Abstract
Objective
The relationship between placental and fetal brain growth is poorly understood, and difficult to assess. The objective of this study was to interrogate placental and fetal brain growth in healthy pregnancies and those complicated by fetal growth restriction (FGR).
Study Design
In a prospective, observational study, pregnant women with normal pregnancies or pregnancies complicated by FGR underwent fetal MR imaging. Placental, global and regional brain volumes were calculated.
Results
114 women (79 controls and 35 FGR) underwent MR imaging (median GA 30 weeks, range 18 –39). All measured volumes increased exponentially with advancing GA. Placental, total brain, cerebral and cerebellar volumes were smaller in FGR compared to controls (p<0.05). Increasing placental volume was associated with increasing cerebral and cerebellar volumes (p<0.05).
Conclusion
Quantitative fetal MRI can accurately detect decreased placental and brain volumes in pregnancies with FGR and may provide insight into the timing and mechanisms of brain injury in FGR.
Introduction
Acute and chronic placental dysfunction is associated with both short- and long-term neurologic injury and developmental delays. Chronic placental dysfunction most commonly presents with fetal growth restriction (FGR) in utero, when it fails to adequately meet the needs of the developing fetus (1). With chronic fetal hypoxemia and nutrient deprivation, the fetal cardiovascular system may adapt with preferential shunting of circulating oxygen and nutrients to the developing brain (2). Initially proposed to be a neuro-protective mechanism for the developing brain, recent evidence suggests that this type of “brain-sparing” detected by Doppler sonography also is associated with short- and long-term neurologic injury (3–6). Nonetheless, delays in brain growth, estimated by standard fetal biometrics, is associated with neurodevelopmental delays, even in the presence of these cardiovascular adaptations (7, 8). It is hypothesized that placental disease may result in deficient trans-placental transport of essential nutrients necessary for adequate brain development and these deficiencies may not be reflected by changes in the feto-placental circulation. While placental disease likely precedes fetal brain maldevelopment, the ability to detect placental failure prior to the onset of FGR remains problematic. Recent evidence suggests that magnetic resonance imaging (MRI) of the placenta offers additional information on qualitative vascular development of the placenta than Doppler analysis alone (9). While placental volumetry is associated with birth weight and the detection of small for gestation (SGA) infants (10, 11), the relationship between placental volumetry and fetal brain development has not been well studied. The objective of this study was to interrogate placental and fetal brain growth in healthy pregnancies as well as pregnancies complicated by FGR. We hypothesized that fetal brain volume would be reduced in FGR, and related to placental volume.
Methods
Subjects
Subjects were recruited prospectively into a longitudinal, observational study on placental-fetal development throughout the second half of gestation in which MRI was performed up to two time points in the fetal period, to acquire data between 18 and 40 weeks gestation; women recruited in the second trimester were eligible to undergo a second MRI in the third trimester This report includes data from the first fetal MRI study and clinical data from the immediate postnatal period. The study was approved by the institutional review board of the Children’s National Health System (Protocol Number 1732) and written informed consent was obtained from all subjects.
Healthy controls were recruited from community obstetric offices and pregnant volunteers were eligible if medical record review confirmed a normal prenatal history, including screening laboratory and ultrasound studies. Women with pregnancies complicated by FGR were recruited from regional Maternal-Fetal Medicine practices if the following criteria were met: singleton pregnancy with estimated fetal weight < 10th percentile (12) and either (1) abnormal Doppler sonography of the umbilical and/or middle cerebral arteries, specifically an umbilical artery pulsatility index > 95% or cerebroplacental ratio <1 or (2) evidence of impaired somatic growth where abdominal circumference lagged head circumference > 1week for expected gestational age (13, 14). Exclusion criteria for both healthy pregnancies and pregnancies complicated by FGR were multiple-gestation pregnancy, known or suspected congenital infection, dysmorphic features or dysgenetic lesions of the fetus, or documented chromosomal abnormalities. These criteria were meant to best identify growth disturbances secondary to placental insufficiency. We also excluded patients with any maternal contraindication to MRI. Enrolled subjects found to have structural abnormalities on fetal MRI or postnatal confirmation of a genetic syndrome were subsequently excluded from the analysis. Healthy controls did not undergo Doppler testing.
Demographic and Clinical Data
Gestational age was calculated based on first-trimester ultrasound measurement or last menstrual period if unavailable; women with uncertain dates were excluded. Clinical and demographic data were collected for each subject through medical chart review and/or subject questionnaire, including maternal parity, maternal health conditions and medications, fetal gender, gestational age at delivery, birth weight, length and head circumference. Anthropomorphic measures collected at birth were corrected for gestational age using the Fenton growth chart calculations (15). Infants with birth weights < 10th centile for gestational age were categorized as small for gestational age (SGA), and those with birth weights 10–90th centile were categorized as appropriate for gestational age (AGA) using the Fenton growth chart for weight and gender (16). Placental pathology findings were collected, and trimmed placental weight at delivery were corrected for gestational age and gender (17).
Fetal Ultrasonography
All pregnancies complicated by FGR underwent complete fetal sonographic assessment of both anatomy and Doppler evaluation as part of the study protocol on the same day as the MRI study using a LOGIQ E9 ultrasound scanner (GE Healthcare, WI). Abdominal circumference, head circumference, femur length and estimated fetal weight were measured and plotted according to gestational age (12). Uterine artery, fetal middle cerebral and umbilical arterial flow velocities were measured using a pulse-wave Doppler, and pulsatility and resistance indices were calculated. The cerebroplacental ratio was calculated by dividing the middle cerebral artery pulsatility index by the umbilical artery pulsatility index (18). All sonographic studies were reviewed by a single attending radiologist (D.B.).
