Abstract
Background
Malondialdehyde (MDA) and acetaldehyde (AA) exist following ethanol metabolism and tobacco pyrolysis. As such, lungs of individuals with alcohol use disorders (AUD) are a target for the effects of combined alcohol and cigarette smoke metabolites. MDA and AA form a stable protein adduct, malondialdehyde-acetaldehyde (MAA) adduct, known to be immunogenic, profibrotic, and proinflammatory. MAA adduct is the dominant epitope in anti-MAA antibody formation. We hypothesized that MAA-adducted protein forms in lungs of those who both abuse alcohol and smoke cigarettes, and that this would be associated with systemically elevated anti-MAA antibodies.
Methods
Four groups were established: AUD subjects who smoked cigarettes (+AUD/+smoke), smokers without AUD (−AUD/+smoke), AUD without smoke (+AUD/−smoke), and non-AUD/nonsmokers (−AUD/−smoke).
Results
We observed a significant increase in MAA adducts in lung cells of +AUD/+smoke vs. −AUD/−smoke. No significant increase in MAA adducts was observed in −AUD/+smoke or in +AUD/−smoke compared to −AUD/−smoke. Serum from +AUD/+smoke had significantly increased levels of circulating anti-MAA IgA antibodies. After one week of alcohol that MAA-adducted protein is formed in the lungs of those who smoke cigarettes and abuse alcohol, leading to a subsequent increase in serum IgA antibodies. MAA-adducted proteins could play a role in pneumonia and other diseases of the lung in the setting of AUD and smoking.
Keywords: Alcohol, cigarette smoke, adduct, macrophage, IgA
Introduction
The vast majority (80%–95%) of people with AUDs are cigarette smokers (Bobo and Husten, 2000; Grucza and Bierut, 2006), and conversely, people who smoke heavily are more likely to drink alcohol. People who smoke cigarettes inhale high concentrations of acetaldehyde and lipid peroxidation-producing free radicals from the pyrolysis of tobacco (Centers for Disease Control and Prevention, 2010). Up to 10%–15% of consumed alcohol goes to the lung, where it can similarly be metabolized into acetaldehyde. Further, when alcohol consumption is chronic, significant amounts of malondialdehyde can be generated via CYP2E1-mediated lipid peroxidation (Kaphalia and Calhoun, 2013). Therefore, co-exposure to alcohol and cigarette smoke could result in the accumulation of high concentrations of these aldehydes within the lung. When these aldehydes combine in high concentrations as a result of prolonged exposure to alcohol and cigarette smoke, they form a very stable hybrid protein adduct known as the malondialdehyde-acetaldehyde (MAA) adduct, resulting in increased lung inflammation in animal models (Sapkota and Wyatt, 2015; McCaskill et al., 2011). Nevertheless, MAA adduct has not been quantified or otherwise evaluated in the lungs of humans previously.
Previously, we demonstrated that the combination of cigarette smoking and alcohol consumption is a co-exposure condition ideal for the formation of MAA adducts in a mouse model (McCaskill et al., 2011). MAA adducts were not significantly formed in the lung tissue of mice exposed to alcohol only or cigarettes only. Our previous studies also identified the anti-microbial collectin, surfactant protein D, to be a biologically relevant in vivo lung target for MAA adduction in alcohol and cigarette smoke co-exposed mice (McCaskill et al., 2011; Berger et al., 2014; Wyatt et al., 2012). Such MAA-adduction of surfactant protein D may potentially impair its normal physiologic anti-microbial functions, drive an aberrant immunologic response, and impact the predisposition for lung injury.
We have shown that MAA-modified proteins elicit isotype-specific antibody responses (Thiele et al., 1998) and induce the expression of pro-inflammatory cytokine, IL-8, in an in vitro model of airway epithelium (Berger et al., 2014). Our previous studies in mice show that inhalation of purified and sterile MAA-adducted proteins alone can produce an “inflammatory phenotype” characterized by the influx of inflammatory neutrophils (Wyatt et al., 2012). No evaluations to date, however, have reported the formation of MAA-adducted protein and antibody responses in the lungs of human subjects, regardless of corresponding alcohol or cigarette consumption.
