Summary
Although tremendous progress has been made in recent years in skin cancer care for organ transplant recipients, significant gaps remain in data-driven clinical guidelines, particularly for the treatment and prevention of cutaneous squamous cell carcinoma (cSCC), the most common malignancy among this population. In this review, we aim to summarize current knowledge around the management of cSCC and highlight the most significant gaps in knowledge that continue to pose challenges in the delivery of skin cancer care for organ transplant recipients. We suggest future directions for research that will bridge existing gaps and establish evidence-driven guidelines for primary prevention, screening and treatment of cSCC in this high-risk patient population.
Cutaneous squamous cell carcinoma (cSCC) is the most common post-transplant neoplasm in organ transplant recipients (OTR).1 Among immunocompetent individuals, basal cell carcinoma (BCC) outnumbers cSCC approximately 4 : 1;2 however, in OTR this ratio is reversed and cSCC is more common with a 65- to 100-fold increased incidence rate (vs. a 10-fold increased incidence rate for BCC).3–5 cSCC carries substantial morbidity and potential mortality in OTR, with reported metastatic rates of up to 8% vs. 1% in the general population,6 and a 3-year mortality rate of 46% for metastatic disease.6–8 The skin cancer-related mortality among OTR is higher than mortality from either breast or colon cancer.9 Given the high burden of disease and associated mortality, evidence-based therapeutic and preventive strategies for cSCC in OTR are important areas of clinical need. This review discusses what is known in the clinical care and control of cSCCs in OTR, and highlights research gaps that could better inform their management and prevention. The first section discusses management of cSCC in terms of surgery, radiotherapy and modification of immunotherapy, whereas the second section focuses on prevention of cSCC and includes chemoprevention, sun protection and screening. A summary of the results is provided in Table 1.
Table 1.
Summary of gaps in management of cutaneous squamous cell carcinoma (cSCC) in organ transplant recipients
| Surgery | Estimation of cure rates of cSCC after MMS and after surgical excision |
|---|---|
|
| |
| Identification of optimal treatment choice of in situ cSCC | |
|
| |
| Assessment of the clinical benefit of sentinel lymph node biopsy | |
|
| |
| Surgical guidelines for cSCC treatment based on prospective clinical trials | |
|
| |
| Management of local aggressive disease | Clinical evidence for the effect of adjuvant radiation for non-head-and-neck cSCC |
|
| |
| Modifying immune suppression | Magnitude of effect of different induction drugs on cSCC risk |
|
| |
| Identification of optimal dose of sirolimus to prevent skin cancer and minimize adverse events | |
|
| |
| Benefit of initiating mTORi treatment before the first post-transplant cSCC in selected patients | |
|
| |
| Impact of belatacept on post-transplant cSCC | |
|
| |
| QoL | Establishment of the relative QoL deficit between OTR with a high number of cSCC and OTR without cSCC, accounting for confounding factors |
|
| |
| Field-based therapies for actinically damaged skin | Identification of the topical treatment modality with highest clearance rate of AK, best adherence and best prevention of cSCC |
|
| |
| Imiquimod | Identification of preferable dose and duration of treatment |
|
| |
| Risk of cytokine release syndrome when used on a large surface area | |
|
| |
| Ingenol mebutate | Establishment of safety and efficacy |
|
| |
| Chemoprevention | |
|
| |
| Retinoids | Establishment of long-term efficacy |
|
| |
| Nicotinamide | Effect on AK and KC development and long-term safety |
|
| |
| Capecitabine | Large RCT of long-term efficacy and safety |
|
| |
| Modifying sun-protection behaviour | Identification of the educational programmes that most effectively induce long-term modifications of sun behavior |
|
| |
| Clarify whether modification of UV protective behaviour prevents AK and cSCC in OTR. If so, identify most effective primary prevention measures | |
|
| |
| Assessment of the safety and efficacy of T4N5 lotion and afamelanotide in preventing cSCC | |
|
| |
| Role of HPV vaccination in prevention of cSCC in OTR | Establish if β-HPV plays a role in skin carcinogenesis |
| Efficacy of pretransplant β-HPV vaccination in preventing post-transplant cSCC | |
|
| |
| Screening frequency | Evaluation of skin cancer screening guidelines for OTR regarding early detection of cSCC, morbidity and mortality |
|
| |
| Assessment of clinical utility of established screening frequency models in different populations | |
|
| |
| Effectiveness of skin self-examinations in early detection of cSCC | |
MMS, Mohs micrographic surgery; mTORi, inhibitor of mammalian target of rapamycin; QoL, quality of life; OTR, organ transplant recipient; AK, actinic keratoses; KC, keratinocyte carcinoma; RCT, randomized controlled trial; UV, ultraviolet; T4N5, T4 endonuclease; HPV, human papillomavirus.