Fetal MRI
All MRI scans were performed on a 1.5T Discovery MR450 scanner (GE Healthcare, Milwaukee, Wisconsin) using an 8-channel surface receive coil (USAI, Aurora, OH). Single shot fast spin echo (SSFSE) T2-weighted images were performed as follows: for the placenta, fat suppressed with TE 160ms, TR 1100ms, FOV 420 × 420mm, 4mm slice thickness and 40 to 60 consecutive slices for full placental coverage in the axial plane. For the fetal brain, TE 160ms, TR 1100ms, FOV 320 ×320 mm, 2mm slice thickness and 40 to 60 consecutive slices for full brain coverage in all 3 orthogonal plans (axial, coronal, sagittal). No contrast or sedation was used for any of the imaging studies. Fetal MRI studies were reviewed by an attending pediatric neuroradiologist who was blinded to FGR case versus control status (G.V.). Any abnormalities of brain development, maturation or the presence of dysgenetic or acquired brain lesions were documented.
Volumetric MRI Analysis
The placenta was manually outlined in all three planes using ITK-SNAP software (19) and the volume was calculated in cm3 (20) (Figure 1). The fetal brain was reconstructed into 3D high resolution images and segmented into parcellated brain volumes using an atlas-based approach, including motion correction (21, 22), and each automated segmentation was visually inspected and manually corrected by a trained expert. Cerebral, cerebellar and brainstem volumes were calculated in cm3, with total brain volume defined as the sum of the previous three volumes.
Figure 1.
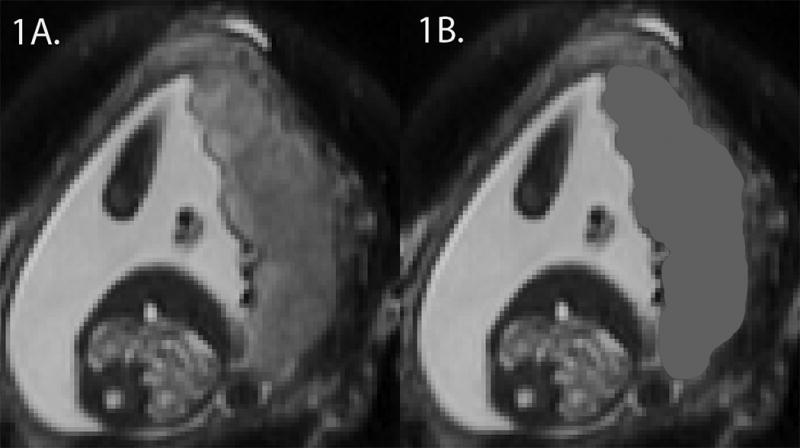
Axial T2-weighted image of the maternal abdomen (1A) and with placental mask in grey (1B) at 25 weeks gestation.
Statistical Analysis
Comparison of demographic and clinical characteristics between cases and controls were performed with t-test analysis. Sample size assessments were completed using PASS assuming a 2-tailed type 1 error of p=0.05; in order to achieve 80% power to detect a moderate difference (0.65 SD) between groups, the study would require a sample size of 40 per group (23, 24). To account for the non-parametric nature of the volumetric measures, median regression analysis was conducted to assess the association between fetal growth restriction (FGR) and placental and brain volumes, adjusting for gestational age at the time of the MRI, and compared to controls. Sub-group analysis was also conducted for abnormal fetal Doppler measures within the FGR group. Logistic regression and median regression analyses were used to determine important associations of in utero MRI volumes with clinical outcomes at birth, including infant size, prematurity, and placental pathology after delivery. All analyses were performed using Stata 13 (25).
Results
Characteristics of our cohort
A total of 114 pregnant women were enrolled, 79 healthy controls and 35 with pregnancies complicated by FGR 16 of which also had abnormal Doppler studies. Fetal MRI studies were performed at a median gestational age (GA) of 30 weeks (range: 18 2/7 to 39 1/7 weeks). Demographic and diagnostic comparisons are presented by group in Table 1. In the group of healthy controls, there were no maternal or neonatal complications noted, and all infants were born at term; median birth weight was 3443 grams (range 2634 – 4483 grams) and mean z-score was 0.1. In the FGR cohort, 7 of the 35 pregnant women were diagnosed with hypertension and/or preeclampsia, although none of the women had other risk factors for FGR, such as diabetes mellitus, renal or auto-immune vascular disease. The median GA at birth for the FGR cohort was 36 completed weeks, although with a notably wider range of 28 to 41 weeks as 29% of the FGR infants were born prematurely. The mean z-score for birth weight was −1.6, 55% of infants were small for gestational age (SGA) at birth, and 42% of infants had birth weights greater than the 10th centile for GA.
Table 1.