In this study, we hypothesized that MAA-adducted protein is formed in the lung in human subjects who have a significant and prolonged exposure to cigarette smoke and alcohol, in association with elevated anti-MAA antibodies. To test this hypothesis, we examined whether MAA-adducted protein was present in lavage cells and fluid of subjects with AUDs who smoke cigarettes. Furthermore, we examined anti-MAA antibody responses in the serum and lavage fluid of subjects who abuse alcohol and smoke cigarettes. Thus, the identification and quantitation of MAA adduct formation in human lung may lead to the identification of these adducts as a biomarker of alcohol and cigarette smoke co-exposure-mediated lung injury and the development of therapeutic targets to address pathways of MAA-adduct mediated pathologic action.
Materials and Methods
Screening, recruitment, and enrollment of study subjects
AUD subjects were recruited between 2008–2016 from the Denver Comprehensive Addictions Rehabilitation and Evaluation Services (Denver CARES) center, an inpatient detoxification facility affiliated with Denver Health and Hospital System in Denver, CO. Eligible AUD subjects met the following criteria: 1) Alcohol Use Disorders Identification Test (AUDIT) score of ≥8; 2) used alcohol < 7 d before enrollment, and 3) were of age ≥21 yr. Exclusion criteria consisted of the following as described previously in detail (Bailey et al., 2015): 1) liver disease; 2) gastrointestinal bleeding; 3) heart disease; 4) renal disease; 5) lung disease; 6) illicit drug use; 7) diabetes mellitus; 8) inability to provide informed consent; 9) human immunodeficiency virus positive; 10) pregnancy; or 11) abnormal nutritional status. Normal subjects (Nonsmokers without AUDs) were recruited from the Denver area to match AUD subjects in terms of age, sex, and smoking history. Pack-year history was calculated in current and former smokers (for both AUD subjects and normals) from collected subject self-reporting. Time since cessation was also recorded for former smokers. All human samples used in this study were obtained after institutional review board approval. Written informed consent was obtained from all subjects before their participation.
Subject demographics
Four subject groups were established: non-AUD/non-cigarette smokers, AUD subjects who did not smoke cigarettes, cigarette smokers without AUDs, and AUD subjects who smoked cigarettes. AUD subjects who smoked were older than subjects without AUD, but median ages of all four groups were within 10 years. There tended to be more men among AUD subjects who smoked. Subjects with AUD had higher AUDIT score when compared to subjects without AUD by design. Packs per day and pack years of smoking did not differ among smokers in the +AUD or −AUD groups (Table 1).
Table 1.
Age, Sex, AUDIT Score (alcohol use), and Cigarette use within the study groups.
| Nonsmokers | Smokers | Non-Smoker + AUD | Smoker + AUD | ||
|---|---|---|---|---|---|
|
| |||||
| Number | 22 | 22 | 20 | 45 | |
|
| |||||
| Age (years ± STD) | 40.7 ±7.4 | 40.5±6.39 | 43.0 ± 5.97 | 44.2 ±7.43 | p=0.64 |
|
| |||||
| % Male | 72.7% | 68.1% | 80.0% | 91% | p=0.099 |
|
| |||||
| Race | p=0.01 | ||||
| -Caucasian | 63.6% | 36.3% | 40.0% | 51.1% | |
| -Hispanic | 31.8% | 27.2% | 20.0% | 20% | |
| -Native American | 0% | 0% | 20.0% | 13.3% | |
| -African American | 4.5% | 18.1% | 10.0% | 15% | |
| -Asian | 0% | 9.1% | 0% | 0% | |
| -Other/Declined to answer | 0% | 9.1% | 10.0% | 0% | |
|
| |||||
| AUDIT score (± STD) | 2.9 ±1.9 | 2.36 ± 2.0 | 30.3 ± 5.8 | 28.53 ± 7.2 | p<0.0001 |
|
| |||||
| Pack-years of smoking (±STD) | 0 | 13.6 ± 15.1 | 0.29 ± 1.29 | 13.63 ± 11.2 | p<0.0001 |
|
| |||||
| FEV1 (Percent predicted) (± STD) | 103.5 ± 14.6 | 92.9 ± 12.3 | 102.7 ± 13.4 | 97.84 ± 11.7 | p=0.02 |
|
| |||||
| Body Mass Index (±STD) | 28.62±4.98 | 28.91±6.03 | 26.71± 5.36 | 25.48±4.60 | p=0.092 |
Race and male sex were analyzed by Chi Square test. All other analyses are ANOVA without comparisons.
