Abstract
Background
Cell and molecular mechanisms mediating the cardiovascular effects of alcohol are not fully understood. Our aim was to determine the effect of moderate Ethanol (EtOH) on Sonic Hedgehog (SHh) signaling in regulating possible Sca1+ progenitor stem cell involvement during pathologic arterial remodeling.
Methods and Results
Partial ligation or sham-operation of the left carotid artery was performed in transgenic Sca1-eGFP mice gavaged with or without ‘daily moderate’ EtOH. The EtOH group had reduced adventitial thickening and less neo-intimal formation, compared to ligated controls. There was expansion of eGFP expressing (i.e., Sca1+) cells in remodeled vessels post-ligation (14d), especially in the neo-intima. Ethanol treatment reduced the number of Sca1+ cells in ligated vessel cross-sections concomitant with diminished remodeling, compared to control ligated vessels. Moreover, EtOH attenuated SHh signaling in injured carotids as determined by immunohistochemical analysis of the target genes patched 1 (Ptch1) and Gli2, and RT-PCR of whole vessel Gli2 mRNA levels. Intraperitoneal injection of ligated Sca1 - eGFP mice with the SHh signaling inhibitor cyclopamine diminished hedgehog target gene expression, reduced the number of Sca1+ cells, and ameliorated carotid remodeling. EtOH treatment of purified Sca1+ adventitial progenitor stem cells in vitro inhibited SHh signaling, and their rSHh-induced differentiation to vascular smooth muscle cells.
Conclusions
EtOH reduces SHh - responsive Sca1+ progenitor cell myogenic differentiation/expansion in vitro and during arterial remodeling in response to ligation injury in vivo. Regulation of vascular Sca1+ progenitor cells in this way may be an important novel mechanism contributing to alcohol's cardiovascular protective effects.
Keywords: Alcohol, atherosclerosis, stem cells, progenitor cells, Hedgehog, Sca1
Introduction
Epidemiologic and laboratory investigations support an association between alcohol consumption and cardiovascular disease (CVD) (Klatsky 2010; Mukamal et al. 2010; Morrow et al. 2010) (Liu et al. 2011) that is a leading cause of death worldwide (Lozano et al. 2012). In particular, regular light-moderate consumption of ethanol (EtOH) is recognized as a negative risk factor for CVD but the cells targeted and signaling mechanisms involved are not fully understood.
Most of the problems associated with CVD are due to disease-related changes resulting in vessel remodeling and atherosclerosis that leads to arterial narrowing and blockage, resulting in heart attack or stroke. Vascular smooth muscle cells (vSMC) play a key role in the pathophysiology of arterial disease, yet the origins of vSMC contributing to the typically seen medial thickening and neointimal formation are still under debate (Tang et al. 2013). Evidence put forth suggests that resident stem cells, present initially in low numbers within the vessel wall, become triggered to differentiate to vSMC and are active contributors to the remodeling and repair of the artery wall (Tang et al. 2012; Majesky et al. 2012; Lin and Lue 2013). This information highlights a putative new cell target to investigate for agents known to affect CVD (e.g., alcohol), as well as a potential novel therapeutic target for treatment of vascular disease. One resident stem cell population implicated in vascular disease is stem cell antigen-1 positive (Sca1+) adventitial progenitor cells (APC) (Hu et al. 2004) (Torsney and Xu 2011; Klein et al. 2014). EtOH is known to regulate vSMC growth and migration (Hendrickson et al. 1998; Hendrickson et al. 1999; Sayeed et al. 2002), and we have previously reported that daily moderate alcohol consumption inhibits injury-induced vSMC hyperplasia and carotid remodeling in mice (Morrow et al. 2010) (Liu et al. 2011). However, the effect of EtOH on the functionality and regenerative capacity of resident vascular stem cells has yet to be ascertained.
In addition to its acknowledged involvement during embryological development, a role for sonic hedgehog (SHh) in adult vascular disease has been purported. The best characterized signaling mechanism involves SHh binding to its receptor Patched 1 (Ptch1) resulting in the removal of its inhibitory effect on the smoothened receptor (SMO). SMO subsequently allows Gli family transcription factors to translocate to the nucleus and affect expression of Hh target genes, including Gli2 and Ptch1. This pathway can be blocked using cyclopamine (11-deoxojervine), which binds to SMO preventing it from signaling downstream(J. K. Chen et al. 2002). Increased SHh signaling has been demonstrated in vSMC responsible for restenotic lesions after vein grafting(F. Li et al. 2010). Targeted local inhibition of the Ptch1 receptor in mouse carotid arteries in vivo attenuates vessel remodeling following injury (Redmond et al. 2013), supporting a permissive role for recapitulated SHh signaling in this context. Moreover, EtOH has been reported to have significant modulatory effects on Hh signaling in the liver(Jung et al. 2008; Y.-X. Li et al. 2007; Chan et al. 2014). The role of SHh in regulating vascular stem cell fate in vessel disease is not known. Of interest, Sca1+ adventitial progenitor cells (APC) reportedly co-localize in the healthy arterial vessel wall where SHh and its receptor Patched 1 are primarily expressed embryologically, i.e., at the adventitial medial boundary(Passman et al. 2008). The aim of our study was to determine any effect of EtOH on Sca1+ APC in vitro and during arterial remodeling in vivo, and to determine the role of Hedgehog signaling in this response.
Methods
Mouse carotid artery partial ligation
Carotid ligation was performed on Sca1-eGFP transgenic mice obtained from JAX labs; Stock #012643, strain name B6.Cg.Tg(Ly6a-EGFP)G5Dzk/j. These transgenic mice have an enhanced green fluorescent protein (eGFP) under the control of murine lymphocyte antigen 6 complex, locus A (Ly6a) promoter. Hemizygous Ly6a-GFP mice are viable, fertile, normal in size and do not display any gross physical or behavioral abnormalities (Ma et al. 2002). The eGFP transgene expression pattern corresponds to that of Sca1 (Ma et al. 2002). The carotid artery ligation model of injury and remodeling was performed as described previously (Korshunov and Berk 2003) (Morrow et al. 2010) (Liu et al. 2011). All procedures were approved by the University of Rochester Animal Care Committee and conform to NIH guidelines (Guide for the care and use of laboratory animals). After buprenorphine analgesia (0.05-0.1 mg/kg SQ), and anesthesia using inhalational isoflurane, the mouse was positioned on a clean operating table, with a warming pad to maintain body temperature. The animal was clipped and the surgical site prepped using betadine solution and alcohol. A midline cervical incision was made. With the aid of a dissecting microscope, the left external and internal carotid arterial branches were isolated and ligated with 6-0 silk suture reducing left carotid blood flow to flow via the patent occipital artery. The neck incision (2 layers, muscle and skin) was sutured closed. Partial ligation of the left carotid artery in this manner resulted in a decrease (∼80%) in blood flow, leaving an intact endothelial monolayer. Buprenorphine was administered at least once post-op 6-12 hrs.
EtOH treatment
For 1 week before ligation, mice received the equivalent of 2 drinks daily by oral gavage; i.e., 0.8 g/kg of 200 proof ethanol (ASC/USP grade), giving a peak BAC of 15 mM or 0.08%. For example, a 20 g mouse was gavaged with 18.75 μl EtOH in 200 μl water. This ‘daily moderate’ alcohol feeding regimen was re-continued 1 day post ligation, and continued daily for up to 2 wks when animals were anesthetized and vessels harvested. The control group was gavaged with a calorically matched water-cornstarch mixture (Morrow et al. 2010). There was no significant effect of EtOH consumption on mouse body weight over the experimental time frame, compared to the cornstarch control group (data not shown).
Cyclopamine treatment
Sca1-eGFP mice were treated with the Smoothened inhibitor, cyclopamine, or the vehicle 2-hydropropyl-β-cyclodextrin (HβCD) (Sigma-Aldrich) alone as a control, (essentially as described by van den Brink (van den Brink et al. 2001)). Mice were injected (250 μl max volume) intraperitoneally (IP) 1 day before ligation, then every other day after ligation at a dose of 10 mg/kg Cyclopamine (Sigma-Aldrich) dissolved in a solution of 45% (w/v) HβCD. There was no effect of Cyclopamine treatment on mouse body weight, compared to the HβCD vehicle control group (data not shown).
Quantitative real time RT-PCR
Total RNA (0.5-1 μg) isolated from whole carotids or from purified rat Sca1+ adventitial progenitor cells (see below) using Qiagen RNeasy Micro kit (Valencia, CA) was reverse-transcribed using iScriptTM cDNA Synthesis kit from BIO-RAD (Carlsbad, CA). The gene-specific oligonucleotide sequences were as previously described(Sweeney et al. 2004) (Redmond et al. 2013). GAPDH was used as a housekeeping gene. Real-Time RT-PCR was performed using the Stratagene Mx3005 machine and the SYBER® Green Jumpstart PCR kit (Sigma, St. Louis, MO) as described by the manufacturer.
