Abstract
Objectives
Histopathological studies have implicated herpes zoster (HZ) as a causative organism of giant cell arteritis (GCA). We assessed the epidemiologic association of HZ events with incident GCA.
Methods
We performed a retrospective cohort study utilizing two, large, independent US administrative datasets (Medicare 5% and Truven MarketScan) with eligible subjects having 12 months of continuous coverage, >50 years old, and without history of GCA or polymyalgia rheumatica. HZ events (complicated and uncomplicated) and GCA were identified by ICD-9 codes from physician visits or hospital discharges. Antiviral therapies and vaccinations were identified as prescription claims and drug codes. Risk of incident GCA was calculated using multivariable Cox proportional hazards regression.
Results
Among 16,686,345 subjects, 5,942 GCA cases occurred with 3.1% (MarketScan) and 6.0% (Medicare) having preceding HZ events. Unadjusted GCA incidence rates were highest in those with complicated and uncomplicated HZ. After multivariable adjustment, complicated HZ was associated with an increased risk of GCA (MarketScan Hazard ratio [HR] 2.16, 95% confidence interval (CI) 1.46–3.18; Medicare HR 1.99, 95% CI 1.32–3.02) as was uncomplicated HZ (MarketScan HR 1.45, 95% CI 1.05–2.01; Medicare HR 1.42, 1.02–1.99). Vaccination and antiviral treatment were not consistently associated with GCA risk, though antivirals were marginally associated with a decreased risk of GCA in Medicare (HR 0.67, 95% CI 0.46–0.99).
Conclusions
HZ is associated with increased GCA risk. The infrequency of HZ in GCA patients suggests it is only one potential trigger for GCA. Antivirals and vaccination did not consistently mitigate this risk.
Keywords: herpes zoster, giant cell arteritis, vasculitis, epidemiology
Introduction
Giant cell arteritis (GCA) is the most common systemic vasculitis among individuals >50 years of age (1) with incidence rates that steadily increase with age and are highest amongst Caucasians (2). Typical symptoms of GCA include headache, jaw claudication, scalp tenderness, and features of polymyalgia rheumatica (PMR) such as shoulder and hip girdle pain (3). One of the most feared clinical complications in GCA, permanent vision loss, can occur early in the disease process and has been attributed to anterior ischemic optic neuropathy, retinal artery occlusion, posterior ischemic optic neuropathy, or cortical ischemia (3). Additional complications of GCA include aortic aneurysms and dissections (3), cardiovascular events (4), and venous thromboembolic events (5). Prolonged, high-dose corticosteroids with or without additional immunomodulatory therapy are the mainstay of management, treatments that are frequently the cause of serious adverse events (6).
Although the etiology of GCA remains to be fully elucidated, dysregulated interactions between the vessel wall and the immune system appear to be critical to its pathogenesis (7). Activated vascular dendritic cells recruit CD4+ T cells and macrophages to the vessel wall, subsequently entering through the vasa vasorum, and penetrating through tissue space from adventitia to intima resulting in granuloma formation (7). Because intracellular pathogens often elicit similar granulomatous inflammation, infections have been postulated to incite this process in at least some patients (8).
Varicella zoster virus (VZV), a human herpes virus, has also been implicated in GCA pathogenesis. Similar to GCA, VZV reactivation (termed herpes zoster [HZ]) occurs in older adults and induces a T cell mediated immune response (9). VZV causes a spectrum of vasculopathies, and pathological specimens of involved vessels demonstrate characteristics similar to GCA such as multinucleated giant cells (10). Upon detailed inspection of temporal artery and aorta specimens from biopsy positive and negative GCA, VZV antigen and DNA has been detected in some investigations (11–15). Moreover, VZV has been located in skip lesions and was most prevalent in the adventitia with decreased detection closer to the intima (12, 14). In contrast, there have been multiple studies that have not identified VZV in GCA temporal artery specimens (16–20), and a recent study suggested the possibility of cross-reactivity of the immunohistochemical staining to muscular elements (21).
To date, the association of VZV with GCA risk has largely been studied using pathological specimens and cross-sectional study designs. One prior case-control study focused primarily on HZ occurring after GCA diagnosis performed limited comparisons of HZ events preceding GCA, finding no association (22). Another recent study found a small but significantly increased risk of GCA after HZ events, as well as infections in general, in a population based case-control study (23). In summary, the epidemiologic relationship between HZ events and incident GCA is not fully understood and yet to be appropriately studied using a cohort design that is necessary in defining the temporal relationship between exposure and disease onset. Moreover, if HZ contributes to GCA risk, then it follows that antiviral therapy or vaccination could mitigate disease risk, effects that have to-date not been investigated. The purpose of this study was to determine the relationship of HZ events with the risk of incident GCA and to examine whether antiviral treatment or HZ vaccination reduces this risk.