Demographics and clinical characteristics of our cohort
| Control (n=79) |
FGR (n=35) |
P-value | |
|---|---|---|---|
| Gestational Age at MRI (weeks) | 30.1 (18 – 40) | 30.8 (22 – 37) | 0.12 |
| Maternal Age (years) | 26 (18–42) | 22 (18 – 41) | 0.06 |
| Male Gender, n (%) | 32 (41%) | 14 (40%) | 0.95 |
| Race or Ethnicity, n (%) | 0.11 | ||
| Black | 45 (57%) | 29 (83%) | |
| White | 18 (23%) | 3 (9%) | |
| Hispanic | 9 (11%) | 2 (6%) | |
| Other or Unknown | 7 (9%) | 1 (2%) | |
| Asymmetric Growth, n (%) | n/a | 25 (71%) | |
| Abnormal Doppler US, n (%) | n/a | 16 (46%) | |
| Gestational Age at Birth (weeks)f | 39 (37–41) | 37 (28 – 41) | < 0.01 |
| Birth Weight (grams)€ | 3443 (2634 – 4483) | 2284 (335 – 3688) | < 0.01 |
| Maternal Hypertensive Disorders, n (%)§ | N/A | 7 (20%) |
Data presented as median (range), unless otherwise noted;
N/A = not applicable;
GA at birth available for 72 controls and 34 FGR;
Birth weight data available for 65 controlsand 34 FGR; 15 patients were lost to follow-up;
Includes chronic hypertension, preeclampsia and HELP syndrome
Comparison of placental volume in FGR versus healthy fetuses
Placental volume increased with gestational age for both control and FGR fetuses (Figure 2). Controlling for gestational age at the time of fetal MRI, the FGR group had significantly smaller median placental volume compared to controls (414.3 cm3 vs 581.2 cm3, p<0.001) (Figure 3). The subset of FGR cases with abnormal fetal umbilical artery Doppler studies had lower median placental volumes compared to FGR cases with normal Doppler studies (385.4 cm3 vs. 485.6 cm3, p< 0.03), however placental volume was not associated with maternal uterine artery Doppler studies (p=0.29), fetal middle cerebral artery Doppler studies (p=0.56) or the CPR measured at the time of fetal MRI study (p = 0.84).
Figure 2.
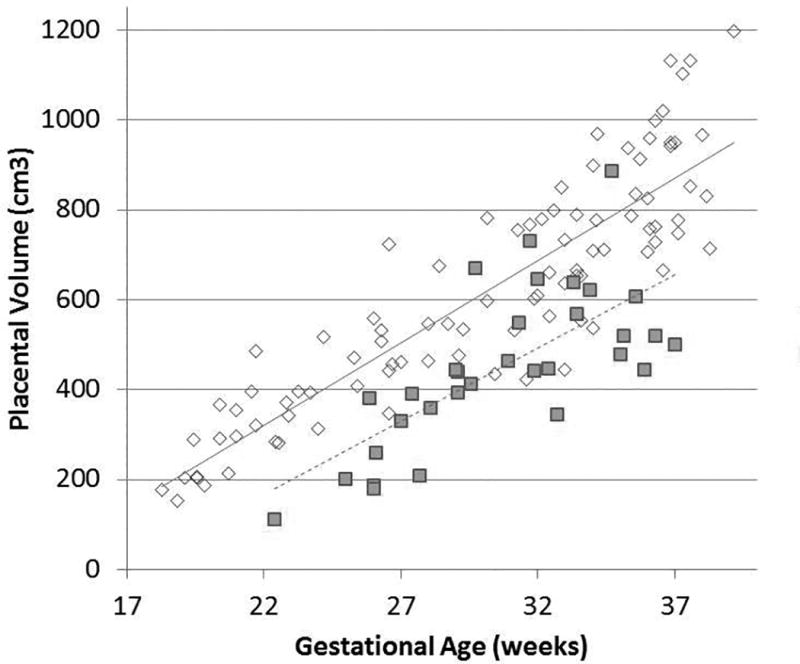
Placental volume, in cm3, increases with advancing gestational age for both populations, however overall placental size is smaller in FGR. Healthy control pregnancies are represented by open diamonds, and pregnancies complicated by FGR are denoted by closed squares.
Figure 3.
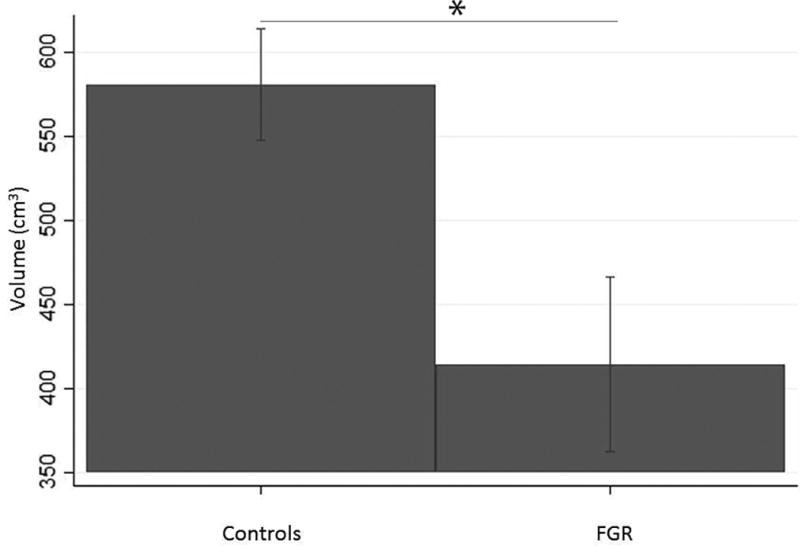
Median placental volume (corrected for gestational age) in healthy controls and FGR pregnancies, demonstrates significantly smaller volumes in FGR compared to controls.
Comparison of brain volume in FGR versus healthy fetuses
All brain volumes (i.e., cerebral, cerebellar, brainstem) increased with gestational age for both control and FGR fetuses. Controlling for gestational age at the time of MRI, compared to controls, the FGR group had significantly smaller median cerebral, cerebellar and total brain volumes (p < 0.001 for all) but no significant difference in brainstem volume (Table 2). FGR cases with abnormal fetal umbilical artery Doppler studies had significantly smaller brain volumes compared to FGR cases with normal Doppler studies. Interestingly, brainstem volume was preserved, without significant differences detected among the three groups. Doppler studies of the middle cerebral arteries (MCA PI) in FGR were not associated with volumetric measures of global or regional brain volumes (p=0.41 – 0.89).
Table 2.