Histomorphometry
On the indicated days post-ligation, mice were anesthetized (ketamine/xylazine) and perfusion fixed with 4% paraformaldehyde in sodium phosphate buffer (pH 7.0). Fixed carotids were embedded in paraffin for sectioning. Starting at the carotid bifurcation landmark (single lumen) a series of cross-sections (10 × 5 μm) were made, every 200 μm through 2 mm length of carotid artery. Cross-sections were de-paraffinized, rehydrated in graded alcohols and stained with Verhoeff-Van Gieson stain for elastic laminae and imaged using a Nikon TE300 microscope equipped with a Spot RT digital camera (Diagnostic Instruments). Digitized images were analyzed using SPOT 5.2 Advanced imaging software. Assuming a circular structure in vivo, the circumference of the lumen was used to calculate the lumen area, the intimal area was defined by the luminal surface and internal elastic lamina (IEL), the medial area was defined by the IEL and external elastic lamina (EEL) and the adventitial area was the area between the EEL and the outer edge, essentially as described previously(Korshunov and Berk 2003) (Morrow et al. 2010) (Liu et al. 2011).
Sca1+ cells in vivo
Sca1+ cells were identified as green fluorescence protein (eGFP)-expressing cells visualized in de-paraffinized Sca1-eGFP mouse carotid cross sections mounted with Sigma Fluoroshield with DAPI, using an FV1000 Olympus laser scanning confocal microscope. Numbers of eGFP expressing cells in carotid cross section images from different experimental groups were analyzed by Fiji ImageJ software. ‘Analyze particles’ function was used to count total cells (DAPI stained, blue) and eGFP (green) cells per section.
Immunohistochemistry
Carotid cross-sections were stained with rabbit polyclonal to alpha-smooth muscle actin (α-SMA) (Abcam ab5694, 1:200); rabbit polyclonal Gli-2 antibody (Novousbio NBP2-23602SS, 1:50); rabbit polyclonal Ptch1 antibody (Abcam, ab53715, 1:100), followed by a goat-anti rabbit IgG secondary Alexa Fluor 594® conjugate (Invitrogen Cat # A-11037). Isotype control, and secondary antibody only control were performed. For antigen retrieval, slides were brought to a boil in 10 mM sodium citrate (pH 6.0) then maintained at a sub-boiling temperature for 10 minutes. Slides were cooled on the bench-top for 30 minutes then washed in deionized water three times for 5 min each before being washed in PBS for 5 min. The antigen retrieval protocol diminished endogenous eGFP transgene signal. Therefore, sections were co-stained with anti-eGFP antibody (1:1000, Thermo Fisher) and donkey anti-mouse secondary alexa fluor 488 (1:2000, Invitrogen). Numbers of Ptch1 or Gli2 expressing cells in whole carotid cross sections from different experimental groups were analyzed by Fiji ImageJ software. ‘Analyze particles’ function was used to count total cells (DAPI stained, blue) and the number of ‘red’ cells per section.
Adventitial Sca1+ cell isolation and purification
Rat thoracic aorta was harvested and placed in Hank's Balanced Salt solution (HBSS) with 1% fetal bovine serum (FBS). The endothelium was removed by scraping with a sterile scalpel blade before the adventitia was detached from the media following brief enzymatic digestion with 2.5 mg/mL collagenase (15 min at 37°C), using forceps under a dissection microscope, as described previously(Cappadona et al. 1999). The adventitia was cut into 1 mm sections, placed in wells of 6 well plates and left to dry for 5 min. EMEM supplemented with 2 mM L-glutamine and 10 % ATCC ESC qualified FBS was added to each well and then plates left undisturbed for a minimum of 2 d in a cell culture incubator (37°C, 5% CO2). Cells that migrated from the explanted tissue were dissociated and placed in EMEM media supplemented with 10 % ATCC ESC qualified FBS and 2 mM L-glutamine. Dissociated cells were pelleted and treated in accordance with the EasySep® Sca1 Positive Selection Kit protocol from STEMCELL Technologies (STEMCELL Technologies, Cambridge, UK). The separated cells were analyzed for Sca1 purity by flow cytometry with IgG-PE cells used as control. Post-purification, Sca1+ APC were expanded and routinely re-purified and eventually cloned using ClonaCell™ FLEX 03818 (STEMCELL Technologies, Cambridge, UK) according to the manufacturers recommendations in order to maintain a pure Sca1+ population. Cell were routinely grown and expanded in maintenance medium III.
Sca1+ adventitial progenitor cell immunohistochemistry
Cells were seeded onto UV sterilized non-coated glass cover slips (20 mm) and grown for 24 hr, then fixed with 3.7% formaldehyde. Samples were permeabilized in 0.025 % Triton X-100 PBS (15 min, RT), blocked using a 5 % BSA, 0.3 M Glycine, 1 % Tween PBS blocking solution (1 hr at room temperature), then incubated with anti-calponin antibody (Cnn1, Sigma Cat No: C2687), anti-myosin heavy chain antibody, (Myh11, Abcam Cat No: ab683), or anti-Gli2 (Gli2, Abcam Cat No: ab167389) primary antibodies at the recommended dilutions at room temperature for 1 hr, or 4 °C overnight. Samples were washed twice in PBS and incubated with the recommended concentration of appropriate secondary antibody in blocking buffer for 1 hr or 4 °C overnight. Cell nuclei were stained using DAPI: PBS (dilution 1:1000) at room temperature for 15 min. An Olympus CK30 microscope and FCell software was used to capture images. A threshold of background staining was defined using the secondary antibody control and exposure rates were limited in order to rule out false positives. At least five images from the Olympus CK30 microscopy per experimental group (minimum n=4) were analyzed using ImageJ software and confocal images were analyzed using Zen 2008 software.
Data Analysis
4-6 animals were used per experimental group. An ANOVA test was performed on cell count data and a Wilcoxon Signed rank test was used for comparison of two groups when compared to normalized control. Results are expressed as mean ± SEM. A value of p≤0.05 was considered significant.
Results
Ethanol (EtOH) attenuates ligation injury-induced carotid remodeling in Sca1-eGFP transgenic mice
Partial ligation or sham-operation was performed on the left carotid of Sca1- eGFP transgenic mice, treated with or without ‘daily moderate’ EtOH by gavage. Carotids were harvested on day 14 post-ligation and morphologic analysis performed. Ligation injury resulted in increased adventitial (Adv) and medial (Med) compartments and neo-intimal formation, resulting in a decreased lumen, when compared to the sham-operated group (Fig 1). Media and neo-intima of the remodeled vessels were composed of alpha-actin positive cells and Sca1+ cells, which did not appear to co-localize (Fig 2). EtOH treatment resulted in significantly reduced adventitia and neo-intima, when compared to calorically-matched ligated controls (Fig 1). Intimal medial thickening (IMT) was significantly reduced in the EtOH group, compared to ligated controls (Fig 1).
Figure 1. Daily moderate EtOH inhibits ligation-induced arterial remodeling.
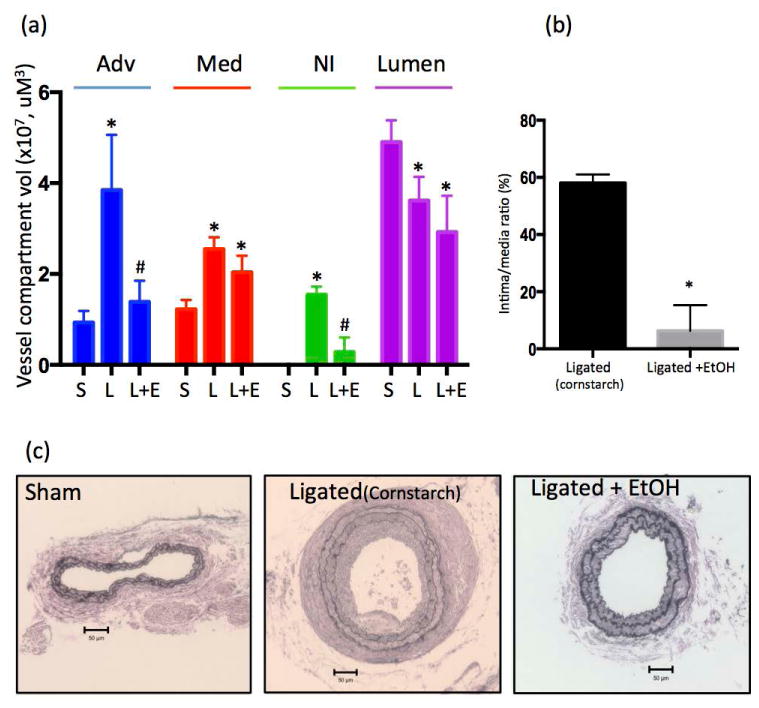
Partial carotid ligation was performed in Sca1-eGFP transgenic mice treated with or without ‘moderate’ amounts of EtOH (0.8 g/kg in 200 μL volume by oral gavage, once daily 1 wk prior and each day after ligation, resulting in a peak BAC of 15 mmol/L). Control animals received an isocaloric cornstarch solution. Vessels harvested 14 days post ligation were used for morphological analysis. (a) Ligation injury increased adventitial (Adv) and medial (Med) compartments, and stimulated neo-intimal (NI) hyperplasia, with a decrease in lumen. Daily moderate EtOH inhibited adventitial and neo-intimal formation, with no significant effect on the media or lumen, compared to ligated controls. Data are given as Mean ± SEM, n=5 animals, (5 section analyzed per animal) S= Sham-operated, L= Ligated, L+E= Ligated + EtOH. *P<0.05 vs Sham; #P<0.05 vs Ligated control. (b) Intima/media ratio was calculated for vessels from ligated mice +/- EtOH. N=5, * P< 0.05 vs control. (c) Representative Van Gieson stained carotid cross sections also shown. Scale bars 50 μM.
