Abstract
The TREAT Consortium has carried out clinical studies on alcoholic hepatitis (AH) for over four years. We encountered problems with participant recruitment, retention, and eligibility for specific protocols. To improve our ability to carry out such trials, we reviewed recruitment screening logs, end of study logs, and surveyed study coordinators to learn the reasons for missing patients, why patients declined enrollment, and the number of patients eligible for treatment trials. Associations of the recruited subjects’ demographics with their adherence to follow-up appointments were examined. 387 patients (AH and heavy drinking controls) were enrolled in the observational study and 55 AH patients were recruited into treatment trials. About half of patients identified with AH could not be recruited; no specific reason could be determined for about two thirds of these. Among the patients who gave a reason for not participating, the most common reasons were: feeling too sick to participate, desire to concentrate on abstinence, and lack of interest in research. Approximately a quarter of the AH patients met eligibility criteria for treatment trials for moderate or severe AH and we were able to recruit half to two thirds of those eligible. Approximately 35% of participants in the observational study returned for both 6 and 12 month follow-up visits. We did not identify biopsychosocial or demographic correlates of retention in the study. This analysis revealed that attempts at recruitment into trials for AH miss some subjects because of structural issues surrounding their hospital admission, and encounter a high rate of patient refusal to participate. Nonetheless, more than half of the patients who met the eligibility criteria for moderate or severe AH were entered into clinical trials. Retention rates for the observational study are relatively low. These findings need to be accounted for in clinical trial design and power analysis.
Keywords: alcoholic hepatitis, recruitment, retention, clinical trial, MELD score
Alcoholic hepatitis (AH) is a serious public health problem with a high mortality rate. When severe, as judged by the MELD over 19 or Maddrey discriminant function over 32, mortality approaches 50% within the first 30–90 days; those surviving the acute phase still carry a high risk of dying in the ensuing year (Mathurin and Bataller, 2015). Analysis of hospital and insurance administrative data suggests that the incidence of (AH) is rising (Jinjuvadia and Liangpunsakul, 2015). The only widely used therapy, corticosteroids, is at best modestly effective and has significant contra-indications and side effects. No new therapies are near introduction into clinical practice. Animal models for AH do not fully recapitulate the human disease; thus, clinical research is vital to this field for the development of new treatment modalities and non-invasive diagnostic techniques.
In response to the dearth of treatment options for these patients, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) has funded four consortia to accelerate AH research. The Translational Research and Evolving Alcoholic hepatitis Treatment (TREAT) consortium launched an observational study (TREAT001) in 2012 for patients with (AH), defined as recent onset of jaundice (bilirubin >2 mg/dL), aspartate transaminase (AST) > 50 U/L, and a history of heavy alcohol consumption (> 40 grams per day on average in women and 60 grams per day in men) for a minimum of 6 months and within the 6 weeks prior to study enrollment. The patients underwent clinical evaluation and appropriate laboratory testing to exclude confounding issues (negative markers for autoimmune liver disease and metabolic liver disease; absence of sepsis, shock, cocaine use, or recent drug use with drug-induced liver injury (DILI) potential within 30 days). Co-existing HCV or HBV infection did not mandate a liver biopsy if other features were consistent with AH. With confounding factors, or atypical laboratory tests (AST <50 or >400 IU/mL, AST/ALT ratio <1.5, ANA >1:160 or SMA > 1:80), a liver biopsy (if clinically feasible and the subject has no contra-indications) was required.
TREAT001 also includes heavily drinking controls who are individuals with no history of alcoholic liver disease, AST and alanine aminotransferase (ALT) ≤ 50 U/L, and normal total bilirubin. This study required completion of several survey instruments, a physical examination with anthropomorphic measurements, and collection of biosamples (blood, urine, stool) for archiving. Participants are scheduled to return for follow-up visits at 6 and 12 months. Study participants receive a payment of up to $255 dollars for completing all study visits and vouchers to cover parking expenses when applicable. TREAT also conducts phase 1 and 2 clinical trials for patients with moderate (MELD < 20) and severe AH (MELD >19 but <29). Clinical trial participants are randomized to placebo or active drug and require more frequent follow-up visits during the treatment phase. All treatment trial participants are concurrently enrolled in the observational study.
