Abstract
Abnormal pulmonary function is prevalent in survivors of allogeneic hematopoietic cell transplantation (HCT). Post-transplantation recovery of pulmonary function, and its effect on survival, in children are not known. This retrospective cohort study of 308 children followed for 10 years after HCT at a single institution included 2 groups of patients. Group 1 comprised 188 patients with 3 or more pulmonary function test (PFT) results, of which at least 1 was abnormal, and group 2 comprised 120 patients with 3 or more PFTs, all of which were normal. Pulmonary function normalized post-transplantation in 51 patients (27%) in group 1. Obstructive lung disease, restrictive lung disease, mixed lung disease, and normal pattern were seen in 43%, 25%, 5%, and 27% of patients, respectively, at a median of 5 years (range, 0.5 to 11.9 years) post-transplantation. Lung volumes recovered better than spirometric indices. Pulmonary complications were seen in 80 patients (43%) in group 1. Patients who recovered pulmonary function had better overall survival (P = .006), which did not differ significantly from that in patients in group 2 with normal lung function post-transplantation (P = .80). After adjusting for duration of follow-up, pulmonary complications (P = .01), and lower pretransplantation forced vital capacity z-scores (P = .01) were associated with poor recovery. T cell depletion (P < .001), lower pretransplantation forced expired volume in 1 second z-scores (P = .006), and chronic graft-versus-host disease (P < .001) increased the risk for pulmonary complications. Nonrecovery of lung function with pulmonary complications (P = .03), acute graft-versus-host disease (P = .004), and mechanical ventilation (P < .001) were risk factors for nonrelapse mortality. Normalization of pulmonary function is possible in long-term survivors of allogeneic HCT. Strategies to decrease the risk of pulmonary complications may improve outcomes.
Keywords: Lung function, Pediatric, Hematopoietic cell transplantation, Recovery, Survival
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is a curative treatment for patients with certain hematologic malignancies and nonmalignant disorders. Impaired pulmonary function and post-transplantation pulmonary complications contribute significantly to the morbidity and mortality associated with this procedure [1,2].
Abnormal pulmonary function is prevalent in survivors of allogeneic HCT. One study of adult HCT survivors found that their single-breath diffusing capacity for carbon monoxide (DLCO) reached a nadir at 5 years but normalized at 10 years post-transplantation [3]. However, normalization of pulmonary function in children, and its effect on survival, are not clear. We hypothesized that poor recovery of lung function increased the risk of late mortality after 1-year post-transplantation.
As the rapidly increasing numbers of HCT survivors age, with potentially decades of life ahead of them, understanding the recovery process may aid the development of strategies to preserve lung function.
METHODS
Study Population
The retrospective initial group consisted of 797 patients aged <21 years who underwent allogeneic HCT during a 25-year period (January 1990 through December 2014, both inclusive) at St Jude Children’s Research Hospital. Patients age <6 years (273 patients) were excluded because of their inability to perform a PFT. Patients with early post-transplantation mortality who were unable to perform more than 2 PFTs (216 patients) were excluded as well. The majority of these patients (57%) were 0 to 6 months post-transplantation. Therefore, the final cohort consisted of 308 patients.
All patients except those who died were followed until 10 years after transplantation. There was no loss to follow-up. Patients age 6 to 8 years were followed up to age 18 years. The mean duration of follow-up for patients in the final group of 308 patients was 4.1 years, with a median duration of 4.5 years (range, 0.4 to 12 years). This study was approved by the St Jude Institutional Review Board.
Patient-related variables were abstracted from a prospectively collected database. The conditioning regimen was classified as myeloablative or reduced intensity. Patients in the myeloablative group included those who received either total body irradiation (TBI) ≥5 Gy in a single dose or ≥8 Gy in fractionated doses or busulfan >8 mg/kg. Patients in the reduced-intensity group received a fludarabine-based regimen.
Haploidentical transplantation was done with T cell–depleted grafts. Ex vivo T cell depletion of the graft was performed using the Miltenyi CliniMACS system (Miltenyi Biotec, Bergisch Gladbach, Germany).
Prophylaxis for graft-versus-host disease (GVHD) was provided with a calcineurin inhibitor and either mycophenolate mofetil or methotrexate. Patients undergoing haploidentical HCT received mycophenolate mofetil for GVHD prophylaxis. The assessment of acute GVHD was based on consensus criteria [4].
All patients received prophylaxis against Pneumocystis jirovecii for up to 1 year after transplantation. Patients at risk for cytomegalovirus or Herpes simplex virus reactivation received acyclovir prophylaxis until 1 year after transplantation. Antifungal prophylaxis was provided with micafungin until engraftment, and with voriconazole thereafter.
An infectious pulmonary complication was defined as the isolation or detection of an organism associated with symptoms suggestive of lower respiratory tract involvement or the detection of a new lung infiltrate by chest radiography. Specimens obtained for analysis included nasopharyngeal wash, tracheal aspirate, bronchoalveolar lavage, blood cultures, and lung autopsy.
Noninfectious pulmonary complications included idiopathic pneumonia syndrome (IPS), diffuse alveolar hemorrhage, bronchiolitis obliterans syndrome (BOS), cryptogenic organizing pneumonia (COP), and interstitial lung disease, as defined previously [5–7]. Management of pulmonary complications in all patients was standardized as described previously [8]. Infectious and noninfectious pulmonary complications were grouped together in our study.
