Abstract
Objective
Exposure therapy is an effective treatment for posttraumatic stress disorder (PTSD), but many patients will not respond. Brain functions governing treatment outcome are not well characterized. Here, we examined brain systems relevant to emotional reactivity and regulation, constructs thought to be central to PTSD and exposure therapy effects, to identify the functional traits of individuals most likely to benefit from treatment.
Methods
Individuals with PTSD underwent functional magnetic resonance imaging (fMRI) while completing three tasks assessing emotional reactivity and regulation. Participants were then randomized to immediate prolonged exposure treatment (N=36) or waitlist (N=30). A random subset of treatment-randomized individuals (N=17) underwent single-pulse transcranial magnetic stimulation (TMS) concurrent with fMRI to examine if predictive activation patterns reflect causal influence within circuits. Linear mixed effects modeling in line with the intent-to-treat principle was used to examine how baseline brain function moderated the treatment effect on PTSD symptoms.
Results
Individuals with larger treatment-related symptom reductions (compared to waitlist) showed at baseline: 1) greater dorsal prefrontal activation and 2) less left amygdala activation, both during emotion reactivity; 3) better inhibition of the left amygdala induced by single TMS pulses to the right dorsolateral prefrontal cortex; and 4) greater ventromedial prefrontal activation during emotional conflict regulation. Reappraisal-related activation was not a significant moderator of the treatment effect.
Conclusions
Capacity to benefit from prolonged exposure for PTSD is gated by the degree to which prefrontal resources are spontaneously engaged when superficially processing threat and adaptively mitigating emotional interference, but not when deliberately reducing negative emotionality.
Posttraumatic stress disorder (PTSD) is a prevalent condition (1) with a large burden of suffering (2). Effective treatments have been developed, with the most widely utilized being trauma-focused psychotherapies such as prolonged exposure (3). Though psychotherapy is widely utilized and highly effective, it requires a considerable investment of time and effort, with about one quarter of individuals not completing treatment and one third to one half of completers still remaining symptomatic and impaired (4). It is therefore critical to identify who will benefit from this treatment and why—information that is still largely unknown. Noting that clinical and demographic characteristics are poor predictors (5), we suggest that brain-based characteristics of individuals may serve as particularly robust indicators of favorable treatment outcome.
Extant data regarding how brain function prior to treatment predict PTSD psychotherapy outcome are sparse. Additionally, prior studies offer limited insights or generalizability due to lack of a patient waitlist or control intervention arm (6), use of an uncommon treatment modality (7), or small samples (8). Critically, no prior study has reported a comprehensive, multi-faceted assessment of brain function and structure in a single study, instead separately presenting data from individual paradigms in partially overlapping participant groups.
Here, in a sample that is large relative to published fMRI treatment studies, we identify brain activation that moderates the relationship between treatment arm and symptom change in a randomized clinical trial of prolonged exposure for PTSD. We utilized a patient waitlist comparison group and examined multiple functional tasks united under a common conceptual theme—emotional reactivity and regulation, i.e. how an individual recognizes an emotionally-charged stimulus, processes that information, and resolves the emotional response. We investigated these processes under the assumption that appropriate reactivity to and regulation of emotion is essential for successful exposure therapy (9). This is consistent with emotional processing theory, the foundation of prolonged exposure, which states that confronting feared stimuli to activate the fear response is needed to incorporate information that is incompatible with its pathological structure (10). This process promotes adaptive learning, which leads to a regulation of fear. Therefore, the intactness of brain mechanisms that optimize balance between these processes is likely necessary to benefit from exposure treatment. Moreover, as treatment imaging studies typically analyze data only in treatment completers, this can also fundamentally bias results (11). Hence, we adopted a full intent-to-treat analysis framework using linear mixed models, thereby incorporating all available imaging data.
Prior PTSD imaging studies examining predictors of psychotherapy treatment response have observed the following, all in the context of single-arm treatment studies. First, greater activation in ventral anterior cingulate cortex and medial prefrontal cortex during non-conscious fear processing was found to predict poorer response to cognitive-behavioral therapy (CBT)(6). Second, greater activation in the dorsal anterior cingulate during non-conscious fear processing (6) and anticipation of negative vs. positive emotional images predicted better CBT response (12), but less dorsal anterior cingulate activation during image presentation also predicted better CBT response (12, 13). Third, less amygdala reactivity to non-conscious fear processing (6) and conscious processing of negative pictures (13) predicted a better response to CBT.
Thus, we formulated the following hypotheses. First, we expected individuals with less amygdala activation during emotion detection at baseline to show a greater reduction in symptoms following treatment. Second, we expected individuals with less ventromedial prefrontal activation during non-conscious fear processing to show a greater reduction in symptoms post-treatment (6). However, we also expected individuals with greater ventromedial prefrontal activation during emotional conflict regulation to show a greater treatment-related reduction in symptoms. This is consistent with prior work implicating the this region in emotional conflict regulation (14) and fear extinction (15), which we anticipated would support exposure habituation and improve treatment efficacy. Third, we predicted activation of the rostral/dorsal anterior cingulate when processing an emotional cue to moderate the relationship between treatment arm and symptom change, consistent with prior work, though we did not have an a priori directional hypothesis given inconsistent prior findings (6, 12). Finally, we hypothesized individuals with greater activation of dorsolateral prefrontal regions during processing and deliberate regulation of negative emotion would demonstrate greater treatment-related symptom reductions given the role of these regions in emotion regulation and their importance to existing models of psychotherapy mechanisms (16).
Methods
Participants, Assessments, and Inclusion Criteria
Individuals, age 18–60, were recruited via advertisement for participation in a psychotherapy treatment study and provided written informed consent.
Behavioral Paradigms
Emotional Reactivity Task
This task (17) probes goal-irrelevant emotional reactivity via conscious and non-conscious (backwardly masked) presentation of fearful and neutral faces. The goal is to identify the color tint of the emotional face.
Emotional Conflict Task
This task (14) induces emotional conflict through pairing fearful and happy faces with congruent or incongruent emotion words, and regulation occurs via an implicit process when conflict trials are preceded by other conflict trials. The task is to identify the facial emotion and ignore the emotion word.
Gender Conflict Task
Participants viewed the same facial stimuli as in the emotional conflict task (18), but here the goal is to identify gender and ignore an overlaid congruent or incongruent gender word.