Recruitment and retention of subjects in clinical trials related to alcohol abuse is difficult. These challenges have been discussed with regard to two very large randomized, controlled trials carried out with NIAAA support over the last several decades, Project MATCH (Zweben et al, 1994) and the COMBINE Study (Zweben et al., 2005). These studies examined various alcohol abuse treatments and required frequent contact as part of their patients’ therapy. To extend these analyses and explore possible additional issues related to recruitment and retention of patients with liver disease in addition to alcohol use disorders, we reviewed four years of experience in TREAT. We examined reasons patients gave for not participating in the studies, and factors correlating with failure to complete 6 and 12 month follow-up visits. To improve patient recruitment for future clinical trials, we analyzed the TREAT001 patients to see what proportion would be eligible for the current trials and our success in recruiting them. Strategies to improve enrollment and retention are suggested, based on the experiences of the study coordinators and investigators.
RECRUITMENT TO THE OBSERVATIONAL STUDY
We retrospectively analyzed our experiences recruiting patients over four years. Recruitment sites included Indiana University, Indianapolis, IN; Mayo Clinic, Rochester MN; and Virginia Commonwealth University, Richmond, VA. We did not initially develop a formal process for tracking possible recruits; we therefore reviewed recruitment screening logs and end of study logs, and surveyed study coordinators to estimate the numbers of patients with AH we encountered and to learn the reasons for missing potentially eligible patients.
We estimated the numbers of patients with AH encountered during the first 4 years of the study (Table 1). There were structural issues in the health systems reported by the coordinators that impacted the study teams’ ability to recruit patients. Most patients were first seen by general medical teams (hospitalists) less familiar with AH (and perhaps with the common overlap between AH and cirrhosis), and who may start therapy before the patients can be seen by consulting hepatologists involved in treatment trials. Additionally, as is the case with many clinical trials, it was difficult to identify AH patients immediately following hospital admission without manual medical record screening. Relying on reminders to the medical teams to refer AH patients for to the study team did not suffice. There were also instances where patients were discharged before study coordinators could speak with them due to the delayed referral. In general, Spanish speaking patients were not enrolled due of the difficulties inherent in working though interpreters and a lack of validated Spanish language study documents and diagnostic instruments.
Table 1.
Reasons for declining to enroll in the TREAT studies
| Total potential alcoholic hepatitis patients | 431 |
| Total alcoholic hepatitis patients enrolled | 218 |
| Total unable to recruit | 213 |
| Reason for declining | Number of individuals (% of total declining) |
| Reason not specified | 136 (63%) |
| Too sick/not feeling well/poor prognosis | 20 (9%) |
| Patient unable to consent | 13 (6%) |
| Not interested in research | 11 (5%) |
| Does not accept alcoholic hepatitis diagnosis /denies drinking/alcoholism | 8 (4%) |
| Homeless | 6 (3%) |
| Wants to focus on sobriety and/or getting better/recovery | 6 (3%) |
| Transportation concerns/unable to complete follow up visits | 5 (2%) |
| Soon to be discharged from the hospital/Going home | 5 (2%) |
| Concerns from patient family or spouse | 5 (2%) |
| Too busy/time concerns/ “too much going on” | 2 (1%) |
| Going to rehab | 3 (1%) |
| No benefit from participating | 2 (1%) |
| Moving soon | 1 (1%) |
| Currently in another research study | 2 (1%) |
This information was gathered from the study coordinators’ recruitment logs. There was variability in what constituted a “potential alcoholic hepatitis patient”, as some hospitalized patients were discharged before a careful chart review could be performed. Thus the total potential patients and total unable to recruit are approximations. Some patients gave more than one reason for not wanting to participate.
We then turned to self-reported reasons for declining to participate in the studies; the patient responses were recorded in recruitment logs and are compiled in Table 1. The study coordinators were also surveyed to capture their recollection of reasons why patients declined participation and the results were similar (Table 2). It is striking that a) only about half of patients identified as likely having AH could be enrolled in a study and b) a reason for declining to participate was available for only about one third, but we did not systematically collect this information or followup with further questioning. In the future, it may be beneficial to ask patients to elaborate on why they are not interested to make sure they understand the information they are given about AH and the study. The other reasons fell into several broad categories that also overlapped with reasons participants failed to complete the study. Common reasons cited by study coordinators for participants being lost to follow-up or withdrawing from the study were inability to contact participants, relocation or lack of transportation, and lack of interest in research.
Table 2.