PFT
Spirometry, lung volume measurement by body plethysmography, and DLCO were performed at St Jude’s PFT laboratory by a certified technician in accordance with American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines [9], using a Platinum Elite DX series body plethysmograph (MedGraphics, St Paul, MN). All patients underwent PFTs pretransplantation, at 3 and 6 months post-transplantation, and yearly thereafter. The pre- and post-HCT PFTs consisted of spirometry, including measurements of the forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC ratio, and forced expiratory flow during the mid-portion of vital capacity (FEF25-75%). Lung volume measurements included total lung capacity (TLC). The DLCO was corrected for hemoglobin content. In 308 patients, 1430 PFTs were performed, with a mean of 4, a median of 5, and a range of 3 to 17 PFTs per patient.
Global Lung Initiative (GLI)-2012 indices were used for all spirometry values, with predictive values, lower limits of normal (LLN), and z-scores for FVC, FEV1, FEV1/FVC, and FEF25-75% [10]. The GLI-2012 equations are based on a very large reference population of patients age 3 to 95 years and produces age-specific spirometric indices for LLN [10]. Lung volumes were normalized according to prediction equations published by Crapo et al. [11] and Miller et al. [12], and were adjusted for race [13].
Statistical Analysis
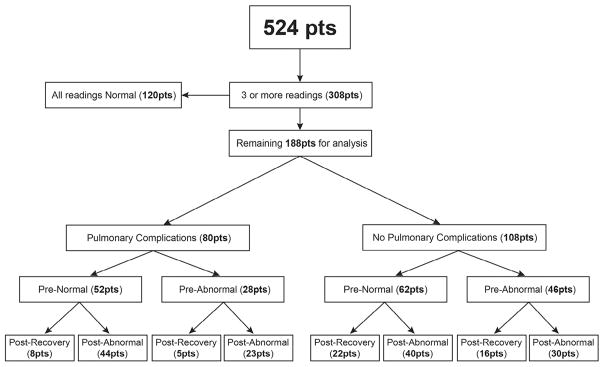
Pulmonary function was classified as normal, obstructive, restrictive, or mixed based on an algorithm published by Pellegrino et al. [14] and the ATS/ ERS task force for the standardization of lung function testing. Patients with FEV1/VC and VC values equal to or greater than the LLN were categorized as normal. Patients with obstructive, restrictive, or mixed defects were categorized as abnormal. Group 1 (188 patients) comprised patients with 3 or more PFT results, of which at least 1 was abnormal, and group 2 (120 patients) comprised patients with 3 or more PFT results, all of which were normal (Figure 1).
Figure 1.
Study population flow chart for the analysis of recovery of pulmonary function after HCT. The results of PFTs were classified as normal or abnormal based on the algorithm published by Pellegrino et al. [14]. Patients with restrictive, obstructive, and mixed defects were categorized as abnormal; all others were categorized as normal. All patients had a minimum of 3 readings, including pretransplantation PFTs, post-transplantation PFTs, and the most recent PFTs. The series of 188 patients were categorized as with or without pulmonary complications and with normal or abnormal pretransplantation and post-transplantation PFTs. Recovery indicates that normal pulmonary function was restored.
Descriptive statistics for patients in groups 1 and 2, and for patients in group 1 who did and did not experience recovery of lung function, were reported and compared using either Pearson’s chi-square test or Fisher’s exact test for categorical variables and the 2-sample t test or Wilcoxon’s rank-sum test for quantitative variables based on the normality assumption criteria. Descriptive statistics for the patients in group 2 and for those who recovered in group 1 were also compared using the same approach.
The distributions of the pre-HCT and the most recent post-HCT z-scores were compared using either a 1-sample t test or Wilcoxon’s signed-rank test based on the normality assumption. The time periods for the PFTs were specified as 0 to 100 days, 100 to 365 days, and >365 days after HCT. These groups reflect PFTs completed at 3 months, 6 months, and then annually after transplantation. The distributions of the changes in PFTs between any 2 time points were compared using the same approach as above.
Logistic regression analyses for group 1 were done using recovery and pulmonary complications as binary outcomes, to evaluate their association with the covariates outlined below. Logistic regression analysis for pulmonary complications was performed for group 2. The overall survival (OS) probabilities for patients in group 1 who recovered and for group 2 were estimated using the Kaplan–Meier method and compared using the log-rank test. In group 1, the OS of patients who recovered or did not recover normal lung function with or without pulmonary complications were estimated and compared using the same method. OS was defined as the time from HCT until death from any cause, censoring those patients who were still alive at the last follow-up. The Cox proportional hazards model was used to evaluate the associations between OS, recovery, pulmonary complications, and covariates outlined below. The assumption of proportional hazard was confirmed in all survival analyses.
The cumulative incidence of an event was estimated by the Kalbfleisch–Prentice method [15] and compared using Gray’s test [16]. In the estimation of cumulative incidence of deaths due to nonrelapse, deaths due to relapse were considered competing events. The Fine and Gray regression model [17] was used to evaluate the associations between cumulative incidence and all other covariates.