Methods
Data sources and study participants
This was a retrospective cohort study utilizing medical and pharmacy claims from the Medicare 5% random sample (January 1, 2006 through December 31, 2013) and Truven MarketScan Commercial Claims and Encounters database (January 1, 2010 through December 31, 2014). The Medicare 5% random sample is a nationally representative 5% random sample of the overall Medicare beneficiary population (Centers for Medicare and Medicaid Services) which consists of individuals age 65 years or older who are not enrolled in Medicare Advantage, as well as those with certain disabling conditions who are younger than age 65. MarketScan (https://marketscan.truvenhealth.com/) includes individuals of all ages covered under a variety of commercial and employee-sponsored benefit plans. Participants were required to have at least 12 months of continuous enrollment to be eligible for analyses and were excluded if they were under 50 years of age or had a physician or hospital diagnosis code for GCA (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 446.5x) or PMR (ICD-9-CM code 725.xx) during this time-period. This study was approved by the institutional review board at the University of Alabama at Birmingham.
Exposures and outcome
Exposures of interest were HZ events, antiviral treatment for a HZ event, and HZ vaccination. HZ events were identified by ICD-9-CM code 053.xx from either a hospital discharge or physician visit. HZ was further classified as complicated (Supplementary Table 1; i.e. HZ with meningitis, other nervous system, ophthalmic, or other complications; ICD-9-CM codes 053.0 to 053.8) or uncomplicated (codes 053.9), similar to a HZ classification used in prior analyses(24). HZ treatment was identified by a combination of a HZ event and an accompanying outpatient pharmacy claim for acyclovir, famciclovir, valacyclovir, ganciclovir, and foscavir within 7 days before or after the HZ event date. HZ vaccination was identified by Current Procedural Terminology (CPT) codes 90736, or National Drug Codes (NDC; 00006496300, 00006496301, 00006496341) plus a vaccine administration code within 7 days (Health Care Common Procedure Coding System [HCPCS] code G03077 or CPT code 90471). Incident GCA was identified using ICD-9-CM code 446.5 from hospital discharge or two physician visits occurring between 7 and 365 days apart. In the HZ-exposed cohort, follow-up began at the index date, defined as the HZ diagnosis date. Patients also had to be observable in the data with at least 365 continuous days of prior medical and pharmacy coverage. For those not HZ-exposed, the index date was set as the date that patients first had 365 continuous days with medical and pharmacy coverage. Patients were followed from the index date until the date of first GCA diagnosis or censoring at disenrollment, death, or end of the study period. Baseline covariates (comorbidities, health screenings, medications, and number of visits) were assessed during the 12 months of continuous enrollment prior to the index date.
Statistical analysis
Descriptive statistics were calculated at the index date stratified by HZ exposure. Crude incidence rates (IR) of GCA per 10,000 person-years (PY) and 95% confidence intervals (CI) were calculated using Poisson regression stratified by HZ exposure. We examined the temporal relationship between HZ and incident GCA by calculating incident rates stratified by the time period since the index date: 0–3 months, 3–6 months, 6–12 months, 1–2 years, and >2 years. We used Cox proportional hazards regression models to calculate unadjusted and adjusted hazard ratios (HR) and 95% CI for GCA. Because of the potential for a non-linear relationship between age and GCA risk, we used age as the time axis, rather than time from the index date. Covariates identified in exploratory analyses and included in multivariable models were sex, race, corticosteroid use in the 12 months prior to the index date, and number of visits during the 12 months prior to the index date. We performed several sensitivity analyses to address potential misclassification of the GCA outcome including (1) requiring a temporal artery biopsy Current Procedure Terminology (CPT) code (CPT 37609) for GCA diagnosis, (2) requiring at least two steroid prescriptions for GCA diagnosis, (3) requiring at least one outpatient GCA ICD-9-CM code to be from a rheumatologist. Additionally, following recent recommendations for observational studies, we calculated E-values as a representation of the minimum strength of association that an unmeasured confounder would need to have with the exposure and outcome to nullify an observed exposure-outcome association (25). The E-value ranges from one to infinity, with a value of one representing an existing null association and increasing values signifying a higher degree of unmeasured confounding needed to reduce the observed association to null. A P value <0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS institute).