Placental Volume and Brain Volumes in FGR and controls
| Control (n=79) |
All FGR (n-35) |
FGR with Abnormal Dopplers (n=16) |
FGR without Abnormal Dopplers (n=19) |
|
|---|---|---|---|---|
| Placenta | 581.2 | 414.3 (p < 0.01) | 354.9 (p < 0.01) | 421.1 (p < 0.01) |
| Total Brain | 169.9 | 147.4 (p < 0.01) | 144.2 (p < 0.01) | 147.4 (p < 0.01) |
| Cerebrum | 157.9 | 138.32 (p < 0.01) | 136.7 (p < 0.01) | 138.4 (p < 0.01) |
| Cerebellum | 7.8 | 6.17 (p < 0.01) | 6.1 (p < 0.01) | 6.34 (p < 0.01) |
| Brainstem | 2.53 | 2.50 (p = 0.88) | 2.00 (p = 0.12) | 2.68 (p = 0.41) |
Median volume of placenta, total brain, cerebrum, cerebellum and brainstem in cm3; significant values in bold. P-values reflect group differences compared to controls.
Relationship between placental and fetal brain volumes
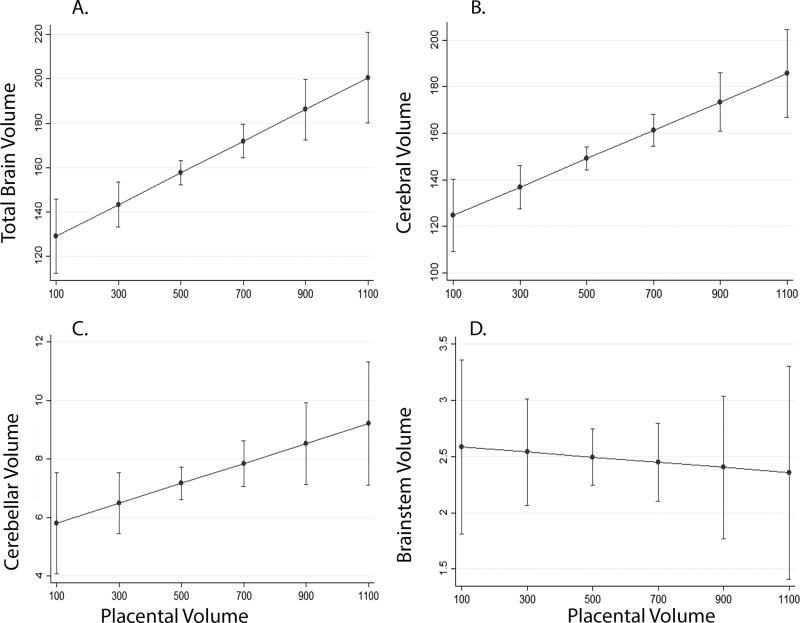
Increasing placental volume was associated with increasing total brain volume (p < 0.01), cerebral (p < 0.01) and cerebellar volume (p = 0.03) but not associated with brainstem volume (p = 0.79) (Figure 4). The interaction between increasing placental volume and increasing total brain volume was similar for both FGR and controls, but the overall volumes were smaller and thus shifted downward, in pregnancies with FGR (Figure 5).
Figure 4.
The association of placental volume (in cm3) to global (A) and regional (B–C) brain volumes (in cm3) in all pregnancies, adjusted for gestational age at MRI. Increasing placental volume was associated with increasing total brain (A), cerebral (B) and cerebellar (C) volumes, but not brainstem (D).
Figure 5.
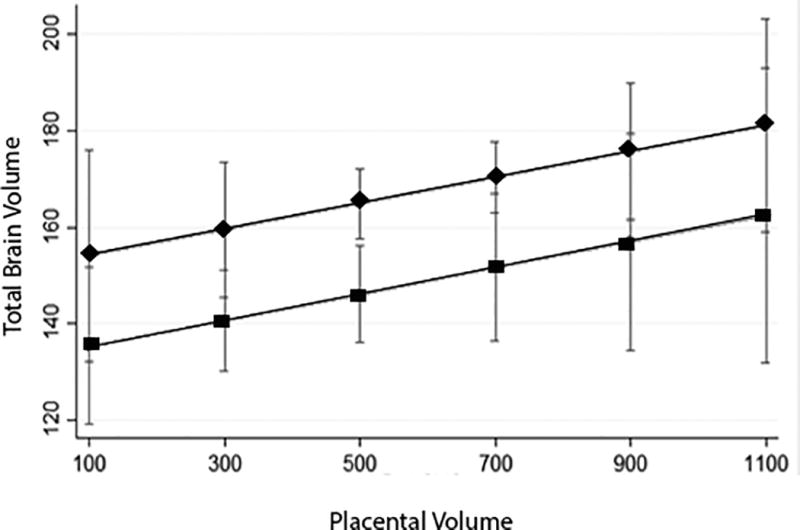
The total brain volume (in cm3) was smaller in FGR compared to controls, however the relationship between placental volume (in cm3) to total brain volume was not statistically different in FGR compared to controls. Healthy control pregnancies are represented by diamonds, FGR by squares.
Relationship between fetal brain and placental volumes and neonatal outcomes in FGR
Placental volume was not associated with any of the anthropomorphic neonatal measures, including birth weight, head circumference or ponderal index despite controlling for GA (p = 0.49 – 0.64). Our data suggest that total brain volume, cerebral volume and cerebellar volume in the fetal period may be associated with birth weight, controlling for GA at the time of delivery, but did not reach statistical significance (p = 0.07, p = 0.10, and p = 0.08, respectively). Brain volumes were not associated with the other anthropomorphic measures of head circumference or ponderal index. Neither brain nor placental volumes were associated with premature delivery (p = 0.38; p=0.80, respectively).