Management of cutaneous squamous cell carcinoma in organ transplant recipients
Surgery
Choosing the optimal treatment for cSCC in OTR involves considering clinical features of the tumour, including the anatomical location, size and stage, as well as patient features, such as comorbidities and patient preferences. Mohs micrographic surgery (MMS) is the most common treatment modality for high-risk cSCC.10 While there is a paucity of data assessing MMS cure rates of cSCC in the OTR population, existing data in immunocompetent patients suggests that MMS offers the highest cure rate.11 Surgical excision with postoperative margin assessment is an alternative option if MMS is unavailable or if patients are unable to tolerate surgery under local anaesthesia. Typically, the optimal recommended surgical margins for excision of cSCC in OTR are between 3 and 10 mm.10 However, there are no clinical data on the proportion of residual or recurrent cSCC in OTR based on these recommended margins. Furthermore, optimal treatment of in situ cSCC is also controversial, and more long-term prospective studies are needed to compare surgery with other modalities, such as destructive treatments with cryotherapy, electrodessication and curettage, as well as topical chemotherapy.12
In high-risk OTR patients, the practical utility of sentinel lymph node biopsies (SNLB) to detect subclinical nodal disease is unclear. Emerging data suggest that SLNB may detect subclinical nodal disease with a high level of accuracy and relatively low risk of adverse effects, even for head and neck lesions.5 However, there are no prospective studies delineating which patients are most likely to benefit from SLNB. There is a significant gap in existing data regarding the actual clinical benefit of SLNB, and further evidence-driven guidelines are needed.
Current treatment guidelines are established based on expert clinical opinion and randomized controlled trial data are very limited.10 Stasko et al. developed the guidelines for cSCC management in OTR after a review of predominantly retrospective case series.10 Further prospective clinical trials are needed to determine optimal surgical management of cSCC in the OTR population, and to enable the establishment of guidelines based on level I clinical data.
Management of eruptive keratoacanthomas/cutaneous squamous cell carcinoma
Keratoacanthomas and/or well-differentiated cSCC can erupt in areas of prior skin trauma, including surgery, radiation and even topical therapies.13 While this is a rare condition, its frequency is increased in immunosuppressed populations.13 Management can be challenging and evidence-based treatment guidelines are lacking. Multiple off-label options aimed at managing ulceration and destruction have been reported, including topical 5-fluorouracil (5-FU) and imiquimod, as well as intralesional 5-FU, methotrexate and bleomycin, for which safety in OTR has not been determined.14–16 Topical 5-FU under zinc oxide-impregnated compression bandages (chemowraps) can be an effective treatment option.17 Systemic retinoids can also be used to treat eruptive cSCC while reducing risk of subsequent cSCC,18 and can be combined with targeted topical or intralesional treatments.
Management of locally aggressive disease with adjuvant radiation
Radiation is a well-accepted alternative for inoperable cSCC or for patients who are nonsurgical candidates. Postoperative radiation is used as adjuvant therapy for certain high-risk cSCC, including those with perineural invasion or incomplete resection as indicated by positive surgical margins.19–21 The largest retrospective review investigating the impact of radiation found that surgery with adjuvant radiation for head-and-neck cSCC was associated with both lower recurrence (20% vs. 43%) and higher 5-year survival (73% vs. 54%) vs. surgery alone.22 There is limited clinical evidence for non-head-and-neck cSCC, particularly in the OTR population.
Management of advanced locoregional and metastatic disease
There is no current agreement on the standard of care for the management of regionally advanced and metastatic cSCC in OTR. Current treatment approaches are similar to that of advanced locoregional or metastatic disease in the general population, which include radiation, chemotherapy and targeted therapy. On a case-by-case basis, additional strategies of immunosuppression reduction include conversion to inhibitors of mammalian target of rapamycin (mTORi) or complete withdrawal of immunosuppressive agents (renal and pancreas transplants). Clinical studies investigating immunosuppression reduction is largely limited owing to the considerable risk of graft failure.
Modifying immune suppression in organ transplant recipients to minimize skin cancer risk
Immunosuppression in OTR is divided into induction, maintenance and rejection therapy. Induction therapy is given around the time of transplantation and in the immediate postoperative period to prevent acute rejection and allow delayed initiation of maintenance therapy, which can cause renal dysfunction. Induction therapy can be categorized into T-cell-depleting (e.g. OKT3, rabbit antithymocyte globulin, alemtuzumab) and nondepleting agents (e.g. daclizumab, basiliximab). The frequency of use of induction differs according to organ transplant type, from 25–30% in liver recipients up to 80% in renal transplant recipients.23,24 Evidence for risk of cSCC following induction therapy is inconclusive, and there is a need for further study of these therapies, including the potential magnitude of the effect of different induction drugs on cSCC and how it relates to other risk factors.
Common regimens for maintenance therapy include a calcineurin inhibitor (e.g. ciclosporin, tacrolimus), an antiproliferative agent (e.g. azathioprine, mycophenolate mofetil) and steroids. More recently, mTORi such as sirolimus and everolimus, have been introduced as alternative immunosuppressant drugs (Fig. 1). Demonstrating both immunosuppressive and anticarcinogenic effects, mTORi are associated with a reduced risk of post-transplant skin cancer.25 Belatacept is a biological immunosuppressive agent, which was approved for renal transplant recipients in 2012. In a recent, long-term, phase III study, belatacept was shown to be significantly associated with a reduction in mortality and graft loss compared with ciclosporin. The impact of belatacept on subsequent cSCC development has not been extensively assessed and needs further study.26
Fig 1.