Results
Baseline characteristics
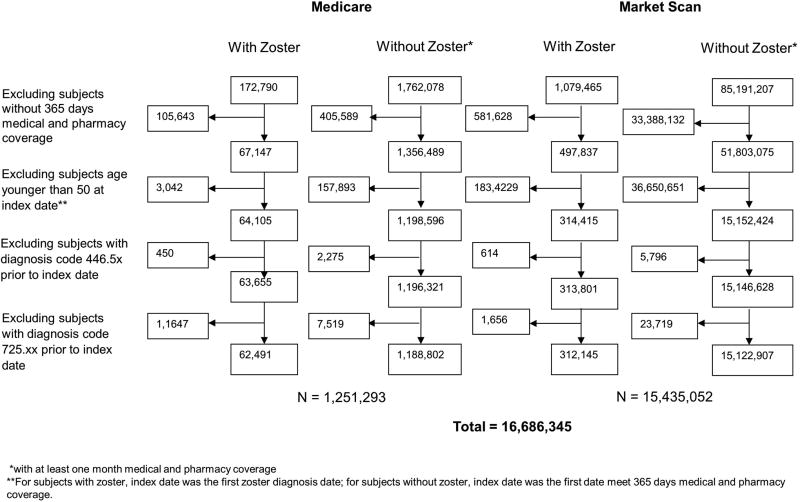
A total of 16,686,345 persons were included in the study with 1,251,293 from Medicare and 15,435,052 from MarketScan (Figure 1). HZ events occurred in 5.3% of Medicare and 2.1% of MarketScan subjects. Of those, 17.6% of Medicare and 15.0% of MarketScan HZ events were complicated. Antiviral treatment was prescribed in 53.0% of complicated HZ and 72.5% of uncomplicated HZ in Medicare, and 58.0% of complicated HZ and 77.2% of uncomplicated HZ in MarketScan. In both datasets, those with HZ were older at the index date and more often female (Table 1). The frequencies of comorbidity and health screenings are shown in Supplementary Table 2. HZ events were more common in Caucasians within the Medicare dataset, while race was not available for the MarketScan dataset. The proportion of patients receiving HZ vaccination in each exposure group (HZ and non-HZ) were low in both datasets (range 1.3 to 4.0% MarketScan, range 2.2 to 7.1% in Medicare).
Figure 1. Study flow diagram.
Study flow diagram illustrating the selection of subjects from Medicare 5% and Truven MarketScan Commercial Claims and Encounters databases for analysis by herpes zoster exposure status at the index date.
Table 1.
Baseline characteristics of Medicare and MarketScan participants by Zoster exposure.
| Medicare | MarketScan | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Zoster with complication N=10,964 |
Zoster without complication N=51,527 |
None N=1,188,802 |
Zoster with complication N=46,784 |
Zoster without complication N=265,361 |
None N=15,122,907 |
|
| Age in year, Mean (SD) | 76.3 (9.6) | 75.5 (9.5) | 72.1 (9.9) | 65.4 (11.1) | 63.4 (10.3) | 60.8 (9.3) |
| Female, % | 72.1 | 72.1 | 63.4 | 62.8 | 63.5 | 53.4 |
| Race, % * | ||||||
| Asian | 2.9 | 2.4 | 2.3 | |||
| Black | 6.0 | 5.9 | 10.6 | |||
| Hispanic | 2.7 | 2.4 | 2.8 | |||
| Other | 2.1 | 1.8 | 2.2 | |||
| White | 86.3 | 87.5 | 82.1 | |||
| Zoster vaccine, % | 6.3 | 7.1 | 2.2 | 4.0 | 3.9 | 1.3 |
| Zoster medication within 7 days, % | 53.0 | 72.5 | NA | 58.0 | 77.2 | NA |
Race not available in MarketScan
GCA incidence rates
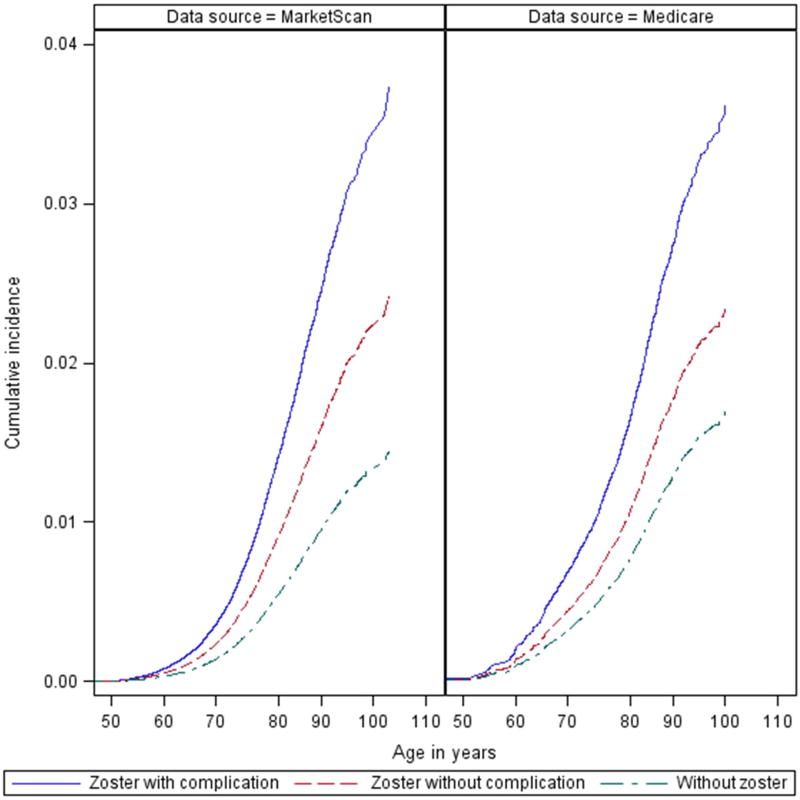
A total of 5,942 incident GCA cases (1,782 Medicare and 4,160 MarketScan) occurred over 37,724,757 patient-years of follow-up (Table 2). Median duration of follow-up ranged from 453 to 730 days in MarketScan and from 858 to 1269 days in Medicare, with those without HZ having the longest duration of follow-up (Supplemental Table 3). Of those who developed GCA, 3.1% had preceding HZ events in MarketScan and 6.0% had preceding HZ events in Medicare. Median duration from HZ events to GCA diagnosis was 177 and 361 days (MarketScan) and 651 and 593 (Medicare) for complicated and uncomplicated events (Supplemental Table 3). In both datasets, crude GCA IRs were highest amongst those with complicated HZ events and lowest in those without a documented HZ event, with uncomplicated HZ having intermediate IRs (Table 2). Cumulative incidence curves demonstrated a “dose-response” between HZ exposure with and without complications, and GCA risk (Figure 2). Stratified by follow-up period, IR estimates were universally numerically higher for HZ exposed groups than unexposed, though estimates were imprecise due to the limited number of GCA cases that occurred during each follow-up period (Supplementary Table 4).
