Abstract
Background
Testing for direct gene/SNP replication of association across studies may not capture the true importance of a candidate locus; rather, we suggest that relevant replication across studies may be found at the level of a biological process. We previously observed that variation in two members of the SWI/SNF chromatin remodeling complex is associated with alcohol dependence (AD) in the Irish Affected Sib Pair Study for Alcohol Dependence. Here, we tested for association with alcohol-related outcomes using a set of genes functioning in the SWI/SNF complex in two independent samples.
Methods
We used a set-based analysis to examine the 29 genes of the SWI/SNF complex for evidence of association with (1) AD in the adult Collaborative Study on the Genetics of Alcoholism (COGA) case/control sample and (2) antisocial behavior, hypothesized to be a genetically-related developmental precursor, in a younger population sample (Spit for Science).
Results
We found evidence for association of the SWI/SNF complex with AD in COGA (p=0.0435) and more general antisocial behavior in Spit for Science (p=0.00026). The genes that contributed most strongly to the signal in COGA were SS18L1, SMARCD1, BRD7, SMARCB1 and BCL11A. In the Spit for Science sample, ACTB, ARID2, BCL11A, BCL11B, BCL7B, BCL7C, DPF2, and DPF3 all contributed strongly to the signal.
Conclusions
We detected associations between the SWI/SNF complex and AD in an adult population selected from treatment-seeking probands and antisocial behavior in an adolescent population sample. This provides strong support for a role for SWI/SNF in the development of alcohol-related problems.
Keywords: Alcohol Dependence, SWI/SNF, COGA, chromatin remodeling, externalizing, antisocial behavior
Introduction
Alcohol dependence (AD) is a significant social and economic problem. The propensity to develop AD is strongly influenced by genetics (Rodriguez et al., 1993, Heath et al., 1999, Schuckit and Smith, 1996, Prescott and Kendler, 1999, Schuckit, 2000), but few of the specific genetic factors underlying this propensity have been identified. One major problem with the identification of strong candidate genes in humans is that promising signals are frequently not replicated across studies at the level of the individual polymorphism or gene. Because variation in different genes that act together in a biological process may yield similar phenotypic outcomes, we suggest that identification of associated variation in different genes controlling the same process across studies strongly supports a role for that biological process in AD. For this reason, biological process-based set analyses may provide more reliable and replicable results than individual gene-specific analyses. Here, we assess the association of all genes known to act together to perform a common biological action with alcohol-related outcomes in an adult sample drawn from six treatment centers, the Collaborative Study on the Genetics of Alcoholism (COGA) study, and in a younger population sample, the Spit for Science (S4S) sample, to determine the importance of a protein complex in AD liability.
SWI/SNF (SWItching defective/Sucrose Non-Fermenting) is a multi-protein complex that regulates chromatin structure by altering the associations between DNA and histones, which can lead to changes in gene expression. Chromatin remodeling is one mechanism of epigenetic gene regulation (Allis and Jenuwein, 2016), and members of the SWI/SNF chromatin remodeling complex have been implicated as epigenetic regulators in the development of cancer (Kadoch and Crabtree, 2015, Nickerson et al., 2017). The complexes contain an enzymatic core of four subunits and several accessory subunits that vary with distinct SWI/SNF complex types (Fig. 1; Son and Crabtree, 2014). Different accessory subunits lend specificity to the function of the different complexes; some accessory subunits are found in all complexes, while others are specific to a particular type of SWI/SNF complex (Kadoch and Crabtree, 2015, Son and Crabtree, 2014).
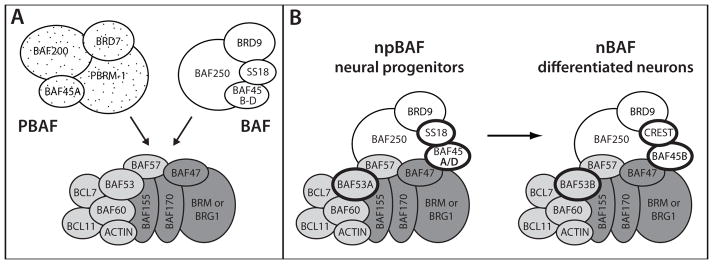
Figure 1. SWI/SNF chromatin remodeling complexes.
A. SWI/SNF complexes result from the combination of core (dark grey), common accessory (light grey), and unique accessory (stipple/PBAF or white/BAF) subunits. More than one gene encodes different versions of many of the subunits: the core ATPase subunit is encoded by SMARCA2 (BRM) or SMARCA4 (BRG1); BAF250 is encoded by ARID1A and ARID1B; BAF155 and BAF170 encode interchangeable core subunits; BAF45A is encoded by PHF10 and BAF45B-D are encoded by DPF1, DPF2 and DPF3; BAF53 is encoded by ACTL6A and ACTL6B; BAF60 is encoded by SMARCD1, SMARCD2, and SMARCD3; BCL7 is encoded by BCL7A, BCL7B, and BCL7C; BCL11 is encoded by BCL11A and BCL11B; SS18 and CREST are encoded by SS18 and SS18L1, respectively. B. BAF complexes purified from neural progenitors (npBAF) and differentiated neurons (nBAF) contain different SWI/SNF subunits; subunits that differ are highlighted with bold lines. npBAF contains BAF53A, BAF45A or BAF45D, and SS18. nBAF contains BAF53B, BAF45B, and CREST.