Relationship between in vivo placental volume and placental pathology in FGR
Twenty (57%) placentas of the FGR cohort were sent for pathologic examination. Of these, 85% had trimmed placental weights less than the 3rd centile for gestational age and gender (17). Seven (35%) were noted to have thrombi and fibrin deposition, 5 (25%) had evidence of inflammation, 4 (20%) had accelerated villous maturation, and 3 (15%) had infarcts. Placental weight and pathologies were equally distributed between FGR pregnancies with and without Doppler abnormalities. Placental volume in the fetal period was significantly associated with placental weight at the time of delivery (p = 0.03).
Discussion
In this prospective study, using quantitative volumetric MRI, we report for the first time that in vivo placental volume is related to global and regional fetal brain volumes in both healthy and growth-restricted fetuses. As expected, placental volume and fetal brain volumes increase with advancing gestational age. In FGR, placental, total brain, cerebral and cerebellar volumes were significantly smaller than in healthy controls. Pregnancies complicated by FGR with fetal Doppler changes had smaller placental, cerebral and cerebellar volumes compared to pregnancies complicated by FGR without Doppler changes. Brainstem volumes, however, did not differ between FGR groups and controls.
Placental insufficiency is a known, independent risk factor for impaired fetal growth and abnormal neurodevelopment (7). In order to examine the impact of placental dysfunction on the developing fetus, we investigated the relationship between fetal brain and placental growth in the growth-restricted fetus. Postnatally, growth restricted and small for gestational age (SGA) infants are at risk for impaired neurodevelopment compared to infants born appropriate for age (AGA) (26–29). Cognitive and behavioral difficulties persist well into childhood, adolescence and young adulthood for both FGR and SGA survivors (30–32). Despite normal intelligence quotient testing in FGR and SGA children, they remain at greater risk for memory and attention deficits (33, 34). Adverse intrauterine environments also are associated with the development of neuropsychiatric disease later in life, thought in part to be due to neuroendocrine and epigenetic influences that may persist across generations (35). For example, modifications in serotonin pathways, including placental production of serotonin, are associated with schizophrenia, bipolar disorders and autism spectrum disorders (36–38). The neuroendocrine anomalies, oxygen and nutrient transfer deficiencies, inflammation and oxidative stress seen in placental disease all act as potential mechanisms for neurodevelopmental compromise (39). And yet, direct effects of placental disease on brain development remain difficult to identify.
To date, morphometric differences in brain development have been reported in survivors of FGR and SGA (30, 40, 41). These differences are evident as early as the immediate neonatal period and persist through adulthood (30, 40). More recently, fetal imaging just prior to delivery demonstrates that volumetric differences in the cerebrum, cerebellum and brainstem can be detected in fetuses with FGR at 37 weeks gestation, and associated with abnormal neurobehavior at birth (42, 43). However, the onset of these volumetric differences in brain development during the fetal period remains largely unknown.
Several studies have shown that placental volumes are associated with birth weight throughout gestation, as well as the development of FGR (44, 45). In pregnancies complicated by preeclampsia, placental volume and vascularization indices calculated by 3D sonography are diminished compared to controls (46). In addition, these in vivo anomalies are associated with significant histopathologic lesions of the placenta detected after delivery, including infarction and calcifications for the smallest placentas, and increased rates of infarction for placentas greater than the 50th centile for volume (46). However, the reproducibility of 3D sonography, along with maternal body habitus and placental insertion site, limit the utility of sonography alone in placental assessment (45, 47, 48). Adjuvant MR imaging of the placenta has been shown to better predict neonatal outcome than umbilical artery Doppler ultrasound alone in high risk pregnancies (9). As in our work, previous MRI analyses of placental volume in pregnancies complicated by FGR have demonstrated reduced placental volume compared to controls (47, 49, 50). It is noted that there is a wide variability in placental volume for FGR, more so than in controls, which likely reflects the heterogeneous nature of this cohort. Nonetheless, decreased placental volume is associated with adverse neonatal outcomes, including increased fetal or neonatal mortality (49). Placental vascular pathologies detected by in vivo MRI are associated with significant neurodevelopmental impairment rates (51). However, there are few studies that study in vivo placental and fetal brain development.
Notably, in our study, we demonstrate that pregnancies complicated by FGR demonstrate markedly decreased placental and brain volumes that can be detected prenatally. The relationship between placental volumes and brain volumes reflect that these volumes increase at a similar rate in both controls and FGR, but that the volumes are shifted downward in FGR; this suggests that the interaction of placental development with brain development is similar for the two groups, but may be compromised in FGR. Only one other study has explored the relationship of placental volume on brain development (20). Our group has previously shown that fetuses with CHD also have significantly smaller brain volumes compared to healthy controls, however, the relationship between placental volume and brain volume differed between CHD and controls, suggesting different mechanisms of compromised neurodevelopment (20).
Identifying the mechanisms of placental failure that result in altered neurodevelopment remains difficult to elucidate. Circulatory changes in the maternal-fetal-placental unit have been well described and remain a mainstay of clinical surveillance of fetal well-being(52). However, we demonstrate significant differences in placental and brain volumes with and without maternal-fetal-placental Doppler changes. In vivo assessment of the feto-placental unit provides unique opportunities to assess the developing placenta and the real-time effects on fetal brain development. As suggested by previous literature, placental support for the developing fetus includes the transport of critical nutrients needed to support the growing brain, but also performs important synthetic and immune-modulatory functions needed for normal brain development. The ability to interrogate the feto-placental unit in vivo helps identify early deviations of normal growth and may provide a window of opportunity for future interventions to protect fetal brain development.