Table 2.
Giant cell arteritis incidence rates by herpes zoster event.
| MarketScan | Medicare | |||||
|---|---|---|---|---|---|---|
| Herpes zoster | GCA cases | Person-years | IR (/10,000 PY) | GCA cases | Person-years | IR (/10,000 PY) |
| Complicated | 32 | 68,388 | 4.68 (3.31, 6.62) | 27 | 29,377 | 9.19 (6.30, 13.40) |
| Uncomplicated | 97 | 382,734 | 2.53 (2.08, 3.09) | 80 | 138,622 | 5.77 (4.64, 7.18) |
| None | 4,031 | 33,273,538 | 1.21 (1.17, 1.25) | 1,675 | 4,451,219 | 3.76 (3.59, 3.95) |
Abbreviations: GCA = giant cell arteritis, IR = incidence rate, PY = person-years
Figure 2. Cumulative incidence of GCA by herpes zoster exposure.
Curves demonstrating the cumulative incidence of giant cell arteritis (GCA) in Medicare 5% and Truven MarketScan Comercial Claims and Encounters databases for those without zoster (dashed green), uncomplicated zoster (dashed red), and complicated zoster (solid blue).
Associations of HZ events with incident GCA
In unadjusted analyses, complicated HZ was associated with a 2.61-fold higher risk and uncomplicated HZ was associated with a 1.68-fold higher risk of GCA referent to no HZ event in the MarketScan analysis (Table 3). In Medicare, complicated HZ was associated with a 2.16-fold higher risk and uncomplicated HZ a 1.39-fold higher risk of GCA referent to no HZ events. Adjustment for sex, race (Medicare only), corticosteroid use, number of visits, antiviral treatment, and zoster vaccination did not meaningfully impact the results. After adjustment, in MarketScan, complicated HZ remained associated with a 2.16-fold higher risk of GCA (95% CI 1.46, 3.18) and uncomplicated HZ associated with a 1.45-fold higher risk of GCA (95% CI 1.05, 2.01) (Table 3). Similarly, after adjustment in the Medicare dataset complicated HZ was associated with a 1.99-fold higher risk of GCA (95% CI 1.32, 3.02) and uncomplicated HZ a 1.42-fold higher risk (95% 1.02–1.99) risk of GCA. Using an inverse-variance weighted, fixed effects model to combine results from both datasets, the adjusted HR for complicated HZ was 2.08 (95% CI 1.57, 2.76) and for uncomplicated HZ was 1.44 (95% CI 1.14, 1.82). Sensitivity analyses with alternate GCA definitions requiring greater specificity supported the original findings (Table 4). E-values from combined dataset estimates were 3.58 for complicated HZ (Supplementary Table 5; 2.52 for the lower confidence interval) and 2.24 for uncomplicated HZ (1.54 for the lower confidence interval). Therefore, for complicated HZ, unmeasured cofounders would need to have an association with complicated HZ and GCA greater than a HR of 3.58 to nullify our observed association between complicated HZ and GCA. These results demonstrate that substantial unmeasured confounding would be needed to reduce the observed associations to null (25).
Table 3.