Bronchoalveolar lavage (BAL)
Bronchoscopy was performed on eligible and consented subjects at the University of Colorado Hospital Clinical and Translational Research Center (CTRC). Subjects were sedated using standard conscious sedation protocols and bronchoscopy was performed as previously described (Gaydos et al., 2016). Briefly, three to four 50-ml aliquots of sterile, room temperature 0.9% saline were sequentially instilled and recovered with gentle aspiration. The first aspirated aliquot was not utilized in experiments for this investigation. After collection, all subsequent aliquots were combined and immediately centrifuged (900g, 10 min) to separate the cellular component. Approximately 4 x 104 cells were cytospun onto glass slides for immunohistochemical staining.
Immunocytochemistry
BAL cytospins were prepared and immunohistochemistry performed. The slides were fixed in 4% paraformaldehyde for 10 minutes. Following fixation, the slides were rinsed 3 times in phosphate buffered saline (PBS; pH 7.4) for 5 min each and incubated with 0.1 % triton X-100 in PBS for 10 min to permeabilize cells. The slides were rinsed 3 times in PBS for 5 min each and incubated in a blocking solution (0.2% instant nonfat milk in PBS-Tween) for 30 min at room temperature (RT). After a final PBS rinse, slides were incubated 1 hr with rabbit anti-MAA adduct antibody (1:1000) in blocking solution. After incubation, slides were rinsed in PBS and further incubated with horseradish peroxidase conjugated goat anti-rabbit (1:1000; Sigma, St. Louis, MO) in blocking solution for 1 hr. The slides were rinsed 3 times in PBS for 5 min each and stained with chromagen substrate (IMMPACT DAB, Vector, Burlingame, CA) for 3 min at RT. After 3 min, slides were submerged in PBS to stop the reaction. The slides were counterstained with hematoxylin for 1 min at RT and later rinsed with de-ionized water. Following this, the slides were incubated in 0.1% sodium bio-carbonate at RT for 1 min, rinsed in de-ionized water and allowed to air-dry. Finally, slides were per mounted with coverslip. Cells staining positive for MAA-adducts were counted in an average of 10 high power fields (20X) per slide and expressed as percent of total cells positive for MAA adduct.
ELISA for MAA adducts
MAA-adducted proteins were assayed by indirect competitive ELISA as previously described (McCaskill et al., 2011; Xu et al., 1998). Briefly, polystyrene flat bottom plates (96-well) were coated overnight at 4°C in a humidified chamber with 2 μg/mL purified malondialdehyde-acetaldehyde-adducted surfactant protein D (SPD-MAA) antigen in a blocking agent consisting of 1% PBS/Tween (Blotto) for 2 hr at RT. Human lung lavage samples were centrifuged (200g) to remove cells and adjusted to equivalent total protein concentration. Lavage supernatant fluid and SPD-MAA standards were added to a 96-well PVC round bottom plate. Samples were then diluted (1:3) in Blotto and incubated with biotinylated anti-MAA IgG (1:100). The contents of the round-bottomed plate were then transferred to the flat-bottomed plate and incubated with a 1:200 dilution of Streptavidin-conjugated horseradish peroxidase (HRP) at room temperature for 45 min. Plates were developed with tetramethylbenzidine (TMB) substrate in the dark at RT before halting the color reaction with 8 M H2SO4. Plates were read at 450 nm using a Bio-Rad plate reader to determine optical density.
ELISA for immunoglobulin (Ig) formed to MAA adducts
Serum samples from all subjects were screened for the presence of the IgM, IgG, IgA, and secretory IgA (sIgA) isotypes of anti-MAA antibodies as previously described (Anderson et al., 2014). Briefly, ELISA plates were coated with 2 μg/well of either MAA-Albumin or unmodified Albumin. Additional wells were coated with known concentrations of human IgM, IgG, IgA, or sIgA isotype standards (Sigma) from which the relative antibody concentrations were extrapolated. Plates were incubated overnight at 4°C and then washed, blocked with 2% bovine serum albumin, and incubated with 200 μL subject serum (diluted 1:1000). Following incubation at 37°C for 1 hr, a secondary horseradish peroxidase-conjugated goat anti-human antibody specific for IgM (5μ Fc fragment–specific), IgG (Fcγ-specific), IgA (α-chain–specific; Jackson ImmunoResearch), or sIgA (mouse anti-human secretory component, Clone GA-1; Sigma) was added. Plates were developed using TMB substrate, and the absorbance at 450 nm was determined using an MRX II microplate reader (Dynatech).