Covariates considered in univariate analysis for pulmonary complications included age at transplantation, diagnosis, disease status, donor type, sex, race, type of product received, T cell depletion, receipt of TBI, conditioning (myeloablative versus reduced intensity), cytomegalovirus donor/recipient serostatus, acute and chronic GVHD, HCT time cohort (1990 to 1999 versus 2000 to 2014), receipt of multiple HCTs, pretransplantation PFT z-scores, and duration of follow-up. Apart from the covariates outlined above, for univariate analysis of recovery, covariates such as pulmonary complications, and ventilator status were considered. Similarly for OS and nonrelapse mortality (NRM), covariates such as recovery, pulmonary complications, and ventilator status were also considered in the analysis. A pretransplantation PFT z-score was considered normal if it was >2 SDs in the left tail of the normal distribution (−2 < z), mildly reduced (−3 < z < −2), moderately reduced (−4 < z < −3), or severely reduced (z < −4) [18].
The parameters associated with outcomes in univariate analyses at a nominal level of .10 were included in their respective multivariate analyses based on a stepwise model selection strategy that used logistic regression, Cox proportional hazards, and Fine and Gray regression models. All reported P values are 2-sided and considered significant at <.05. Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC), and R version 2.13.1 (R Institute for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
Demographic data of patients in group 1 with abnormal PFTs were compared with those of patients in group 2 with normal PFTs (Table 1). The patients in group 2 were younger (P = .003) and more likely to have received a marrow product (P = .004). The demographic data of patients in group 1 in whom pulmonary function normalized were not significantly different from that of patients with persistently abnormal PFT results (Table 1).
Table 1.
Characteristics of Patients in Group 1 with and without Normalization of Pulmonary Function and Patients in Group 2
| Characteristic | Group 1 (n = 188) | Normalization
|
P Value* | Group 2 (n = 120) | P Value† | |
|---|---|---|---|---|---|---|
| Yes (n = 51) | No (n = 137) | |||||
| Age, yr | .16 | .003 | ||||
| Mean (SD) age at HCT | 13.54 (3.49) | 12.90 (3.60) | 13.77 (3.44) | 12.28 (3.78) | ||
| Median | 13.82 | 13.45 | 13.91 | 12.50 | ||
| Range | 6.60–20.45 | 6.60–18.83 | 6.64–20.45 | 6.21–20.08 | ||
| Race, n (%) | .63 | .58 | ||||
| White | 136 (72) | 37 (72) | 99 (72) | 93 (78) | ||
| African-American | 35 (19) | 8 (16) | 27 (20) | 17 (14) | ||
| Other | 17 (9) | 6 (12) | 11 (8) | 10 (8) | ||
| Male sex, n (%) | 105 (56) | 25 (49) | 80 (58) | .32 | 67 (56) | 1.00 |
| Underlying disease, n (%) | .74 | .30 | ||||
| Hematologic malignancy | 152 (81) | 39 (76) | 113 (83) | 93 (77) | ||
| Heme disorders | 29 (15) | 10 (20) | 19 (14) | 24 (20) | ||
| Immune disorders | 3 (2) | 1 (2) | 2 (1) | 3 (3) | ||
| Solid tumors | 4 (2) | 1 (2) | 3 (2) | 0 (0) | ||
| Remission at HCT, n (%) | 120 (64) | 28 (55) | 92 (67) | .13 | 77 (64) | 1.00 |
| Donor match, n (%) | .23 | .30 | ||||
| Fully matched | 129 (69) | 40 (78) | 89 (65) | 92 (77) | ||
| Haploidentical | 44 (23) | 9 (18) | 35 (25) | 20 (17) | ||
| Mismatched | 15 (8) | 2 (4) | 13 (10) | 8 (6) | ||
| Multiple transplants, n (%) | 39 (21) | 6 (12) | 33 (24) | .07 | 6 (5) | .39 |
| Product type, n (%) | .04 | .004 | ||||
| HPC-A | 55 (29) | 11 (21) | 44 (32) | 17 (14) | ||
| HPC-M | 131 (70) | 38 (75) | 93 (68) | 102 (85) | ||
| HPC-C | 2 (1) | 2 (4) | 0 (0) | 1 (1) | ||
| Time cohort, n (%) | .29 | .90 | ||||
| Before 2000 | 54 (29) | 18 (35) | 36 (26) | 36 (30) | ||
| On or after 2000 | 134 (71) | 33 (65) | 101 (74) | 84 (70) | ||
| T cell depletion, n (%) | 66 (35) | 15 (29) | 51 (37) | .39 | 35 (29) | .32 |
| Reduced-intensity conditioning, n (%) | 50 (27) | 11 (22) | 39 (29) | .45 | 36 (30) | .52 |
HPC-M indicates human progenitor cell, marrow; HPC-A, human progenitor cell, apheresis or peripheral blood stem cell product; HPC-C, human progenitor cell, cord.
Group 1 includes patients with 3 or more PFT results, of which at least 1 was abnormal. Group 2 includes patients with 3 or more PFT results, all of which were normal.
P value comparing patients in group 1 who did and did not normalize lung function.
P value comparing patients in group 1 and group 2.
Recovery of Pulmonary Function
Pretransplantation PFTs were abnormal in 74 of the 188 patients (39%) in group 1 (Figure 1). Obstructive, restrictive, and mixed lung disease was seen in 45 patients (24%), 27 patients (14%), and 2 patients (1%), respectively. Patients with abnormal PFTs post-transplantation (group 1) had lower pretransplantation FEV1 (P < .001), FVC (P < .001), FEF25-75% (P < .001), and TLC z-scores (P < .001) compared with patients with all normal PFTs (group 2). Patients who did not recover lung function in group 1 had lower pretransplantation FEV1 (P = .01), FVC (P < .001), FEV1/FVC (P = .003), and TLC z-scores (P = .04).