Associations of herpes zoster, antiviral treatment, and vaccination with GCA.
| Unadjusted | Sex adjusted | Fully adjusted | |
|---|---|---|---|
| MarketScan | |||
|
| |||
| Herpes zoster event | |||
| Complicated | 2.61 (1.84, 3.70) | 2.52 (1.78, 3.57) | 2.16 (1.46, 3.18) |
| Uncomplicated | 1.68 (1.38, 2.06) | 1.62 (1.32, 1.98) | 1.45 (1.05, 2.01) |
| None | Referent | Referent | Referent |
| Female | 1.86 (1.74, 1.99) | 1.82 (1.70, 1.95) | |
| Antiviral treatment | 0.90 (0.64, 1.28) | ||
| Zoster vaccination | 0.85 (0.65, 1.11) | ||
| Corticosteroid use | 1.88 (1.75, 2.02) | ||
| Number of visits | 1.02 (1.02, 1.02) | ||
|
| |||
| Medicare | |||
|
| |||
| Herpes zoster event | |||
| Complicated | 2.16 (1.48, 3.16) | 2.09 (1.43, 3.06) | 1.99 (1.32, 3.02) |
| Uncomplicated | 1.39 (1.11, 1.74) | 1.34 (1.07, 1.68) | 1.42 (1.02, 1.99) |
| None | Referent | Referent | Referent |
| Female | 1.78 (1.58, 2.00) | 1.74 (1.54, 1.95) | |
| Non-white race | 0.91 (0.80, 1.04) | 0.93 (0.81, 1.06) | |
| Antiviral treatment | 0.67 (0.46, 0.99) | ||
| Zoster vaccination | 1.30 (0.93, 1.80) | ||
| Corticosteroid use | 1.88 (1.68, 2.10) | ||
| Number of visits | 1.02 (1.02, 1.03) | ||
Results are hazard ratio (95% confidence interval) from Cox regression models where age served as time axis. Adjustment was performed for each variable where an effect estimate was provided in that column. Race not available in MarketScan database.
Abbreviations: GCA = giant cell arteritis
Table 4.
Sensitivity analyses with additional specificity for GCA diagnosis
| Medicare+ HR (95% CI) |
MarketScan± HR (95% CI) |
|
|---|---|---|
| Requiring a temporal artery biopsy CPT code for GCA diagnosis | ||
| Complicated HZ | 3.03 (1.85, 4.97) ** | 2.28 (1.32, 3.94) ** |
| Uncomplicated HZ | 1.69 (1.09, 2.60) * | 2.03 (1.35, 3.06) ** |
| Requiring two steroid prescriptions for GCA diagnosis | ||
| Complicated HZ | 3.29 (1.84, 5.89) ** | 1.86 (0.89, 3.86) |
| Uncomplicated HZ | 1.75 (1.05, 2.92) * | 2.09 (1.28, 3.42) ** |
| Requiring ≥1 outpatient ICD-9 for GCA to be from a rheumatologist | ||
| Complicated HZ | 2.80 (1.74, 4.52) ** | 1.99 (1.13, 3.51) * |
| Uncomplicated HZ | 1.70 (1.13, 2.56) * | 1.37 (0.86, 2.19) |
includes gender, race, antiviral, vaccination
includes gender, antiviral, vaccination
p < 0.05;
p < 0.01
Associations of antivirals and HZ vaccination with incident GCA
In the same multivariable model used to assess the associations between HZ and GCA, we examined the associations of antiviral treatment and HZ vaccination. Antiviral treatment was not associated with GCA in MarketScan (HR 0.90, 95% CI 0.64–1.28), although there was a marginally significant protective association between antiviral treatment and GCA (HR 0.67, 95% CI 0.46–0.99) in Medicare (Table 3). Recognizing that few patients received HZ vaccination, vaccination was not associated with GCA in either MarketScan (HR 0.85, 95% CI 0.65–1.11) or Medicare (HR 1.30, 95% CI 0.93–1.80).
Discussion
In this study, we constructed two retrospective cohorts using the Medicare and MarketScan administrative databases comprising nearly 17 million subjects to investigate an epidemiologic association between HZ and GCA. Even after accounting for known GCA risk factors (age, sex, and race) we observed a strong association between HZ and incident GCA – with complicated HZ increasing GCA risk by 2-fold and uncomplicated HZ increasing GCA risk by nearly 1.5-fold. These results are in accordance with recent findings from a population-based case-control study where a small, but significant increased incidence of GCA was observed after prior HZ events (23). They also build upon conflicting cross-sectional studies of temporal artery specimens from patients with GCA (11–21).
While observational studies including this one do not conclusively demonstrate causality, Hill, in his classic paper, described many facets needed to construct an argument for causation (26). We aimed to address many of these facets in this study. By identifying HZ events and subsequent incident GCA, we have established a temporal relationship between HZ and GCA. We then categorized HZ into complicated and uncomplicated HZ and found crude incidence rates and adjusted HRs demonstrated a “dose response” association between HZ and GCA risk, with complicated HZ (which includes cranial nerve involvement) associated with higher risk. By using two, separate administrative datasets we also demonstrated reproducibility of our findings, with similar estimates for HZ events and GCA risk and nearly identical “dose response” (greater strength of association with complicated HZ) curves in both datasets. Outside the scope of the current study, others have previously addressed the potential biologic plausibility of this relationship. In addition to the aforementioned pathologic findings, VZV elicits robust T cell responses (9), which are known to be critical to the pathogenesis of GCA, and causes a CNS vasculopathy with giant cell formation (10).