Statistics
Based on our preclinical data from mice (McCaskill et al., 2011), we observed that BAL measures of malondialdehyde in BAL were 2.8 ± 2 μM in smoke-exposed animals, 8±3 μM in alcohol-exposed animals, and 12.3±4 μM in co-exposed animals. Extrapolating from these animal data, we assumed that human BAL from non-smoking controls would contain 1 μM malondialdehyde; smoking controls’ BAL would contain 3 μM malondialdehyde; AUD subjects’ BAL would contain 6 μM; and subjects with combined exposure would contain 12 μM, with a standard deviation of 5. To achieve power of 90% to detect differences between the groups, an alpha of 0.05 requires ~25 total subjects be enrolled across the 4 groups, or 6–7 individuals per group. Given differences between animals and humans, we planned to enroll at least 10 subjects per group to be sufficiently powered to detect these differences.
Results
MAA positive staining in BAL cells
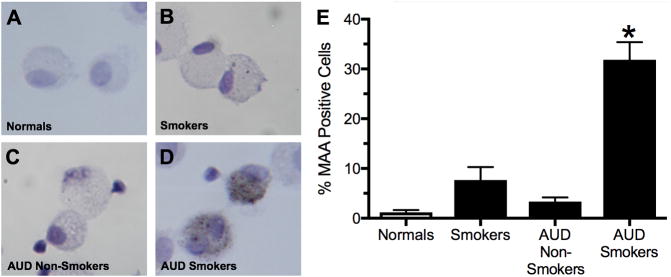
Immunohistochemistry was performed on BAL cytospins using rabbit anti-MAA adduct antibodies to detect those cells staining positive for MAA-adducted proteins (Figure 1). A significant (p<0.05) increase in the percentage of MAA-positive cells were observed in the BAL of AUD subjects who smoked cigarettes when compared to normal non-AUD non-cigarette smokers (Figure 1E). No significant increase in the percentage of MAA-positive cells were observed in normal non-AUD/non-cigarette smokers or in AUD subjects who did not smoke cigarettes (Figure 1E).
Figure 1. Immunohistochemical staining for MAA adducted protein.
BAL cells (mostly macrophages) from non-AUD/non-cigarette smokers (−AUD/−Smoker; panel A), AUD subjects who did not smoke cigarettes (AUD/−Smoker; panel B), cigarette smokers without AUDs (−AUD/+Smoker; panel C) and AUD subjects who smoked cigarettes (+AUD/+Smoker; panel D) stained for MAA adducted protein. Cells stained positive for MAA adduct were counted under microscope and calculated as % of MAA positive cells in the total number of cells counted for each group (D). Values are presented as the mean ± SEM and analyzed by One-Way ANOVA with Tukey’s post hoc multiple comparisons. Subject numbers were as follows: normal nonsmokers (22), smokers (22), AUD nonsmokers (20), and AUD smokers (45). *P ≤ 0.05 vs. other groups.
Detection of antibodies to MAA adducts in serum and BAL fluid samples