Figure 2.
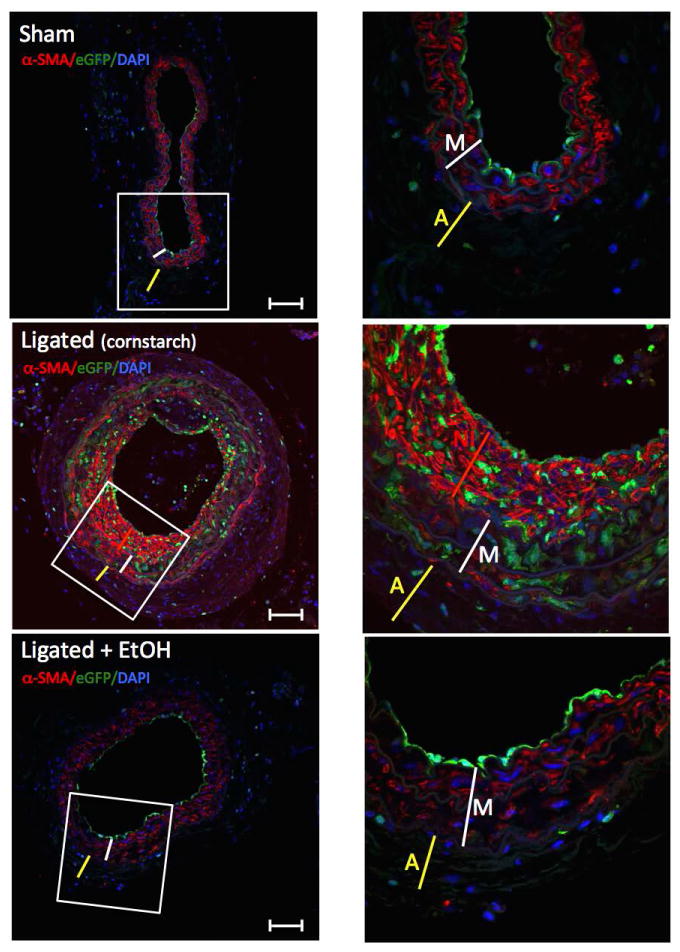
SMC α-actin expression in Sca1-eGFP transgenic mice carotid cross sections from sham-operated (Sham), ligated (cornstarch), and ligated treated with Ethanol (Ligated + EtOH) groups. Immunohistochemistry for smooth muscle specific α-actin was performed on sections from vessels harvested 14 days post ligation or sham-operation, using an anti alpha-smooth muscle actin (α-SMA) antibody (Abcam ab5694). Representative images shown; blue = Dapi nuclear stain, Green = eGFP (i.e., Sca1+), red = α-actin. ×20 magnification on left (scale bars 50 μM); ×60, of boxed portion, shown on right. Width of Adventitia ‘A’ denoted by yellow line, Media: ‘M’ white line, Neo-intima: ‘NI’ red line.
Daily moderate EtOH feeding reduces Sca1+ cell expansion following ligation injury
Cross-sections from paraformaldehyde fixed and paraffin-embedded carotids from sham-operated and ligated mice, treated with or without EtOH, were evaluated by confocal microscopy for cells with eGFP fluorescence, indicative of Sca1 expression. In sham-operated carotids there was a small number of eGFP-expressing cells present in the adventitia and at the lumen lining of the vessel (Fig 3). There were markedly increased numbers of eGFP-expressing cells (i.e., Sca1+) in the vessel wall post-ligation (day 14), particularly in the media and neo-intima (Fig 3). Daily EtOH treatment attenuated vessel remodeling (Fig 3a) concomitant with a reduction in Sca1+ cells (assessed as a percentage of total cells) (Fig 3a and c).
Figure 3. Daily moderate EtOH consumption reduces the number of Sca1+ cells in ligated carotids.
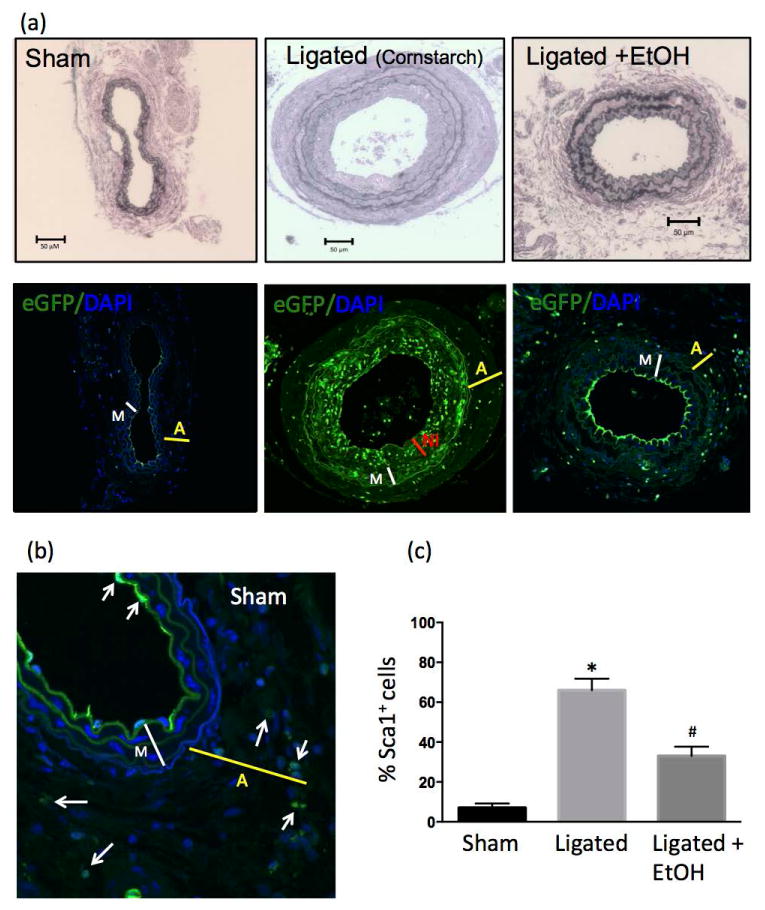
Partial carotid ligation or sham-operation was performed in Sca1-eGFP transgenic mice treated daily with or without ‘moderate’ amounts of EtOH (0.8 g/kg, oral gavage). Control ligated animals received an isocaloric cornstarch solution. Vessels harvested on day 14 were fixed, sectioned and imaged for eGFP, indicative of Sca1 expression. Daily moderate EtOH inhibited adventitial and intimal formation concomitant with a reduction in the number of Sca1+ cells per section as analyzed by Fiji ImageJ software. (a) Representative images (×20 magnification, scale bar 50 μM.); Verhoeff-Van Gieson stained sections (top) and corresponding confocal immunofluorescence pics (bottom) for sham, ligated, and ligated + EtOH groups. Thickness of Adventitia: ‘A’ yellow line, Media: ‘M’ white line, Neo-intima: ‘NI’ red line. (b) Higher magnification view (×60) of sham-operated carotid with a Sca1+ cells present in adventitia and intimal layer indicated by arrows. (c) Bar graph shows cumulative data for Sca1+ cells as a percentage of total cells (determined by DAPI); Sham n=5, Ligated n=6, Ligated + EtOH, n=6, *p<0.05 vs sham, #p<0.05 vs ligated).
EtOH reduces sonic hedgehog (SHh) signaling in ligation-injured mouse carotids
To determine the effect of daily moderate EtOH treatment on SHh signaling in mouse carotids immunohistochemistry (IHC) for SHh target genes Patched1 (Ptch1) and Gli2 was performed in cross sections from sham-operated, ligated, and ligated + EtOH experimental groups. IHC data show that both Ptch1 and Gli2 expression were markedly increased in ligated vessels compared to sham-operated vessels, especially in the neo-intima and media, and that some cells expressing these target genes co-localized with Sca1(eGFP)-expressing cells (Fig 4 and 5a). EtOH treatment attenuated both Ptch1 and Gli2 expression in ligated vessels (Fig 4 and 5a). Moreover, whole vessel Gli2 mRNA levels were significantly reduced in ligated vessels from wildtype C57Bl/6 mice treated with EtOH, compared to no alcohol controls (Fig 5b). Together, these data indicate that EtOH inhibits ligation-injury induced SHh signaling in mouse carotids.
Figure 4. EtOH inhibits Patched1 expression in ligated carotids.