Recognizing the association of HZ with GCA observed in this study, it should be noted that only a small minority of those with GCA had a prior HZ event (3.1% in MarketScan and 6.0% in Medicare). Thus, while HZ may play a role in the pathogenesis of GCA, if it does so, it likely does so only for a limited proportion of patients. Moreover, while we demonstrated HZ prior to GCA, the incidence rates for GCA were stable over time after HZ infection. This may suggest that altered immunity, such as immunosenescence (27), may be the critical mechanism underlying GCA pathogenesis and also predispose to HZ infection.
Our findings do not discount the potential for other infectious agents or environmental antigens to trigger GCA – which were observed to increase GCA risk in a recent study (23). We did not study other specific infectious agents previously proposed (e.g. parvovirus, parainfluenza) given the limitations of the available data sources and study design. Identification of parvovirus or parainfluenza with administrative data would be highly prone to misclassification. Our study also does not address whether HZ selectively increases the risk of only GCA. The potential for HZ to trigger other diseases has been previously demonstrated, with HZ events being associated with an increased risk of stroke in the general population (28, 29) and among patients with autoimmune disease (24). While important to understand if PMR risk is also increased following HZ events, we anticipated that administrative datasets would also be prone to misclassification of PMR given one study found a false positive rate of more than 20% for PMR diagnostic codes (30). Furthermore, because PMR and GCA often overlap, we excluded those with a baseline history of PMR from these analyses.
Given the serious complications that can result from GCA and its treatments, mechanisms to prevent GCA would be clearly be preferred and universally welcomed. If indeed HZ is a trigger of GCA, then perhaps antivirals or vaccination could prevent GCA. In our study, no consistent benefit was observed for antivirals in reducing GCA risk, though there was a trend towards a reduced risk of GCA in those receiving antivirals in the Medicare dataset. The assessment of these associations was complicated by no standardization regarding patient selection for antiviral therapy or the timing of antiviral therapy. The lower proportion of complicated HZ receiving antiviral therapy compared to uncomplicated HZ has several potential explanations, including 1) patients with complicated HZ may receive inpatient anti-viral treatment (not captured in these data); 2) patients presenting with late complications of HZ may be deemed ineligible for treatment if they seek care outside the typical three day treatment window; or 3) as a result of reverse causation, such that HZ became complicated because it was not treated with antivirals. We did not find vaccination to be protective of GCA. However, fewer than 10% of people were vaccinated, HZ vaccination is only about 50% effective in preventing shingles (31), and vaccine responses attenuate over time (32). Future study will be needed as vaccination becomes a more common practice and will require careful assessments relevant to the timing of vaccination in relationship to GCA risk.
There are limitations to this study. Most notably with the use of administrative data is the potential for misclassification of the exposures and outcome. As an optimal approach to identifying GCA with administrative data is unknown, we attempted to control for the misclassification of GCA by limiting eligible subjects to those over 50 years of age and requiring at least two outpatient encounters with the diagnostic code or the inclusion on the hospital discharge. Others have used similar GCA definitions (33, 34). In sensitivity analyses, we also made our GCA definition more specific in several ways: by requiring a CPT code for temporal artery biopsy, by requiring at least two steroid prescriptions, or requiring at least one outpatient diagnostic code for GCA to be assigned by a rheumatologist. Across all of these analyses, our results were consistent. Also, our observed incidence rates of GCA within MarketScan are similar to those previously reported in other Caucasian predominant populations (2). We observed a higher GCA incidence rate in the Medicare dataset, likely as a result of older age and its female predominance. Moreover, since the same GCA identification approach was used across HZ exposure groups, we would expect any resulting misclassification to be non-differential and thus bias our results towards the null. Additionally, HZ is frequently identified using administrative databases with reasonable performance observed compared to gold-standard chart review for both uncomplicated and complicated HZ, with the positive predictive values for HZ ranging from 85–100% (35–37).
Given our observational study design, unmeasured confounders may exist. Following recent recommendations, we calculated E-values as sensitivity analyses for unmeasured confounding. For complicated HZ, we found an E value of 3.58, which means that unmeasured confounder(s) would have to be associated at this magnitude or greater with both our exposure (complicated HZ) and outcome (GCA) in order to nullify our findings (25). Last, this is an epidemiologic study that identifies associations, but does not establish causation, nor does it identify the pathophysiologic mechanisms that link HZ and GCA.
Despite these limitations, there are many strengths to our study including our epidemiologic approach that addresses many unanswered questions regarding HZ, antivirals, vaccination and GCA risk. Additionally, we utilized a cohort study design with a median duration of follow-up greater than 1–2 years to determine GCA risk following HZ events. The use of two separate US-wide administrative databases suggests a high degree of generalizability in addition to demonstrating the reproducibility of these findings. Last, several sensitivity analyses were performed and support the main findings.