Two major families of SWI/SNF complexes are BAF (Brg1-Associated Factors) and PBAF (Polybromo-associated BAF), which incorporate different sets of unique accessory subunits to produce molecularly distinct complexes (Fig. 1A). We previously found that BAF and PBAF affect different components of the acute response to ethanol in the genetic model Caenorhabditis elegans (Mathies et al., 2015): BAF is required for normal initial sensitivity to ethanol, while PBAF is required for the development of acute functional tolerance to ethanol (AFT). We found that PBAF functions in differentiated neurons for the development of AFT (Mathies et al., 2015). Importantly, variation in two human SWI/SNF complex genes, SMARCA2 and BRD7, was associated with AD in the Irish Affected Sib Pair Study for Alcohol Dependence (IASPSAD) (Mathies et al., 2015).
In the current study, we had the goal of expanding this literature in two ways: (1) by testing whether genes involved in the SWI/SNF complex were associated with AD in a second independent human adult sample, similarly ascertained through probands in treatment centers, like the IASPSAD sample; and (2) by testing whether the complex influenced developmental precursors of alcohol-related outcomes in a younger adolescent sample. A large portion of the genetic predisposition to alcohol dependence has been shown to be via a general predisposition toward externalizing behavior (Kendler et al., 2003, Krueger et al., 2002, Dick et al., 2005, Slutske et al., 1998), that manifests as different phenotypes at different developmental stages (Dick et al., 2006). It has been robustly demonstrated that conduct problems in children and adolescents are associated with the subsequent development of AD (Molina et al., 2002, Kuperman et al., 2001) and, importantly, that childhood conduct problems are genetically related to adult AD (Trucco et al., 2016, Kendler et al., 2003, Slutske et al., 1998). Several genes that have been associated with adult AD have been shown to influence behavior problems in younger adolescents before the onset of alcohol problems, suggesting that behavior problems in younger individuals are an earlier phenotypic manifestation of a genetic predisposition that subsequently influences alcohol problems in adults (Dick et al., 2013, Dick et al., 2016). Accordingly, we hypothesized that in our younger population sample of adolescents, the SWI/SNF gene set may impact antisocial behavior rather than alcohol dependence per se.
In this study, we examined all 29 genes encoding known subunits of the SWI/SNF chromatin remodeling complex in humans for association with AD in the COGA adult case-control sample (Bierut et al., 2002) and with antisocial behavior in the younger S4S sample (Dick et al., 2014). We further hypothesized that we would find evidence for replication of association of SWI/SNF genes at the level of the biological complex, rather than at the level of individual SNPs or genes, which may vary from sample to sample.
Materials and Methods
COGA case-control sample
Sample
The Collaborative Study on the Genetics of Alcoholism (COGA) is a multi-site project, with the goal of identifying genes contributing to alcoholism and related phenotypes. Probands were identified through inpatient or outpatient alcohol treatment programs at six sites around the United States and were invited to participate if they had a sufficiently large family (usually sibships > 3, with parents available) with two or more members living in a COGA catchment area (Begleiter et al., 1995). The institutional review boards of all participating centers approved the study. Written consent was obtained from all study participants. Additional details about the study have been published previously (Foroud et al., 2000, Edenberg et al., 2004, Reich et al., 1998). The sample has n=1905 individuals (1205 cases and 700 controls). The sample is 46% female; 74% of the sample self-identified as European American, and 26% as African American.
Genotyping
For COGA case control samples, genotyping was performed by the Center for Inherited Disease Research (CIDR). DNA sources included blood (n=1453) and lymphoblastoid cell lines (LCL, n=492). Genotyping was performed using the Illumina Infinium II assay protocol (Gunderson et al., 2006) with hybridization to Illumina HumanHap1M BeadChips (Illumina, San Diego, CA, USA), which contain 1,199,187 markers with a mean spacing of 2.4 kb. Allele cluster definitions for each SNP were determined using Illumina BeadStudio Genotyping Module version 3.1.14 and the combined intensity data from 96% of study samples. The resulting cluster definition file was used on all study samples to determine genotype calls and quality scores. Genotype calls were made when a genotype yielded a quality score (Gencall value) of 0.15 or higher. The final raw dataset with 1,041,465 SNPs for 1,945 unique DNA samples from case and control subjects was imputed to 1000G platform phase 3, b37, October 2014 and filtered based on info criterion<0.3, genotyping rate<0.9, MAF<0.005 and HWE<10−6.
Phenotype
Assessment was based on the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994). DSM-IV alcohol dependence was defined by the clustering of 3 or more dependence criteria in a 12-month period.
Analyses
We used the program PLINK (Purcell et al., 2007, Chang et al., 2015) to run set-based analyses for alcohol dependence diagnosis. The p-value threshold used in set-based analyses was 0.01, and the r2 threshold for LD pruning was set to 0.5. Gender and two race principal components were used as covariates.
S4S sample
Sample
Spit for Science (S4S) is a longitudinal study of college students enrolled in a large, urban university in the Mid-Atlantic region of the United States, as previously described (Dick et al., 2014). Briefly, all incoming students age 18 or older were invited to complete surveys with content related to alcohol use and related behavioral and emotional health outcomes in the fall of their first semester. Study data were collected and managed using REDCap electronic data capture tools (Harris et al., 2009). Participants who completed the phenotypic assessments were eligible to provide a DNA sample. The current study includes three cohorts, which matriculated in fall 2011 (n = 2,714), 2012 (n = 2,486), and 2013 (n = 2,403), for a total n = 7,603. Of these, 98% provided a DNA sample. The average (±SD) age was 18.59 (±0.61), and 61% of the sample was female. Self-report race/ethnicity for the sample was 15% Asian, 20% African American, 7% Hispanic, 6% more than 1 race, and 50% White, which closely maps onto overall university demographics (Dick et al., 2014).