Limitations
Our study is powered to detect moderate differences in placental volume between the FGR and control groups, but is less likely to detect smaller differences, which may still be clinically meaningful. Post-hoc power analysis suggested that at an alpha level of 0.05 and 80% power, the study was sufficiently powered to detect relatively large differences in placental volume (1 SD) among sub-groups of the FGR cohort specifically. Our study cohort of fetuses with FGR represents a heterogeneous group that reflects the difficulties in identifying placental insufficiency in utero – nearly 50% of the prospectively enrolled cohort was born AGA, despite the earlier period of growth restriction. The ability to distinguish growth restriction in the non-SGA infant remains difficult to confirm postnatally, just as the ability to distinguish the constitutionally small infant from the pathologically growth-restricted newborn in the SGA group. These distinctions require long-term monitoring for potential neurodevelopmental compromise, which is ongoing. In addition, slightly more than half of FGR subjects had available placental pathology to confirm placental insufficiency postnatally. Larger studies of placental pathology of both healthy and high-risk pregnancies are warranted to confirm in vivo findings of placental development and histopathologic signs of placental function after delivery.
Conclusions
We report that decreased placental volume as well as global and regional brain volumes can be accurately detected in both the second and third trimesters of pregnancy using quantitative MRI in fetuses with and without fetal Doppler changes. In pregnancies complicated by FGR with fetal Doppler changes, placental and brain volumes demonstrated more significant growth delays compared to pregnancies with FGR but without Doppler changes. Despite diminished volumes in FGR, the interaction between placental volume and brain volumes were similar, suggesting a common mechanism between placental development and fetal brain growth, in FGR. These studies provide important insight into the real-time impact of placental dysfunction on the developing brain. Future studies of the feto-placental unit are needed to further elucidate the mechanisms behind intrauterine placental dysfunction and the effects on neurodevelopment.
Acknowledgments
Funding sources: Supported by the Canadian Institutes of Health Research (MOP-81116 to C. Limperopoulos); National Institutes of Health (UL1TR000075 and KL2TR000076, Clinical-Translational Science Institute-Children’s National to N. Andescavage).
We thank our families who participated in this study.
Footnotes
Conflicts of interest:
The authors have no financial interest or conflicts of interest to disclose.
References
- 1.Neerhof MG, Thaete LG. The fetal response to chronic placental insufficiency. Semin Perinatol. 2008;32(3):201–5. doi: 10.1053/j.semperi.2007.11.002. Epub 2008/05/17. [DOI] [PubMed] [Google Scholar]
- 2.Cohen E, Baerts W, van Bel F. Brain-Sparing in Intrauterine Growth Restriction: Considerations for the Neonatologist. Neonatology. 2015;108(4):269–76. doi: 10.1159/000438451. Epub 2015/09/04. [DOI] [PubMed] [Google Scholar]
- 3.Brandt I, Sticker EJ, Lentze MJ. Catch-up growth of head circumference of very low birth weight, small for gestational age preterm infants and mental development to adulthood. J Pediatr. 2003;142(5):463–8. doi: 10.1067/mpd.2003.149. Epub 2003/05/21. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Martinez R, Figueras F, Oros D, Padilla N, Meler E, Hernandez-Andrade E, et al. Cerebral blood perfusion and neurobehavioral performance in full-term small-for-gestational-age fetuses. American journal of obstetrics and gynecology. 2009;201(5):474 e1–7. doi: 10.1016/j.ajog.2009.05.028. Epub 2009/07/28. [DOI] [PubMed] [Google Scholar]
- 5.Eixarch E, Meler E, Iraola A, Illa M, Crispi F, Hernandez-Andrade E, et al. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet Gynecol. 2008;32(7):894–9. doi: 10.1002/uog.6249. Epub 2008/11/28. [DOI] [PubMed] [Google Scholar]
- 6.Guellec I, Marret S, Baud O, Cambonie G, Lapillonne A, Roze JC, et al. Intrauterine Growth Restriction, Head Size at Birth, and Outcome in Very Preterm Infants. J Pediatr. 2015;167(5):975–81 e2. doi: 10.1016/j.jpeds.2015.08.025. Epub 2015/09/20. [DOI] [PubMed] [Google Scholar]
- 7.Baschat AA. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet Gynecol. 2011;37(5):501–14. doi: 10.1002/uog.9008. Epub 2011/04/27. [DOI] [PubMed] [Google Scholar]
- 8.Baschat AA. Neurodevelopment after fetal growth restriction. Fetal diagnosis and therapy. 2014;36(2):136–42. doi: 10.1159/000353631. Epub 2013/07/28. [DOI] [PubMed] [Google Scholar]
- 9.Messerschmidt A, Baschat A, Linduska N, Kasprian G, Brugger PC, Bauer A, et al. Magnetic resonance imaging of the placenta identifies placental vascular abnormalities independently of Doppler ultrasound. Ultrasound Obstet Gynecol. 2011;37(6):717–22. doi: 10.1002/uog.8891. Epub 2010/11/26. [DOI] [PubMed] [Google Scholar]