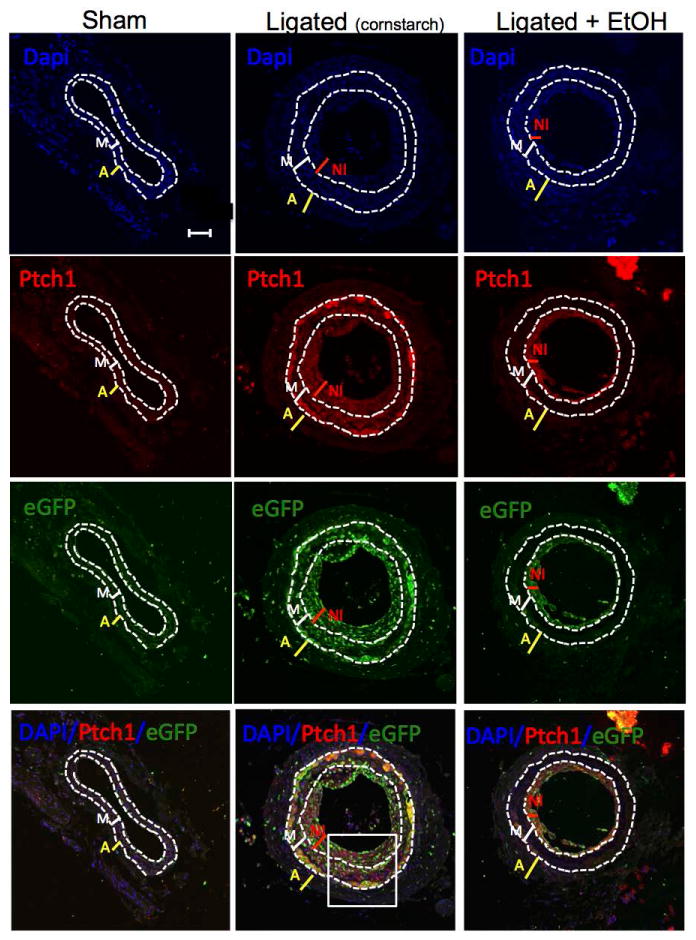
The protein expression of Sonic hedgehog target gene Patched 1 (Ptch1) in carotid cross sections from the different experimental groups [sham-operated, ligated (cornstarch control), ligated + EtOH] was assessed by immunohistochemistry using a rabbit polyclonal anti-Ptch1 primary antibody and an Alexa fluor 594-conjugated secondary antibody. Representative images (×20 magnification, scale bar 50 μM) shown. DAPI (blue); Ptch1 (red), eGFP (green, i.e., Sca1+), merged image (bottom). Dotted lines trace internal and external elastic laminae.
Figure 5. EtOH inhibits Gli2 expression in ligated carotids.
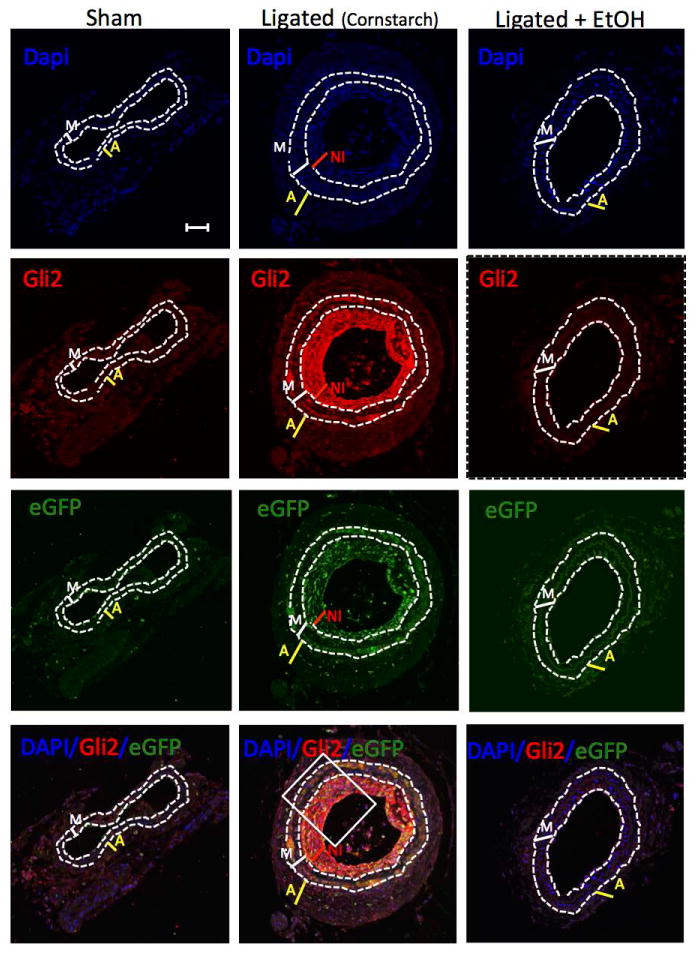
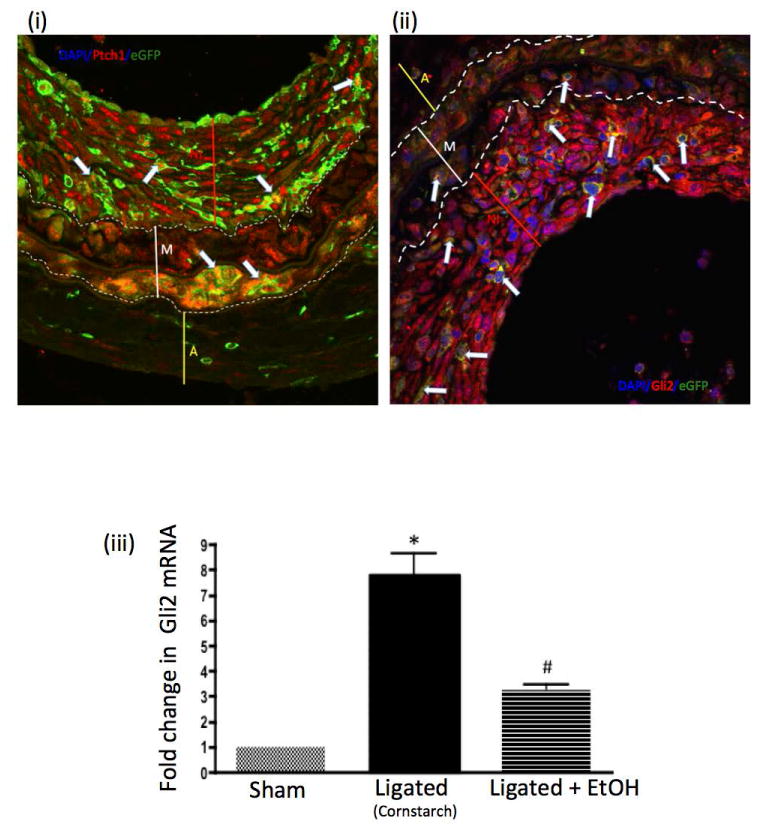
(a) The protein expression of Sonic hedgehog target gene Gli2 in carotid cross sections from the different experimental groups [sham-operated, ligated (cornstarch control), ligated + EtOH] was assessed by immunohistochemistry using a rabbit polyclonal anti-Gli2 primary antibody and an Alexa fluor 594-conjugated secondary antibody. Representative images (×20 magnification, scale bar 50 μM) shown. DAPI (blue); Gli2 (red), eGFP (green, i.e., Sca1+), merged image (bottom). Dotted lines trace internal and external elastic laminae. (b) Higher magnification view (×60) of boxed portions from Figure 4 and 5. Arrows indicate sample cells co-expressing (i) Ptch1 and Sca1 or (ii) Gli2 and Sca1. (iii) Quantitative RT-PCR analysis of Gli2 mRNA isolated 2 wks post-ligation or sham operation from whole carotid arteries of control (cornstarch) or EtOH-gavaged C57Bl/6 mice. N=5, p<0.05 vs sham, #P<0.05 vs ligated.
Cyclopamine reduces Ptch1 expression, inhibits Sca1+ cell expansion and attenuates injury-induced vessel remodeling
Carotid ligation was performed in Sca1 - eGFP mice treated with the SHh signaling inhibitor cyclopamine (10 mg/kg, IP, every other day) or the vehicle control 2-hydroxypropyl-β-cyclodextrin (HβCD). Expression of the SHh target gene Ptch1, determined by immunohistochemistry, in ligated carotid cross sections was decreased by cyclopamine treatment (Fig 6). Similar to EtOH, cyclopamine treatment significantly reduced the amount of Sca1+ cells found in vessels post ligation, compared to control ligated vessels; 42±3% vs 65±5% (percent of total number of cells) n=5. Moreover, vessel remodeling was attenuated in the cyclopamine treated group; carotid intimal-media ratio was significantly less in cyclopamine treated mice compared to ligated controls; 11±3.5% vs 58±1.73% (intima/media ratio) (Fig 7).
Figure 6. The Hedgehog inhibitor Cyclopamine inhibits Patched 1 (Ptch1) expression in ligated carotids.