Genotyping, Pre-imputation QC, and Imputation
There were 6534 samples passing DNA and initial genotyping quality control (QC). Genotyping was performed at Rutgers University Cell and DNA Repository (RUCDR) using the Affymetrix BioBank array (653K variants) which contains both common GWAS framework variants (296K) for imputation and functional variants (357K) including rare high impact exome variants (272K), indels (18K), eQTLs (16K), and miscellaneous variants (51K). QC excluded Off Target Variants found by SNPolisher, single nucleotide polymorphisms (SNPs) missing >5% of genotypes, samples missing >2% of genotypes, and SNPs missing >2% of genotypes after sample filtering, similar to the Psychiatric Genomics Consortium (PGC; Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). This pre-imputation QC removed 209 samples, leaving 6,325 samples and 560,138 variants for imputation. Imputation was conducted using SHAPEIT2 (Delaneau et al., 2013), IMPUTE2 (Howie et al., 2009) and the 1000 genomes phase 3 reference panel (n = 2,504) (Sudmant et al., 2015, 1000 Genomes Project Consortium et al., 2015).
Phenotype
Antisocial behavior was assessed in the fall surveys via a six-item scale in which individuals retrospectively reported on behavior during the high school period. The score was created as a standardized sum of six responses (I skipped school, I ran away from home overnight, I stole things from a store or from someone I knew, I used a weapon in a fight, I robbed or mugged someone, I started physical fights) with four response options (3=6 or more times, 2=3–5 times, 1=1–2 times, 0=never). Missing data was prorated. Individuals not answering 50% of questions were assigned as missing.
Analyses
We used the program PLINK to run set-based analyses for the antisocial behavior phenotype using the same options as in the COGA sample. Race, cohort, gender and six non-collinear race principal components were used as covariates.
Results
COGA case/control sample
We began by testing if the entire set of 29 genes encoding members of the SWI/SNF complex was associated as a whole with a phenotype of AD in the COGA case/control sample. We found that, using a set-based analysis, the 29 genes in the SWI/SNF complex was associated with alcohol dependence at p=0.0435. We next asked which of the genes in the set of 29 contributed to this association by performing individual gene-based tests on each gene. Table 1 shows the results for individual gene-based tests. We found that SS18L1, SMARCD1, BRD7, SMARCB1 and BCL11A were all significant at p<0.05.
Table 1.
Gene-based tests of association with alcohol dependence in the COGA case/control sample
| Gene | Protein | NSNP | NSIG | ISIG | p-value |
|---|---|---|---|---|---|
| ACTB | ACTIN | 4 | 0 | 0 | 1.000 |
| ACTL6A | BAF53A | 24 | 3 | 2 | 0.163 |
| ACTL6B | BAF53B | 16 | 0 | 0 | 1.000 |
| ARID1A | BAF250A | 116 | 4 | 3 | 0.342 |
| ARID1B | BAF250B | 810 | 19 | 9 | 0.957 |
| ARID2 | BAF200 | 119 | 2 | 1 | 0.712 |
| BCL11A | CTIP1 | 153 | 5 | 3 | 0.038* |
| BCL11B | CTIP2 | 150 | 2 | 2 | 0.739 |
| BCL7A | BCL7A | 57 | 7 | 3 | 0.262 |
| BCL7B | BCL7B | 59 | 1 | 1 | 0.042* |
| BCL7C | BCL7C | 11 | 0 | 0 | 1.000 |
| BRD7 | BRD7 | 85 | 3 | 1 | 0.021* |
| BRD9 | BRD9 | 71 | 2 | 2 | 0.315 |
| DPF1 | BAF45B | 30 | 0 | 0 | 1.000 |
| DPF2 | BAF45D | 36 | 11 | 1 | 0.066 |
| DPF3 | BAF45C | 523 | 28 | 10 | 0.303 |
| PBRM1 | PBRM1 | 262 | 36 | 2 | 0.300 |
| PHF10 | BAF45A | 37 | 0 | 0 | 1.000 |
| SMARCA2 | hBRM | 289 | 22 | 11 | 0.233 |
| SMARCA4 | BRG1 | 280 | 11 | 1 | 0.928 |
| SMARCB1 | BAF47/INI1 | 202 | 136 | 5 | 0.042* |
| SMARCC1 | BAF155 | 207 | 14 | 6 | 0.184 |
| SMARCC2 | BAF170 | 10 | 0 | 0 | 1.000 |
| SMARCD1 | BAF60A | 19 | 3 | 1 | 0.016* |
| SMARCD2 | BAF60B | 20 | 0 | 0 | 1.000 |
| SMARCD3 | BAF60C | 77 | 0 | 0 | 1.000 |
| SMARCE1 | BAF57 | 33 | 2 | 2 | 0.405 |
| SS18 | SS18 | 119 | 3 | 3 | 0.294 |
| SS18L1 | CREST | 123 | 52 | 2 | 0.008** |
Note: For each gene, NSNP= number of SNPs; NSIG= number of significant SNPs; ISIG= number of independent significant SNPs.
p-value <0.05;
p-value ≤ 0.01.