Results of survey of research coordinators
| What were the reasons given by patients for not participating in the observational study (TREAT001) and for treatment trials? | This will not benefit me. Not interested. I wasn’t drinking that much. I am going to quit. I quit drinking already. Sometimes family members advise against enrolling. No transportation. Denial of active drinking. Sounds like too much work. Wants nothing to do with research. Participated in research in the past and doesn’t want to do again. Want to focus on getting better. Going home to drink. |
| How were patients identified for possible enrollment? | Hepatology consults, messages from admitting fellows, referring clinics, emergency department, hepatology inpatient service, hospital admission logs. |
| Did you encounter difficulties connecting with patients referred for possible enrollment? | Failing follow-up appointments. |
| What questions did families and patients frequently raise about enrolling in one of the studies? | What is the benefit for me? How much travel time will this take? How will this affect my care? Will I be able to maintain privacy of my records? |
| Do you think that the patients and families understood the serious nature of alcoholic hepatitis? | No (reported from all sites). |
| Did the desire of the patient or family for alcoholism treatment overshadow their willingness to participate in the trial (would incorporating alcoholism treatment more explicitly in the alcoholic hepatitis trials improve recruitment?) | Probably. Maybe. No, treatment wouldn’t increase enrollment. |
| What factors seemed to increase the patients’ willingness to participate? | Monetary compensation (including for transportation or cell phones). Extra medical care. Patients facing legal issues may wish to enroll as a show of good intentions. |
| Were there actions of the primary team caring for the patients that resulted in them being ineligible or un-recruitable for studies you were recruiting for? | Starting various treatments prior to consultation (steroids, antibiotics). |
| What would make your job as a research coordinator easier, more efficient, and more rewarding? | Less searching for patients to enroll. More physician involvement with recruiting. |
One category of responses reflected a poor understanding of AH and research by patients. Noted issues included the observation by coordinators that patients did not understand the severity of their disease, felt too sick to participate, and did not see any personal benefit from participation. A second category revolved around social issues: homelessness, lack of transportation, anticipated moving to another locale, and study coordinators noted that financial issues also played an important role. Third, it was interesting that a number of patients did not accept the diagnosis of AH because they did not feel they drank excessively (or at all!). There were overtones of distrust for researchers or research studies, including a concern about the privacy of the records. Privacy concerns could reflect the stigma of alcohol use disorders or concern about the impact of this diagnosis on family or employers. One interesting observation that touches on the consent process was the comment that patients may feel that their show of altruism (by participating in medical research) might be beneficial to them in pending legal proceedings: such patients might need to be considered vulnerable in the sense that prisoners are vulnerable.
ELIGIBILITY FOR CLINICAL TRIALS
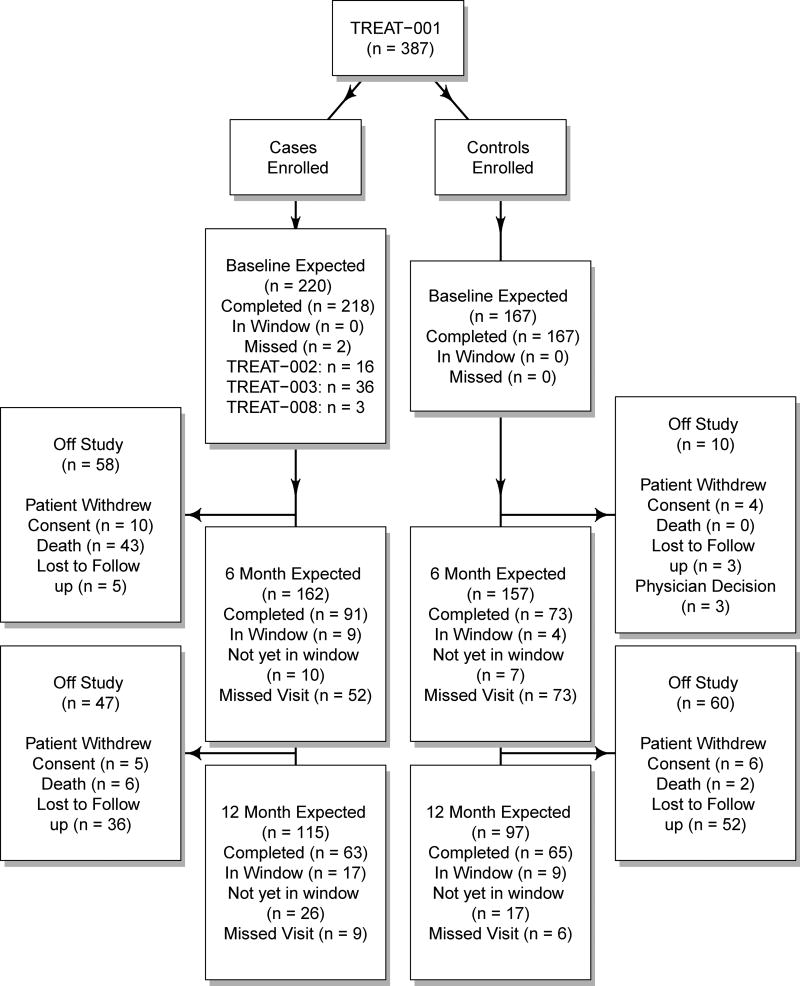