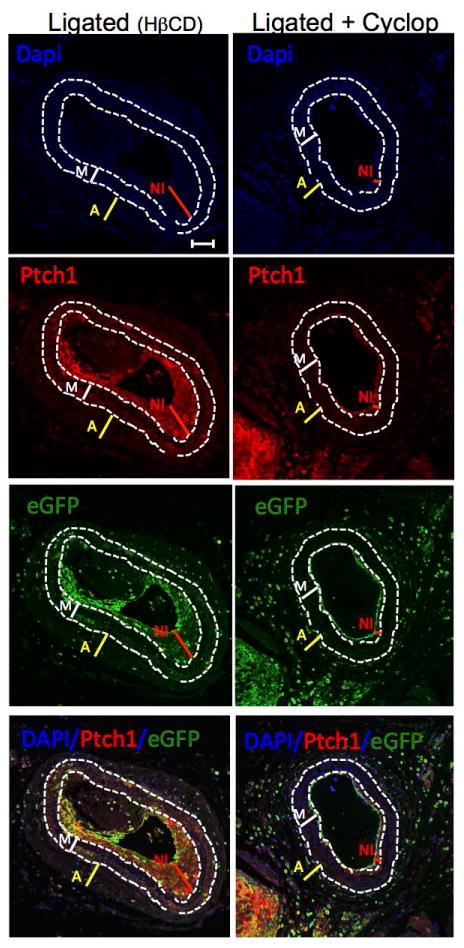
The protein expression of Sonic hedgehog target gene Ptch1 in carotid cross sections from Ligated (HβCD vehicle control) and Ligated + Cyclopamine (10 mg/kg, IP, every other day) was assessed using a rabbit polyclonal anti-Ptch1 primary antibody and an Alexa fluor 594-conjugated secondary antibody. Representative images (×20 magnification, scale bar 50 μM) shown. DAPI (blue); Ptch1 (red), eGFP (green, i.e., Sca1+), merged image (bottom). Dotted lines trace internal and external elastic laminae.
Figure 7. Cyclopamine attenuates ligation-induced carotid remodeling concomitant with inhibition of Sca1+ cell expansion.
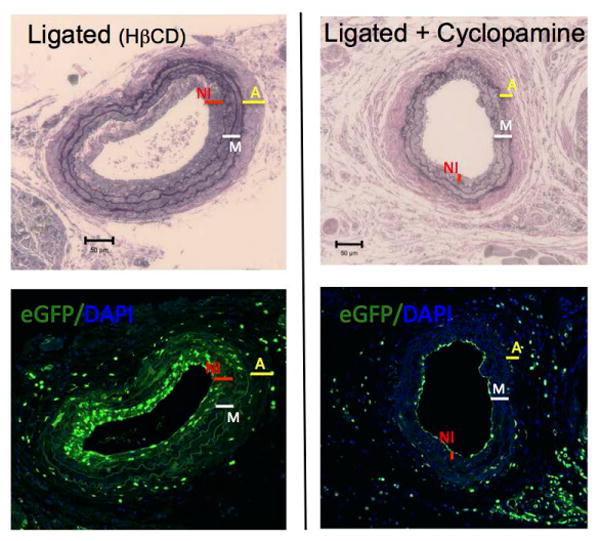
Ligation was performed in Sca1-eGFP transgenic mice treated with or without the SHh signaling inhibitor Cyclopamine (10 mg/kg) or the vehicle control 2-hydropropyl-β-cyclodextrin (HβCD). Carotids harvested 14 d post ligation were assessed for morphology and imaged for enhanced green fluorescent protein (eGFP), indicative of Sca1 expression. Thickness of Adventitia: ‘A’ yellow line, Media: ‘M’ white line, Neo-intima: ‘NI’ red line. Cyclopamine reduced the number of eGFP expressing (Sca1+) cells per cross-section, compared to controls and inhibited neo-intima formation. Representative images (×20, scale bars 50 μM) (Verhoeff-Van Gieson stained sections (top) and corresponding confocal immunofluorescence pics showing eGFP (bottom).
EtOH inhibits SHh-induced myogenic differentiation of Sca1+ adventitial progenitor cells (APC) in vitro
Treatment of purified and cloned rat Sca1+ adventitial progenitor cells in vitro with rShH (0.5 μg/ml) stimulated Gli2 target gene mRNA levels and promoted myogenic differentiation to smooth muscle cells as mRNA for the smooth muscle-specific markers Myosin heavy chain 11 (Myh11) and Calponin (Cnn1) were both significantly increased (Figure 8a). Treatment of cells with rSHh also increased the fraction expressing Cnn1 as determined by immunocytochemistry (Figure 8b). These effects were markedly attenuated by EtOH treatment (25 mM), similar to the effect of the SHh inhibitor cyclopamine (15 μM) (Figure 8). These experiments were repeated in murine Sca1+ C3H10T1/2 stem cells, with quantitatively similar results (data not shown). These data indicate that Sca1+ progenitor stem cells are SHh responsive, and that EtOH inhibits SHh-induced myogenic differentiation of Sca1+ progenitor cells.
Figure 8. EtOH inhibits SHh-induced myogenic differentiation of Sca1+ adventitial progenitor cells in vitro.
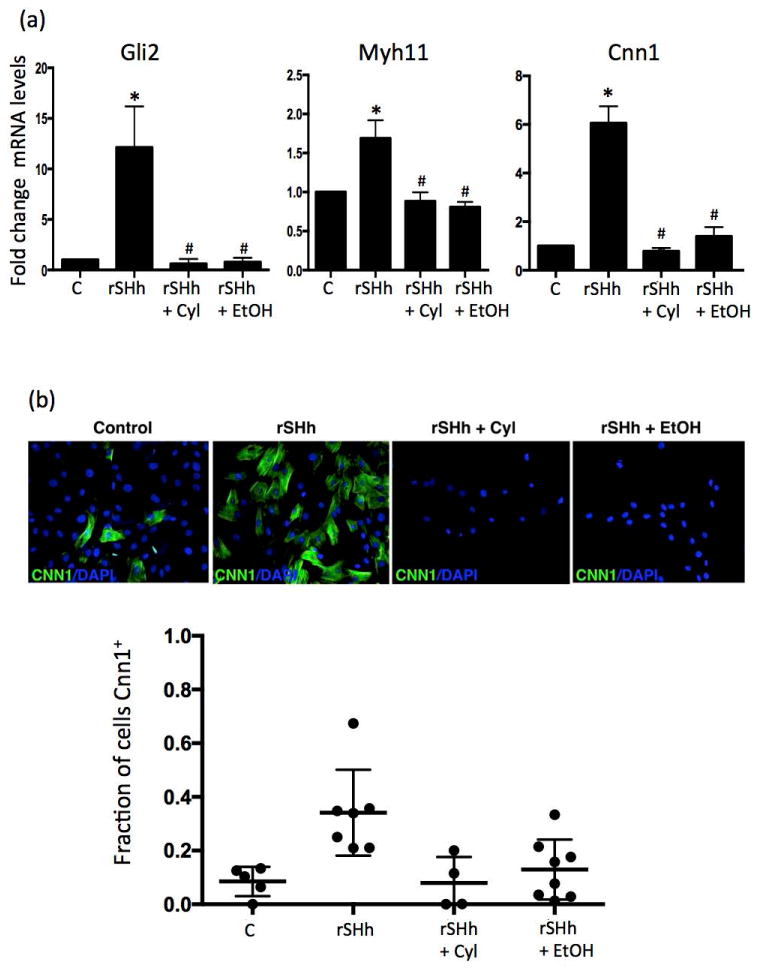
(a) Rat Sca1+ adventitial progenitor cells in maintenance media were treated in vitro with or without rShH (0.5 μg/ml), in the absence or presence of EtOH (25 mM), or cyclopamine (15 μM), for 7 days before mRNA levels of the SHh target gene Gli2, and smooth muscle specific markers Myosin heavy chain 11 (Myh11) and Calponin (Cnn1) were assessed by RT-PCR. Data are from a representative experiment of 3 with similar results. (b) rSHh treatment of rat Sca1+ APC cells increased the fraction expressing Cnn1 as determined by immunocytochemistry, an effect markedly attenuated by either EtOH (25 mM), or cyclopamine (15 μM) (Figure 8). Representative images shown, together with cumulative data showing fraction of Cnn1+ cells in each experimental group. Mean ± SEM, n= 3.
Discussion
We report that ethanol (EtOH) feeding inhibits Sonic hedgehog-stimulated Sca1+ progenitor cell expansion and attenuates carotid remodeling in ligation-injured Sca1 - eGFP transgenic mice. This novel effect of EtOH on vascular stem cells may contribute, in part, to the cardiovascular protective effects of moderate alcohol consumption.
Numerous epidemiological studies have investigated the relationship between imbibing alcohol and general overall health. With respect to cardiovascular disease (CVD) specifically, meta analysis reveals that compared with abstinence, regular light to moderate consumption of alcohol is associated with the lowest risk for CVD incidence and mortality (Corrao et al. 2000; Bagnardi et al. 2008; Ronksley et al. 2011; Roerecke and Rehm 2014). ‘Light to moderate’ alcohol consumption is generally considered to be in the range of 1-3 drinks/day, giving rise to blood alcohol levels (BAC) of approximately 5-25 mM. Conversely, episodic binge drinking (i.e., 5 or more drinks in less than 2 h) or chronic excessive alcohol use resulting in BAC up to 50 mM are associated with a higher incidence of cardiovascular disorders and increased mortality(Thun et al. 1997; Ruidavets et al. 2010). In apparent agreement with such epidemiologic findings, we have previously reported differential effects, beneficial and deleterious, respectively, of ‘daily moderate’ vs ‘2-day binge’ alcohol feeding on arteriosclerosis in a mouse model (Liu et al. 2011).