Accordingly, test statistics are corrected for multiple testing with each gene, but not across all genes. We note that these analyses are presented as secondary analyses to supplement the primary test of the overall gene set and provide insight into the genes in the set most strongly driving the overall association signal for the SWI/SNF complex.
S4S Sample
The Spit for Science sample members are young, and therefore appropriate for examining the genetic contributions to an antisocial behavior phenotype that has been described as an early predictor of alcohol problems (Kuperman et al., 2001, Kessler et al., 1997, Zucker, 2008). We tested if there was an association between SWI/SNF complex members and antisocial behavior. We began by testing the set of 29 known SWI/SNF genes as a whole, and found that the set-based analysis of the SWI/SNF complex genes showed significant association with antisocial behavior in the Spit for Science sample (p=0.00026). We next used gene-based analyses to identify the members of the set that were contributing to this signal. We found that ACTB, ARID2, BCL11A, BCL11B, BCL7B, BCL7C, DPF2, and DPF3 all yielded p<0.05, contributing to the highly significant association of the set (Table 2).
Table 2.
Gene-based tests of association between SWI/SNF genes and externalizing behavior in the S4S sample
| Gene | Protein | NSNP | NSIG | ISIG | p-value |
|---|---|---|---|---|---|
| ACTB | ACTIN | 4 | 3 | 1 | 0.007** |
| ACTL6A | BAF53A | 22 | 0 | 0 | 1.000 |
| ACTL6B | BAF53B | 6 | 0 | 0 | 1.000 |
| ARID1A | BAF250A | 53 | 0 | 0 | 1.000 |
| ARID1B | BAF250B | 270 | 30 | 4 | 0.272 |
| ARID2 | BAF200 | 71 | 20 | 2 | 0.042* |
| BCL11A | CTIP1 | 53 | 2 | 1 | 0.003** |
| BCL11B | CTIP2 | 98 | 42 | 10 | 0.002** |
| BCL7A | BCL7A | 29 | 0 | 0 | 1.000 |
| BCL7B | BCL7B | 4 | 2 | 1 | 0.039* |
| BCL7C | BCL7C | 1 | 1 | 1 | 0.008** |
| BRD7 | BRD7 | 46 | 0 | 0 | 1.000 |
| BRD9 | BRD9 | 3 | 0 | 0 | 1.000 |
| DPF1 | BAF45B | 0 | 0 | 0 | NA |
| DPF2 | BAF45D | 1 | 1 | 1 | 0.015* |
| DPF3 | BAF45C | 231 | 118 | 10 | 0.001*** |
| PBRM1 | PBRM1 | 169 | 0 | 0 | 1.000 |
| PHF10 | BAF45A | 23 | 0 | 0 | 1.000 |
| SMARCA2 | hBRM | 140 | 12 | 5 | 0.059 |
| SMARCA4 | BRG1 | 39 | 2 | 1 | 0.250 |
| SMARCB1 | BAF47/INI1 | 185 | 0 | 0 | 1.000 |
| SMARCC1 | BAF155 | 116 | 0 | 0 | 1.000 |
| SMARCC2 | BAF170 | 0 | 0 | 0 | NA |
| SMARCD1 | BAF60A | 2 | 1 | 1 | 0.071 |
| SMARCD2 | BAF60B | 15 | 0 | 0 | 1.000 |
| SMARCD3 | BAF60C | 17 | 0 | 0 | 1.000 |
| SMARCE1 | BAF57 | 15 | 0 | 0 | 1.000 |
| SS18 | SS18 | 76 | 3 | 2 | 0.089 |
| SS18L1 | CREST | 63 | 0 | 0 | 1.000 |
Note: For each gene, NSNP= number of SNPs; NSIG= number of significant SNPs; ISIG= number of independent significant SNPs.
p-value ≤ 0.05;
p-value ≤ 0.01;
p-value ≤ 0.001.
Accordingly, test statistics are corrected for multiple testing with each gene, but not across all genes. We note that these analyses are presented as secondary analyses to supplement the primary test of the overall gene set and provide insight into the genes in the set most strongly driving the overall association signal for the SWI/SNF complex.
Discussion
The propensity to develop AD is strongly influenced by genetics (Rodriguez et al., 1993, Heath et al., 1999, Schuckit and Smith, 1996, Prescott and Kendler, 1999, Schuckit, 2000), and human genetic association studies have identified a variety of candidate loci that are associated with the propensity to develop AD in individual populations (Tawa et al., 2016, Hart and Kranzler, 2015). However, very few of these associations, excepting loci in the ADH and ALDH genes, have been replicated across different studies. One possible reason for the difficulty that has been observed in replicating associations for AD is that traditional replication analysis, in which the same allele or gene is examined in more than one study, presupposes that identical or equivalent mutational events in a given gene must occur across populations to cause the phenotype. This stringent requirement may not appropriately capture the potential role of the gene in AD because it fails to take into account how broader gene networks function. Because genes act together in biological networks to perform a particular function, we reasoned that if a biological process is important in modulating the propensity to develop AD, then variation in any genes similarly affecting that process might cause a similar change in the liability to develop AD. We therefore tested all members of the SWI/SNF chromatin remodeling complex as a set to evaluate association with AD and a related phenotype in two human samples because we had evidence from our model organism work that this complex is involved in the behavioral response to ethanol, and we had previously observed an association with two members of the SWI/SNF complex and AD in the IASPSAD study (Mathies et al., 2015).