Reappraisal Task
Participants viewed either negative or neutral IAPS pictures under two conditions: “Look” (for negative and neutral) and “Decrease” (negative only). During “Look” trials, participants could experience their natural emotional response, while during “Decrease” reduced their emotional responses by interpreting the picture differently (19).
MRI Data Acquisition
See Supplemental Methods.
Randomization
Following clinical assessments and fMRI scans, participants were individually randomized to one of two arms: 1) Immediate treatment with prolonged exposure (N=36); or 2) Treatment waitlist (N=30).
Concurrent TMS-fMRI causal mapping
As an experimental probe of brain circuitry, a random subset of treatment-randomized individuals (N=17) underwent concurrent single pulse TMS-fMRI conducted prior to treatment according to established protocols (20). This session occurred about two weeks following the task-based fMRI session. Given our task-related moderation findings and existing evidence for efficacy of repetitive TMS (rTMS) to right dorsolateral prefrontal cortex in alleviating PTSD symptoms (21), we focused analyses on two right dorsolateral prefrontal sites: an anterior site in the middle frontal gyrus (part of the resting state salience network), and a more posterior site in the middle frontal gyrus (part of the resting state executive control network)(22). The primary site of interest was the right posterior middle frontal gyrus, given its proximity to our task-based moderator findings and to the location “5cm anterior to the motor cortex” used in prior PTSD rTMS treatment studies (23). The anterior middle frontal gyrus was utilized as a comparison site to control for the subjective effects of prefrontal stimulation.
Prolonged Exposure Treatment, Therapist Competency, and Supervision
Treatment sessions occurred either once or twice-weekly for 9 to 12 90-minute sessions, according to manualized procedures (24).
Post-Treatment Clinical Assessment
Approximately 4 weeks after the final treatment session, participants completed a post-treatment clinical assessment. This period was chosen in order to allow treatment changes to consolidate and symptom levels to equilibrate prior to the post-treatment assessment.
Image Preprocessing
See Supplemental Methods.
Individual-Level Analysis of Task Data
For the Emotional Reactivity task, the a priori contrasts of interest were conscious fear vs. neutral and non-conscious (masked) fear vs. neutral. For the Emotional Conflict task, contrasts of interest were Incongruent vs. Congruent trials (conflict), Post-incongruent Incongruent trials vs. Post-congruent Incongruent trials (an established measure of conflict regulation (14)), and Congruent Fear vs. Congruent Happy trials, an additional probe of emotional reactivity. For the Gender Conflict task, contrasts of interest were those capturing conflict and conflict regulation. For the Reappraisal paradigm, contrasts of interest were Look Negative vs. Neutral and Reappraise Negative vs. Look Negative.
Assessing Treatment Moderation Effects
To identify brain activation moderating the relationship between treatment arm and symptom change, we employed the MacArthur approach (25) embedded in our longitudinal linear mixed effects models on a voxel-wise level, treating baseline brain activation as a potential moderator of differential changes by treatment arm on our primary outcome measure of PTSD symptoms—total scores from the Clinician Administered PTSD Scale (CAPS) for DSM-IV (26). All region of interest analyses utilized the same anatomical mask (Supplemental Figure 2).
Assessing Utility of Activation Moderators for Predicting Clinical Remission
See Supplemental Methods. Linear discriminant functions with leave one out cross-validation were utilized to determine the classification accuracy of brain activation moderators for predicting remission from PTSD.
Results
Sample Characteristics, Task Behavior, and Treatment Response
The randomized sample encompassed 66 individuals, with 36 randomized to immediate treatment and 30 randomized to waitlist. The groups were well matched on all relevant clinical and demographic variables (Table 1). See companion paper (27) for a complete discussion of treatment outcome results. Briefly, the immediate treatment group demonstrated a significantly greater reduction in PTSD symptoms relative to the patient waitlist condition.
Table 1.
Participant demographics and treatment outcome.
| Measure | Immediate Treatment (N=36) | Patient Waitlist (N=30) | F/χ2 (p value) | Cohen’s d |
|---|---|---|---|---|
|
| ||||