Under certain conditions atherosclerotic plaques develop following interactions between circulating inflammatory cells, lipids, and the cells (i.e., endothelial cells, smooth muscle cells, adventitial fibroblasts) comprising the artery wall. Ultimately, hyperplasia of vascular smooth muscle cells (vSMC) in the media and their accumulation in the tunica intima plays a key role in wall thickening seen in atherosclerosis and in restenosis following stenting(Doran, Meller, and McNamara 2008). The classic hypothesis was that quiescent contractile medial vSMC switch to a synthetic phenotype capable of proliferation and translocation. Moreover, there is often significant adventitial remodeling in response to vessel injury and adventitial myofibroblasts are believed capable of translocating to the neo-intima and differentiating to vSMC and in this way also contribute to vascular lesion formation (Scott et al. 1996; Shi et al. 1996). In addition to alcohol effects on lipoproteins(Wakabayashi 2015; Hao et al. 2015), we and others have described potentially important effects of alcohol on vSMC growth and migration(Sayeed et al. 2002) (Cullen et al. 2005; Cahill and Redmond 2012; Shirpoor et al. 2013), as well as on vascular endothelial cells(Morrow et al. 2008) and monocytes (Cullen et al. 2005; Muralidharan et al. 2014). The emergence of the concept that resident vascular stem cells, in addition to de-differentiation of vSMC and myofibroblasts as mentioned above, may become activated and contribute to arterial pathology(Orlandi 2015), together with the lack of information as to whether alcohol can regulate stem cells in the adult vessel, provoked our present study.
Stem cell antigen-1 (Sca1) is a candidate marker used in the search for tissue resident stem cells (Holmes and Stanford 2007) and it is recognized as an antigen of adult vascular wall-resident stem cells (Hu et al. 2004) (Torsney and Xu 2011) (Orlandi 2015). Our data show Sca1+ cells are present, albeit sparsely, in healthy mouse carotids; in the adventitia and lining the lumen. The Sca1+ cells lining the lumen stained positively for endothelial nitric oxide synthase (eNOS) (data not shown) and were negative for α-SMA. Of note, vascular endothelial cells reportedly express Sca1 (Holmes and Stanford 2007) (Kotton et al. 2003), and they can display plasticity in disease. Recently, endothelial cells were shown to undergo transformation (i.e., endothelial-mesenchymal transition) to smooth muscle-like cells that contribute to neointimal formation (P.-Y. Chen et al. 2012)(reviewed in (Lao, Zeng, and Xu 2015)). This may also be the case in our study but it will require further investigation for a definitive answer.
After ligation injury, there was a marked expansion of the Sca1+ cell population in remodeled vessels, especially in the neo-intimal compartment, but also in the media and adventitia. In contrast, in carotids of mice that were ligated but received alcohol, there were significantly less Sca1+ cells in vessel cross-sections, concomitant with attenuated vessel remodeling (i.e., reduced adventitial enlargement, and less neo-intima formation). These data suggest that alcohol feeding attenuates neo-intima thickening by inhibiting resident Sca1+ progenitor cell expansion. Of note, reduced neo-intimal thickening in moderate EtOH consumers would be expected to result in less atherosclerotic plaque and, thus, reduced vascular occlusive events leading to fewer heart attacks and strokes.
The precise source of the Sca1+ cell population that accumulates within the medial and intimal layers following injury is unknown. Sca-1 expression has been identified on putative stem/progenitor cell populations within numerous tissues (Holmes and Stanford 2007). Whether these Sca-1 positive cell populations are truly tissue-specific precursor/stem cells or represent hematopoietic, mesenchymal, or endothelial precursor/stem cells associated with these tissues is not known in all cases. Sca1+ progenitor cells could potentially arise locally or be recruited from the circulation in response to injury. However, a recent study using wildtype and c-mybh/h mice lethally irradiated and reconstituted with eGFP+ bone marrow indicated that >99% of all carotid adventitial CD45−Lin−Sca1+ cells were host vessel-derived (Shikatani et al. 2016). Several potential stem/progenitor cell populations have been identified as residing in the intima, media and adventitia including stem cell antigen-1+ (Sca1+) CD45+ adventitial macrophage progenitor cells (Psaltis et al. 2014) and Sca1+CD45− progenitor cells that give rise to endothelial and mesenchymal cell lineages (Tigges, Komatsu, and Stallcup 2013; Naito et al. 2012). Although our study does not fully clarify the relative contribution of adventitial progenitor cells to vessel remodeling, it clearly identifies the expansion of a Sca1+ / α-SMA negative progenitor cell population within the media and intima in response to vessel injury. Moreover, the accumulation of this population, irrespective of its source, is subject to significant inhibition following treatment with EtOH. Additional lineage tracing experiments of Sca1+ cells using transgenic mice will help definitively identify Sca1+ progeny following ligation injury ± EtOH; this strategy has been successful in distinguishing a contribution of Sca1-derived cells to cardiomyocytes during normal aging and following ischemic damage and pressure overload (Uchida et al. 2013).
We, and others, have implicated the sonic hedgehog pathway in vessel disease pathogenesis. SHh signaling regulates vSMC growth in vitro (Morrow et al. 2007; Morrow et al. 2009; Walshe et al. 2011; H. Li et al. 2012) and in vivo (F. Li et al. 2010; Walshe et al. 2011; Passman et al. 2008; Morrow et al. 2007; Morrow et al. 2009). Components of the SHh pathway are induced after vascular injury (Morrow et al. 2009) and during vein graft intimal hyperplasia (F. Li et al. 2010), and inhibition of SHh signaling using Ptch1 siRNA applied locally at the site of ligation injury attenuates intimal-medial thickening (Redmond et al. 2013). Moreover, a role for SHh in stem cell regulation and maintenance in other contexts has been described (Siggins et al. 2009) (Huang and Kalderon 2014) (Mooney et al. 2015). In the present study EtOH inhibited SHh-induced signaling and myogenic differentiation of Sca1+ adventitial progenitor stem cells in vitro. EtOH treatment in vivo, that reduced injury-induced Sca1+ cell expansion and vessel remodeling, inhibited SHh target gene (Ptch1, Gli2) expression that co-localized with Sca1+ expressing cells in ligated carotids. Moreover, in separate experimental groups, pharmacological inhibition of SHh signaling by cyclopamine deterred Sca1+ cell expansion and remodeling of injured mouse carotids, to an extent similar to the EtOH treatment groups.
In conclusion, these data indicate that daily moderate alcohol consumption attenuates pathologic arterial remodeling by inhibiting SHh-dependent expansion of Sca1+ vascular stem cells. Further, these data highlight resident progenitor cells as additional vascular ‘targets’ for alcohol. Targeting of these progenitor stem cells represents a novel mechanism whereby beneficial alcohol consumption patterns may lessen vessel neointimal lesions and, thus, cardiovascular disease.
Acknowledgments
We thank Diana Scott for histological tissue processing, and Drs. Linda Callahan and Paivi Jordan for confocal microscope expertise. We also thank Dr Heli Hamalainen-Laanaya for critical review of the manuscript.
Funding: This work was supported by grants from the National Institutes of Health [R21AA020365 and R21AA023213 to EMR] and the Science Foundation Ireland (SFI) [SFI 11/PI/1128 to PAC].