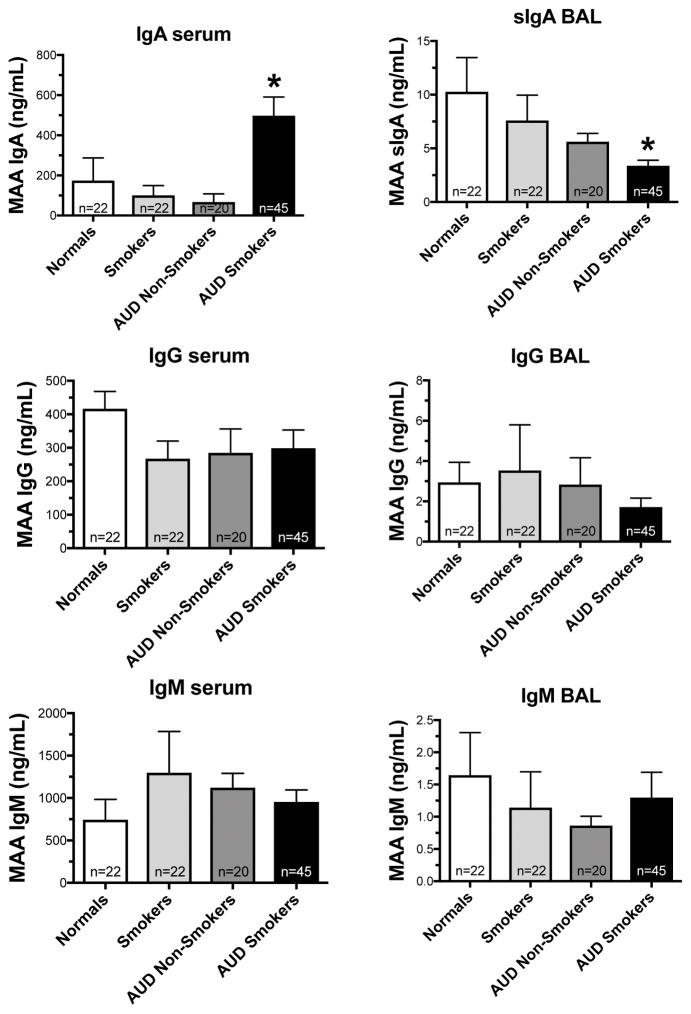
Serum and BAL fluid (without BAL cells) from all subjects were screened for the presence of the immunoglobulins IgM, IgG, and IgA isotypes of anti-MAA antibodies. Serum samples from subjects with alcohol use disorders who smoked (AUD smokers) had significantly increased (p<0.05) levels of serum anti-MAA IgA antibodies compared to −AUD/−smoke (Figure 2A). Conversely, secretory IgA antibodies (sIgA) to MAA in the BAL fluid were significantly lower in AUD smokers (p<0.05) (Figure 2D). No differences were observed in sera or BAL fluid for IgG (Figure 2B, E) or IgM levels (Figure 2C, F).
Figure 2. Detection of antibodies to MAA in serum and BAL fluid samples.
Serum samples from normal, smokers, AUD Nonsmokers and AUD smokers were measured for antibodies isotypes to MAA (panel A- IgA; panel B- IgM; panel C- IgG). BAL fluid samples from the above subjects were measured for antibody isotypes to MAA (panel D- sIgA; panel E- IgM; panel F- IgG) using ELISA. Values are presented as the mean ± SEM and analyzed by One-Way ANOVA with Tukey’s post hoc multiple comparisons. Subject numbers were as follows: normal nonsmokers (22), smokers (22), AUD nonsmokers (20), and AUD smokers (45). *P ≤ 0.05 vs. other groups.
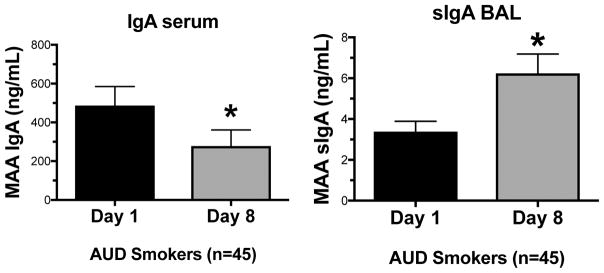
Detection of IgA antibody to MAA in serum and BAL fluid samples after alcohol cessation
Serum samples from AUD smokers were screened for the presence of the immunoglobulin, IgA, at day 1 and after 1 week of alcohol cessation (Figure 3A). Serum samples from subjects with alcohol use disorders who smoke (AUD smokers) had significantly less (p<0.05) circulating anti-MAA IgA antibodies after alcohol cessation compared to day 1 levels (Figure 3A). Conversely, BAL fluid samples from subjects with alcohol use disorders who smoke (AUD smokers) had significantly higher (p<0.05) levels of secretory anti-MAA IgA antibody (sIgA) after alcohol cessation compared to day 1 levels (Figure 3B).
Figure 3. IgA antibodies level after 1 week of alcohol cessation.
Serum and BAL samples from AUD smokers were measured for IgA and sIgA levels, respectively, at day 1 and 1 wk following alcohol cessation using ELISA. Values are presented as the mean ± SEM and analyzed by Student’s t-test. Subject numbers were as follows: AUD smokers (45). *P ≤ 0.05.