Here, our set-based analyses provide additional evidence for association between allelic variation in subunits of the SWI/SNF chromatin remodeling complex and AD in the COGA adult case-control sample, and with behavior problems in a younger sample of adolescents. We have now observed association between multiple, different, members of the SWI/SNF complex and AD, as well as a genetically related developmental precursor to AD, in three independent human populations. As hypothesized, secondary analyses of the individual genes in the set indicated that it was not always the same genes contributing to the association signal observed with the complex. In the IASPSAD sample, SWI/SNF complex members SMARCA2 and BRD7 were associated with AD (Mathies et al., 2015). In COGA, we found that the strongest association signals came from SS18L1, SMARCD1, BRD7, SMARCB1 and BCL11A. When we examined the Spit for Science sample, we found that the strongest signals came from ACTB, ARID2, BCL11A, BCL11B, BCL7B, BCL7C, DPF2, and DPF3. This combination of data provides growing support for the involvement of SWI/SNF chromatin remodeling in the development of AD. Importantly, our analyses indicate that association should be evaluated at the level of the complex, which is made up of the protein products of multiple genes, and that variation in different genes may be altering SWI/SNF activity across different samples.
How do SWI/SNF complex variants influence both the liability to develop AD in adults and antisocial behavior in adolescents? In current models of AD, a significant component of the genetic liability to develop AD in adults is shared with the genetic components that influence antisocial behavior, impulsivity, and conduct disorders in younger people (Young et al., 2000). We found that the SWI/SNF gene complex impacted AD in an affected adult sample, and influenced adolescent antisocial behavior in a younger community-based sample. There are two major models to explain how these phenotypes may be related to SWI/SNF: First, variation in the SWI/SNF complex may have effects on nervous system function that are manifested in youth as a predisposition to conduct disorders. Those conduct phenotypes may themselves predispose people to developing AD when they are older and alcohol is readily available. In this case, the effects on AD liability are a consequence of the effects on conduct phenotypes in adolescents, and there are data that support this model (Cho et al., 2014). A second possibility is that there may be shared or distinct effects of SWI/SNF on nervous system function that are manifested in youth as a predisposition to conduct disorders and are independently revealed in adults as an increased liability to develop AD. Importantly, these models are not mutually exclusive, and in the complex landscape of genetic effects on these human behavioral phenotypes, both mechanisms may be working simultaneously.
Our data currently cannot distinguish between these and other possible models. However, the original observations that led to the current studies do suggest that the effects of variation in SWI/SNF complex members on AD may be, at least in part, through the modulation of the physiological response to the drug itself. We previously found that in the model organism C. elegans, the acute physiological response to alcohol is altered in animals with altered SWI/SNF function. Animals with loss of function of the BAF complex have decreased initial sensitivity to ethanol, while loss of function of the PBAF complex eliminates the ability of animals to develop AFT to ethanol (Mathies et al., 2015). Although the direct relationship between acute ethanol response phenotypes in C. elegans and human AD is not clear, empirically, a finding that a gene has an effect on acute alcohol response phenotypes in invertebrate model organisms very strongly predicts that variation in that gene will be implicated in human AD (Grotewiel and Bettinger, 2015). This empirically observed relationship between genes affecting acute alcohol responses in model organisms and AD in humans strongly suggests that at least some shared molecular mechanisms influence physiological responses to the drug and development of AD. That said, AD is frequently comorbid with antisocial behavior, creating difficulty teasing apart these phenotypes in human genetic analyses. Secondary analyses of log-transformed DSM-IV adult antisocial personality disorder symptom counts in the COGA AD case-control sample yielded a trend toward association with the gene set (p=0.098) despite this being a non-ideal test.
SWI/SNF complexes have important and diverse actions during development (Ho and Crabtree, 2010). They are important in the regulation of neuronal development (Son and Crabtree, 2014), where they are required for neural tube closure and the regulation of brain size in mouse (Bultman et al., 2000, Kim et al., 2001). Furthermore, there is strong evidence in humans for functions of SWI/SNF in the nervous system. Recently, several mutations in a member of the SWI/SNF complex, ARID2, were identified in individuals with intellectual disability (Shang et al., 2015). Additionally, a transcription factor (ADNP) that directly interacts with the SWI/SNF complex is mutated in syndromic autism (Helsmoortel et al., 2014, Vandeweyer et al., 2014).
It is well established that mutations in different genes encoding proteins that act together can cause the same genetic disorder in humans. In the case of the SWI/SNF complex, variation in five different BAF genes, SMARCB1, SMARCA4, SMARCE1, ARID1A, and ARID1B, has been linked to Coffin-Siris Syndrome (CSS), a sporadic intellectual disability syndrome (Santen et al., 2012, Tsurusaki et al., 2012, Tsurusaki et al., 2014). Furthermore, in 87% of CSS patients studied, a mutation in a BAF complex subunit was identified. Together, these data underscore how mutations in different genes encoding members of the same SWI/SNF protein complex can cause similar clinical outcomes.
SWI/SNF complexes have demonstrated functions in differentiated cells. These complexes are required in differentiated neurons for dendritic outgrowth, synaptic plasticity, and memory (Vogel-Ciernia et al., 2013, Olave et al., 2002, Wu et al., 2007, Staahl et al., 2013). Of particular interest in this study was our finding that allelic variation in SS18L1 is associated with AD. SS18L1 encodes CREST, which is found specifically in SWI/SNF complexes biochemically purified from differentiated neurons (Fig. 1; Staahl et al., 2013), and is required for dendritic outgrowth (Aizawa et al., 2004). Together, these data suggest that the SWI/SNF complex members identified in our association studies have a clear potential to influence important biological events underlying behavioral problems and the development of AD, because they are functional during both adolescence and adulthood at the place and time during which relevant events would be expected to occur with disease progression.