Abbreviations
- EtOH
Ethanol
- SHh
sonic hedgehog
- Sca1
stem cell antigen 1
- Ptch1
patched 1
- APC
adventitial progenitor cells
- eGFP
enhanced green fluorescent protein
- vSMC
vascular smooth muscle cells
Footnotes
Conflict of Interest: none declared
References
- Bagnardi V, Zatonski W, Scotti L, La Vecchia C, Corrao G. Does Drinking Pattern Modify the Effect of Alcohol on the Risk of Coronary Heart Disease? Evidence From a Meta-Analysis. Journal of Epidemiology and Community Health. 2008;62(7):615–619. doi: 10.1136/jech.2007.065607. [DOI] [PubMed] [Google Scholar]
- Cahill Paul A, Redmond Eileen M. Alcohol and Cardiovascular Disease-Modulation of Vascular Cell Function. Nutrients. 2012;4(4):297–318. doi: 10.3390/nu4040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappadona C, Redmond EM, Theodorakis NG, McKillop IH, Hendrickson R, Chhabra A, Sitzmann JV, Cahill PA. Phenotype Dictates the Growth Response of Vascular Smooth Muscle Cells to Pulse Pressure in Vitro. Experimental Cell Research. 1999;250(1):174–186. doi: 10.1006/excr.1999.4502. [DOI] [PubMed] [Google Scholar]
- Chan Isaac S, Guy Cynthia D, Machado Mariana V, Wank Abigail, Kadiyala Vishnu, Michelotti Gregory, Choi Steve, Swiderska-Syn M, Karaca G, Pereira TA, Yip-Schneider MT, Max Schmidt C, Diehl AM. Alcohol Activates the Hedgehog Pathway and Induces Related Procarcinogenic Processes in the Alcohol-Preferring Rat Model of Hepatocarcinogenesis. Alcoholism, Clinical and Experimental Research. 2014;38(3):787–800. doi: 10.1111/acer.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen James K, Taipale Jussi, Cooper Michael K, Beachy Philip A. Inhibition of Hedgehog Signaling by Direct Binding of Cyclopamine to Smoothened. Genes & Development. 2002;16(21):2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Pei-Yu, Qin Lingfeng, Barnes Carmen, Charisse Klaus, Yi Tai, Zhang Xinbo, Ali Rahmat, et al. FGF Regulates TGF-B Signaling and Endothelial-to-Mesenchymal Transition via Control of Let-7 miRNA Expression. Cell Reports. 2012;2(6):1684–1696. doi: 10.1016/j.celrep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and Coronary Heart Disease: a Meta-Analysis. Addiction (Abingdon, England) 2000;95(10):1505–1523. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- Cullen John P, Sayeed Shariq, Kim Youngrin, Theodorakis Nicholas G, Sitzmann James V, Cahill Paul A, Redmond Eileen M. Ethanol Inhibits Pulse Pressure-Induced Vascular Smooth Muscle Cell Migration by Differentially Modulating Plasminogen Activator Inhibitor Type 1, Matrix Metalloproteinase-2 and -9. Thrombosis and Haemostasis. 2005;94(3):639–645. doi: 10.1160/TH05-03-0174. [DOI] [PubMed] [Google Scholar]
- Doran Amanda C, Nahum Meller, McNamara Coleen A. Role of Smooth Muscle Cells in the Initiation and Early Progression of Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(5):812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Guang, Wang Zengwu, Zhang Linfeng, Chen Zuo, Wang Xin, Guo Min, Tian Ye, Shao Lan, Zhu Manlu. Relationship Between Alcohol Consumption and Serum Lipid Profiles Among Middle-Aged Population in China: a Multiple-Center Cardiovascular Epidemiological Study. Angiology. 2015;66(8):753–758. doi: 10.1177/0003319714549557. [DOI] [PubMed] [Google Scholar]
- Hendrickson RJ, Cahill PA, McKillop IH, Sitzmann JV, Redmond EM. Ethanol Inhibits Mitogen Activated Protein Kinase Activity and Growth of Vascular Smooth Muscle Cells in Vitro. European Journal of Pharmacology. 1998;362(2-3):251–259. doi: 10.1016/s0014-2999(98)00771-7. [DOI] [PubMed] [Google Scholar]
- Hendrickson RJ, Okada SS, Cahill PA, Yankah E, Sitzmann JV, Redmond EM. Ethanol Inhibits Basal and Flow-Induced Vascular Smooth Muscle Cell Migration in Vitro. The Journal of Surgical Research. 1999;84(1):64–70. doi: 10.1006/jsre.1999.5605. [DOI] [PubMed] [Google Scholar]
- Holmes Christina, Stanford William L. Concise Review: Stem Cell Antigen-1: Expression, Function, and Enigma. Stem Cells (Dayton, Ohio) 2007;25(6):1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- Hu Yanhua, Zhang Zhongyi, Torsney Evelyn, Afzal Ali R, Davison Fergus, Metzler Bernhard, Xu Qingbo. Abundant Progenitor Cells in the Adventitia Contribute to Atherosclerosis of Vein Grafts in ApoE-Deficient Mice. The Journal of Clinical Investigation. 2004;113(9):1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Jianhua, Kalderon Daniel. Coupling of Hedgehog and Hippo Pathways Promotes Stem Cell Maintenance by Stimulating Proliferation. The Journal of Cell Biology. 2014;205(3):325–338. doi: 10.1083/jcb.201309141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Youngmi, Brown Kevin D, Witek Rafal P, Omenetti Alessia, Yang Liu, Vandongen Margon, Milton Richard J, et al. Accumulation of Hedgehog-Responsive Progenitors Parallels Alcoholic Liver Disease Severity in Mice and Humans. Gastroenterology. 2008;134(5):1532–1543. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatsky Arthur L. Alcohol and Cardiovascular Health. Physiology & Behavior. 2010;100(1):76–81. doi: 10.1016/j.physbeh.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Klein Diana, Meissner Nicole, Kleff Veronika, Jastrow Holger, Yamaguchi Masahiro, Ergün Süleyman, Jendrossek Verena. Nestin(+) Tissue-Resident Multipotent Stem Cells Contribute to Tumor Progression by Differentiating Into Pericytes and Smooth Muscle Cells Resulting in Blood Vessel Remodeling. 2014;4:169. doi: 10.3389/fonc.2014.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov Vyacheslav A, Berk Bradford C. Flow-Induced Vascular Remodeling in the Mouse: a Model for Carotid Intima-Media Thickening. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(12):2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- Kotton Darrell N, Summer Ross S, Sun Xi, Ma Bei Yang, Fine Alan. Stem Cell Antigen-1 Expression in the Pulmonary Vascular Endothelium. American Journal of Physiology Lung Cellular and Molecular Physiology. 2003;284(6):L990–L996. doi: 10.1152/ajplung.00415.2002. [DOI] [PubMed] [Google Scholar]
- Lao Ka Hou, Zeng Lingfang, Xu Qingbo. Endothelial and Smooth Muscle Cell Transformation in Atherosclerosis. Current Opinion in Lipidology. 2015 Jul; doi: 10.1097/MOL.0000000000000219. [DOI] [PubMed] [Google Scholar]
- Li Fenghe, Duman-Scheel Molly, Yang Dong, Du Wei, Zhang Jian, Zhao Chenchao, Qin Lingfeng, Xin Shijie. Sonic Hedgehog Signaling Induces Vascular Smooth Muscle Cell Proliferation via Induction of the G1 Cyclin-Retinoblastoma Axis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(9):1787–1794. doi: 10.1161/ATVBAHA.110.208520. [DOI] [PubMed] [Google Scholar]
- Li H, Li J, Li Y, Singh P, Cao L, Xu Lj, Li D, et al. Sonic Hedgehog Promotes Autophagy of Vascular Smooth Muscle Cells. AJP: Heart and Circulatory Physiology. 2012;303(11):H1319–H1331. doi: 10.1152/ajpheart.00160.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Yin-Xiong, Yang Hai-Tao, Zdanowicz Marzena, Sicklick Jason K, Qi Yi, Camp Terese J, Diehl Anna Mae. Fetal Alcohol Exposure Impairs Hedgehog Cholesterol Modification and Signaling. Laboratory Investigation; a Journal of Technical Methods and Pathology. 2007;87(3):231–240. doi: 10.1038/labinvest.3700516. [DOI] [PubMed] [Google Scholar]
- Lin Ching-Shwun, Lue Tom F. Defining Vascular Stem Cells. Stem Cells and Development. 2013;22(7):1018–1026. doi: 10.1089/scd.2012.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Weimin, Redmond Eileen M, Morrow David, Cullen John P. Differential Effects of Daily-Moderate Versus Weekend-Binge Alcohol Consumption on Atherosclerotic Plaque Development in Mice. Atherosclerosis. 2011;219(2):448–454. doi: 10.1016/j.atherosclerosis.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and Regional Mortality From 235 Causes of Death for 20 Age Groups in 1990 and 2010: a Systematic Analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Xiaoqian, Robin Catherine, Ottersbach Katrin, Dzierzak Elaine. The Ly-6A (Sca-1) GFP Transgene Is Expressed in All Adult Mouse Hematopoietic Stem Cells. Stem Cells (Dayton, Ohio) 2002;20(6):514–521. doi: 10.1634/stemcells.20-6-514. [DOI] [PubMed] [Google Scholar]
- Majesky Mark W, Dong Xiu Rong, Hoglund Virginia, Daum Gunter, Mahoney William M. Cells, Tissues, Organs. 1-2. Vol. 195. Karger Publishers; 2012. The Adventitia: a Progenitor Cell Niche for the Vessel Wall; pp. 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney Ciaran J, Hakimjavadi Roya, Fitzpatrick Emma, Kennedy Eimear, Walls Dermot, Morrow David, Redmond Eileen M, Cahill Paul A. Hedgehog and Resident Vascular Stem Cell Fate. Stem Cells International. 2015;2015:468428. doi: 10.1155/2015/468428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow David, Sweeney Catherine, Birney Yvonne A, Guha Shaunta, Collins Nora, Cummins Philip M, Murphy Ronan, Walls Dermot, Redmond Eileen M, Cahill Paul A. Biomechanical Regulation of Hedgehog Signaling in Vascular Smooth Muscle Cells in Vitro and in Vivo. American Journal of Physiology Cell Physiology. 2007;292(1):C488–C496. doi: 10.1152/ajpcell.00337.2005. [DOI] [PubMed] [Google Scholar]