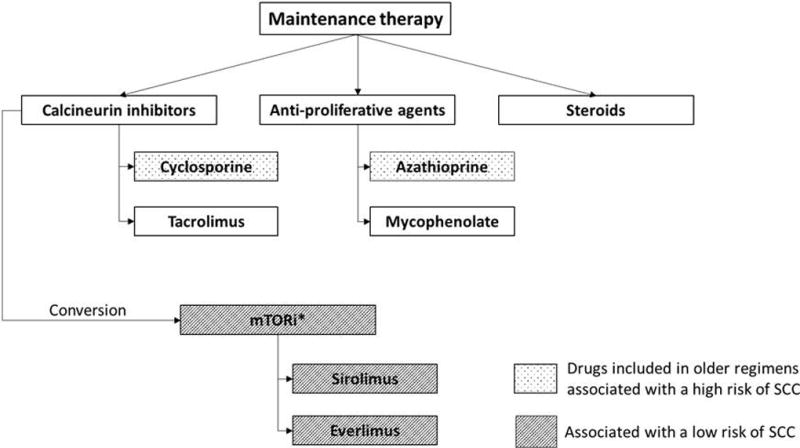
Agents commonly used for maintenance therapy after solid organ transplantation.
SCC: squamous cell carcinoma, mTORi: mammalian target of rapamycin inhibitors.
*mTORi may replace calcineurin inhibitors
Revision of maintenance therapy should be considered in the prevention of additional primary cSCC after multiple or aggressive cSCC, but may also be effective against skin cancer development after a single cSCC.27,28 Revision can be achieved by discontinuing, changing the type or modifying the intensity of immune-suppressive therapy. As compared with newer immunosuppressive regimens, older immunosuppressive agents, including azathioprine and ciclosporin, have been associated with a higher risk of cSCC (Fig. 1).29–31 This is well-documented for azathioprine,29 whereas the evidence is more controversial for ciclosporin.31,32 Conversion from azathioprine to mycophenolate mofetil should always be considered for OTR with multiple cSCC, but azathioprine and ciclosporin are now rarely used in standard maintenance immunosuppressive regimens.30,32,33 Another strategy for revision of immunosuppression is conversion from calcineurin inhibitors to mTORi. Growing evidence now suggests that it may be preferable to convert to mTORi to prevent subsequent cSCC after the first post-transplant cSCC but does currently not support a beneficial effect for mTORi before initial post-transplant skin cancer.28,34,35 However, significant gaps still exist in our understanding of mTORi: their use is limited by a relatively high rate of discontinuation, mainly because of side effects.25,36,37 In addition, an increased risk of death [hazard ratio (HR) 1.43, 95% confidence interval (CI) 1.21–1.71], primarily attributed to infection and cardiovascular disease, was recently identified in a review and meta-analysis of 21 randomized controlled trials (RCTs) of sirolimus (n = 5876),34 although when restricted to low-dose sirolimus trials, the reduced risk of keratinocyte carcinoma (KC) with sirolimus persisted (HR 0.43, 95% CI 0.24–0.78), whereas the risk of death was no longer statistically significant (HR 1.07, 95% CI 0.81–1.41).34 Accordingly, sirolimus may have a specific threshold at which prevention of skin cancer is balanced with minimization of adverse events, and it may be useful for highly selected patients, for example those with aggressive cSCC disease, where benefits outnumber disadvantages. Future studies should test these hypotheses and be designed to elucidate how prior number of actinic keratoses (AK)/cSCC and aggressiveness of cSCC should influence choice and timing of immune modulation. Well-designed observational studies based on data from solid-organ transplant registries capturing KC and AK outcomes could be valuable, because they may have enough power to detect both treatment effects in subpopulations and rare side-effects in general.
Assessing quality of life in organ transplant recipients with cutaneous squamous cell carcinoma
Quality of life (QoL) is a construct that expresses subjective well-being, lack of disease- or treatment-related symptoms, positive social function and freedom from psychological distress. It is measured by observing or interviewing the patient or via questionnaires, although such measurement studies have been few in patients with KC overall and even fewer in OTR. Existing evidence from immunocompetent patients suggests that treatment of AK or occasional KC does not reduce QoL,38,39 but those with high morbidity, for example after extensive reconstruction, are more likely to suffer poor QoL.40 Thus, long-term OTR with an exceptionally high number of cSCC appear likely to suffer poorer QoL than OTR without cSCC, although research is needed to establish the relative deficit in QoL, taking account of potential confounding factors, such as age and duration of immunosuppression. Information on QoL is needed to inform treating physicians attempting to balance the strong wishes of OTR to avoid dialysis and maintain stable, satisfactory transplant function vs. the carcinogenic effects of long-term immunosuppressive therapy.18 As always, the ultimate decision is one that would be jointly determined by the patient and physician.