The clinical characteristics of the patients recruited into the TREAT001 study have been previously reported (Liangpunsakul et al., 2016). The patients were mainly middle-aged white men who were very heavy drinkers. Over half had severe AH (MELD >19). Twelve month mortality was under 2% for control subjects, and about 25% for AH cases. All patients in the clinical trials were first enrolled in TREAT001, so in order to assess the feasibility of enrolling subjects into future clinical trials, the entry characteristics and laboratory values of AH patients recruited through 6/27/2016 into TREAT001 were compared to exclusion and inclusion criteria for TREAT002, TREAT003, and TREAT008, three ongoing clinical trials for assessing efficacy of therapeutic agents for AH. The flow of patients into these studies, and their retention, is summarized in Figure 1.
Figure 1.
CONSORT diagram of the recruitment and retention of patients with alcoholic hepatitis (cases) and heavy drinking controls (controls). The windows for completing a followup visit were returning between 3 and 9 months after enrollment for the 6 month time point, and returning between 9 and 15 months for the 12 month time point.
TREAT002 sought to enroll patients with moderately severe AH (MELD between 11 and 19) for treatment with an FXR agonist. TREAT003 enrolled patients with severe AH (MELD >19) for treatment with oral immunoglobulin against lipopolysaccharide in addition to steroids. TREAT008 studies the effect of IL-22 in patients with moderate or severe AH. Each study had exclusion criteria related to renal function, treatment with other agents prior to enrollment, and other co-morbidities. There were 218 AH cases in the TREAT001 study with at least some data collected at their baseline visit. Of this entire group, 58 (27%) met eligibility criteria for TREAT 002 and 41 (19%) were eligible for TREAT003 at baseline. TREAT001 began about 2 years before the treatment trials, and there were slightly over 100 AH patients enrolled while the treatment trials were recruiting patients. After accrual for TREAT002 began, 24 of 102 (23.5%) were eligible and 13 (54.2%) of these were enrolled. Two other subjects eligible for TREAT002 were enrolled in TREAT008. After accrual for TREAT003 began, 27 of 109 (24.8%) of TREAT001 individuals were eligible for entry, and 18 (67%) of these individuals were successfully enrolled. The most common cause of ineligibility for TREAT002 was a high MELD score. For TREAT003 candidates with severe AH, ineligibility was most commonly due to the treating physicians not intending to treat with steroids, creatinine elevated above 1.5 mg/dl, and co-existing viral hepatitis. The TREAT008 study was activated most recently and therefore a smaller number of AH patients were encountered. Ten of 21 (67.1%) were eligible for entry into TREAT008 and we enrolled 3 (30%) of them; 3 individuals who were eligible for TREAT008 were enrolled in either TREAT002 or TREAT003. The numbers of patient eligible for the clinical trials at the time of recruitment into TREAT001 is lower than the numbers actually recruited (Figure 1), because the clinical status of some patients changed after their initial enrollment; in particular, 18 patients who were not eligible for TREAT003 at baseline suffered worsening of their AH severity scores and became eligible.
RETENTION OF PATIENTS IN THE STUDIES
We compared baseline demographic and biopsychosocial information between the patients in the TREAT001 cohort who returned for follow-up at 12 months vs those who did not return to determine patient and social factors that reduced the likelihood of the participant completing follow-up visits. We also reviewed research coordinator logs to learn of other factors which interfered with retention. For continuous variables, a t-test was employed to test for a difference in means by AH status, while for categorical variables, a Chi-square test was used.