Conclusions
In conclusion, in two, large, independent US administrative datasets we used a cohort study design to demonstrate an increased risk of GCA following HZ events. However, the low frequency of HZ events in those who developed GCA suggests that HZ events likely contributes meaningfully to GCA risk in only a small portion of patients. Additional studies examining pathophysiological mechanisms linking HZ and GCA as well as the potential benefits of antivirals or vaccination are warranted.
Supplementary Material
Acknowledgments
Funding sources:
BRE and TRM: Nebraska Arthritis Outcomes Research Center.
BRE: UNMC Mentored Scholars Program.
TRM: Receives support from NIH/NIGMS.
JRC: Receives salary support from NIAMS (UM1AR065705) and the Patient Centered Outcomes Research Institute (PCORI).
Dr. Curtis received salary support from NIAMS (UM1AR065705) and PCORI.
Abbreviations
- CCAE
Commercial Claims and Encounters
- CI
Confidence interval
- CPT
Current procedural terminology
- GCA
Giant cell arteritis
- HCPCS
Health Care Common Procedure Coding System
- HR
Hazard ratio
- HZ
Herpes zoster
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IR
Incidence rate
- PMR
Polymyalgia rheumatica
- PY
Person years
Footnotes
The authors report no conflicts of interest.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J, et al. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Care Res. 2009;61(10):1454–61. doi: 10.1002/art.24459. [DOI] [PubMed] [Google Scholar]
- 3.Salvarani C, Pipitone N, Versari A, Hunder GG. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol. 2012;8(9):509–21. doi: 10.1038/nrrheum.2012.97. [DOI] [PubMed] [Google Scholar]
- 4.Tomasson G, Peloquin C, Mohammad A, Love TJ, Zhang Y, Choi HK, et al. Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: A cohort study. Ann Intern Med. 2014;160(2):73–80. doi: 10.7326/M12-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avina-Zubieta JA, Bhole VM, Amiri N, Sayre EC, Choi HK. The risk of deep venous thrombosis and pulmonary embolism in giant cell arteritis: a general population-based study. Ann Rheum Dis. 2016 Jan;75(1):148–54. doi: 10.1136/annrheumdis-2014-205665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proven A, Gabriel SE, Orces C, O'Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003 Oct 15;49(5):703–8. doi: 10.1002/art.11388. [DOI] [PubMed] [Google Scholar]
- 7.Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol. 2013;9(12):731–40. doi: 10.1038/nrrheum.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo MG, Waxman J, Abdoh AA, Serebro LH. Correlation between infection and the onset of the giant cell (temporal) arteritis syndrome. A trigger mechanism? Arthritis Rheum. 1995 Mar;38(3):374–80. doi: 10.1002/art.1780380312. [DOI] [PubMed] [Google Scholar]
- 9.Steain M, Sutherland JP, Rodriguez M, Cunningham AL, Slobedman B, Abendroth A. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. J Virol. 2014 Mar;88(5):2704–16. doi: 10.1128/JVI.03445-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani B, Schiller A, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008 Mar 11;70(11):853–60. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell BM, Font RL. Detection of varicella zoster virus DNA in some patients with giant cell arteritis. Invest Ophthalmol Vis Sci. 2001 Oct;42(11):2572–7. [PubMed] [Google Scholar]
- 12.Nagel MA, White T, Khmeleva N, Rempel A, Boyer PJ, Bennett JL, et al. Analysis of Varicella-Zoster Virus in Temporal Arteries Biopsy Positive and Negative for Giant Cell Arteritis. JAMA Neurol. 2015 Nov;72(11):1281–7. doi: 10.1001/jamaneurol.2015.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilden D, White T, Khmeleva N, Katz BJ, Nagel MA. Blinded search for varicella zoster virus in giant cell arteritis (GCA)-positive and GCA-negative temporal arteries. J Neurol Sci. 2016 May 15;364:141–3. doi: 10.1016/j.jns.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilden D, White T, Khmeleva N, Heintzman A, Choe A, Boyer PJ, et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology. 2015 May 12;84(19):1948–55. doi: 10.1212/WNL.0000000000001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilden D, White T, Boyer PJ, Galetta KM, Hedley-Whyte ET, Frank M, et al. Varicella Zoster Virus Infection in Granulomatous Arteritis of the Aorta. J Infect Dis. 2016 Mar 31; doi: 10.1093/infdis/jiw101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy PG, Grinfeld E, Esiri MM. Absence of detection of varicella-zoster virus DNA in temporal artery biopsies obtained from patients with giant cell arteritis. J Neurol Sci. 2003 Nov 15;215(1–2):27–9. doi: 10.1016/s0022-510x(03)00167-9. [DOI] [PubMed] [Google Scholar]