Prevention of cutaneous squamous cell carcinoma in organ transplant recipients
Field-based therapies for actinically damaged skin
AK are precancerous lesions and a marker of chronic sun damage. AK may progress to cSCC, providing a rationale for the potential benefit of field-based therapies for actinically damaged skin. Imiquimod, 5-FU and diclofenac have all been investigated as topical treatment options for AK in OTR. Imiquimod is a topical immune modulator deemed safe for use in the transplant population with no adverse effects of systemic immune activation leading to subsequent organ rejection.41 In one study of heart, lung and liver transplants, the application of imiquimod 5% therapy three times per week for 16 weeks on a 100 cm2 area of skin led to a 62% clearance rate.42 Many different dosing regimens and treatment durations of imiquimod may be effective for the treatment of AK, but the optimal dosing and duration of treatment has not been established in OTR. Additionally, there is a risk of cytokine release syndrome when applied over a large surface area and further studies are needed to determine this risk.43 Imiquimod is used in OTR with caution owing to the possible risk of increasing immunoreactivity and organ rejection.44,45 5-FU interferes with cellular DNA synthesis, with resultant cell death. Ingham and Weightman examined the safety and efficacy of 5% 5-FU cream applied twice daily for 3 weeks in OTR and reported that 12 months after the initiation of treatment, 71% of patients had a 75% or greater clearance AK numbers.46 However, the use of 5-FU is limited by patient compliance because treatment duration is often long (3–6 weeks) and cyclical use is required for ongoing efficacy. The nonsteroidal anti-inflammatory agent diclofenac inhibits the cyclo-oxygenase pathway and diclofenac 3% gel twice daily for 16 weeks led to a 41% clearance of AK in OTR.47 Ingenol mebutate, a macrocyclic diterpene ester, is the newest available agent, demonstrating AK clearance rates of up to 34–42% but has yet to be fully evaluated in OTR.48 The notable advantages of ingenol mebutate are the short duration of treatment, which may increase adherence and relative rapid resolution of local irritation. However, the cost of ingenol mebutate for patients must be considered, as well as adverse cutaneous effects. Clinical trials determining safety and efficacy of ingenol mebutate treatment of AK in OTR are currently ongoing (www.clinicaltrials.gov; identifier NCT02473848 and NCT02866695).
Photodynamic therapy is a highly effective method that combines the use of a photosensitizing agent with targeted phototherapy and is a safe, well-tolerated approach for treatment of AK in OTR but appears to have lower long-term effectiveness in OTR.49 Further head-to-head studies are needed to determine the treatment that is associated with the highest clearance rate and best adherence in OTR. There is also a need for longer-term studies that can demonstrate that topical approaches to treating field damage can lead to prevention of cSCC.
Chemoprevention
Retinoids
Systemic retinoids, including acitretin, isotretinoin and etretinate, have been used in systemic chemoprevention of OTR cSCC. A systematic review suggests they are safe for short-term use in the organ transplant population.18 Systemic retinoids are generally reserved for OTR who are actively developing skin cancers (e.g. >5 per year).5 In a retrospective study, there was a significant reduction in cSCC development for the first 3 years after acitretin treatment,50 and three RCTs in OTR have confirmed a significant reduction in AK and/or cSCC up to 2 years post-transplant.18 In general, systemic retinoid therapy must be maintained long-term to achieve effectiveness, and discontinuation may lead to a significant rebound in cSCC.50 Future studies are needed to establish the long-term efficacy of systemic retinoids as chemopreventive agents in the organ transplant population.
Nicotinamide
Nicotinamide is an amide form of vitamin B3 that prevents ATP depletion and enhances DNA repair. Oral nicotinamide reduces ultraviolet radiation (UVR)-induced immunosuppression,50 and in a phase III RCT reduced the incidence of AK and KC in immunocompetent patients.51 However, the study population did not include OTR and those who were immunosuppressed were also excluded from the trial. It is unclear whether similar benefits would be generalizable to the OTR cohort as a result. In a recent phase II RCT, nicotinamide was associated with a statistically nonsignificant reduction of KC and AK in OTR.52 Nicotinamide is typically well tolerated with a minimal side-effect profile and is widely available over the counter, although there are no long-term safety data in OTR. Larger phase III RCT studies investigating the role of nicotinamide in OTR would significantly contribute to filling this important gap.
Capecitabine
Capecitabine is a prodrug of 5-FU that is metabolized by thymidine phosphorylase, which is often overexpressed in certain carcinomas. Low-dose oral capecitabine has been used for chemoprevention in OTR who develop more than two cSCC in 6 months.53 It is also used in those who continue to develop KC despite treatments with oral retinoids, photodynamic therapy, topical chemotherapies or after optimization of immunosuppressive regimens.53 One small case series demonstrated that low-dose capecitabine significantly reduced the incidence of cSCC in OTR, with relatively manageable adverse effects.53,54 These data are encouraging, but the data are derived from small study cohorts. Further large, randomized studies are now necessary to determine the long-term efficacy and safety of capecitabine as a chemopreventive agent in OTR.
Modifying sun-protection behaviour in organ transplant recipients
Sun exposure is the most significant modifiable environmental risk factor for the development of cSCC in OTR. It has been well documented that OTR have insufficient knowledge about the consequences of sun exposure, as well as inadequate sun-protective behaviour,55 highlighting the need for more effective educational interventions. Factors that affect sun-protective behaviour have been identified in OTR,56 and several educational interventions have been evaluated for short-term efficacy (≤ 2 years). Successful strategies that have promoted sun-protective habits in OTR include counselling from dermatologists, written information pamphlets, word books with and without repetitive text/e-mail reminders tailored to weather conditions and mobile medical applications.57,58 However, the proportion of sun-protective behaviour compliance among OTR postintervention differed widely across studies, and may differ even more long term. Further identification of effective education programmes that encourage sustainable, long-term modifications in sun behaviour for the OTR population is needed.
There is also a critical lack of primary prevention studies with clinically significant end points, such as the presence of precancerous lesions.57 Indeed, the evidence that modifying ultraviolet protective behaviour prevents cSCC in OTR is very limited. Only one prospective, nonrandomized study from Germany has considered these end points among 120 OTR and found that 2 years of regular vs. discretionary sunscreen use prevented development of AK and subsequent cSCC in OTR.59 Although this study provides an encouraging signal that regular sunscreen use may reduce cSCC risk in OTR, it will need to be replicated in randomized studies, one of which is in progress (www.clinicaltrials.gov; identifier NCT01532453) along with studies of effectiveness of other primary prevention measures besides sunscreen application in reducing AK and cSCC risk.