From the entire TREAT cohort (AH cases and heavy drinking controls), a total of 310 participants had been followed long enough to either miss their 6 month visit or successfully complete it. Of those participants, 164 or 52.9% (91 (58%) of cases and 73 (48%) of controls) returned for the 6 month follow-up visit (which we prospectively defined as returning between 3 and 9 months after recruitment). A total of 263 participants had been followed long enough to either miss their 12 month visit or successfully complete it. Of these participants, 128 or 48.7% (63 (49.6%) of cases and 65 (47.8%) of controls) returned for a 12 month follow-up visit (which we prospectively defined as returning between 9 and 15 months after recruitment), although 32 (19 cases, 13 controls) of these individuals failed to return for the 6 month visit. Only 96 or 36.5% (44 (34.6%) of cases and 52 (38.2%) of controls) returned for both the 6 and 12 month visits.
We examined demographic and other characteristics of the study participants for correlations with failure to return for follow-up visits. There was no evidence of an effect for sex, age, or race of the subject on the likelihood of dropping out by 12 months. Similarly, two social factors we examined were not found to correlate with drop-out: marital status (married, divorced or widowed, or single/never married) or level of educational achievement (less than year 10, completed high school, trade school/some college, standard college/university, and completed graduate/professional). Several additional biopsychosocial factors were examined: self-rating of health using the first question of the SF36 questionnaire, treatment arm (cases vs controls), MELD score, and regular use of marijuana. There was no statistical evidence that these factors were associated with the participant returning for 12 month visit.
RECOMMENDATIONS TO IMPROVE RECRUITMENT AND RETENTION IN TRAILS FOR ALCOHOLIC HEPATITIS
Alcoholic hepatitis and heavy drinking controls were difficult to identify early in their medical trajectory, to recruit, and to retain for follow-up visits. Fewer than half of the patients identified as having probable AH were enrolled in any study, including a purely observational study. The proportion eligible for trials was rather low (20–25% based on TREAT002 and 003 criteria) and our ability to recruit them was fair at best (50–67%). Only about a third of those enrolled in the observational study completed both 6 and 12 months visits. It was striking that there was no difference in retention rates between the heavy drinking controls and the patients with AH, indicating that the underlying problem with retention is related to the alcohol use disorder, not the liver disease.
These challenges will need to be overcome to conduct larger phase 3 studies. We perceive three different types of problems to be addressed: identification of the patients, recruitment into trials, and retention in the studies. These issues can be addressed with revised study design, improved communication by the study teams during the enrollment and throughout the study, and development of educational materials for patients and families.
The structural issues in the health systems which interfere with identifying patients may be the easiest to address. Identification of AH patients admitted to the hospital can be facilitated using IRB-approved automated screening of patients’ admission laboratory data by the electronic health record. Once possible participants are identified, research teams can contact the patient’s physician for permission to engage the patient. This approach facilitates finding patients admitted over the weekend/holidays or leaving the hospital after a short stay (in particular those with moderately severe AH). It appears that there is insufficient appreciation by hospital medicine teams that AH commonly coexists with alcoholic cirrhosis, leading to misclassification as simply decompensated cirrhosis, rather than AH. The study coordinators noted that the involvement of consulting physicians leading the trial was very important in recruitment, which could reflect the need for greater patient education about this little known liver disease.
The recruitment challenges seem to be related to individual patient characteristics, but with specific reasons for declining to participate available for only one third of the patients, we suggest future studies systematically explore these reasons to improve our ability to recruit. Improved communication between study teams and potential participants may overcome this. Knowledge of the serious nature of AH (40–50% 90 day mortality) by patients and families may generate interest in clinical research participation to improve their outcome. Some patients declined to participate because they did not feel the study would provide any benefit or they wanted to prioritize sobriety and recovery. To address those concerns, incorporation of medication-assisted treatment, possibly with baclofen (Addolorato et al., 2007), could be considered in future trials. A double blind study of the effectiveness of baclofen in patients with alcoholic liver disease is underway in Australia (Morley et al., 2013); it will be important to clarify the effect of AH on the pharmaco-dynamics and -kinetics of baclofen. The added emphasis on treatment of alcoholism per se could rightly be promoted as a benefit of participation in the trial, and reinforced as one of the reasons follow-up is essential. Trial sponsors would need to support this treatment phase of the trial, if the participant’s insurance plan does not.