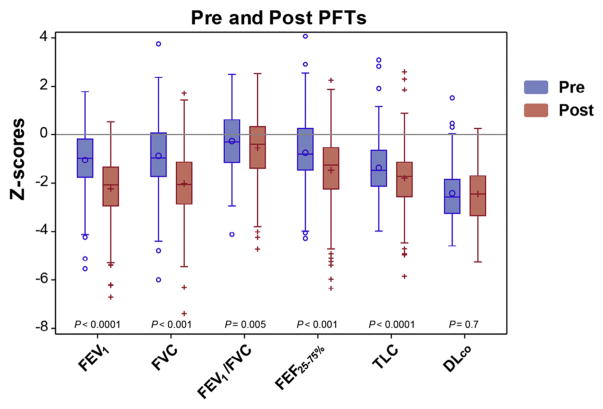
FEV1, FVC, FEV1/FVC, FEF25-75%, and TLC z-scores in group 1 patients were significantly lower in the most recent post-transplantation PFTs compared with the pretransplantation baseline; however, the corrected DLCO z-score was unchanged (Figure 2). Obstructive, restrictive, and mixed lung disease and normal pattern was seen in 80 patients (43%), 48 patients (25%), 9 patients (5%), and 51 patients (27%), respectively, at a median follow-up of 5 years (range, 0.4 to 11.9 years) post-transplantation.
Figure 2.
Boxplots for z-scores of patients in group 1 with the pretransplantation and the most recent post-transplantation PFTs.
Pulmonary function normalized in 51 patients (27%) (Table 2). Changes in pulmonary function were compared at 0 to 100 days, 101 to 365 days, and more than 365 days post-transplantation in patients with and without normalization of pulmonary function (Table 3). Patients who recovered had improved FVC, TLC, and corrected DLCO z-scores. Patients who did not recover had worse FEV1, FVC, FEV25-75%, and FEV1/FVC z-scores, but an improved corrected DLCO z-score (Table 3).
Table 2.
Outcomes of Patients in Group 1 with and without Normalization of Pulmonary Function and Patients in Group 2
| Characteristic | Group 1 (n = 188) | Normalization
|
P Value* | Group 2 (n = 120) | P Value† | |
|---|---|---|---|---|---|---|
| Yes (n = 51) | No (n = 137) | |||||
| Acute GVHD, n (%)‡ | 57 (30) | 16 (31) | 41 (30) | .86 | 31 (26) | .44 |
| Chronic GVHD, n (%) | 53 (28) | 12 (24) | 41 (30) | .47 | 25 (21) | .18 |
| PC, n (%) | 80 (43) | 13 (26) | 67 (49) | .005 | 37 (31) | .04 |
| Noninfectious PC, n (%) | 14 (8) | 0 (0) | 14 (10) | .01 | 1 (1) | .007 |
| Infectious PC, n (%) | 66 (35) | 13 (26) | 53 (39) | .09 | 36 (30) | .39 |
| Survival, n (%) | .006 | <.001 | ||||
| Alive | 143 (76) | 46 (90) | 97 (71) | 110 (92) | ||
| Died | 45 (24) | 5 (10) | 40 (29) | 10 (8) | ||
| Death due to PC, n (%) | 12 (6) | 0 (0) | 12 (9) | .04 | 0 (0) | .004 |
PC indicates pulmonary complications.
P value comparing patients in group 1 who did and did not normalize lung function.
P value comparing patients in group 1 and group 2.
Severe acute GVHD grade II-IV.
Table 3.
z-Scores of Pulmonary Function Variables of Patients in Group 1 with Lung Function Did or Did Not Normalize, by Time Period Post-Transplantation
| Characteristic | Statistic | Recovery (n = 51)
|
No Recovery (n = 137)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–100 d | 101–365 d | >365 d | P Value | 0–100 d | 101–365 d | >365 d | P Value | ||
| FEV1 | NS | P 0–100 versus 101–365 = .001 ↓ | |||||||
| Number | 21 | 26 | 45 | 68 | 71 | 106 | |||
| Mean (SD) | −1.46 (1.40) | −1.56 (1.44) | −.91 (.58) | −1.67 (1.31) | −2.33 (1.26) | −2.42 (1.20) | P 101–365 versus >365 = .009 ↓ | ||
| Median (range) | −.95 (−4.14~.78) | −1.39 (−4.49~2.35) | −1.01 (−2.11~.53) | −1.53 (−4.68~1.97) | −2.38 (−6.16~.88) | −2.22 (−6.45~.08) | P 0–100 versus >365 < .001 ↓ | ||
| FVC | P 101–365 versus >365 =.005 ↑ | P 0–100 versus 101–365 = .02 ↓ | |||||||
| Number | 21 | 26 | 45 | 68 | 71 | 106 | P 0–100 versus >365 = .001↓ | ||
| Mean (SD) | −1.23 (1.59) | −1.41 (1.46) | −.70 (.78) | −1.54 (1.40) | −2.17 (1.42) | −2.17 (1.36) | |||
| Median (range) | −1.43 (−4.08~1.45) | −1.52 (−3.67~2.95) | −.84 (−1.75~1.35) | −1.57 (−5.11~2.63) | −2.28 (−5.21~2.09) | −2.19 (−7.28~1.79) | |||
| FEV1/FVC | NS | P 101–365 versus >365 = .008↓ | |||||||
| Number | 21 | 26 | 45 | 67 | 71 | 106 | P 0–100 versus >365 = .02↓ | ||
| Mean (SD) | −.39 (1.24) | −.44 (1.05) | −.36 (.77) | −.37 (1.11) | −.45 (1.32) | −.64 (1.37) | |||
| Median (range) | −.20 (−2.83~1.53) | −.42 (−2.74~1.34) | −.34 (−2.15~1.18) | −.35 (−3.31~1.59) | −0.14 (−4.72~1.85) | −0.55 (−4.24~2.21) | |||
| FEF25-75% | NS | P 0–100 versus 101–365 = .003↓ | |||||||