Novel approaches to photoprotection have been investigated in OTR, but results from clinical trials are not yet available: T4 endonuclease V (T4N5) is an enzyme involved in the repair of DNA damage after exposure to UVR, and a phase II study investigating the safety and efficacy of T4N5 lotion in preventing KC in OTR is underway (www.clinicaltrials.gov; identifier NCT00089180). Afamelanotide is a more potent and longer-acting chemical analogue of α-melanocyte-stimulating hormone administered by subcutaneous pellet and results of a multicentre, randomized, double-blind, placebo-controlled phase II trial to investigate its efficacy in reducing AK and cSCC in OTR are also awaited (www.clinicaltrials.gov; identifier NCT00829192).
Glucocorticoid treatment increases the destruction of 25-hydroxyvitamin D [25(OH)D], and may therefore affect vitamin D levels in OTR. Given that OTR are at increased risk of vitamin D deficiency, one potential adverse effect of advocating stringent sun-protective measures is the resultant exacerbation of existing vitamin D deficiency.60 Supplements are safe and efficacious in improving 25(OH)D blood levels in this patient group,61 and a multicentre, randomized, controlled vitamin D supplementation trial (n = 640) with several clinical end points, including cancer, is ongoing (www.clinicaltrials.gov; identifier NCT01431430). Dermatologists should be aware that OTR are at increased risk for vitamin D deficiency, and work with the transplant care team to ensure that OTR are appropriately monitored.
Role of human papillomavirus vaccination in the prevention of cutaneous squamous cell carcinoma in organ transplant recipients
β-Human papillomavirus (HPV) DNA is commonly associated with cutaneous cSCC in immunosuppressed patients and is detectable in > 80% cSCC in OTR,62 but the role of HPV in cSCC pathogenesis is still not well established. Current HPV vaccines target high-risk α-HPV types that cause cervical, anogenital and oropharyngeal cancers.62 Although available HPV vaccines show high efficacy in preventing high-risk α-HPV infection and partial cross-protection against the other α-HPV types,63 it is unclear whether these vaccines cross-protect against β-HPV types. Currently, novel vaccines that offer cross-reactivity against β-HPV types are being developed, and may have promise in prevention of cSCC.64 It is critical to determine if there is a role of β-HPV in skin carcinogenesis in OTR independent of UVR, because β-HPV vaccines could be efficacious in helping prevent post-transplant cSCC in OTR along with sun protection, particularly if administered prior to organ transplantation.
Early detection: screening frequency
The U.S. Preventive Services Task Force recently stated that there is insufficient evidence to recommend screening for skin cancer in the general population.65 The goal of screening and surveillance in OTR is to facilitate early detection and treatment of KC, and potentially improve prognosis. Ideally, the time interval of surveillance should be short enough to detect true precursors of malignant lesions, and long enough to avoid overuse of available resources.
Most available guidelines of KC screening intervals in OTR are based on retrospective data, and risk stratification is determined by history of cutaneous malignancy.5,66,67 The American Society of Transplantation recommends full-body skin examinations by ‘a qualified health professional, with experience in diagnosing skin cancer’ every 12 months,68 and full-body skin examinations are also commonly recommended in other expert consensus guidelines every 12 months for OTR with no skin cancer history, 6 months for AK or one KC, 3 months for those with multiple KC or high-risk cSCC and 1–3 months for metastatic cSCC.5,66,67 However, with the growing patient populations of OTR, the challenge for healthcare resources of such an approach could prove considerable, and evaluation of the current guidelines for OTR in prospective clinical studies is important to ensure that surveillance protocols are optimally deployed.
A few studies have addressed this area prospectively and indicate that the surveillance interval of selected patients could be increased without compromising safety.69,70 In these studies, models were developed to predict the optimum interval of cutaneous surveillance, based on several predictors of KC, including history of skin cancer, age, Fitzpatrick skin type, and episodes of sunburn. However, the relative importance of risk factors may differ across countries with different ethnic distributions and geographical locations. There is a need to assess the clinical utility of these models in other populations and the role of pretransplant screening has yet to be validated. In addition, the effectiveness of regular skin self-examinations in early detection of cSCC in OTR has not yet been addressed.
Conclusion
This review highlights key areas and gaps in clinical evidence for management and prevention strategies of cSCC in OTR. The OTR population is at high risk for keratinocyte carcinogenesis and important data-driven advancements in therapeutic and preventives strategies to reduce cSCC risk are urgently needed. Furthermore, findings from the transplant population have the potential to inform the management and therapy in the general population, in whom keratinocyte cancers are the most common human malignancy.
What’s already known about this topic?
Cutaneous squamous cell carcinoma is the most common malignancy in solid-organ transplant recipients and is associated with substantial morbidity.
Gaps exist in our knowledge of treatment and prevention of squamous cell carcinoma in solid-organ transplant recipients
What does this study add?
We identify knowledge gaps in skin cancer management among solid-organ transplant recipients to help guide future research enabling the development of evidence-based guidelines.
Acknowledgments
Funding sources
This work was supported by a grant from the United States National Institute of Health (R01 CA166672 to M.M.A.).