What molecular mechanisms might underlie how allelic variation in members of the SWI/SNF complex influence antisocial behavior and progression to AD? Because SWI/SNF complexes remodel chromatin structure, they alter gene expression by changing the accessibility of regulatory sequences to the transcriptional machinery. We speculate that allelic variation in these chromatin regulators may result in important changes in their function, either by subtly changing the strength or specificity of protein interactions or enzymatic activity, or by altering the interaction with specific DNA regulatory elements. An alternative mechanism may be that genetic variation may alter the levels of expression of the SWI/SNF component. In any of these cases, such variation could have important effects on the transcriptional regulation of downstream targets of SWI/SNF, either by increasing or decreasing the expression of the target sequences. We anticipate that these targets include the mediators of both of these phenotypes in the brain. Our findings that several SWI/SNF complex members are associated with AD in different human populations and with antisocial behavior in a younger, community sample, that represents a genetically-linked developmental precursor phenotype, argue strongly for efforts to identify the downstream targets of SWI/SNF that are important for mediating these important phenotypes.
Acknowledgments
The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, H. Edenberg, L. Bierut, includes eleven different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., T. Foroud); University of Iowa (S. Kuperman, J. Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, A. Brooks); Department of Biomedical and Health Informatics, The Children’s Hospital of Philadelphia; Department of Genetics, Perelman School of Medicine, University of Pennsylvania, Philadelphia PA (L. Almasy), Virginia Commonwealth University (D. Dick), Icahn School of Medicine at Mount Sinai (A. Goate), and Howard University (R. Taylor). Other COGA collaborators include: L. Bauer (University of Connecticut); J. McClintick, L. Wetherill, X. Xuei, Y. Liu, D. Lai, S. O’Connor, M. Plawecki, S. Lourens (Indiana University); G. Chan (University of Iowa; University of Connecticut); J. Meyers, D. Chorlian, C. Kamarajan, A. Pandey, J. Zhang (SUNY Downstate); J.-C. Wang, M. Kapoor, S. Bertelsen (Icahn School of Medicine at Mount Sinai); A. Anokhin, V. McCutcheon, S. Saccone (Washington University); J. Salvatore, F. Aliev, B. Cho (Virginia Commonwealth University); and Mark Kos (University of Texas Rio Grande Valley). A. Parsian and M. Reilly are the NIAAA Staff Collaborators.
We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
We would like to thank the VCU students for making this study a success, as well as the many VCU faculty, students, and staff who contributed to the design and implementation of the project. Spit for Science: The VCU Student Survey has been supported by Virginia Commonwealth University, P20 AA017828, R37AA011408, K02AA018755 (to DMD), and P50 AA022537 from the National Institute on Alcohol Abuse and Alcoholism, and UL1RR031990 from the National Center for Research Resources and National Institutes of Health Roadmap for Medical Research. These funding sources had no role in the analysis or interpretation of the data, writing of the manuscript, or the decision to submit the paper for publication. JCB, LDM, and AGD are supported by NIH RO1AA016837 and P50AA022537.
References
- 1000 Genomes Project Consortium. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Hu SC, Bobb K, Balakrishnan K, Ince G, Gurevich I, Cowan M, Ghosh A. Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science. 2004;303:197–202. doi: 10.1126/science.1089845. [DOI] [PubMed] [Google Scholar]
- Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nature reviews Genetics. 2016;17:487–500. doi: 10.1038/nrg.2016.59. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Reich T, Hesselbrock V, Porjesz B, Li TK, Schuckit M, Edenberg H, Rice JP. The Collaborative Study on the Genetics of Alcholism. Health & Research World. 1995;19:228–236. [Google Scholar]
- Bierut LJ, Saccone NL, Rice JP, Goate A, Foroud T, Edenberg H, Almasy L, Conneally PM, Crowe R, Hesselbrock V, Li TK, Nurnberger J, Jr, Porjesz B, Schuckit MA, Tischfield J, Begleiter H, Reich T. Defining alcohol-related phenotypes in humans. The Collaborative Study on the Genetics of Alcoholism. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2002;26:208–213. [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of studies on alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell. 2000;6:1287–1295. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SB, Heron J, Aliev F, Salvatore JE, Lewis G, Macleod J, Hickman M, Maughan B, Kendler KS, Dick DM. Directional relationships between alcohol use and antisocial behavior across adolescence. Alcohol Clin Exp Res. 2014;38:2024–2033. doi: 10.1111/acer.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaneau O, Zagury JF, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods. 2013;10:5–6. doi: 10.1038/nmeth.2307. [DOI] [PubMed] [Google Scholar]