| Number | 21 | 26 | 45 | 68 | 71 | 106 | P 101–365 vs. >365 = .01↓ | ||
| Mean (SD) | −1.03 (1.24) | −.99 (1.20) | −.64 (.76) | −1.07 (1.24) | −1.49 (1.34) | −1.64 (1.47) | P 0–100 versus >365 = .002↓ | ||
| Median (Range) | −1.14 (−3.44~0.53) | −.97 (−4.12~0.78) | −.79 (−2.26~1.02) | −1.16 (−4.29~1.03) | −1.21 (−6.25~0.71) | −1.62 (−5.76~1.59) | |||
| TLC | P 101–365 versus >365 =.04 ↑ | NS | |||||||
| Number | 21 | 26 | 45 | 68 | 71 | 103 | |||
| Mean (SD) | −1.70 (1.44) | −1.62 (1.08) | −1.10 (.90) | −1.72 (1.20) | −1.98 (1.31) | −1.79 (1.20) | |||
| Median (Range) | −1.68 (−4.77~1.12) | −1.72 (−4.18~.12) | −1.21 (−2.69~.91) | −1.92 (−3.85~1.63) | −2.00 (−4.92~1.88) | −1.89 (−4.38~2.17) | |||
| DLCO | P 101–365 versus >365 <.0001 ↑ | P 101–365 versus >365 = .002 ↑ | |||||||
| Number | 21 | 25 | 45 | 66 | 70 | 102 | P 0–100 versus >365 = .0006 ↑ | ||
| Mean (SD) | −2.72 (1.46) | −2.94 (.78) | −2.05 (.82) | P 0–100 vs. >365 =.04 ↑ | −3.08 (.82) | −3.10 (.87) | −2.57 (.97) | ||
| Median (Range) | −3.06 (−4.54~1.66) | −3.05 (−4.93~−1.47) | −2.12 (−3.74~−.08) | −3.26 (−4.55~−.49) | −3.28 (−4.77~−1.07) | −2.58 (−4.49~.46) | |||
NS indicates not significant.
Time periods include 0 to 100, 101 to 365, and >365 days post-transplantation.
Univariate logistic regression analysis for patients in group 1 showed that patients with pulmonary complications (P = .005; odds ratio [OR], .36; 95% confidence interval [CI], .18 to .73), and those with mildly reduced pretransplantation FVC z-score (P = .02; OR, .09; 95% CI, .01 to .65) were less likely to experience normalization of pulmonary function.
Multivariate logistic regression analysis showed that after adjusting for duration of follow-up, patients with pulmonary complications (P = .01; OR, .39; 95% CI, .19 to .82), and those with mildly reduced pretransplantation FVC z-score (P = .01; OR, .07; 95% CI, .01 to .57) were less likely to experience normalization of pulmonary function.
Pulmonary Complications
Pulmonary complications were seen in 80 patients (43%) in group 1 and in 37 patients (31%) in 2 (P = .04). A univariate logistic regression analysis of pulmonary complications for patients in group 1 is presented in Supplementary Table S2. On multivariate analysis, T cell depletion (P < .001; OR, 3.27; 95% CI, 1.69 to 6.33), moderately to severely reduced pretransplantation FEV1 z-score (P = .006; OR, 9.97; 95% CI, 1.92 to 51.84), and chronic GVHD (P < .001; OR, 3.42; 95% CI, 1.70 to 6.89) were associated with increased risk for pulmonary complications.
Multivariate logistic regression analysis of pulmonary complications for patients in group 2 showed an association between severe acute GVHD and increased risk for pulmonary complications (P = .02; OR, 2.86; 95% CI, 1.22 to 6.70).
Fourteen patients in group 1 had noninfectious pulmonary complications, including BOS in 5 patients, diagnosed by high-resolution chest computed tomography and pulmonary function tests; diffuse alveolar hemorrhage in 2 patients, diagnosed by clinical findings and bronchoalveolar lavage; and COP 6 patients and interstitial lung disease in 1 patient, respectively, diagnosed by lung biopsy. Two patients were diagnosed with IPS at 4 months and 7 months before being diagnosed with COP. Noninfectious pulmonary complications were preceded by acute grade III-IV GVHD in 9 patients. (Supplementary Table S1). Sixty-six patients in group 1 had only infectious complications (Supplementary Table S1).
OS and NRM
Patients in group 1 had worse OS compared with those in group 2 (P < .001). Patients in group 1 who recovered pulmonary function had better OS (P = .006), which was not different from that of patients in group 2 (P = .80). Twelve patients (8%) died of pulmonary complications in group 1. No patients in group 1 who had normalized pulmonary function or patients in group 2 died of pulmonary complications.