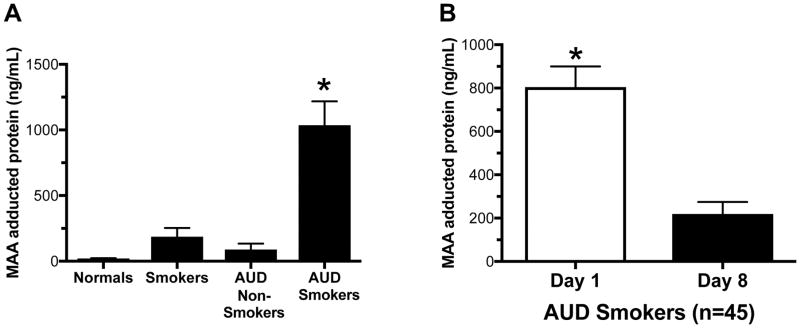
Detection of MAA-adducted protein in BAL fluid samples
Total MAA-adducted proteins in BAL fluid were measured in the established four subject groups. Similar to BAL cells that bound and stained positive for MAA-adducted protein, significant (p>0.0001) concentrations of MAA-adducted proteins were only observed in the BAL fluid of AUD smokers (Figure 4A). MAA adducts were detectable, but not significant, in smoker-only and alcohol-only groups. Consistent with the previous alcohol cessation data, a significant (p<0.0001) reduction in MAA-adducted protein was detected one week after alcohol cessation in the AUD smoker group BAL fluid.
Figure 4. Detection of MAA adducted protein in BALF.
BAL fluid from Non-smoking, No-AUD (normals), AUD subjects who did not smoke cigarettes (drinkers), cigarette smokers without AUDs (AUD nonsmokers) and AUD subjects who smoked cigarettes (AUD smokers) measured for MAA adducted protein by ELISA (A). Values are presented as the mean ± SEM. Subject numbers were as follows: control nonsmokers (22), control smokers (22), AUD nonsmokers (20), and AUD smokers (45). *P ≤ 0.05. BAL samples from the AUD smokers were measured for MAA adducted protein at day 1 and 1 wk following alcohol cessation by ELISA. Values are presented as the mean ± SEM and analyzed by (A) One-Way ANOVA with Tukey’s post hoc multiple comparisons or (B) Student’s t-test. Subject numbers were as follows: AUD smokers (23). *P ≤ 0.05.
Discussion
In this study, for the first time, we show that MAA-adducted protein is formed in the lungs of subjects who abuse alcohol and smoke cigarettes. We measured MAA-adducted protein in BAL fluid of such subjects. We also showed that a significant percentage of BAL cells (mostly macrophages) stained positive for MAA-adducted protein in the AUD smoker group. In addition, we were able to detect antibodies to MAA-adducted protein in both the serum and BAL fluid of subjects who abuse alcohol and smoke cigarettes.
Our previous studies in mice show high levels of acetaldehyde and malondialdehyde in lung after chronic exposure to alcohol and cigarette smoke (McCaskill et al., 2011). We concluded that MAA adducts are detected only in mouse lung of the alcohol and smoke co-exposure group because of the accumulation of high concentrations of these reactive aldehydes. Consistent with this finding, our human subjects study revealed that MAA-adducted protein only could be detected in significant amounts on the lavage macrophages of the AUD smokers group. This furthers supports our previous finding that a co-exposure condition is necessary to generate sufficient amounts of reactive aldehyde leading to the formation of MAA adducted proteins. Likewise, we only measured significant concentrations of MAA-adducted protein in the BAL fluid of AUD smokers, also suggesting that this adduct is formed under unique co-exposure conditions in the lung.
Our study, for the first time, shows that MAA-adducted proteins formed in human lung bind to macrophages. Consistent with our mouse study (McCaskill et al., 2011) positive MAA-adduct staining was observed only in BAL macrophages from AUD subjects who smoked cigarettes. This result also supports our previous in vitro finding that MAA-adducted protein, once formed, rapidly binds to both macrophages (Sapkota et al., 2016) and airway epithelium (Berger et al., 2014). This rapid uptake of MAA-adducted protein by mouse macrophages requires the expression of scavenger receptor A (Sapkota et al., 2016). Several previous studies report that scavenger receptor A (CD204) is the major receptor that binds MAA-adducted proteins (Duryee et al., 2005; Berger et al., 2014). In the absence of the receptor, the effect of MAA is decreased. Future studies examining the potential of CD204 polymorphisms on macrophage receptor expression and MAA-binding function as an indicator of MAA adducted protein-mediated lung injury are warranted.