- 10.Quant HS, Sammel MD, Parry S, Schwartz N. Second-Trimester 3-Dimensional Placental Sonography as a Predictor of Small-for-Gestational-Age Birth Weight. J Ultrasound Med. 2016;35(8):1693–702. doi: 10.7863/ultra.15.06077. Epub 2016/06/24. [DOI] [PubMed] [Google Scholar]
- 11.Effendi M, Demers S, Giguere Y, Forest JC, Brassard N, Girard M, et al. Association between first-trimester placental volume and birth weight. Placenta. 2014;35(2):99–102. doi: 10.1016/j.placenta.2013.11.015. Epub 2013/12/19. [DOI] [PubMed] [Google Scholar]
- 12.Hadlock FP, Deter RL, Harrist RB, Park SK. Estimating fetal age: computer-assisted analysis of multiple fetal growth parameters. Radiology. 1984;152(2):497–501. doi: 10.1148/radiology.152.2.6739822. Epub 1984/08/01. [DOI] [PubMed] [Google Scholar]
- 13.Poljak B, Agarwal U, Jackson R, Alfirevic Z, Sharp A. Diagnostic accuracy of individual antenatal tools for prediction of small-for-gestational age at birth. Ultrasound Obstet Gynecol. 2017;49(4):493–9. doi: 10.1002/uog.17211. Epub 2016/08/04. [DOI] [PubMed] [Google Scholar]
- 14.Stirnemann J, Villar J, Salomon LJ, Ohuma E, Ruyan P, Altman DG, et al. International estimated fetal weight standards of the INTERGROWTH-21st Project. Ultrasound Obstet Gynecol. 2017;49(4):478–86. doi: 10.1002/uog.17347. Epub 2016/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. Epub 2003/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. Epub 2013/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almog B, Shehata F, Aljabri S, Levin I, Shalom-Paz E, Shrim A. Placenta weight percentile curves for singleton and twins deliveries. Placenta. 2011;32(1):58–62. doi: 10.1016/j.placenta.2010.10.008. Epub 2010/11/03. [DOI] [PubMed] [Google Scholar]
- 18.Ebbing C, Rasmussen S, Kiserud T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet Gynecol. 2007;30(3):287–96. doi: 10.1002/uog.4088. Epub 2007/08/28. [DOI] [PubMed] [Google Scholar]
- 19.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31(3):1116–28. doi: 10.1016/j.neuroimage.2006.01.015. Epub 2006/03/21. [DOI] [PubMed] [Google Scholar]
- 20.Andescavage N, Yarish A, Donofrio M, Bulas D, Evangelou I, Vezina G, et al. 3-D volumetric MRI evaluation of the placenta in fetuses with complex congenital heart disease. Placenta. 2015;36(9):1024–30. doi: 10.1016/j.placenta.2015.06.013. Epub 2015/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE transactions on medical imaging. 2010;29(6):1310–20. doi: 10.1109/TMI.2010.2046908. Epub 2010/04/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serag A, Aljabar P, Ball G, Counsell SJ, Boardman JP, Rutherford MA, et al. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. NeuroImage. 2012;59(3):2255–65. doi: 10.1016/j.neuroimage.2011.09.062. Epub 2011/10/12. [DOI] [PubMed] [Google Scholar]
- 23.Machin D. Sample size tables for clinical studies. 2. Oxford England; Malden, MA, USA: Blackwell Science; 1997. p. x.p. 315. [Google Scholar]
- 24.Hintze J. PASS 11. 11. NCSS, LLC; Kaysville, Utah, USA: 2011. [Google Scholar]
- 25.StataCorp. Stata Statistical Software: Release. 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 26.Arcangeli T, Thilaganathan B, Hooper R, Khan KS, Bhide A. Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound Obstet Gynecol. 2012;40(3):267–75. doi: 10.1002/uog.11112. Epub 2012/02/04. [DOI] [PubMed] [Google Scholar]
- 27.Bickle Graz M, Tolsa JF, Fischer Fumeaux CJ. Being Small for Gestational Age: Does it Matter for the Neurodevelopment of Premature Infants? A Cohort Study. PLoS One. 2015;10(5):e0125769. doi: 10.1371/journal.pone.0125769. Epub 2015/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarton CM, Wallace IF, Divon M, Vaughan HG., Jr Cognitive and neurologic development of the premature, small for gestational age infant through age 6: comparison by birth weight and gestational age. Pediatrics. 1996;98(6 Pt 1):1167–78. Epub 1996/12/01. [PubMed] [Google Scholar]
- 29.Gutbrod T, Wolke D, Soehne B, Ohrt B, Riegel K. Effects of gestation and birth weight on the growth and development of very low birthweight small for gestational age infants: a matched group comparison. Arch Dis Child Fetal Neonatal Ed. 2000;82(3):F208–14. doi: 10.1136/fn.82.3.F208. Epub 2000/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogne T, Engstrom AA, Jacobsen GW, Skranes J, Ostgard HF, Martinussen M. Fetal growth, cognitive function, and brain volumes in childhood and adolescence. Obstet Gynecol. 2015;125(3):673–82. doi: 10.1097/AOG.0000000000000694. Epub 2015/03/03. [DOI] [PubMed] [Google Scholar]
- 31.Lohaugen GC, Ostgard HF, Andreassen S, Jacobsen GW, Vik T, Brubakk AM, et al. Small for gestational age and intrauterine growth restriction decreases cognitive function in young adults. J Pediatr. 2013;163(2):447–53. doi: 10.1016/j.jpeds.2013.01.060. Epub 2013/03/05. [DOI] [PubMed] [Google Scholar]
- 32.Tideman E, Marsal K, Ley D. Cognitive function in young adults following intrauterine growth restriction with abnormal fetal aortic blood flow. Ultrasound Obstet Gynecol. 2007;29(6):614–8. doi: 10.1002/uog.4042. Epub 2007/05/25. [DOI] [PubMed] [Google Scholar]
