Parkinson’s Disease substantia nigra. Exp Neurol. 2000;166:127–135. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Jacovina AT, Zhong F, Khazanova E, Lev E, Deora AB, Hajjar KA. Neuritogenesis and the nerve growth factor-induced differentiation of PC-12 cells requires annexin II-mediated plasmin generation. J Biol Chem. 2001;276:49350–49358. doi: 10.1074/jbc.M106289200. [DOI] [PubMed] [Google Scholar]
- Je HS, Yang F, Ji Y, Potluri S, Fu XQ, Luo ZG, Nagappan G, Chan JP, Hempstead B, Son YJ, Lu B. ProBDNF and mature BDNF as punishment and reward signals for synapse elimination at mouse neuromuscular junctions. J Neurosci. 2013;33:9957–9962. doi: 10.1523/JNEUROSCI.0163-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno D, Crumiller M, Ohnishi YN, Ohnishi YH, Mouzon E, Dietz DM, Lobo MK, Neve RL, Russo SJ, Han MH, Nestler EJ. BDNF is a negative modulator of morphine action. Science. 2012;338:124–128. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun-Julien F, Bertrand MJ, De Backer O, Stellwagen D, Morales CR, Di Polo A, Barker PA. ProNGF induces TNFalpha-dependent death of retinal ganglion cells through a p75NTR non-cell-autonomous signaling pathway. Proc Natl Acad Sci U S A. 2010;107:3817–3822. doi: 10.1073/pnas.0909276107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lim ST, Esfahani K, Avdoshina V, Mocchetti I. Exogenous gangliosides increase the release of brain-derived neurotrophic factor. Neuropharmacology. 2011;60:1160–1167. doi: 10.1016/j.neuropharm.2010.10.012. S0028-3908(10)00282-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Zhong XL, Zhou FH, Li JY, Zhou P, Xu JM, Song B, Li CQ, Zhou XF, Dai RP. Peripheral Brain Derived Neurotrophic Factor Precursor Regulates Pain as an Inflammatory Mediator. Sci Rep. 2016;6:27171. doi: 10.1038/srep27171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi FJ, Rasti M, Rahvar M, Mokarram P, Namavar MR, Owji AA. Expression levels of the BDNF gene and histone modifications around its promoters in the ventral tegmental area and locus ceruleus of rats during forced abstinence from morphine. Neurochem Res. 2012;37:1517–1523. doi: 10.1007/s11064-012-0746-9. [DOI] [PubMed] [Google Scholar]
- Matys T, Pawlak R, Matys E, Pavlides C, McEwen BS, Strickland S. Tissue plasminogen activator promotes the effects of corticotropin-releasing factor on the amygdala and anxiety-like behavior. Proc Natl Acad Sci U S A. 2004;101:16345–16350. doi: 10.1073/pnas.0407355101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada K, Yoshimura M, Ishikawa K, Miyamoto Y, Hashimoto K, Noda Y, Nitta A, Nabeshima T. The tissue plasminogen activator-plasmin system participates in the rewarding effect of morphine by regulating dopamine release. Proc Natl Acad Sci U S A. 2004;101:3650–3655. doi: 10.1073/pnas.0306587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagappan G, Zaitsev E, Senatorov VV, Jr, Yang J, Hempstead BL, Lu B. Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc Natl Acad Sci U S A. 2009;106:1267–1272. doi: 10.1073/pnas.0807322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan S, Lane-Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB, Nestler EJ. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci. 1998;18:10700–10708. doi: 10.1523/JNEUROSCI.18-24-10700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. 306/5695/487 [pii] [DOI] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. The cell biology of the plasminogen system. FASEB J. 1995;9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluger JH, Bart G, Green M, Ho A, Kreek MJ. Corticotropin-releasing factor testing reveals a dose-dependent difference in methadone maintained vs control subjects. Neuropsychopharmacology. 2003;28:985–994. doi: 10.1038/sj.npp.1300156. [DOI] [PubMed] [Google Scholar]
- Seeds NW, Basham ME, Haffke SP. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proc Natl Acad Sci U S A. 1999;96:14118–14123. doi: 10.1073/pnas.96.24.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Serra GP, Puddu MC, Foddai M, Diana M. Morphine withdrawal-induced abnormalities in the VTA: confocal laser scanning microscopy. Eur J Neurosci. 2003;17:605–612. doi: 10.1046/j.1460-9568.2003.02435.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Lim Y, Li F, Liu S, Lu JJ, Haberberger R, Zhong JH, Zhou XF. ProBDNF collapses neurite outgrowth of primary neurons by activating RhoA. PLoS One. 2012;7:e35883. doi: 10.1371/journal.pone.0035883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Momose Y, Murata M, Tamiya G, Yamamoto M, Hattori N, Inoko H. Toward identification of susceptibility genes for sporadic Parkinson’s disease. J Neurol. 2003;250(Suppl 3):III40–43. doi: 10.1007/s00415-003-1307-6. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Endogenous Opioids: The Downside of Opposing Stress. Neurobiol Stress. 2015;1:23–32. doi: 10.1016/j.ynstr.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, Ting AKR, Walton CH, Hansen DM, Razavi R, Clarke L, Bufalino MR, Allison DW, Steffensen SC, van der Kooy D. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science. 2009;324:1732–1734. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Steward DJ. Neuropsychopathology in the SIV/macaque model of AIDS. Front Biosci. 2005;10:710–727. doi: 10.2741/1566. [DOI] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75(NTR) by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/Nn1510. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nagai T, Nabeshima T. Drug dependence, synaptic plasticity, and tissue plasminogen activator. J Pharmacol Sci. 2005;97:157–161. doi: 10.1254/jphs.cp0040014. [DOI] [PubMed] [Google Scholar]
- Yang F, Je HS, Ji Y, Nagappan G, Hempstead B, Lu B. Pro-BDNF-induced synaptic depression and retraction at developing neuromuscular synapses. J Cell Biol. 2009;185:727–741. doi: 10.1083/jcb.200811147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Lim Y, Li X, Zhong JH, Zhou XF. Precursor of brain-derived neurotrophic factor (proBDNF) forms a complex with Huntingtin-associated protein-1 (HAP1) and sortilin that modulates proBDNF trafficking, degradation, and processing. J Biol Chem. 2011;286:16272–16284. doi: 10.1074/jbc.M110.195347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ishikawa M, Niitsu T, Nakazato M, Watanabe H, Shiraishi T, Shiina A, Hashimoto T, Kanahara N, Hasegawa T, Enohara M, Kimura A, Iyo M, Hashimoto K. Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One. 2012;7:e42676. doi: 10.1371/journal.pone.0042676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Representative proBDNF standard curve obtained using the ELISA kit, as described in Materials and Methods. B. Cross reactivity of different concentrations (ng/ml) of BDNF as detected by the proBDNF ELISA.