| Mean or N and % of Group (SD) | Mean or N and % of Group (SD) | |||
| Age (yrs) | 34.42 (10.23) | 39.03 (10.35) | -- | -- |
|
| ||||
| Education (yrs) | 14.72 (2.17) | 15.17 (2.78) | -- | -- |
|
| ||||
| Sex | Male (N=13; 36%) | Male (N=10; 33%) | -- | -- |
| Female (N=23; 64%) | Female (N=20; 66%) | |||
|
| ||||
| WASI Full Scale IQ | 109.03 (9.09) | 112.81 (11.57) | -- | -- |
|
| ||||
| SSRI/SNRI Meds | Sertraline (N=1; 3%) | Duloxetine (N=1; 3%) | -- | -- |
| Citalopram (N=2; 5%) | Sertraline (N=1; 3%) | |||
|
| ||||
| MDD Diagnosis at Intake | Yes (N=18; 50%) | Yes (N=17; 57%) | -- | -- |
| No (N=18; 50%) | No (N=13; 43%) | |||
|
| ||||
| Dropout | Completed (N=25; 69%) | Completed (N=26; 87%) | -- | -- |
| Did not complete (N=11; 31%) | Did not complete (N=4; 13%) | |||
|
| ||||
| CAPS Index Trauma | Natural disaster (N=3; 8%) | Natural disaster (N=1; 3%) | -- | -- |
| Physical Assault (N=9; 25%) | Physical assault (N=7; 23%) | |||
| Assault w/ weapon (N=3; 8%) | Assault w/ weapon (N=2; 7%) | |||
| Sexual assault (N=12; 33%) | Sexual assault (N=9; 30%) | |||
| Combat exposure (N=4; 11%) | Combat exposure (N=4; 13%) | |||
| Injury/illness/suffering (N=5; 14%) | Injury/illness/suffering (N=7; 23%) | |||
|
| ||||
| Pre-Treatment Symptom/Quality of Life Measures | ||||
|
| ||||
| CAPS: Developmental Stage at Time of Index Trauma | Adult (N=20; 56%) | Adult (N=14; 47%) | -- | -- |
| Teen (N=8; 22%) | Teen (N=11; 37%) | |||
| Child (N=8; 22%) | Child (N=5; 17%) | |||
|
| ||||
| CAPS: How Exposed to Index Trauma | Experienced (N=27; 75%) | Experienced (N=17; 57%) | -- | -- |
| Witnessed (N=9; 25%) | Witnessed (N=13; 43%) | |||
|
| ||||
| CAPS: Index Trauma Repeated? | No (N=25; 69%) | No (N=20; 66%) | -- | -- |
| Yes (N=11; 31%) | Yes (N=10; 33%) | |||
|
| ||||
| CAPS: Multiple Criterion A Events? | No (N=24; 66%) | No (N=20; 66%) | -- | -- |
| Yes (N=12; 33%) | Yes (N=10; 33%) | |||
|
| ||||
| CAPS Total | 66.33 (15.17) | 71.37 (14.99) | -- | -- |
|
| ||||
| CAPS ReExp | 17.53 (6.40) | 18.73 (6.02) | -- | -- |
|
| ||||
| CAPS Avd | 26.94 (7.86) | 28.77 (8.89) | -- | -- |
|
| ||||
| CAPS Hyper | 21.86 (6.28) | 23.87 (4.91) | -- | -- |
|
| ||||
| BDI-II Total | 23.69 (8.68) | 23.17 (8.60) | -- | -- |
|
| ||||
| PCL-C Total | 56.16 (10.61) | 57.36 (12.04) | -- | -- |
|
| ||||
| PCL-C ReExp | 16.47 (3.83) | 16.29 (3.98) | -- | -- |
|
| ||||
| PCL-C Avd | 22.78 (5.05) | 23.04 (6.02) | -- | -- |
|
| ||||
| PCL-C Hyper | 16.91 (4.22) | 18.04 (4.19) | -- | -- |
|
| ||||
| WHO-QoL Physical | 12.46 (2.99) | 12.43 (3.11) | -- | -- |
|
| ||||
| WHO-QoL Psych | 10.04 (2.29) | 10.83 (2.34) | -- | -- |
|
| ||||
| WHO-QoL SocRx | 9.71 (4.06) | 9.29 (3.51) | -- | -- |
|
| ||||
| WHO-QoL Envir | 12.30 (3.48) | 12.79 (3.37) | -- | -- |
|
| ||||
| Post-Treatment Symptom/Quality of Life Measures | ||||
|
| ||||
| CAPS Total | 29.60 (21.26) | 64.23 (21.77) | 32.99 (< 0.001)*** | 1.61 |
|
| ||||
| CAPS ReExp | 6.20 (6.49) | 16.92 (7.97) | 27.62 (< 0.001)*** | 1.48 |
|
| ||||
| CAPS Avd | 10.60 (9.50) | 24.50 (11.30) | 22.51 (< 0.001)*** | 1.33 |
|
| ||||
| CAPS Hyper | 12.80 (8.75) | 22.81 (7.00) | 20.43 (< 0.001)*** | 1.26 |
|
| ||||
| BDI-II Total | 9.69 (7.77) | 17.87 (9.27) | 11.23 (0.002)** | 0.96 |
|
| ||||
| PCL-C Total | 26.13 (7.80) | 49.00 (13.35) | 45.55 (< 0.001)*** | 2.09 |
|
| ||||
| PCL-C ReExp | 7.41 (2.63) | 14.38 (5.14) | 31.76 (< 0.001)*** | 1.71 |
|
| ||||
| PCL-C Avd | 10.36 (3.36) | 19.24 (6.32) | 33.46 (< 0.001)*** | 1.75 |
|
| ||||
| PCL-C Hyper | 8.41 (3.11) | 15.38 (4.15) | 39.05 (< 0.001)*** | 1.90 |
|
| ||||
| WHO-QoL Physical | 14.63 (3.29) | 12.65 (3.19) | 4.09 (0.049)* | 0.61 |
|
| ||||
| WHO-QoL Psych | 13.19 (2.59) | 11.94 (2.52) | 2.63 (0.11) | 0.49 |
|
| ||||
| WHO-QoL SocRx | 11.83 (3.20) | 10.73 (3.20) | 1.29 (0.26) | 0.34 |
|
| ||||
| WHO-QoL Envir | 14.59 (2.42) | 13.57 (2.99) | 1.55 (0.22) | 0.38 |
|
| ||||
Avd = avoidance/numbing subscale; BDI-II = Beck Depression Inventory-II; CAPS = Clinician-Administered PTSD Scale for DSM-IV; Hyper = hyperarousal subscale; MDD = major depressive disorder; PCL = PTSD Checkist for DSM-IV Civilian Version; ReExp = reexperiencing subscale; SSRI = selective serotonin reuptake inhibitor; SNRI = serotonin/norepinephrine reuptake inhibitor; WASI = Wechsler Abbreviated Scale of Intelligence; WHO-QoL = WHO Quality of Life BREF Scale; WHO-Qol Physical = physical health subscale; WHO-QoL Psych = psychological health subscale; WHO-QoL SocRx = social relationships subscale; WHO-QoL Environ = environment subscale;
p < 0.05;
p < 0.01,
p < 0.001.
Assessing Demographic/Clinical Variables as Moderators
See Supplemental Results.