The KeraCon Immunosuppression Working Group (Sarah Arron, Maryam Asgari, Maria Blomberg, Jan Nico Bouwes Bavinck, Eric Engels, Adele Green, Catherine Harwood, Günther Hofbauer, Margaret Madeline, Priya Nagarajan, Luigi Naldi, Nishit Patel, Charlotte Proby, Amanda Ewart Toland) provided discussion on topics for review and feedback on the manuscript.
M.M.A. has received grant funding from Pfizer and Valeant Pharmaceuticals, but they are not relevant to the topics reviewed.
Footnotes
Conflicts of interest
All remaining authors report no conflicts of interest.
References
- 1.Madeleine MM, Patel NS, Plasmeijer E, et al. Epidemiology of keratinocyte cancers after organ transplant. Br J Dermatol. 2017 doi: 10.1111/bjd.15931. (in press) [DOI] [PubMed] [Google Scholar]
- 2.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–80. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 3.Bouwes Bavinck JN, Harwood CA, Genders RE, et al. Pain identifies squamous cell carcinoma in organ transplant recipients: the SCOPE-ITSCC PAIN study. Am J Transplant. 2014;14:668–76. doi: 10.1111/ajt.12587. [DOI] [PubMed] [Google Scholar]
- 4.Krynitz B, Edgren G, Lindelöf B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008 – a Swedish population-based study. Int J Cancer. 2013;132:1429–38. doi: 10.1002/ijc.27765. [DOI] [PubMed] [Google Scholar]
- 5.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263–279. doi: 10.1016/j.jaad.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 6.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957–66. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Silberstein E, Sofrin E, Bogdanov-Berezovsky A, et al. Lymph node metastasis in cutaneous head and neck squamous cell carcinoma. Dermatol Surg. 2015;41:1126–9. doi: 10.1097/DSS.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 8.Berg D, Otley CC. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1–17. doi: 10.1067/mjd.2002.125579. [DOI] [PubMed] [Google Scholar]
- 9.Garrett GL, Lowenstein SE, Singer JP, et al. Trends of skin cancer mortality after transplantation in the United States: 1987 to 2013. J Am Acad Dermatol. 2016;75:106–12. doi: 10.1016/j.jaad.2016.02.1155. [DOI] [PubMed] [Google Scholar]
- 10.Stasko T, Brown MD, Carucci JA, et al. Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Dermatol Surg. 2004;30:642–50. doi: 10.1111/j.1524-4725.2004.30150.x. [DOI] [PubMed] [Google Scholar]
- 11.Ad Hoc Task Force. Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531–50. doi: 10.1016/j.jaad.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 12.de Graaf YGL, Basdew VR, van Zwan-Kralt N, et al. The occurrence of residual or recurrent squamous cell carcinomas in organ transplant recipients after curettage and electrodesiccation. Br J Dermatol. 2006;154:493–7. doi: 10.1111/j.1365-2133.2005.07069.x. [DOI] [PubMed] [Google Scholar]
- 13.Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220–33. doi: 10.1016/j.jaad.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Panther D, Nino T, Macknet KD, et al. Eruptive keratoacanthomas as a complication of fractionated CO2 laser resurfacing and combination therapy with imiquimod and intralesional methotrexate. Dermatol Surg. 2015;41:172–5. doi: 10.1097/DSS.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 15.Ribero S, Balagna E, Sportoletti Baduel E, et al. Efficacy of electrochemotherapy for eruptive legs keratoacanthomas. Dermatol Ther. 2016;29:345–8. doi: 10.1111/dth.12374. [DOI] [PubMed] [Google Scholar]
- 16.Metterle L, Nelson C, Patel N. Intralesional 5-fluorouracil (FU) as a treatment for nonmelanoma skin cancer (NMSC): a review. J Am Acad Dermatol. 2016;74:552–7. doi: 10.1016/j.jaad.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Tallon B, Turnbull N. 5% fluorouracil chemowraps in the management of widespread lower leg solar keratoses and squamous cell carcinoma. Australas J Dermatol. 2013;54:313–16. doi: 10.1111/ajd.12055. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Craig JC, Shumack S. Oral retinoids for the prevention of skin cancers in solid organ transplant recipients: a systematic review of randomized controlled trials. Br J Dermatol. 2005;152:518–23. doi: 10.1111/j.1365-2133.2005.06347.x. [DOI] [PubMed] [Google Scholar]
- 19.Jambusaria-Pahlajani A, Miller CJ, Quon H, et al. Surgical Monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35:574–84. doi: 10.1111/j.1524-4725.2009.01095.x. [DOI] [PubMed] [Google Scholar]
- 20.Ross AS, Miller Whalen F, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859–66. doi: 10.1111/j.1524-4725.2009.01354.x. [DOI] [PubMed] [Google Scholar]
- 21.Jennings L, Schmults CD. Management of high-risk cutaneous squamous cell carcinoma. J Clin Aesthetic Dermatol. 2010;3:39–48. [PMC free article] [PubMed] [Google Scholar]
- 22.Veness MJ, Palme C, Smith M, Kalnins I. Cutaneous head and neck squamous cell carcinoma metastatic to cervical lymph nodes (nonparotid): a better outcome with surgery and adjuvant radiotherapy. Laryngoscope. 2003;113:1827–33. doi: 10.1097/00005537-200310000-00031. [DOI] [PubMed] [Google Scholar]