- Morrow David, Cullen John P, Cahill Paul A, Redmond Eileen M. Ethanol Stimulates Endothelial Cell Angiogenic Activity via a Notch- and Angiopoietin-1-Dependent Pathway. Cardiovascular Research. 2008;79(2):313–321. doi: 10.1093/cvr/cvn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow David, Cullen John P, Liu Weimin, Cahill Paul A, Redmond Eileen M. Alcohol Inhibits Smooth Muscle Cell Proliferation via Regulation of the Notch Signaling Pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(12):2597–2603. doi: 10.1161/ATVBAHA.110.215681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow David, Cullen John P, Liu Weimin, Guha Shaunta, Sweeney Catherine, Birney Yvonne A, Collins Nora, Walls Dermot, Redmond Eileen M, Cahill Paul A. Sonic Hedgehog Induces Notch Target Gene Expression in Vascular Smooth Muscle Cells via VEGF-a. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(7):1112–1118. doi: 10.1161/ATVBAHA.109.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamal Kenneth J, Chen Chiung M, Rao Sowmya R, Breslow Rosalind A. Alcohol Consumption and Cardiovascular Mortality Among U.S. Adults, 1987 to 2002. Journal of the American College of Cardiology. 2010;55(13):1328–1335. doi: 10.1016/j.jacc.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan Sujatha, Ambade Aditya, Fulham Melissa A, Deshpande Janhavee, Catalano Donna, Mandrekar Pranoti. Moderate Alcohol Induces Stress Proteins HSF1 and Hsp70 and Inhibits Proinflammatory Cytokines Resulting in Endotoxin Tolerance. Journal of Immunology (Baltimore, Md : 1950) 2014;193(4):1975–1987. doi: 10.4049/jimmunol.1303468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Hisamichi, Kidoya Hiroyasu, Sakimoto Susumu, Wakabayashi Taku, Takakura Nobuyuki. Identification and Characterization of a Resident Vascular Stem/Progenitor Cell Population in Preexisting Blood Vessels. 4. Vol. 31. EMBO Press; 2012. pp. 842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi Augusto. The Contribution of Resident Vascular Stem Cells to Arterial Pathology. International Journal of Stem Cells. 2015;8(1):9–17. doi: 10.15283/ijsc.2015.8.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A Sonic Hedgehog Signaling Domain in the Arterial Adventitia Supports Resident Sca1+ Smooth Muscle Progenitor Cells. Proceedings of the National Academy of Sciences. 2008;105(27):9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaltis Peter J, Puranik Amrutesh S, Spoon Daniel B, Chue Colin D, Hoffman Scott J, Witt Tyra A, Delacroix Sinny, Kleppe LS, Mueske CS, Pan S, Gulati R, Simari RD. Characterization of a Resident Population of Adventitial Macrophage Progenitor Cells in Postnatal Vasculature. 3. Vol. 115. Lippincott Williams & Wilkins; 2014. pp. 364–375. [DOI] [PubMed] [Google Scholar]
- Redmond Eileen M, Hamm Katie, Cullen John P, Hatch Ekaterina, Cahill Paul A, Morrow David. Inhibition of Patched-1 Prevents Injury-Induced Neointimal Hyperplasia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(8):1960–1964. doi: 10.1161/ATVBAHA.113.301843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke Michael, Rehm Jürgen. Alcohol Consumption, Drinking Patterns, and Ischemic Heart Disease: a Narrative Review of Meta-Analyses and a Systematic Review and Meta-Analysis of the Impact of Heavy Drinking Occasions on Risk for Moderate Drinkers. BMC Medicine. 2014;12(1):182. doi: 10.1186/s12916-014-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronksley Paul E, Brien Susan E, Turner Barbara J, Mukamal Kenneth J, Ghali William A. Association of Alcohol Consumption with Selected Cardiovascular Disease Outcomes: a Systematic Review and Meta-Analysis. BMJ (Clinical Research Ed) 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruidavets Jean-Bernard, Ducimetière Pierre, Evans Alun, Montaye Michèle, Haas Bernadette, Bingham Annie, Yarnell John, et al. Patterns of Alcohol Consumption and Ischaemic Heart Disease in Culturally Divergent Countries: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) BMJ (Clinical Research Ed) 2010;341:c6077. doi: 10.1136/bmj.c6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed Shariq, Cullen John P, Coppage Myra, Sitzmann James V, Redmond Eileen M. Ethanol Differentially Modulates the Expression and Activity of Cell Cycle Regulatory Proteins in Rat Aortic Smooth Muscle Cells. European Journal of Pharmacology. 2002;445(3):163–170. doi: 10.1016/s0014-2999(02)01761-2. [DOI] [PubMed] [Google Scholar]
- Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN. Identification of a Potential Role for the Adventitia in Vascular Lesion Formation After Balloon Overstretch Injury of Porcine Coronary Arteries. Circulation. 1996;93(12):2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial Myofibroblasts Contribute to Neointimal Formation in Injured Porcine Coronary Arteries. Circulation. 1996;94(7):1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- Shikatani Eric A, Chandy Mark, Besla Rickvinder, Li Cedric C, Momen Abdul, El-Mounayri Omar, Robbins Clinton S, Husain Mansoor. C-Myb Regulates Proliferation and Differentiation of Adventitial Sca1+ Vascular Smooth Muscle Cell Progenitors by Transactivation of Myocardin. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016 May; doi: 10.1161/ATVBAHA.115.307116. [DOI] [PubMed] [Google Scholar]
- Shirpoor Alireza, Salami Siamak, Ansari Mohammad-Hasan Khadem, Ilkhanizadeh Behrouz, Abdollahzadeh Naseh. Ethanol Promotes Rat Aortic Vascular Smooth Muscle Cell Proliferation via Increase of Homocysteine and Oxidized-Low-Density Lipoprotein. Journal of Cardiology. 2013;62(6):374–378. doi: 10.1016/j.jjcc.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Siggins Sarah L, Nguyen Nhu-Y N, McCormack Matthew P, Vasudevan Sumitha, Villani Rehan, Jane Stephen M, Wainwright Brandon J, Curtis David J. The Hedgehog Receptor Patched1 Regulates Myeloid and Lymphoid Progenitors by Distinct Cell-Extrinsic Mechanisms. Blood. 2009;114(5):995–1004. doi: 10.1182/blood-2009-03-208330. [DOI] [PubMed] [Google Scholar]
- Sweeney Catherine, Morrow David, Birney Yvonne A, Coyle Seamus, Hennessy Colm, Scheller Agnieszka, Cummins Philip M, Walls Dermot, Redmond Eileen M, Cahill Paul A. Notch 1 and 3 Receptor Signaling Modulates Vascular Smooth Muscle Cell Growth, Apoptosis, and Migration via a CBF-1/RBP-Jk Dependent Pathway. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2004;18(12):1421–1423. doi: 10.1096/fj.04-1700fje. [DOI] [PubMed] [Google Scholar]
- Tang Zhenyu, Wang Aijun, Wang Dong, Li Song. Smooth Muscle Cell Plasticity: Fact or Fiction? 2013;112(1):17–22. doi: 10.1161/CIRCRESAHA.112.281048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Zhenyu, Wang Aijun, Yuan Falei, Yan Zhiqiang, Liu Bo, Chu Julia S, Helms Jill A, Li Song. Nature Communications. June. Vol. 3. Nature Publishing Group; 2012. Differentiation of Multipotent Vascular Stem Cells Contributes to Vascular Diseases; p. 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Doll R. Alcohol Consumption and Mortality Among Middle-Aged and Elderly U.S. Adults. The New England Journal of Medicine. 1997;337(24):1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- Tigges Ulrich, Komatsu Masanobu, Stallcup William B. Adventitial Pericyte Progenitor/Mesenchymal Stem Cells Participate in the Restenotic Response to Arterial Injury. Journal of Vascular Research. 2013;50(2):134–144. doi: 10.1159/000345524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsney Evelyn, Xu Qingbo. Resident Vascular Progenitor Cells. Journal of Molecular and Cellular Cardiology. 2011;50(2):304–311. doi: 10.1016/j.yjmcc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Uchida Shizuka, De Gaspari Piera, Kostin Sawa, Jenniches Katharina, Kilic Ayse, Izumiya Yasuhiro, Shiojima Ichiro, Grosse Kreymborg K, Renz H, Walsh K, Braun T. Sca1-Derived Cells Are a Source of Myocardial Renewal in the Murine Adult Heart. Stem Cell Reports. 2013;1(5):397–410. doi: 10.1016/j.stemcr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink GR, Hardwick JC, Tytgat GN, Brink MA, Ten Kate FJ, Van Deventer SJ, Peppelenbosch MP. Sonic Hedgehog Regulates Gastric Gland Morphogenesis in Man and Mouse. Gastroenterology. 2001;121(2):317–328. doi: 10.1053/gast.2001.26261. [DOI] [PubMed] [Google Scholar]
- Wakabayashi I. Light-to-Moderate Alcohol Intake Reduces Lipid Accumulation Product and Attenuates Its Relation to Hypertension. Journal of Human Hypertension. 2015;29(6):359–365. doi: 10.1038/jhh.2014.97. [DOI] [PubMed] [Google Scholar]
- Walshe Tony E, Connell Paul, Cryan Lorna, Ferguson Gail, Gardiner Tom, Morrow David, Redmond Eileen M, O'Brien Colm, Cahill Paul A. Microvascular Retinal Endothelial and Pericyte Cell Apoptosis in Vitro: Role of Hedgehog and Notch Signaling. Investigative Ophthalmology & Visual Science. 2011;52(7):4472–4483. doi: 10.1167/iovs.10-7061. [DOI] [PubMed] [Google Scholar]