Other barriers to enrollment include ineligibility because of incarceration and cognitive impairment from encephalopathy. Language barriers with Spanish-speaking patients also hinder recruitment. Hispanic patients may have an increased risk of AH (the PNLPA3 variant, common in Hispanics, which confers risk of non-alcoholic steatohepatitis (Romeo et al., 2008) and alcoholic cirrhosis (Stickel et al., 2011; Buch et al. 2015) also increases risk for AH (Liangpunsakul, et al, 2016). Future studies could incorporate validated and IRB-approved Spanish translations of consent documents, surveys, and other data collection instruments (e.g., the Time Line Follow-Back survey, and instruments used to detect concomitant mental health disorders).
The social issues of patients with alcoholism are more difficult to address. Lack of transportation, parking costs, and other financial issues could be dealt with through gift cards given at each visit. Involvement of family and friends in the review of drinking history, and counseling of the patient, might overcome the denial of hazardous drinking. Regular contact with participants between study visits can help keep study coordinators aware of changing contact information and relocation plans. To address distrust of research, study teams must explain the human subjects’ protection and privacy regulations. A certificate of confidentiality (COC) might reassure the patients that the diagnosis of an alcohol use disorder could not be used against them at work or in legal situations. However, there may by limitations that would prevent investigators from obtaining a COC. The TREAT consortium was unable to obtain a COC due to federally mandated documentation required of investigators that conflicted with the guidelines of the COC.
A number of patients were excluded from trials because of concomitant viral hepatitis or other medical illness (HIV infection, advanced heart failure, chronic obstructive pulmonary disease, renal failure, active substance abuse). These issues are difficult to avoid because such exclusions may be required by sponsors for specific trials. Even those eligible for a clinical trial commonly declined enrollment. The barriers to trial participation mirrored those affecting enrollment into the observational study. A more significant challenge is the increased time commitment required of clinical trial participants. The additional study visits required for participation can be too burdensome for working individuals and those with transportation challenges. Offering study visits outside of standard working hours and providing additional assistance with transportation may lessen the participant burden of participating in clinical trials. Study coordinators also noted cases where eligible patients were unable to participate due to homelessness and the resulting inability to store investigational products in a secure and temperature-stable location.
Lastly, retention in the TREAT001 study was rather poor, with about half of participants returning at 6 months and only 35% completing both 6 and 12 month visits. We were surprised that none of the demographic factors we assessed were associated with study retention. The end of study logs revealed that the patients move often, change telephone numbers, and have less reliable contact information. Family support may be lacking because of the destructive effect of alcohol dependence on relationships. Participants are, of course, at risk of relapse to heavy drinking during the follow-up period. An interesting observation was that many participants missed the 6 month visit but returned at 12 months. This suggests that visits are not simply missed because individuals decided against further participation in the study. With improved and more frequent communication between study coordinators and participants, it may be possible to improve followup attendance. Future efforts may explore how to manage the participants remotely for some of the follow-up visits. A long distance approach would address the inconvenience of return visits.
These issues have been analyzed in detail by the leaders of Project MATCH and the COMBINE Study. Project MATCH excluded individuals with ongoing use of other substances, low social stability (i.e., being unable to provide a “locator” who would be able to help investigators follow-up), and dual diagnoses of other mental health problems (Zweben et al., 1994). The COMBINE study tested pharmacological agents to reduce drinking reported their experience in more detail (Zweben et al., 2005). They attempted to risk stratify the potential enrollees, which included assessment of the patients’ insight into their drinking, possible negative attitudes about pharmacotherapy, interest in reducing drinking (four days of abstinence were required before enrollment), and willingness to participate in a placebo-controlled study. Red flags during recruitment included the patient complaining about demands of the study, failing to return calls, or being unable to schedule appointments. Much attention was devoted to maintaining high retention, including reducing the time demands of follow-up visits, asking for explanations for why participants missed follow-up visits, and addressing the potential embarrassment of patients who had relapsed into heavy drinking.
In conclusion, we offer the strategies described in Table 3 as means to improve the ability to recruit and retain patients with AH and heavy drinking controls for future studies. The experience of the TREAT consortium provides data that will be useful in power analysis and the design of these much needed trials.
Table 3.
Recommended strategies for recruitment and retention of AH patients in clinical trials
| To improve recruitment | To improve retention |
|---|---|
|
| |
|
|
Acknowledgments
Financial support/Sources of funding: This study is supported by NIH/NIAAA 5U01AA021840, AA 021893, AA021908, and AA 021886 to the TREAT (Translational Research and Evolving Alcoholic hepatitis Treatment) consortium.