- 23.Laftavi MR, Sharma R, Feng L, et al. Induction therapy in renal transplant recipients: a review. Immunol Invest. 2014;43:790–806. doi: 10.3109/08820139.2014.914326. [DOI] [PubMed] [Google Scholar]
- 24.Petite SE, Bollinger JE, Eghtesad B. Antithymocyte globulin induction therapy in liver transplant old drug, new uses. Ann Pharmacother. 2016;50:592–8. doi: 10.1177/1060028016647974. [DOI] [PubMed] [Google Scholar]
- 25.Geissler EK. Skin cancer in solid organ transplant recipients: are mTOR inhibitors a game changer? Transplant Res. 2015;4:1. doi: 10.1186/s13737-014-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374:333–43. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 27.Hanlon A, Colegio OR. The cutting edge of skin cancer in transplant recipients: scientific retreat of international transplant skin cancer collaborative and skin cancer in organ transplant patients Europe. Am J Transplant. 2014;14:1012–15. doi: 10.1111/ajt.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell SB, Walker R, Tai SS, et al. Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am J Transplant. 2012;12:1146–56. doi: 10.1111/j.1600-6143.2012.04004.x. [DOI] [PubMed] [Google Scholar]
- 29.Jiyad Z, Olsen CM, Burke MT, et al. Azathioprine and risk of skin cancer in organ transplant recipients: systematic review and meta-analysis. Am J Transplant. 2016;16:3490–503. doi: 10.1111/ajt.13863. [DOI] [PubMed] [Google Scholar]
- 30.O’Neill JO, Edwards LB, Taylor DO. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:1186–91. doi: 10.1016/j.healun.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Coghill AE, Johnson LG, Berg D, et al. Immunosuppressive medications and squamous cell skin carcinoma: nested case-control study within the Skin Cancer after Organ Transplant (SCOT) cohort. Am J Transplant. 2016;16:565–73. doi: 10.1111/ajt.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wisgerhof HC, van der Boog PJM, de Fijter JW, et al. Increased risk of squamous-cell carcinoma in simultaneous pancreas kidney transplant recipients compared with kidney transplant recipients. J Invest Dermatol. 2009;129:2886–94. doi: 10.1038/jid.2009.181. [DOI] [PubMed] [Google Scholar]
- 33.Einollahi B, Nemati E, Lessan-Pezeshki M, et al. Skin cancer after renal transplantation: results of a multicenter study in Iran. Ann Transplant. 2010;15:44–50. [PubMed] [Google Scholar]
- 34.Knoll GA, Kokolo MB, Mallick R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ. 2014;49:g6679. doi: 10.1136/bmj.g6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533–40. doi: 10.1001/jamadermatol.2015.5548. [DOI] [PubMed] [Google Scholar]
- 36.Asgari MM, Arron ST, Warton EM, et al. Sirolimus use and risk of cutaneous squamous cell carcinoma (SCC) in solid organ transplant recipients (SOTRs) J Am Acad Dermatol. 2015;73:444–50. doi: 10.1016/j.jaad.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 37.Su L, Tam N, Deng R, et al. Everolimus-based calcineurin-inhibitor sparing regimens for kidney transplant recipients: a systematic review and meta-analysis. Int Urol Nephrol. 2014;46:2035–44. doi: 10.1007/s11255-014-0783-1. [DOI] [PubMed] [Google Scholar]
- 38.Weinstock MA, Lee KC, Chren M-M, et al. Quality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) Trial. J Am Acad Dermatol. 2009;61:207–15. doi: 10.1016/j.jaad.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chren M-M, Sahay AP, Bertenthal DS, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351–7. doi: 10.1038/sj.jid.5700740. [DOI] [PubMed] [Google Scholar]
- 40.Rhee JS, Matthews BA, Neuburg M, et al. The skin cancer index: clinical responsiveness and predictors of quality of life. Laryngoscope. 2007;117:399–405. doi: 10.1097/MLG.0b013e31802e2d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovach BT, Stasko T. Use of topical immunomodulators in organ transplant recipients. Dermatol Ther. 2005;18:19–27. doi: 10.1111/j.1529-8019.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- 42.Ulrich C, Bichel J, Euvrard S, et al. Topical immunomodulation under systemic immunosuppression: results of a multicentre, randomized, placebo-controlled safety and efficacy study of imiquimod 5% cream for the treatment of actinic keratoses in kidney, heart, and liver transplant patients. Br J Dermatol. 2007;157:25–31. doi: 10.1111/j.1365-2133.2007.08269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trakatelli M, Katsanos G, Ulrich C, et al. Efforts to counteract locally the effects of systemic immunosupression: a review on the use of imiquimod, a topical immunostimulator in organ transplant recipients. Int J Immunopathol Pharmacol. 2010;23:387–96. doi: 10.1177/039463201002300201. [DOI] [PubMed] [Google Scholar]
- 44.Benson E. Imiquimod: potential risk of an immunostimulant. Australas J Dermatol. 2004;45:123–4. doi: 10.1111/j.1440-0960.2004.00060.x. [DOI] [PubMed] [Google Scholar]
- 45.Santos-Juanes J, Esteve A, Mas-Vidal A, et al. Acute renal failure caused by imiquimod 5% cream in a renal transplant patient: review of the literature on side effects of imiquimod. Dermatology. 2011;222:109–12. doi: 10.1159/000323737. [DOI] [PubMed] [Google Scholar]