Baseline Task Effects
Baseline Functional Brain Moderators in Regions of Interest
Emotional Reactivity Task
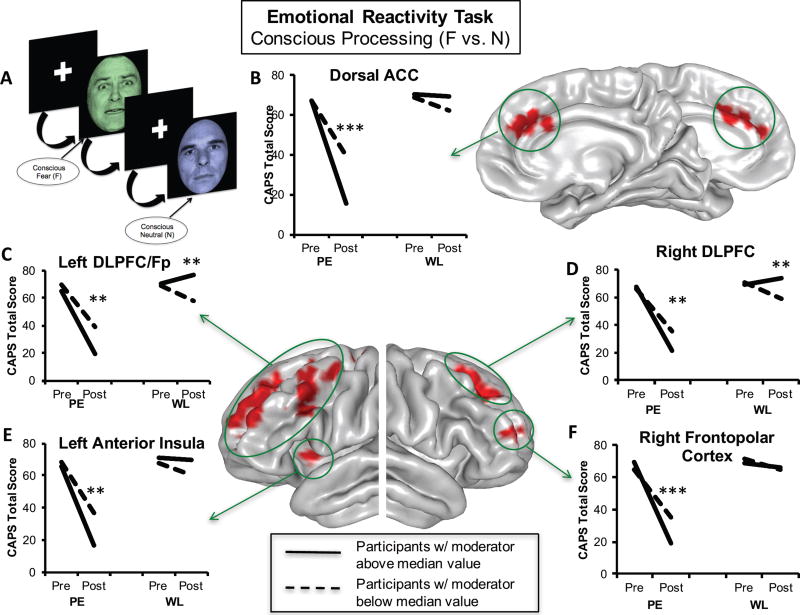
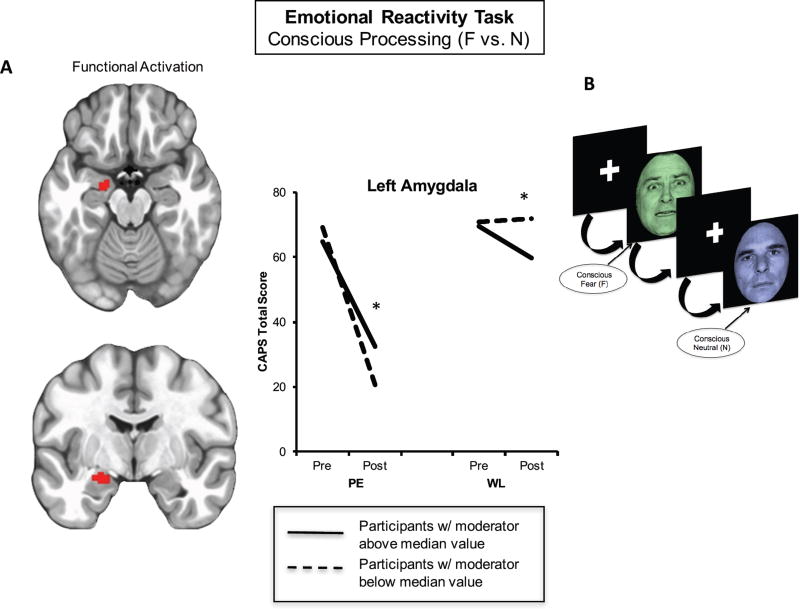
Conscious processing of fearful compared to neutral faces yielded significant moderation effects within our a priori mask, including large portions of the bilateral dorsolateral prefrontal and frontopolar cortex (inferior, middle, and superior frontal gyri; Brodmann areas 6, 8, 9, 10, 46; Fig. 1C, 1D, 1F), the dorsal anterior cingulate (Brodmann area 32; Fig. 1B), the left anterior insula (Brodmann areas 13 and 44; Fig. 1E), and the left amygdala (Fig. 2A)(Supplemental Table 2). Consistent with our hypotheses, for all prefrontal regions and the left anterior insula, greater baseline activation to fear vs. neutral was associated with greater symptom reduction in the immediate treatment group (p’s<0.01) relative to waitlist. The waitlist group showed the opposite effect, whereby greater prefrontal activation was associated with less symptom improvement (all p’s<0.03). As hypothesized, in the fear vs. neutral contrast, less left amygdala activation was associated with greater symptom improvement in the treatment group (p=0.012; Fig. 2A), with the waitlist group again displaying the opposite pattern (p=0.03). The amygdala effect arose from activation during the fear condition (F = 7.821, p = 0.006) but not the neutral condition (F = 3.170, p = 0.08). Non-conscious processing of masked fearful vs. neutral faces did not yield significant moderation effects.
Figure 1. Prefrontal Emotional Reactivity Baseline Activation Moderators Treatment-Related Symptom Change.
During emotional reactivity, i.e. conscious processing of fearful vs. neutral facial stimuli (A), greater degree of activation in the dorsal anterior cingulate (B), left dorsolateral prefrontal/frontopolar cortex (C), right dorsolateral prefrontal cortex (D), left anterior insula (E), and right frontopolar cortex (F) predicted better treatment outcomes for individuals randomized to immediate treatment vs. those randomized to waitlist. Separate lines within each group represent individuals above (solid line) or below (dotted line) the median level of activation across the entire sample for the purposes of visualizing disparate symptom change trajectories within each group. ACC = anterior cingulate cortex; BOLD = blood oxygenation level-dependent response; CAPS = Clinician-Administered PTSD Scale for DSM-IV; DLPFC = dorsolateral prefrontal cortex; F = fear; FDR = false discovery rate; Fp = frontopolar cortex; N = neutral; PE = prolonged exposure group; WL = waitlist group; *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 2. Left Amygdala Activation During Emotional Reactivity Moderates Symptom Change Following Prolonged Exposure.
In individuals randomized to immediate prolonged exposure vs. those randomized to waitlist, less left amygdala activation (A) to consciously-processed fearful vs. neutral faces (B) predicted a better treatment response. Separate lines within each group represent individuals above (solid line) or below (dotted line) the median level of activation across the entire sample for the purposes of visualizing disparate symptom change trajectories within each group. BOLD = blood oxygenation level-dependent response; CAPS = Clinician-Administered PTSD Scale for DSM-IV; F = fear; N = neutral; PE = prolonged exposure group; WL = waitlist group; *p < 0.05; **p < 0.01.
Emotional Conflict Task
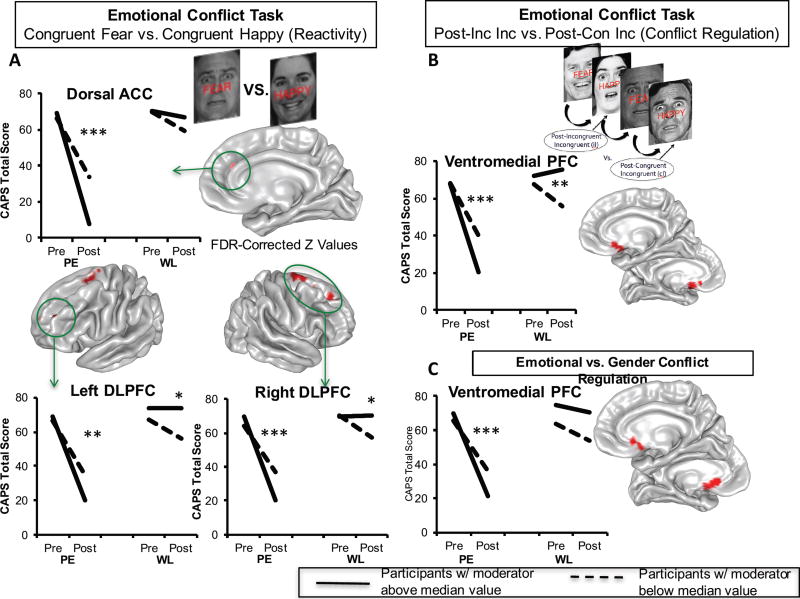
We next analyzed the emotional conflict task beginning with the congruent fear vs. happy contrast, which isolates valence in the absence of conflict, in order to test the generalization of the emotional reactivity results above. Bilateral dorsolateral prefrontal (middle and superior frontal gyri; Brodmann areas 6, 8, 9, 10) and dorsal anterior cingulate activation (Brodmann area 32) moderated the relationship between treatment arm and symptom change (Fig. 3A)(Supplemental Table 3). Moreover, these effects overlapped with the conceptually similar effects in the Emotional Reactivity task. Consistent with our hypotheses, moderation effects in all dorsolateral prefrontal clusters were driven primarily by greater baseline activation being associated with larger symptom reductions in the treatment condition (all p’s<0.003) compared to waitlist. Less baseline activation was additionally associated with greater symptom reduction in the waitlist group in two right dorsolateral prefrontal clusters and two left dorsolateral prefrontal clusters (p’s<0.03). Finally, greater dorsal anterior cingulate activation at baseline was associated with greater reductions in symptoms in the treatment group (p<0.001) but not in the waitlist group (p=0.074).