Abbreviations
- AH
Alcoholic hepatitis
- MELD
Model for end stage liver disease
Footnotes
Conflicts of interest: None
Roles of authors
All authors have read and approved the manuscript for submission. All have made a substantial contribution to the conception, design, gathering, analysis and/or interpretation of data and a contribution to the writing and intellectual content of the article; and acknowledge that they have exercised due care in ensuring the integrity of the work
References
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D'Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–1922. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- Buch S, Stickel F, Trepo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M, Rietschel M, McQuillin A, Frank J, Kiefer F, Schreiber S, Lieb W, Soyka M, Semmo N, Aigner E, Datz C, Schmelz R, Brückner S, Zeissig S, Stephan AM, Wodarz N, Devière J, Clumeck N, Sarrazin C, Lammert F, Gustot T, Deltenre P, Völzke H, Lerch MM, Mayerle J, Eyer F, Schafmayer C, Cichon S, Nöthen MM, Nothnagel M, Ellinghaus D, Huse K, Franke A, Zopf S, Hellerbrand C, Moreno C, Franchimont D, Morgan MY, Hampe J. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47:1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- Burza MA, Molinaro A, Attilia ML, Rotondo C, Attilia F, Ceccanti M, Ferri F, Maldarelli F, Maffongelli A, De SA, Attili AF, Romeo S, Ginanni CS. PNPLA3 I148M (rs738409) genetic variant and age at onset of at-risk alcohol consumption are independent risk factors for alcoholic cirrhosis. Liver Int. 2014;34:514–520. doi: 10.1111/liv.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro AJ, Torres JL, Miron-Canelo JA, Gonzalez-Sarmiento R, Laso FJ, Marcos M. Systematic review with meta-analysis: the I148M variant of patatin-like phospholipase domain-containing 3 gene (PNPLA3) is significantly associated with alcoholic liver cirrhosis. Aliment Pharmacol Ther. 2014;40:571–581. doi: 10.1111/apt.12890. [DOI] [PubMed] [Google Scholar]
- Jinjuvadia R, Liangpunsakul S Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in Alcoholic Hepatitis-related Hospitalizations, Financial Burden, and Mortality in the United States. J Clin Gastroenterol. 2015;49:506–511. doi: 10.1097/MCG.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liangpunsakul S, Puri P, Shah VH, Kamath P, Sanyal A, Urban T, Ren X, Katz B, Radaeva S, Chalasani N, Crabb DW. Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Effects of Age, Sex, Body Weight, and Quantity of Alcohol Consumption on Occurrence and Severity of Alcoholic Hepatitis. Clin Gastroenterol Hepatol. 2016;14:1831–1838. doi: 10.1016/j.cgh.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015;62:S38–46. doi: 10.1016/j.jhep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley KC, Leung S, Baillie A, Haber PS. The efficacy and biobehavioural basis of baclofen in the treatment of alcoholic liver disease (BacALD): study protocol for a randomised controlled trial. Contemp Clin Trials. 2013;36:348–355. doi: 10.1016/j.cct.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickel F, Buch S, Lau K, Meyer zu Schwabedissen H, Berg T, Ridinger M, Rietschel M, Schafmayer C, Braun F, Hinrichsen H, Günther R, Arlt A, Seeger M, Müller S, Seitz HK, Soyka M, Lerch M, Lammert F, Sarrazin C, Kubitz R, Häussinger D, Hellerbrand C, Bröring D, Schreiber S, Kiefer F, Spanagel R, Mann K, Datz C, Krawczak M, Wodarz N, Völzke H, Hampe J. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology. 2011;53:86–95. doi: 10.1002/hep.24017. [DOI] [PubMed] [Google Scholar]
- Zweben A, Donovan DM, Randall CL, Barrett D, Dermen K, Kabela E, McRee B, Meyers R, Rice C, Rosengren D, et al. Issues in the development of subject recruitment strategies and eligibility criteria in multisite trials of matching. J Stud Alcohol Suppl. 1994;12:62–69. doi: 10.15288/jsas.1994.s12.62. [DOI] [PubMed] [Google Scholar]
- Zweben A, Barrett D, Berger L, Murray KT. Recruiting and retaining participants in a combined behavioral and pharmacological clinical trial. J Stud Alcohol Suppl. 2005;(15):72–81. doi: 10.15288/jsas.2005.s15.72. [DOI] [PubMed] [Google Scholar]