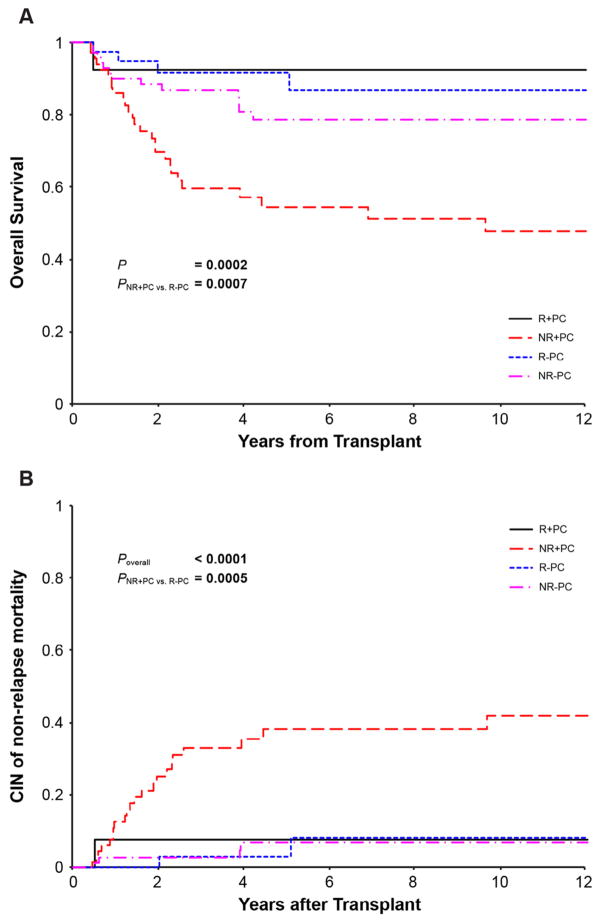
The mean and median durations of follow-up for the 188 patients in group 1 were 4.86 and 4.02 years, respectively (range, 0.43 to 11.56 years). The probabilities of OS and NRM for patients who did not recover lung function and had pulmonary complications were 47.6% (95% CI, 32.1% to 61.4%) and 42% (95% CI, 27.5% to 56%), respectively, compared with 86.6% (95% CI, 66.6% to 95%) and 8.1% (95% CI, 1.2% to 24%) for patients who recovered lung function and had no pulmonary complications (Figure 3).
Figure 3.
(A) OS of patients in group 1 who recovered (R) or did not recover (NR) normal lung function with (+) or without (−) pulmonary complications (PCs). (B) Cumulative incidence (CIN) of NRM of patients in group 1 who recovered (R) or did not recover (NR) normal lung function, with (+) or without (−) PCs.
The results of univariate analysis of OS and NRM for patients in group 1 are shown in Supplementary Table S2. On multivariate analysis, mechanical ventilation (P < .001; hazard ratio [HR], 7.71; 95% CI, 3.79 to 15.71) and nonrecovery of lung function with pulmonary complications (P = .02; HR, 2.18; 95% CI, 1.12 to 4.23) were associated with increased risk for overall mortality.
Mechanical ventilation (P < .001; HR, 17.41; 95% CI, 6.89 to 43.97), nonrecovery of lung function with pulmonary complications (P = .03; HR, 3.31; 95% CI, 1.12 to 9.78), and severe acute GVHD (P = .004; HR, 2.68; 95% CI, 1.37 to 5.27) were associated with increased risk for NRM.
Univariate and multivariate analyses of OS and NRM for patients in group 2 were inconclusive due to insufficient power. Only 10 patients died in this group, 4 due to relapse.
DISCUSSION
Our study of a large cohort of children who underwent allogeneic HCT with long follow-up at a single institution reveals that normalization of pulmonary function is possible in long-term survivors of allogeneic HCT, and that normalization is associated with improved survival. Patients with pulmonary complications were less likely to develop normal pulmonary function.
Lung volumes recovered better than spirometric indices. Significant improvements in TLC and DLCO at more than 1 year post-transplantation were seen in patients whose pulmonary function normalized. A decline in spirometric indices at more than 1 year post-transplantation has been corroborated by other studies [19–22]. Previous studies also have identified older age at the time of HCT [23] and receipt of a peripheral blood stem cell graft [21] as risk factors for PFT decline, which was confirmed in our present study.
Kaya et al. [19], in a smaller cohort of patients, reported declines in spirometric indices and lung volumes at 3 to 6 months post-transplantation. In that study, spirometric indices were improved at 12 to 24 months post-transplantation but remained significantly below pretransplantation values, and lung volumes recovered to baseline during that period. Recovery of spirometric indices and lung volumes to normal values in children at long-term follow-up is a novel finding. Approximately one-quarter of patients with abnormal lung function pretransplantation recovered to normal on post-transplantation follow-up in our study. OS was not significantly different between patients whose lung function normalized and patients who had no deterioration in lung function post-transplantation. This is a novel finding.