Figure 3. Emotional Conflict Task Activation Moderators of Treatment Response.
During conflict-free trials of congruent fear vs. congruent happy, individuals randomized to immediate treatment (but not to waitlist) displaying greater activation in the dorsal anterior cingulate and the bilateral dorsolateral prefrontal cortices demonstrated greater reductions in symptoms (A). When examining emotional conflict regulation, greater activation in the ventromedial prefrontal cortex in the immediate treatment group predicted greater symptom reduction, but not in those randomized to waitlist (B). This effect continued to hold when contrasting emotional conflict regulation with gender conflict regulation, indicating emotional specificity of the conflict regulation effect (C). Separate lines within each group represent individuals above (solid line) or below (dotted line) the median level of activation across the entire sample for the purposes of visualizing disparate symptom change trajectories within each group. ACC = anterior cingulate cortex; CAPS = Clinician-Administered PTSD Scale for DSM-IV; Con = congruent; DLPFC = dorsolateral prefrontal cortex; FDR = false discovery rate; Inc = incongruent; PE = prolonged exposure group; PFC = prefrontal cortex; WL = waitlist group; *p < 0.05; **p < 0.01; ***p < 0.001.
Examining the conflict regulation contrast, we observed that baseline activation in a posterior portion of the ventromedial prefrontal cortex extending into the rostroventral striatum (olfactory cortex, mid-orbital gyrus, caudate nucleus, and anterior cingulate; Brodmann area 25) moderated the relationship between treatment arm and symptom change (Fig. 3B)(Supplemental Table 3). As predicted, this was driven primarily by greater ventromedial prefrontal/ventral striatal activation predicting a greater reduction in PTSD symptoms in the treatment group (p<0.001). This effect was also significant within the waitlist group, but with less activation at baseline predicting greater symptom reduction (p=0.006). We next examined the emotional specificity of this effect by contrasting activation in the emotional conflict task to the gender conflict task. Prior work has shown that only the emotional conflict task engages and requires the ventromedial prefrontal cortex for conflict regulation (18, 28). Importantly, our prediction effect was indeed specific for emotional compared to gender conflict regulation (Fig. 3C)(Supplemental Table 3).
Lastly, we examined conflict-related activation for treatment moderation effects. No significant effects were observed.
Reappraisal Task
For both contrasts of interest, we observed no brain activation that moderated the relationship between treatment arm and symptom change.
Baseline Functional Brain Moderators: Exploratory Whole Brain Analyses
See Supplemental Results.
Brain-Behavior Relationships
To assess the clinical significance of brain moderators, we conducted exploratory analyses of the relationship of between brain activation moderators and measures of task behavior and self-reported emotion regulation. As detailed in the Supplemental Results, greater activation in the dorsal anterior cingulate and right dorsolateral prefrontal cortex during conscious fear vs. neutral in the Emotional Reactivity Task and during congruent fear vs. happy in the Emotional Conflict Task was associated with less frequent deficits in emotion regulation. Consistent with prior work (14), greater ventromedial prefrontal cortex/ventral striatum activation during emotional conflict regulation was associated with better behavioral regulation of emotional conflict, i.e. larger decrease in reaction times, as well as lower distress ratings during the Reappraisal Task for Look Negative vs. Neutral.
Assessing Utility of Task Activation Moderators for Predicting Clinical Remission
See Supplemental Methods and Results for details. In brief, the best combination of moderators across tasks was able to predict remission from PTSD with 95.5% leave one out cross-validated accuracy, which was significantly better than a predictive model that omitted brain measures.