- Dick DM, Adkins AE, Kuo SI. Genetic influences on adolescent behavior. Neuroscience and biobehavioral reviews. 2016;70:198–205. doi: 10.1016/j.neubiorev.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Latendresse S, Porjesz B, Schuckit M, Rangaswamy M, Hesselbrock V, Edenberg H, Nurnberger J, Agrawal A, Bierut L, Wang J, Bucholz K, Kuperman S, Kramer J. How phenotype and developmental stage affect the genes we find: GABRA2 and impulsivity. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2013;16:661–669. doi: 10.1017/thg.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Jr, Xuei X, Edenberg HJ, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behavior genetics. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dick DM, Nasim A, Edwards AC, Salvatore JE, Cho SB, Adkins A, Meyers J, Yan J, Cooke M, Clifford J, Goyal N, Halberstadt L, Ailstock K, Neale Z, Opalesky J, Hancock L, Donovan KK, Sun C, Riley B, Kendler KS. Spit for Science: launching a longitudinal study of genetic and environmental influences on substance use and emotional health at a large US university. Front Genet. 2014;5:47. doi: 10.3389/fgene.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Understanding the covariation among childhood externalizing symptoms: genetic and environmental influences on conduct disorder, attention deficit hyperactivity disorder, and oppositional defiant disorder symptoms. Journal of abnormal child psychology. 2005;33:219–229. doi: 10.1007/s10802-005-1829-8. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. American journal of human genetics. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Edenberg HJ, Goate A, Rice J, Flury L, Koller DL, Bierut LJ, Conneally PM, Nurnberger JI, Bucholz KK, Li TK, Hesselbrock V, Crowe R, Schuckit M, Porjesz B, Begleiter H, Reich T. Alcoholism susceptibility loci: confirmation studies in a replicate sample and further mapping. Alcohol Clin Exp Res. 2000;24:933–945. [PubMed] [Google Scholar]
- Grotewiel M, Bettinger JC. Drosophila and Caenorhabditis elegans as Discovery Platforms for Genes Involved in Human Alcohol Use Disorder. Alcohol Clin Exp Res. 2015;39:1292–1311. doi: 10.1111/acer.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson KL, Steemers FJ, Ren H, Ng P, Zhou L, Tsan C, Chang W, Bullis D, Musmacker J, King C, Lebruska LL, Barker D, Oliphant A, Kuhn KM, Shen R. Whole-genome genotyping. Methods in enzymology. 2006;410:359–376. doi: 10.1016/S0076-6879(06)10017-8. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, Kranzler HR. Alcohol Dependence Genetics: Lessons Learned From Genome-Wide Association Studies (GWAS) and Post-GWAS Analyses. Alcohol Clin Exp Res. 2015;39:1312–1327. doi: 10.1111/acer.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychological medicine. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J, Schuurs-Hoeijmakers JH, Marcelis CL, Willemsen MH, Vissers LE, Yntema HG, Bakshi M, Wilson M, Witherspoon KT, Malmgren H, Nordgren A, Anneren G, Fichera M, Bosco P, Romano C, de Vries BB, Kleefstra T, Kooy RF, Eichler EE, Van der Aa N. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet. 2014;46:380–384. doi: 10.1038/ng.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoch C, Crabtree GR. Mammalian SWI/SNF chromatin remodeling complexes and cancer: Mechanistic insights gained from human genomics. Science advances. 2015;1:e1500447. doi: 10.1126/sciadv.1500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kim JK, Huh SO, Choi H, Lee KS, Shin D, Lee C, Nam JS, Kim H, Chung H, Lee HW, Park SD, Seong RH. Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol Cell Biol. 2001;21:7787–7795. doi: 10.1128/MCB.21.22.7787-7795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Kramer JR, Bucholz K, Hesselbrock V, Reich T, Reich W. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. Am J Psychiatry. 2001;158:2022–2026. doi: 10.1176/appi.ajp.158.12.2022. [DOI] [PubMed] [Google Scholar]
- Mathies LD, Blackwell GG, Austin MK, Edwards AC, Riley BP, Davies AG, Bettinger JC. SWI/SNF chromatin remodeling regulates alcohol response behaviors in Caenorhabditis elegans and is associated with alcohol dependence in humans. Proc Natl Acad Sci U S A. 2015;112:3032–3037. doi: 10.1073/pnas.1413451112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Bukstein OG, Lynch KG. Attention-deficit/hyperactivity disorder and conduct disorder symptomatology in adolescents with alcohol use disorder. Psychol Addict Behav. 2002;16:161–164. doi: 10.1037//0893-164x.16.2.161. [DOI] [PubMed] [Google Scholar]
- Nickerson JA, Wu Q, Imbalzano AN. Mammalian SWI/SNF Enzymes and the Epigenetics of Tumor Cell Metabolic Reprogramming. Front Oncol. 2017;7:49. doi: 10.3389/fonc.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olave I, Wang W, Xue Y, Kuo A, Crabtree GR. Identification of a polymorphic, neuron-specific chromatin remodeling complex. Genes & development. 2002;16:2509–2517. doi: 10.1101/gad.992102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI, Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H. Genome-wide search for genes affecting the risk for alcohol dependence. American journal of medical genetics. 1998;81:207–215. [PubMed] [Google Scholar]