- 33.Tanis JC, Van Braeckel KN, Kerstjens JM, Bocca-Tjeertes IF, Reijneveld SA, Bos AF. Functional outcomes at age 7 years of moderate preterm and full term children born small for gestational age. J Pediatr. 2015;166(3):552–8 e1. doi: 10.1016/j.jpeds.2014.11.043. Epub 2015/01/13. [DOI] [PubMed] [Google Scholar]
- 34.de Bie HM, de Ruiter MB, Ouwendijk M, Oostrom KJ, Wilke M, Boersma M, et al. Using fMRI to Investigate Memory in Young Children Born Small for Gestational Age. PLoS One. 2015;10(7):e0129721. doi: 10.1371/journal.pone.0129721. Epub 2015/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babenko O, Kovalchuk I, Metz GA. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. Epub 2014/12/03. [DOI] [PubMed] [Google Scholar]
- 36.Yang CJ, Tan HP, Du YJ. The developmental disruptions of serotonin signaling may involved in autism during early brain development. Neuroscience. 2014;267:1–10. doi: 10.1016/j.neuroscience.2014.02.021. Epub 2014/03/04. [DOI] [PubMed] [Google Scholar]
- 37.Debnath M, Venkatasubramanian G, Berk M. Fetal programming of schizophrenia: select mechanisms. Neurosci Biobehav Rev. 2015;49:90–104. doi: 10.1016/j.neubiorev.2014.12.003. Epub 2014/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paquette AG, Marsit CJ. The developmental basis of epigenetic regulation of HTR2A and psychiatric outcomes. J Cell Biochem. 2014;115(12):2065–72. doi: 10.1002/jcb.24883. Epub 2014/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker CK, Ashwood P, Hertz-Picciotto I. Preeclampsia, placental insufficiency, autism, and antiphospholipid antibodies-reply. JAMA Pediatr. 2015;169(6):606–7. doi: 10.1001/jamapediatrics.2015.0345. Epub 2015/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostgard HF, Lohaugen GC, Bjuland KJ, Rimol LM, Brubakk AM, Martinussen M, et al. Brain morphometry and cognition in young adults born small for gestational age at term. J Pediatr. 2014;165(5):921–7 e1. doi: 10.1016/j.jpeds.2014.07.045. Epub 2014/09/14. [DOI] [PubMed] [Google Scholar]
- 41.Keunen K, Kersbergen KJ, Groenendaal F, Isgum I, de Vries LS, Benders MJ. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome: a systematic review. J Matern Fetal Neonatal Med. 2012;25(Suppl 1):89–100. doi: 10.3109/14767058.2012.664343. Epub 2012/02/22. [DOI] [PubMed] [Google Scholar]
- 42.Sanz-Cortes M, Egana-Ugrinovic G, Zupan R, Figueras F, Gratacos E. Brainstem and cerebellar differences and their association with neurobehavior in term small-for-gestational-age fetuses assessed by fetal MRI. American journal of obstetrics and gynecology. 2014;210(5):452 e1–8. doi: 10.1016/j.ajog.2013.12.008. Epub 2013/12/10. [DOI] [PubMed] [Google Scholar]
- 43.Egana-Ugrinovic G, Sanz-Cortes M, Figueras F, Couve-Perez C, Gratacos E. Fetal MRI insular cortical morphometry and its association with neurobehavior in late-onset small-for-gestational-age fetuses. Ultrasound Obstet Gynecol. 2014;44(3):322–9. doi: 10.1002/uog.13360. Epub 2014/03/13. [DOI] [PubMed] [Google Scholar]
- 44.Bozkurt N, Basgul Yigiter A, Gokaslan H, Kavak ZN. Correlations of fetal-maternal outcomes and first trimester 3-D placental volume/3-D power Doppler calculations. Clin Exp Obstet Gynecol. 2010;37(1):26–8. Epub 2010/04/28. [PubMed] [Google Scholar]
- 45.Hata T, Tanaka H, Noguchi J, Hata K. Three-dimensional ultrasound evaluation of the placenta. Placenta. 2011;32(2):105–15. doi: 10.1016/j.placenta.2010.11.001. Epub 2010/12/01. [DOI] [PubMed] [Google Scholar]
- 46.Moran MC, Mulcahy C, Zombori G, Ryan J, Downey P, McAuliffe FM. Placental volume, vasculature and calcification in pregnancies complicated by pre-eclampsia and intra-uterine growth restriction. European journal of obstetrics, gynecology, and reproductive biology. 2015;195:12–7. doi: 10.1016/j.ejogrb.2015.07.023. Epub 2015/10/16. [DOI] [PubMed] [Google Scholar]
- 47.Gowland P. Placental MRI. Semin Fetal Neonatal Med. 2005;10(5):485–90. doi: 10.1016/j.siny.2005.05.001. Epub 2005/07/20. [DOI] [PubMed] [Google Scholar]
- 48.Dekan S, Linduska N, Kasprian G, Prayer D. MRI of the placenta - a short review. Wien Med Wochenschr. 2012;162(9–10):225–8. doi: 10.1007/s10354-012-0073-4. Epub 2012/06/22. [DOI] [PubMed] [Google Scholar]
- 49.Damodaram M, Story L, Eixarch E, Patel A, McGuinness A, Allsop J, et al. Placental MRI in intrauterine fetal growth restriction. Placenta. 2010;31(6):491–8. doi: 10.1016/j.placenta.2010.03.001. Epub 2010/03/30. [DOI] [PubMed] [Google Scholar]
- 50.Ohgiya Y, Nobusawa H, Seino N, Miyagami O, Yagi N, Hiroto S, et al. MR Imaging of Fetuses to Evaluate Placental Insufficiency. Magn Reson Med Sci. 2016;15(2):212–9. doi: 10.2463/mrms.mp.2015-0051. Epub 2015/11/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linduska N, Knoezinger A, Dekan S, Weber M, Hayde M, Prayer D, et al. Placental pathologies on fetal MRI are associated with high impairment rates: a prospective long-term outcome study. J Matern Fetal Neonatal Med. 2015;28(10):1219–23. doi: 10.3109/14767058.2014.947952. Epub 2014/07/24. [DOI] [PubMed] [Google Scholar]
- 52.Baschat AA. Examination of the fetal cardiovascular system. Semin Fetal Neonatal Med. 2011;16(1):2–12. doi: 10.1016/j.siny.2010.09.002. Epub 2010/09/25. [DOI] [PubMed] [Google Scholar]