Testing Dorsolateral Prefrontal Causal Control Over the Amygdala as a Mechanism Moderating Treatment-Related Symptom Reductions
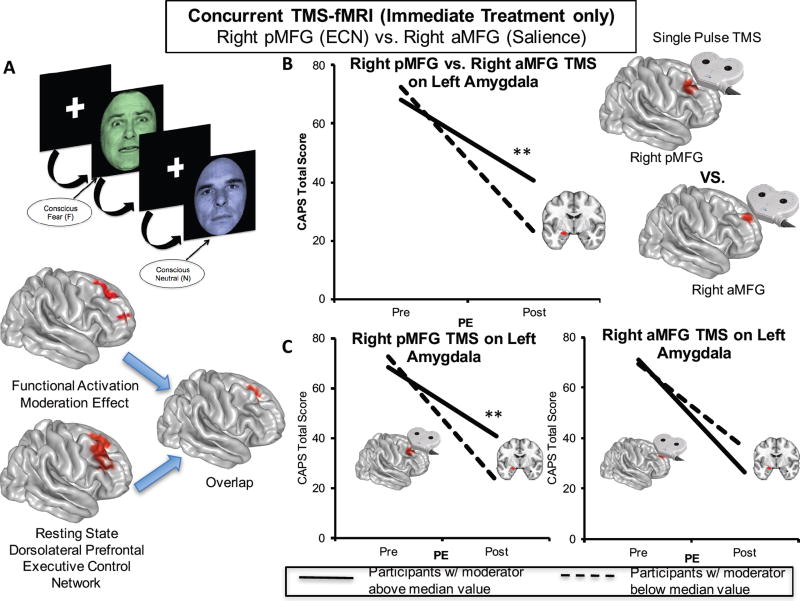
We hypothesized that inverse prefrontal-amygdala moderation effects in the Emotional Reactivity task might reflect lateral prefrontal control over amygdala reactivity. To test this, we used concurrent TMS-fMRI in a random subset of treatment-randomized individuals to examine whether right dorsolateral prefrontal stimulation modulated left amygdala function in a way that predicted symptom reduction following treatment. The right dorsolateral prefrontal activation moderation effect in the Emotional Reactivity task greatly overlapped (369 voxels) with the resting state executive control network (Fig. 4A)(22), which we targeted with single pulse TMS during fMRI. As an active control condition, we utilized a more anterior site in the right middle frontal gyrus, which is part of the resting-state salience network (22) and was more distant to the treatment-moderating clusters. Examining the effect of TMS to the right posterior middle frontal gyrus contrasted with TMS pulses to the right anterior middle frontal gyrus on left amygdala activation defined by the moderation effect from the Emotional Reactivity task, we observed that TMS-evoked activation in the left amygdala was associated with change in PTSD symptoms with treatment (p=0.003; Fig. 4B). Specifically, individuals in the treatment group that showed greater reductions in activation to right posterior middle frontal vs. right anterior middle frontal gyrus TMS single pulses in the same left amygdala region observed to moderate treatment-related symptom reductions during emotional reactivity demonstrated greater reductions in PTSD symptoms. This arose from the effect of right posterior middle frontal gyrus stimulation (p=0.003; Fig. 4C) and not right anterior middle frontal gyrus stimulation (p=0.141).
Figure 4. Single-Pulse TMS and Concurrent fMRI Reveals Right Dorsolateral Prefrontal Causally-Induced Left Amygdala Inhibition at Baseline Predicts Treatment Response to Prolonged Exposure.
The right dorsolateral prefrontal region observed to moderate treatment response during emotional reactivity largely overlapped with the right prefrontal node (posterior middle frontal gyrus) of the canonical resting state executive control network (A). A random subset of individuals (N = 17) randomized to immediate treatment underwent concurrent single pulse TMS to this executive control network node as well as another right prefrontal node (anterior middle frontal gyrus) of the canonical resting state salience network. The area of the left amygdala in which less activation during emotional reactivity was found to moderate treatment response was also modulated by single pulse TMS to the right posterior vs. anterior middle frontal gyrus, such that individuals displaying greater inhibition of the left amygdala to right posterior vs. anterior middle frontal gyrus stimulation at rest displayed better treatment outcomes (B). This effect arose entirely from right posterior middle frontal stimulation and not right anterior middle frontal gyrus stimulation (C). The red areas representing the TMS targets in (B) are 8mm spheres centered on the cluster centers of mass for the right executive control and right salience network prefrontal nodes independently-derived from a separate healthy control dataset. Separate lines represent individuals above (solid line) or below (dotted line) the median level of activation for the purposes of visualizing disparate symptom change trajectories within the immediate treatment group. aMFG = anterior middle frontal gyrus; CAPS = Clinician-Administered PTSD Scale for DSM-IV; ECN = executive control network; PE = prolonged exposure group; pMFG = posterior middle frontal gyrus; TMS = transcranial magnetic stimulation; **p < 0.01.
Discussion
Here, we undertook a rigorous investigation of the brain characteristics that moderate differential symptom change from prolonged exposure therapy vs. waitlist during emotional reactivity and regulation. We also incorporated causality-focused TMS-fMRI manipulations to enhance interpretability of task findings. The primary results are as follows. First, individuals with greater baseline recruitment of the dorsal anterior cingulate, anterior insula, and dorsolateral prefrontal cortex as well as less amygdala activation when incidentally processing an emotional stimulus showed larger symptom reductions after treatment. TMS-fMRI findings recapitulated this dynamic, demonstrating that the magnitude of downstream inhibition on the left amygdala from right dorsolateral prefrontal stimulation moderated the effect of treatment on symptoms. Second, individuals with greater baseline ventromedial prefrontal/ventral striatal activation during implicit regulation of emotional conflict demonstrated larger symptom reductions after treatment. Importantly, this effect was specific for regulation of emotional (vs. non-emotional) content. Thus, an individual’s capacity to benefit from exposure therapy is gated by: a) degree of spontaneous prefrontal control over amygdalar threat detection signals during incidental processing of a fear-conveying stimulus; and b) the brain’s capacity to reduce interference from an emotional cue in the environment.
Interestingly, brain activation when deliberately instructed to alter one’s emotional state did not predict treatment-related symptom change. This observation dovetails with clinical research emphasizing emotional engagement during an exposure without attempting to consciously attenuate emotional responses (24, 29). The capability for this type of emotional engagement may actually depend upon one’s capacity to devote attention towards both the emotional experience itself as well as other simultaneous aspects of one’s experience (such as goals and intentions). We believe this capacity is engaged by the emotional reactivity task used here, which induces a goal orientation (color tint identification) concurrent with the emotional stimulus. Prefrontal engagement during this process may be indicative of greater top-down resources devoted to the appraisal of the emotional stimulus and modulation of attention towards non-emotional components (30), perhaps indexing an individual’s capability to attend to goal-relevant processes in the presence of perceived environmental threat, e.g. sustaining an exposure exercise in the presence of fear. This is consistent with the roles of the dorsal anterior cingulate in appraisal of fear (31) and the dorsolateral prefrontal cortex in top-down attentional control (30). Thus, this uninstructed individual tendency towards engaging greater prefrontal control when appraising an emotional stimulus and modulating attention in relation to it may be a type of “spontaneous” emotion regulation that augurs well for exposure engagement and therapeutic benefit. This interpretation is consistent with the results of single pulse TMS manipulations, which likewise provide a potential mechanism for the efficacy of repetitive TMS to the right dorsolateral prefrontal cortex in treating PTSD (23).