Factors associated with the normalization of lung function have not been previously studied in children or adults. In our study, patients with pulmonary complications were less likely to experience lung function normalization and had a high mortality. Patients without pulmonary complications experienced recovery of lung function and had significantly better survival. T cell depletion was associated with an increased risk of pulmonary complications. This may be related to poor immune reconstitution with an increased risk of respiratory infections [24]. A reduced pretransplantation FEV1 z-score also was associated with increased risk for pulmonary complications. Horak et al. [25] recognized reduced FEV1 as a strong risk factor for the development of viral-associated interstitial pneumonia in HCT recipients [25]. Damage to airways as a result of previous therapy, alloreactivity due to GVHD, and recurrent infections may lead to irreversible steroid-unresponsive airway obstruction and bronchiectasis. Hoffmeister et al. [26] reported that patients surviving more than 20 years after HCT and those with chronic GVHD had a substantially higher risk of obstructive lung disease [26]. The association between GVHD and BOS is well recognized [27,28].
Although lung volumes recovered better than spirometric indices, restrictive lung disease was associated with higher mortality. Because we censored patients with early NRM who were unable to perform more than 2 PFTs, the number of patients with restrictive lung disease, particularly IPS, is likely underrepresented, highlighting the importance of restrictive lung disease on OS. Cooke et al. have reported high mortality in patients with IPS [29], and noted that early recognition and therapy with etanercept and corticosteroids may improve outcomes [30]. Associations between acute GVHD, chronic GVHD, and the subsequent development of COP have been reported [31]. Modification of transplantation strategies to reduce chronic GVHD may reduce the risk of pulmonary complications.
In this study, lower pretransplantation FVC z-scores were associated with poor recovery of lung function. Lower FEV1 or FVC increased the risk of early respiratory failure, as also observed by Kaya et al [19], and acute respiratory failure and mechanical ventilation decreased OS and increased the risk for NRM. A recent prospective multicenter study on the outcome of invasive mechanical ventilation after pediatric allogeneic HCT reported a 52% mortality rate [32], which was clearly improved compared with previous studies [33].
Strengths of our study include a large retrospective cohort of children with a long follow-up. PFTs were standardized using the same instrumental method for the duration of the study. The ATS/ERS standardized algorithm for defining lung function abnormality using the FEV1/VC ratio was used for the first time in this population.
Previous long-term studies of pulmonary function have used percent predicted values rather than the recent GLI-2012 indices. Using percent predicted equations and fixed thresholds for FEV1/FVC has been reported to result in the misdiagnosis of more than 20% of patients undergoing PFTs [34]. The ATS and ERS recommend using the 5th percentile to define LLN (i.e., −1.64 z-score). Unlike percent predicted, z-scores allow for uniform interpretation of test results.
Previous studies also have included autologous transplants and patients at risk for increased transplantation-related mortality, thereby precluding accurate assessment of recovery of pulmonary function. Both NRM and OS were included as outcome measures.
Our study is limited by its retrospective nature. PFTs were compared over broad time spans rather than at specific time points. Children age <6 years were excluded. Causes of pretransplatation abnormal lung function were not determined, and although therapy was standardized for pulmonary complications, individual differences in patient management may have contributed to outcomes. Further studies are needed to validate our findings in an independent, larger retrospective cohort, and to evaluate modified transplantation strategies aimed at reducing the risk of pulmonary complications. Close follow-up of airway obstruction post-transplantation will be important as this population of survivors continues to age.
Supplementary Material
Acknowledgments
The authors thank Paul W. Mackert (Pulmonary Function Laboratory at St. Jude) for performing or supervising pulmonary function testing and for assisting with data collection and Keith A. Laycock (Scientific Editing at St. Jude) for editing the manuscript.
Funding sources: This work was supported by National Cancer Institute (NCI) Grant 5R25CA02394, NCI Cancer Center CORE Support Grant P30 CA 21765, and ALSAC.
Footnotes
Financial disclosure: See Acknowledgments on page 8.
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
Supplementary data related to this article can be found online at doi:10.1016/j.bbmt.2017.08.025.
References
- 1.Eikenberry M, Bartakova H, Defor T, et al. Natural history of pulmonary complications in children after bone marrow transplantation. Biol Blood Marrow Transplant. 2005;11:56–64. doi: 10.1016/j.bbmt.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan A, Srinivasan S, Sunthankar S, et al. Pre-hematopoietic stem cell transplant lung function and pulmonary complications in children. Ann Am Thorac Soc. 2014;11:1576–1585. doi: 10.1513/AnnalsATS.201407-308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain NA, Pophali PA, Klotz JK, et al. Repair of impaired pulmonary function is possible in very-long-term allogeneic stem cell transplantation survivors. Biol Blood Marrow Transplant. 2014;20:209–213. doi: 10.1016/j.bbmt.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 5.Michelson PH, Goyal R, Kurland G. Pulmonary complications of haematopoietic cell transplantation in children. Paediatr Respir Rev. 2007;8:46–61. doi: 10.1016/j.prrv.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Gower WA, Collaco JM, Mogayzel PJ., Jr Pulmonary dysfunction in pediatric hematopoietic stem cell transplant patients: non-infectious and long-term complications. Pediatr Blood Cancer. 2007;49:225–233. doi: 10.1002/pbc.21060. [DOI] [PubMed] [Google Scholar]