- 17.Nordborg C, Nordborg E, Petursdottir V, LaGuardia J, Mahalingam R, Wellish M, et al. Search for varicella zoster virus in giant cell arteritis. Ann Neurol. 1998 Sep;44(3):413–4. doi: 10.1002/ana.410440323. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Lafuente R, Fernandez-Gutierrez B, Jover JA, Judez E, Loza E, Clemente D, et al. Human parvovirus B19, varicella zoster virus, and human herpes virus 6 in temporal artery biopsy specimens of patients with giant cell arteritis: analysis with quantitative real time polymerase chain reaction. Ann Rheum Dis. 2005 May;64(5):780–2. doi: 10.1136/ard.2004.025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helweg-Larsen J, Tarp B, Obel N, Baslund B. No evidence of parvovirus B19, Chlamydia pneumoniae or human herpes virus infection in temporal artery biopsies in patients with giant cell arteritis. Rheumatology (Oxford) 2002 Apr;41(4):445–9. doi: 10.1093/rheumatology/41.4.445. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Pla A, Bosch-Gil JA, Echevarria-Mayo JE, Rossello-Urgell J, Solans-Laque R, Huguet-Redecilla P, et al. No detection of parvovirus B19 or herpesvirus DNA in giant cell arteritis. J Clin Virol. 2004 Sep;31(1):11–5. doi: 10.1016/j.jcv.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Pisapia DJ, Lavi E. VZV, temporal arteritis, and clinical practice: False positive immunohistochemical detection due to antibody cross-reactivity. Exp Mol Pathol. 2016 Feb;100(1):114–5. doi: 10.1016/j.yexmp.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Schafer VS, Kermani TA, Crowson CS, Hunder GG, Gabriel SE, Ytterberg SR, et al. Incidence of herpes zoster in patients with giant cell arteritis: a population-based cohort study. Rheumatology (Oxford) 2010 Nov;49(11):2104–8. doi: 10.1093/rheumatology/keq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee RL, Grayson PC, Merkel PA, Tomasson G. Infections and the risk of incident giant cell arteritis: a population-based, case-control study. Ann Rheum Dis. 2016 Nov 28; doi: 10.1136/annrheumdis-2016-210152. [DOI] [PubMed] [Google Scholar]
- 24.Calabrese LH, Xie F, Yun H, Winthrop KL, Baddley JW, Calabrese C, et al. Herpes Zoster and the Risk of Stroke in Patients With Autoimmune Diseases. Arthritis Rheumatol. 2017 Feb;69(2):439–46. doi: 10.1002/art.39855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017 Jul 11; doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 26.HILL AB. The Environment and Disease: Association Or Causation? Proc R Soc Med. 1965 May;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 27.Mohan SV, Liao YJ, Kim JW, Goronzy JJ, Weyand CM. Giant cell arteritis: immune and vascular aging as disease risk factors. Arthritis Res Ther. 2011 Aug 2;13(4):231. doi: 10.1186/ar3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009 Nov;40(11):3443–8. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 29.Breuer J, Pacou M, Gauthier A, Brown MM. Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology. 2014 Jan 21;82(3):206–12. doi: 10.1212/WNL.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernatsky S, Linehan T, Hanly JG. The accuracy of administrative data diagnoses of systemic autoimmune rheumatic diseases. J Rheumatol. 2011 Aug;38(8):1612–6. doi: 10.3899/jrheum.101149. [DOI] [PubMed] [Google Scholar]
- 31.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005 Jun 2;352(22):2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 32.Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney DJ, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015 Mar 15;60(6):900–9. doi: 10.1093/cid/ciu918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broder MS, Sarsour K, Chang E, Collinson N, Tuckwell K, Napalkov P, et al. Corticosteroid-related adverse events in patients with giant cell arteritis: A claims-based analysis. Semin Arthritis Rheum. 2016 Oct;46(2):246–52. doi: 10.1016/j.semarthrit.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Abel AS, Yashkin AP, Sloan FA, Lee MS. Effect of diabetes mellitus on giant cell arteritis. J Neuroophthalmol. 2015 Jun;35(2):134–8. doi: 10.1097/WNO.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yawn BP, Wollan P, St Sauver J. Comparing shingles incidence and complication rates from medical record review and administrative database estimates: how close are they? Am J Epidemiol. 2011 Nov 1;174(9):1054–61. doi: 10.1093/aje/kwr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klompas M, Kulldorff M, Vilk Y, Bialek SR, Harpaz R. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc. 2011 Dec;86(12):1146–53. doi: 10.4065/mcp.2011.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pimentel MA, Browne EN, Janardhana PM, Borkar DS, Tham VM, Uchida A, et al. Assessment of the Accuracy of Using ICD-9 Codes to Identify Uveitis, Herpes Zoster Ophthalmicus, Scleritis, and Episcleritis. JAMA Ophthalmol. 2016 Sep 1;134(9):1001–6. doi: 10.1001/jamaophthalmol.2016.2166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.