- 46.Ingham AI, Weightman W. The efficacy and safety of topical 5% 5-fluorouracil in renal transplant recipients for the treatment of actinic keratoses. Australas J Dermatol. 2014;55:204–8. doi: 10.1111/ajd.12158. [DOI] [PubMed] [Google Scholar]
- 47.Ulrich C, Johannsen A, Röwert-Huber J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482–8. doi: 10.1684/ejd.2010.1010. [DOI] [PubMed] [Google Scholar]
- 48.Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010–19. doi: 10.1056/NEJMoa1111170. [DOI] [PubMed] [Google Scholar]
- 49.Wlodek C, Ali FR, Lear JT. Use of photodynamic therapy for treatment of actinic keratoses in organ transplant recipients. BioMed Res Int. 2013;2013:e349526. doi: 10.1155/2013/349526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harwood CA, Leedham-Green M, Leigh IM, et al. Low-dose retinoids in the prevention of cutaneous squamous cell carcinomas in organ transplant recipients: a 16-year retrospective study. Arch Dermatol. 2005;141:456–64. doi: 10.1001/archderm.141.4.456. [DOI] [PubMed] [Google Scholar]
- 51.Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101–15. doi: 10.1093/carcin/bgn248. [DOI] [PubMed] [Google Scholar]
- 52.Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618–26. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 53.Chen AC, Martin AJ, Dalziell RA, et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br J Dermatol. 2016;75:1073–5. doi: 10.1111/bjd.14662. [DOI] [PubMed] [Google Scholar]
- 54.Endrizzi B, Ahmed RL, Ray T, et al. Capecitabine to reduce nonmelanoma skin carcinoma burden in solid organ transplant recipients. Dermatol Surg. 2013;39:634–45. doi: 10.1111/dsu.12049. [DOI] [PubMed] [Google Scholar]
- 55.Robinson JK, Rigel DS. Sun protection attitudes and behaviors of solid-organ transplant recipients. Dermatol Surg. 2004;30:610–15. doi: 10.1111/j.1524-4725.2004.30145.x. [DOI] [PubMed] [Google Scholar]
- 56.Mihalis EL, Wysong A, Boscardin WJ, et al. Factors affecting sunscreen use and sun avoidance in a U. S. national sample of organ transplant recipients. Br J Dermatol. 2013;168:346–53. doi: 10.1111/j.1365-2133.2012.11213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238–44. doi: 10.1016/j.jaad.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 58.Robinson JK, Turrisi R, Mallett KA, et al. Efficacy of an educational intervention with kidney transplant recipients to promote skin self-examination for squamous cell carcinoma detection. Arch Dermatol. 2011;147:689–95. doi: 10.1001/archdermatol.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulrich C, Jürgensen JS, Degen A, et al. Prevention of non-melanoma skin cancer in organ transplant patients by regular use of a sunscreen: a 24 months, prospective, case-control study. Br J Dermatol. 2009;161:78–84. doi: 10.1111/j.1365-2133.2009.09453.x. [DOI] [PubMed] [Google Scholar]
- 60.Taweesedt PT, Disthabanchong S. Mineral and bone disorder after kidney transplantation. World J Transplant. 2015;5:231–42. doi: 10.5500/wjt.v5.i4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barros X, Rodríguez NY, Fuster D, et al. Comparison of two different vitamin D supplementation regimens with oral calcifediol in kidney transplant patients. J Nephrol. 2016;29:703–9. doi: 10.1007/s40620-015-0237-6. [DOI] [PubMed] [Google Scholar]
- 62.Chockalingam R, Downing C, Tyring SK. Cutaneous squamous cell carcinomas in organ transplant recipients. J Clin Med. 2015;4:1229–39. doi: 10.3390/jcm4061229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herrero R. Human papillomavirus (HPV) vaccines: limited cross-protection against additional HPV types. J Infect Dis. 2009;199:919–22. doi: 10.1086/597308. [DOI] [PubMed] [Google Scholar]
- 64.Kwak K, Jiang R, Wang JW, et al. Impact of inhibitors and L2 antibodies upon the infectivity of diverse alpha and beta humanpapillomavirus types. PLOS ONE. 2014;9:e97232. doi: 10.1371/journal.pone.0097232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.US Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, et al. Screening for Skin Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429–35. doi: 10.1001/jama.2016.8465. [DOI] [PubMed] [Google Scholar]
- 66.Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407–15. doi: 10.4414/smw.2009.12725. [DOI] [PubMed] [Google Scholar]
- 67.Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients – where do we stand today? Am J Transplant. 2008;8:2192–8. doi: 10.1111/j.1600-6143.2008.02386.x. [DOI] [PubMed] [Google Scholar]
- 68.KDIGO clinical practice guideline for the care of kidney transplant recipients. Kidney disease: Improving Global Outcomes (KDIGO) Transplant Work Group. Chapter 18. Cancer of the skin and lip. Am J Transplant. 2009;9:S84–5. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 69.Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667–71. doi: 10.1097/TP.0b013e3181a5ce2e. [DOI] [PubMed] [Google Scholar]
- 70.Harwood CA, Mesher D, McGregor JM, et al. A surveillance model for skin cancer in organ transplant recipients: a 22-year prospective study in an ethnically diverse population. Am J Transplant. 2013;13:119–29. doi: 10.1111/j.1600-6143.2012.04292.x. [DOI] [PubMed] [Google Scholar]