- Rodriguez LA, Wilson JR, Nagoshi CT. Does psychomotor sensitivity to alcohol predict subsequent alcohol use? Alcohol Clin Exp Res. 1993;17:155–161. doi: 10.1111/j.1530-0277.1993.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Santen GW, Aten E, Sun Y, Almomani R, Gilissen C, Nielsen M, Kant SG, Snoeck IN, Peeters EA, Hilhorst-Hofstee Y, Wessels MW, den Hollander NS, Ruivenkamp CA, van Ommen GJ, Breuning MH, den Dunnen JT, van Haeringen A, Kriek M. Mutations in SWI/SNF chromatin remodeling complex gene ARID1B cause Coffin-Siris syndrome. Nat Genet. 2012;44:379–380. doi: 10.1038/ng.2217. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Genetics of the risk for alcoholism. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2000;9:103–112. doi: 10.1080/10550490050173172. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Shang L, Cho MT, Retterer K, Folk L, Humberson J, Rohena L, Sidhu A, Saliganan S, Iglesias A, Vitazka P, Juusola J, O’Donnell-Luria AH, Shen Y, Chung WK. Mutations in ARID2 are associated with intellectual disabilities. Neurogenetics. 2015;16:307–314. doi: 10.1007/s10048-015-0454-0. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol. 1998;107:363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- Son EY, Crabtree GR. The role of BAF (mSWI/SNF) complexes in mammalian neural development. Am J Med Genet C Semin Med Genet. 2014;166C:333–349. doi: 10.1002/ajmg.c.31416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staahl BT, Tang J, Wu W, Sun A, Gitler AD, Yoo AS, Crabtree GR. Kinetic analysis of npBAF to nBAF switching reveals exchange of SS18 with CREST and integration with neural developmental pathways. J Neurosci. 2013;33:10348–10361. doi: 10.1523/JNEUROSCI.1258-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudmant PH, Rausch T, Gardner EJ, Handsaker RE, Abyzov A, Huddleston J, Zhang Y, Ye K, Jun G, Hsi-Yang Fritz M, Konkel MK, Malhotra A, Stutz AM, Shi X, Paolo Casale F, Chen J, Hormozdiari F, Dayama G, Chen K, Malig M, Chaisson MJ, Walter K, Meiers S, Kashin S, Garrison E, Auton A, Lam HY, Jasmine Mu X, Alkan C, Antaki D, Bae T, Cerveira E, Chines P, Chong Z, Clarke L, Dal E, Ding L, Emery S, Fan X, Gujral M, Kahveci F, Kidd JM, Kong Y, Lameijer EW, McCarthy S, Flicek P, Gibbs RA, Marth G, Mason CE, Menelaou A, Muzny DM, Nelson BJ, Noor A, Parrish NF, Pendleton M, Quitadamo A, Raeder B, Schadt EE, Romanovitch M, Schlattl A, Sebra R, Shabalin AA, Untergasser A, Walker JA, Wang M, Yu F, Zhang C, Zhang J, Zheng-Bradley X, Zhou W, Zichner T, Sebat J, Batzer MA, McCarroll SA, Genomes Project C, Mills RE, Gerstein MB, Bashir A, Stegle O, Devine SE, Lee C, Eichler EE, Korbel JO. An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526:75–81. doi: 10.1038/nature15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawa EA, Hall SD, Lohoff FW. Overview of the Genetics of Alcohol Use Disorder. Alcohol Alcohol. 2016;51:507–514. doi: 10.1093/alcalc/agw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Hicks BM, Villafuerte S, Nigg JT, Burmeister M, Zucker RA. Temperament and externalizing behavior as mediators of genetic risk on adolescent substance use. J Abnorm Psychol. 2016;125:565–575. doi: 10.1037/abn0000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurusaki Y, Okamoto N, Ohashi H, Kosho T, Imai Y, Hibi-Ko Y, Kaname T, Naritomi K, Kawame H, Wakui K, Fukushima Y, Homma T, Kato M, Hiraki Y, Yamagata T, Yano S, Mizuno S, Sakazume S, Ishii T, Nagai T, Shiina M, Ogata K, Ohta T, Niikawa N, Miyatake S, Okada I, Mizuguchi T, Doi H, Saitsu H, Miyake N, Matsumoto N. Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat Genet. 2012;44:376–378. doi: 10.1038/ng.2219. [DOI] [PubMed] [Google Scholar]
- Tsurusaki Y, Okamoto N, Ohashi H, Mizuno S, Matsumoto N, Makita Y, Fukuda M, Isidor B, Perrier J, Aggarwal S, Dalal AB, Al-Kindy A, Liebelt J, Mowat D, Nakashima M, Saitsu H, Miyake N, Matsumoto N. Coffin-Siris syndrome is a SWI/SNF complex disorder. Clin Genet. 2014;85:548–554. doi: 10.1111/cge.12225. [DOI] [PubMed] [Google Scholar]
- Vandeweyer G, Helsmoortel C, Van Dijck A, Vulto-van Silfhout AT, Coe BP, Bernier R, Gerdts J, Rooms L, van den Ende J, Bakshi M, Wilson M, Nordgren A, Hendon LG, Abdulrahman OA, Romano C, de Vries BB, Kleefstra T, Eichler EE, Van der Aa N, Kooy RF. The transcriptional regulator ADNP links the BAF (SWI/SNF) complexes with autism. Am J Med Genet C Semin Med Genet. 2014;166C:315–326. doi: 10.1002/ajmg.c.31413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel-Ciernia A, Matheos DP, Barrett RM, Kramar EA, Azzawi S, Chen Y, Magnan CN, Zeller M, Sylvain A, Haettig J, Jia Y, Tran A, Dang R, Post RJ, Chabrier M, Babayan AH, Wu JI, Crabtree GR, Baldi P, Baram TZ, Lynch G, Wood MA. The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nature neuroscience. 2013;16:552–561. doi: 10.1038/nn.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JI, Lessard J, Olave IA, Qiu Z, Ghosh A, Graef IA, Crabtree GR. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56:94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American journal of medical genetics. 2000;96:684–695. [PubMed] [Google Scholar]
- Zucker RA. Anticipating problem alcohol use developmentally from childhood into middle adulthood: what have we learned? Addiction. 2008;103(Suppl 1):100–108. doi: 10.1111/j.1360-0443.2008.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]