Anterior insula activation during emotional reactivity also moderated the relationship between treatment arm and symptom change. Although this region is involved in processing fear and is known to be hyperactive across anxiety manifestations (32, 33), it is involved in numerous processes, including attention, working memory, language, and perceptual processing (34, 35). The insula can be functionally subdivided into a dorsal cognitive region and a ventral emotional subdivision (34, 36). The effect we detected was located in the more dorsal portion (z coordinate of cluster center of mass = 6), which is consistent with the role of this more dorsal anterior insular region in attentional allocation (36). Conversely, emotion-related meta-analytic insular activations tend to be more ventrally located (34). We therefore interpret this effect to signify greater processing resources being devoted towards allocating attention away from the emotional content of the face and towards the color tint (the focus of the task), consistent with the observed concomitant moderating activation of the dorsal anterior cingulate and dorsolateral prefrontal—regions heavily implicated in attention shifting (37) and in facilitating attentional control in conjunction with the insula (38).
Emotional conflict regulation normally recruits the ventromedial prefrontal cortex (14), is perturbed in individuals with ventromedial prefrontal lesions (28), is abnormal in some affective disorders (39), and is thought to index implicit regulation of interference from an irrelevant emotional stimulus (40). We found that ventromedial prefrontal/ventral striatal recruitment during emotional conflict regulation moderated the relationship between treatment arm and symptom change in an emotion-specific manner. Activation here was also correlated with behavioral indices of emotional conflict regulation at baseline. Localization of this effect to the posterior portion of the ventromedial prefrontal cortex (Brodmann area 25, subgenual cingulate) and adjoining rostroventral striatum may reflect how attunement to goal-relevant emotional information and reduction of perturbation from a salient stimulus results in reduced arousal or vigilance. This is consistent with the positive relationship between subgenual cingulate activation and parasympathetic processes (41) and the crucial role of nucleus accumbens shell (rostroventral striatum) in mediating the resistance of the brain to associating a previously-encountered harmless stimulus with a salience signal for a future aversive outcome (42). This is also consistent with translational neuroscience findings that implicate the infralimbic cortex in rats (ventromedial prefrontal cortex in humans) in facilitating fear extinction (43), as conflict regulation and fear extinction share some conceptual and empirical overlap (31). In relation to exposure, we interpret this effect to be a marker of the brain’s capacity to attenuate heightened arousal or vigilance following stimulus-cued fear responses.
It is notable that many of the brain activation moderator effects were predictive of outcomes in both immediate treatment and patient waitlist arms in opposite directions. We speculate that these effects reflect regulatory mechanisms that are engaged differently by “long-term” and “short-term” symptom coping techniques. When we refer to “long-term” techniques, we denote therapeutic exercises such as in-vivo and imaginal exposure, which promote recovery and lasting adaptive change. By “short-term” coping, we refer to techniques that are readily available and have a lower time and energy cost for the participant, such as active avoidance and distraction. Given the emphasis of the treatment on emotional processing via exposure (10) and minimization of avoidance, these opposite mechanistic relationships are therefore enforced by the randomization. Though “short-term” coping (the only type available to participants on waitlist) may provide some limited symptom relief, we note that none of the participants in waitlist demonstrated naturalistic recovery from PTSD, and only about half of those completing waitlist (N = 13) showed any decrease in PTSD symptoms. Ultimately, naturalistic recovery would need to be studied in a controlled context without treatment, over a longer period of time, and in a larger sample for valid inferences to be made.
This study has several limitations. First, we did not examine a trauma-exposed healthy control sample, which may provide insight regarding how compensatory adaptations or pathological markers interact with treatment to guide outcomes. Second, we did not investigate trauma-specific domains such as symptom provocation and/or experimental constructs of proposed etiological pathways such as fear conditioning/extinction. These are likely to provide useful complementary information. Third, the sample size, although large for a PTSD imaging treatment study, is relatively small for a randomized clinical trial and for examining moderation effects. Therefore, additional studies are needed to replicate and extend these findings and validate their utility for clinical decision-making. This is particularly true of the TMS-fMRI findings, for which only a subset of the sample contributed. Fourth, we did not counterbalance task order across participants since it was not possible to ensure balanced administrations across randomized groups. This could reduce generalizability of brain moderation effects if the order of administration exerted habituation effects on brain dynamics that moderated the effect of treatment on symptoms.
In conclusion, we highlight three primary insights from this study regarding the importance of targeted brain assessments in identifying individuals likely to benefit from exposure treatment and how knowledge derived from brain assessments can improve clinical outcomes. First, assessing individuals’ brain activation patterns can greatly improve our ability to predict remission from PTSD with treatment, beyond typical clinical and demographic measures. Second, these findings identify neurostimulation-accessible cortical regions which could serve as treatment targets for augmenting brain function prior to or concurrent with psychotherapy, thereby potentially “conditioning” the brain to respond to therapy. Third, these findings highlight the relevant behavioral constructs likely to provide a useful predictive signal of an individual’s response to treatment. If further developed with clinic-friendly measurement tools, e.g., using electroencephalography, the neural mechanisms identified in this study can be used to determine an individual’s suitability for prolonged exposure in a clinic setting. The current findings thus inform future efforts at individualized treatment selection and provide much-needed mechanistic insights regarding the neural phenotypes that respond best to exposure therapy.
Supplementary Material
Acknowledgments
This study was funded by a grant from the National Institute of Mental Health R01 MH091860 to AE. GF was partially supported by a grant from the National Institute of Mental Health T32 MH019938.
AE has served as a consultant to Takaeda, Otsuka and Acadia, received a research grant from Brain Resource, Inc. for work unrelated to the present study, and owns equity in Akili Interactive, Inc. BOR owns equity in Virtually Better, Inc. that creates virtual reality products. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. BOR has funding from Wounded Warrior Project, Department of Defense Clinical Trial Grant No. W81XWH-10-1-1045, “Enhancing Exposure Therapy for PTSD: Virtual Reality and Imaginal Exposure with a Cognitive Enhancer”, National Institute of Mental Health Grant No. 1R01MH094757-01, “Prospective Determination of Psychobiological Risk Factors for Posttraumatic Stress,” Brain and Behavior Research Foundation (NARSAD) Distinguished Investigator Grant, “Optimal Dose of early intervention to prevent PTSD”, and McCormick Foundation “Brave Heart: MLB’s Welcome Back Veterans SouthEast Initiative.” BOR receives royalties from Oxford University Press, Guilford, APPI, and Emory University and received one advisory board payment from Genentech.
Footnotes
Previously Presented at: Conference of the American College of Neuropsychopharmacology (ACNP) Hollywood, FL, USA, Dec. 6th–10th 2015
Disclosures
All other authors report no financial conflicts of interest.