MAA-adducted proteins have been previously reported to be immunogenic in nature (Thiele et al., 1998). Our study showed a significant increase in the formation of anti-MAA IgA in serum of subjects with AUD who also smoke cigarettes. Previously published studies also report higher levels of anti-MAA IgA in the sera of atherosclerotic patients (Antoniak et al., 2015). Here, for the first time, we measured elevated anti-MAA IgA in serum of AUD subjects who smoke cigarettes. Interestingly, the levels of sIgA in BAL fluid of AUD subjects who smoke cigarettes was significantly lower than those of normal (non-smoking, no AUD) subjects in spite of the higher level of anti-MAA IgA in serum of those same subjects. Although the mechanism of such a reduction in lung sIgA in AUD subjects who smoke cigarettes is unknown, our results suggest that transcytosis of IgA by the epithelium is decreased, leading to the subsequent accumulated level of serum IgA. It is also well established that sIgA plays an important role in antimicrobial lung defense (Mantis et al., 2011). Because people who abuse alcohol and smoke cigarettes are more prone to respiratory infections, a reduced level of sIgA in lung could contribute to the increased incidence of respiratory infections in this patient population. Future studies will address the specific mechanism and impact of MAA-adducted protein formation in the reduction of lung sIgA.
Our study is limited to a relatively small population size and availability of biological samples. We were only able to measure MAA-adducted protein and antibodies levels in serum and BAL of four different groups categorized based upon their alcohol drinking patterns and cigarette smoking habits. While our alcohol cessation data are interesting, further studies involving longer cessation time of both alcohol and cigarette smoking is necessary to fully characterize the role of MAA adducts in such lung injury. We monitored subjects for alcohol abstinence on an inpatient unit. We did not stipulate that these people had to be abstinent from smoking, but did request for them not to smoke during their time in the inpatient setting. Moreover, the study was conducted on a non-smoking campus.
Our study also does not identify a role for Cannabis usage. We did not exclude cannabis users given its legal status in Colorado (where the study subjects were recruited), so marijuana may have impacted the results. In this study, the percentage of marijuana smokers was similar across both smoking groups. Also, smoked cannabis contains many of the same combustion products as tobacco; the two differ based on nicotine and cannabinoids only. However, it would be interesting to look at impact of cannabis only, or combined use of alcohol and cannabis. We are hopeful to expand future studies in this direction. In addition, it is still unknown if the presence of MAA-adducted proteins and decreased formation of secretory anti-IgA antibodies in lung could have a role on progression of respiratory infections and chronic obstructive pulmonary disease. Future studies are required to further establish the role of MAA-adducted protein and anti-MAA antibody formation as mechanistic biomarkers of lung disease in response to cigarette and alcohol exposure. It would be interesting to determine if such would prove useful for the early detection and treatment of lung disease such as ARDS and COPD, both of which have been associated with alcohol abuse (Moss et al., 1996; Sisson, 2007).
In summary, MAA-adducted protein was detected only in the lungs of those AUD subjects who also smoked cigarettes. Furthermore, these adducts were detected on the surface of BAL macrophages in samples obtained from subjects with AUD who also smoke cigarettes. Anti-MAA IgA levels were elevated in serum samples only in subjects with alcohol use disorders who also smoke cigarettes. In contrast to serum, a small, but significant decrease in secreted IgA is observed in the bronchoalveolar lavage fluid of these same subjects. After alcohol cessation for a week, however, the anti-MAA IgA level returned to normal in AUD subjects who smoke cigarettes. This suggests that alcohol and cigarette smoking contribute to the development of unique immunologic reactions possibly associated with alcohol-related lung injury. In conclusion, the presence of MAA-adducted protein in lung and elevated serum IgA should be explored as potential biomarkers for alcohol and cigarette smoke co-exposure injury.
Acknowledgments
This work is supported by the Clinical Resource for Lung Alcohol Investigators (NIAAA R24 AA019661 to ELB), NIAAA R01 AA008769 (to JHS), NIAAA R01 AA017663 (to TAW) and VA I01 BX003635 (to TAW). TAW is the recipient of a Research Career Scientist Award (IK6 BX003781) from the Department of Veterans Affairs.