- 7.Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183:1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbahlawan L, Srinivasan A, Morrison RR. A critical care and transplantation-based approach to acute respiratory failure after hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplantation. 2016;22:617–626. doi: 10.1016/j.bbmt.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MR, Crapo RO, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 10.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18:419–425. [PubMed] [Google Scholar]
- 12.Miller A, Thornton JC, Warshaw R, Anderson H, Tierstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state. Predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Am Rev Respir Dis. 1983;127:270–277. doi: 10.1164/arrd.1983.127.3.270. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, Christoph K, Yu Z, Eigen H, Tepper RS. Pulmonary diffusing capacity in healthy African-American and Caucasian children. Pediatr Pulmonol. 2016;51:84–88. doi: 10.1002/ppul.23205. [DOI] [PubMed] [Google Scholar]
- 14.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 15.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 16.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Ginsberg JP, Aplenc R, McDonough J, Bethel J, Doyle J, Weiner DJ. Pre-transplant lung function is predictive of survival following pediatric bone marrow transplantation. Pediatr Blood Cancer. 2010;54:454–460. doi: 10.1002/pbc.22337. [DOI] [PubMed] [Google Scholar]
- 19.Kaya Z, Weiner DJ, Yilmaz D, Rowan J, Goyal RK. Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant. 2009;15:817–826. doi: 10.1016/j.bbmt.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Wieringa J, van Kralingen KW, Sont JK, Bresters D. Pulmonary function impairment in children following hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2005;45:318–323. doi: 10.1002/pbc.20304. [DOI] [PubMed] [Google Scholar]
- 21.Inaba H, Yang J, Pan J, et al. Pulmonary dysfunction in survivors of childhood hematologic malignancies after allogeneic hematopoietic stem cell transplantation. Cancer. 2010;116:2020–2030. doi: 10.1002/cncr.24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruno B, Souillet G, Bertrand Y, Werck-Gallois MC, So Satta A, Bellon G. Effects of allogeneic bone marrow transplantation on pulmonary function in 80 children in a single paediatric centre. Bone Marrow Transplant. 2004;34:143–147. doi: 10.1038/sj.bmt.1704549. [DOI] [PubMed] [Google Scholar]
- 23.Ringdén O, Remberger M, Ruutu T, et al. Nordic Bone Marrow Transplantation Group. Increased risk of graft-versus-host disease, obstructive bronchiolitis and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic bone marrow recipients with leukemia. Blood. 1999;93:2196–2201. [PubMed] [Google Scholar]
- 24.Chakrabarti S, Collingham KE, Marshall T, et al. Respiratory virus infections in adult T cell-depleted transplant recipients: the role of cellular immunity. Transplantation. 2001;72:1460–1463. doi: 10.1097/00007890-200110270-00024. [DOI] [PubMed] [Google Scholar]
- 25.Horak DA, Schmidt GM, Zaia JA, Niland JC, Ahn C, Forman SJ. Pretransplant pulmonary function predicts cytomegalovirus-associated interstitial pneumonia following bone marrow transplantation. Chest. 1992;102:1484–1490. doi: 10.1378/chest.102.5.1484. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmeister PA, Madtes DK, Storer BE, Sanders JE. Pulmonary function in long-term survivors of pediatric hematopoietic cell transplantation. Pediatr Blood Cancer. 2006;47:594–606. doi: 10.1002/pbc.20531. [DOI] [PubMed] [Google Scholar]
- 27.Schwarer AP, Hughes JM, Trotman-Dickenson B, Krausz T, Goldman JM. A chronic pulmonary syndrome associated with graft-versus-host-disease after allogeneic marrow transplantation. Transplantation. 1992;54:1002–1008. doi: 10.1097/00007890-199212000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Efrati O, Toren A, Duskin H, et al. Pulmonary function studies in children treated by chemoradiotherapy and stem cell transplantation. Pediatr Blood Cancer. 2008;51:684–688. doi: 10.1002/pbc.21722. [DOI] [PubMed] [Google Scholar]
- 29.Cooke KR, Yanik G. Acute lung injury after allogeneic stem cell transplantation: is the lung a target of acute graft-versus-host disease? Bone Marrow Transplant. 2004;34:753–765. doi: 10.1038/sj.bmt.1704629. [DOI] [PubMed] [Google Scholar]
- 30.Yanik GA, Grupp SA, Pulsipher MA, et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children’s Oncology Group Study (ASCT0521) Biol Blood Marrow Transplant. 2015;21:67–73. doi: 10.1016/j.bbmt.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freudenberger TD, Madtes DK, Curtis JR, Cummings P, Storer BE, Hackman RC. Association between acute and chronic graft-versus-host disease and bronchiolitis obliterans organizing pneumonia in recipients of hematopoietic stem cell transplants. Blood. 2003;102:3822–3828. doi: 10.1182/blood-2002-06-1813. [DOI] [PubMed] [Google Scholar]
- 32.van Gestel JP, Bierings MB, Dauger S, et al. Outcome of invasive mechanical ventilation after pediatric allogeneic hematopoietic SCT: results from a prospective, multicenter registry. Bone Marrow Transplant. 2014;49:1287–1292. doi: 10.1038/bmt.2014.147. [DOI] [PubMed] [Google Scholar]
- 33.Tamburro RF, Barfield RC, Shaffer ML, et al. Changes in outcomes (1996–2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med. 2008;9:270–277. doi: 10.1097/PCC.0b013e31816c7260. [DOI] [PubMed] [Google Scholar]
- 34.Miller MR, Quanjer PH, Swanney MP, Ruppel G, Enright PL. Interpreting lung function data using 80% predicted and fixed thresholds misclassifies more than 20% of patients. Chest. 2011;139:52–59. doi: 10.1378/chest.10-0189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.