ClinicalTrials.gov Name: Brain Imaging of Psychotherapy for Posttraumatic Stress Disorder (PTSD)
ClinicalTrials.gov Identifier: NCT01507948
ClinicalTrials.gov url: https://clinicaltrials.gov/ct2/show/NCT01507948
References
- 1.Seal KH, Metzler TJ, Gima KS, Bertenthal D, Maguen S, Marmar CR. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002–2008. American journal of public health. 2009;99:1651–1658. doi: 10.2105/AJPH.2008.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25:18–24. doi: 10.1007/s11606-009-1117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foa EB, Gillihan SJ, Bryant RA. Challenges and Successes in Dissemination of Evidence-Based Treatments for Posttraumatic Stress: Lessons Learned From Prolonged Exposure Therapy for PTSD. Psychological science in the public interest : a journal of the American Psychological Society. 2013;14:65–111. doi: 10.1177/1529100612468841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162:214–227. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- 5.Cloitre M, Petkova E, Su Z, Weiss B. Patient characteristics as a moderator of post-traumatic stress disorder treatment outcome: combining symptom burden and strengths. British Journal of Psychiatry Open. 2016;2:101–106. doi: 10.1192/bjpo.bp.115.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A, Williams L. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychological medicine. 2008;38:555–561. doi: 10.1017/S0033291707002231. [DOI] [PubMed] [Google Scholar]
- 7.Lindauer RJ, Booij J, Habraken JB, van Meijel EP, Uylings HB, Olff M, Carlier IV, den Heeten GJ, van Eck-Smit BL, Gersons BP. Effects of psychotherapy on regional cerebral blood flow during trauma imagery in patients with post-traumatic stress disorder: a randomized clinical trial. Psychological medicine. 2008;38:543–554. doi: 10.1017/S0033291707001432. [DOI] [PubMed] [Google Scholar]
- 8.Falconer E, Allen A, Felmingham KL, Williams LM, Bryant RA. Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder. The Journal of clinical psychiatry. 2013;74:895–901. doi: 10.4088/JCP.12m08020. [DOI] [PubMed] [Google Scholar]
- 9.Jaycox LH, Foa EB, Morral AR. Influence of emotional engagement and habituation on exposure therapy for PTSD. Journal of consulting and clinical psychology. 1998;66:185–192. doi: 10.1037//0022-006x.66.1.185. [DOI] [PubMed] [Google Scholar]
- 10.Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychol Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 11.Lachin JM. Statistical considerations in the intent-to-treat principle. Controlled clinical trials. 2000;21:167–189. doi: 10.1016/s0197-2456(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 12.Aupperle RL, Allard CB, Simmons AN, Flagan T, Thorp SR, Norman SB, Paulus MP, Stein MB. Neural responses during emotional processing before and after cognitive trauma therapy for battered women. Psychiatry research. 2013;214:48–55. doi: 10.1016/j.pscychresns.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 13.van Rooij SJ, Kennis M, Vink M, Geuze E. Predicting Treatment Outcome in PTSD: A Longitudinal Functional MRI Study on Trauma-Unrelated Emotional Processing. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:1156–1165. doi: 10.1038/npp.2015.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Messina I, Sambin M, Palmieri A, Viviani R. Neural correlates of psychotherapy in anxiety and depression: a meta-analysis. PloS one. 2013;8:e74657. doi: 10.1371/journal.pone.0074657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- 19.Minkel JD, McNealy K, Gianaros PJ, Drabant EM, Gross JJ, Manuck SB, Hariri AR. Sleep quality and neural circuit function supporting emotion regulation. Biology of mood & anxiety disorders. 2012;2:22. doi: 10.1186/2045-5380-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, Glover GH, Deisseroth K, Etkin A. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A. 2013;110:19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berlim MT, Van Den Eynde F. Repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex for treating posttraumatic stress disorder: an exploratory meta-analysis of randomized, double-blind and sham-controlled trials. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2014;59:487–496. doi: 10.1177/070674371405900905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boggio PS, Rocha M, Oliveira MO, Fecteau S, Cohen RB, Campanha C, Ferreira-Santos E, Meleiro A, Corchs F, Zaghi S, Pascual-Leone A, Fregni F. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. The Journal of clinical psychiatry. 2010;71:992–999. doi: 10.4088/JCP.08m04638blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD. Oxford: Oxford University Press; 2007. [Google Scholar]
- 25.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of general psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 26.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of traumatic stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 27.Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, Weiss ME, Thompson AL, Zack SE, Mills-Finnerty CE, Rosenberg BM, Edelstein R, Wright RN, Kole CA, Lindley SE, Arnow BA, Jo B, Gross JJ, Rothbaum BO, Etkin A. Selective Effects of Psychotherapy on Frontopolar Cortical Function in Post-Traumatic Stress Disorder. Am J Psychiatry. doi: 10.1176/appi.ajp.2017.16091073. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maier ME, di Pellegrino G. Impaired conflict adaptation in an emotional task context following rostral anterior cingulate cortex lesions in humans. Journal of cognitive neuroscience. 2012;24:2070–2079. doi: 10.1162/jocn_a_00266. [DOI] [PubMed] [Google Scholar]
- 29.Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Comte M, Schon D, Coull JT, Reynaud E, Khalfa S, Belzeaux R, Ibrahim el C, Guedj E, Blin O, Weinberger DR, Fakra E. Dissociating Bottom-Up and Top-Down Mechanisms in the Cortico-Limbic System during Emotion Processing. Cereb Cortex. 2016;26:144–155. doi: 10.1093/cercor/bhu185. [DOI] [PubMed] [Google Scholar]
- 31.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 33.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 36.Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Structure and Function. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondo H, Osaka N, Osaka M. Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. Neuroimage. 2004;23:670–679. doi: 10.1016/j.neuroimage.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168:968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 40.Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn Emot. 2011;25:400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane RD, Weidenbacher H, Smith R, Fort C, Thayer JF, Allen JJ. Subgenual anterior cingulate cortex activity covariation with cardiac vagal control is altered in depression. J Affect Disord. 2013;150:565–570. doi: 10.1016/j.jad.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Weiner I, Feldon J. The switching model of latent inhibition: an update of neural substrates. Behav Brain Res. 1997;88:11–25. doi: 10.1016/s0166-4328(97)02314-0. [DOI] [PubMed] [Google Scholar]
- 43.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.