References
- Anderson DR, Duryee MJ, Shurmur SW, Um JY, Bussey WD, Hunter CD, Garvin RP, Sayles HR, Mikuls TR, Klassen LW. Unique antibody responses to malondialdehyde-acetaldehyde (MAA)-protein adducts predict coronary artery disease. PloS one. 2014;9:e107440. doi: 10.1371/journal.pone.0107440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniak DT, Duryee MJ, Mikuls TR, Thiele GM, Anderson DR. Aldehyde-modified proteins as mediators of early inflammation in atherosclerotic disease. Free Radical Biology and Medicine. 2015;89:409–418. doi: 10.1016/j.freeradbiomed.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Bailey KL, Romberger DJ, Katafiasz DM, Heires AJ, Sisson JH, Wyatt TA, Burnham EL. TLR2 and TLR4 Expression and Inflammatory Cytokines are Altered in the Airway Epithelium of Those with Alcohol Use Disorders. Alcoholism: Clinical and Experimental Research. 2015;39:1691–1697. doi: 10.1111/acer.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JP, Simet SM, DeVasure JM, Boten JA, Sweeter JM, Kharbanda KK, Sisson JH, Wyatt TA. Malondialdehyde-acetaldehyde (MAA) adducted proteins bind to scavenger receptor A in airway epithelial cells. Alcohol. 2014;48:493–500. doi: 10.1016/j.alcohol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo JK, Husten C. Sociocultural influences on smoking and drinking. Alcohol Research and Health. 2000;24:225–232. [PMC free article] [PubMed] [Google Scholar]
- Duryee MJ, Freeman TL, Willis MS, Hunter CD, Hamilton BC, Suzuki H, Tuma DJ, Klassen LW, Thiele GM. Scavenger receptors on sinusoidal liver endothelial cells are involved in the uptake of aldehyde-modified proteins. Mol Pharmacol. 2005;68:1423–1430. doi: 10.1124/mol.105.016121. [DOI] [PubMed] [Google Scholar]
- Gaydos J, McNally A, Guo R, Vandivier RW, Simonian PL, Burnham EL. Alcohol abuse and smoking alter inflammatory mediator production by pulmonary and systemic immune cells. Am J Physiol Lung Cell Mol Physiol. 2016;310:L507–18. doi: 10.1152/ajplung.00242.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Bierut LJ. Cigarette smoking and the risk for alcohol use disorders among adolescent drinkers. Alcoholism: Clinical and Experimental Research. 2006;30:2046–2054. doi: 10.1111/j.1530-0277.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphalia L, Calhoun WJ. Alcoholic lung injury: metabolic, biochemical and immunological aspects. Toxicol Lett. 2013;222:171–179. doi: 10.1016/j.toxlet.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis NJ, Rol N, Corthésy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal immunology. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill ML, Kharbanda KK, Tuma DJ, Reynolds JD, DeVasure JM, Sisson JH, Wyatt TA. Hybrid malondialdehyde and acetaldehyde protein adducts form in the lungs of mice exposed to alcohol and cigarette smoke. Alcoholism: Clinical and Experimental Research. 2011;35:1106–1113. doi: 10.1111/j.1530-0277.2011.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. [PubMed] [Google Scholar]
- on Smoking O, Centers for Disease Control and Prevention. Chemistry and Toxicology of Cigarette Smoke and Biomarkers of Exposure and Harm. 2010. [Google Scholar]
- Sapkota M, Kharbanda KK, Wyatt TA. Malondialdehyde–Acetaldehyde-Adducted Surfactant Protein Alters Macrophage Functions Through Scavenger Receptor A. Alcoholism: Clinical and Experimental Research. 2016;40:2563–2572. doi: 10.1111/acer.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota M, Wyatt TA. Alcohol, aldehydes, adducts and airways. Biomolecules. 2015;5:2987–3008. doi: 10.3390/biom5042987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41:293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele GM, Tuma DJ, Willis MS, Miller JA, McDonald TL, Sorrell MF, Klassen LW. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcoholism: Clinical and Experimental Research. 1998;22:1731–1739. [PubMed] [Google Scholar]
- Wyatt T, Kharbanda K, McCaskill M, Tuma D, Yanov D, DeVasure J, Sisson J. Malondialdehyde-acetaldehyde (MAA) adducted protein inhalation causes lung injury. Alcohol. 2012;46:51–59. doi: 10.1016/j.alcohol.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Thiele GM, Beckenhauer JL, Klassen LW, Sorrell MF, Tuma DJ. Detection of circulating antibodies to malondialdehyde-acetaldehyde adducts in ethanol-fed rats. Gastroenterology. 1998;115:686–692. doi: 10.1016/s0016-5085(98)70148-9. [DOI] [PubMed] [Google Scholar]