Abstract
Asthma is a chronic inflammatory disorder of the airways. While the local infiltration of eosinophils and mast cells, and their role in the disease, have long been recognized, neutrophil infiltration has also been assessed in many clinical studies. In these studies, airway neutrophilia was associated with asthma severity. Importantly, neutrophilia also correlates with asthma that is refractory to corticosteroids, the mainstay of asthma treatment. However, it is now increasingly recognized that neutrophils are a heterogeneous population, and a more precise phenotyping of these cells may help delineate different subtypes of asthma. Here, we review the current knowledge on the role of neutrophils in asthma and highlight future avenues of research in this field.
Epidemiology of Neutrophilic Asthma
Asthma has long been associated with eosinophilic inflammation as well as IgE-mediated mast cell activation, commonly described as a component of the “atopic march.” Due to the sensitivity of this type 2 inflammation to corticosteroids, inhibition of airway inflammation by inhaled corticosteroids has been the cornerstone of asthma therapy. However, neutrophilic inflammation is observed during asthma exacerbations and, importantly, in subgroups of patients with severe asthma that is more steroid-refractory as well. Indeed, studies performed two decades ago reported a higher neutrophil burden compared to eosinophils in the lung tissue of patients who had died from an asthma exacerbation [1]. Subsequent studies identified two broad inflammatory phenotypes in the airways of asthmatics-those with eosinophilic inflammation and others with neutrophil-dominated inflammation [2, 3], suggesting that these may represent two subtypes of the disease. More generally, an increase in neutrophil counts in the sputum of adults with persistent asthma [4] and in that of children with acute asthma exacerbations was reported [5]. However there is controversy whether solely the presence of eosinophils or neutrophils can be used as a binary disease classifier as there may be overlap between these two phenotypes. In adults, a subset of asthmatics was identified with high sputum neutrophil counts who responded poorly to treatment with inhaled corticosteroids [6]. Also, sputum neutrophil counts were associated with disease severity [7]. Notably, an increased number of airway neutrophils does not denote prolonged disease since the numbers do not differ among those with early versus late onset of disease [8]. Altogether, these studies hinted at a potentially important role of neutrophils in asthma, and a likely association with a distinct, more steroid-refractory subtype of the disease, even though these preliminary observations did not always distinguish between a bystander versus a driver role for this cell population.
Type 2 and Non-type 2 Asthma and the Association of Neutrophils with Asthma Severity and Lung Function
Woodruff and colleagues broadly classified the immune response in asthma into two groups-Th2high and Th2low based on gene expression in the airways [9]. It appears that approximately 50% of patients, whether diagnosed with mild or severe asthma, harbors a type 2 inflammatory response in their airways [9-12]. Whereas in mild asthma, a type 2 inflammatory response is generally linked to early-onset, atopic/allergic disease, in severe asthma, the relationship to atopy/allergy is less clear [13-15]. Despite the decreasing prevalence of atopy in severe disease, studies of various type 2 inflammation-directed therapies are beginning to link the adult onset/nasal polyp-associated phenotype with type 2 cytokine pathways, which include pathways traditionally associated with atopy/allergy (IL-4 and IL-13) and eosinophils (IL-5) [12, 16, 17]. Whether these clinical responses to agents targeting type 2 inflammation in less allergic/atopic patients imply efficacy disassociated from allergy or whether IgE responses exist locally or to non-traditional allergens is not known. Recent studies show the presence of a complex inflammatory process in severe asthma despite treatment with high doses of inhaled or systemic corticosteroids. In one study, clustering analysis of the Severe Asthma Research Program (SARP) cohort was performed using 112 variables, including immune-inflammatory cell counts from blood and bronchoalveolar lavage (BAL) fluid, allergy skin tests, and IgE. This study led to the identification of a severe asthma cluster, in which the patients showed the most impaired lung function with persistent eosinophils in the BAL fluid in combination with high neutrophil counts and exhaled nitric oxide (FeNO, fractional exhaled nitric oxide) [12]. These data suggest that eosinophilic inflammation can be also refractory to steroids and perhaps combined eosinophilic/neutrophilic inflammation may be a biomarker of the most severe form of the disease.
The presence of high FeNO in this most severe asthma cluster was surprising since corticosteroids are typically effective in reducing FeNO levels in milder asthma. However, this finding of high FeNO in patients with severe asthma maintained on high doses of corticosteroids was also observed in other studies [18, 19]. The enzyme inducible nitric oxide synthase (iNOS/NOS2), which catalyzes the generation of NO, is strongly induced by type 2 cytokines, and thus would be expected to be responsive to treatment [20]. However, NOS2 is also induced in human airway epithelial cells by the type I cytokine IFN-γ [21]. To further explore the complex role of FeNO in asthma, particularly in severe asthma, an epithelial cell gene profiling study was conducted to identify genes strongly correlated with FeNO [11]. The resulting FeNO-associated 589 genes were then used to cluster 155 patients. This exercise yielded five different clusters, one of which was associated with more severe disease [11]. In addition to elevation of type 2-related genes, one of three high FeNO clusters also revealed elevations in IFN-related genes. Recent studies in both mice and humans have highlighted increased Th1/IFN-γ and Th17/IL-17 responses in asthma, particularly in corticosteroid-resistant severe asthma, which is often associated with neutrophil infiltration of the airways [22, 23]. Transcripts for IL17A were found to be elevated in the sputum of patients with asthma and were correlated with CXCL8 (IL8) transcripts and sputum neutrophils as well as asthma severity [24]. In a pre-clinical model, adoptive transfer of antigen-specific Th17 cells as opposed to Th2 cells mediated neutrophilic airway inflammation, and airway hyperreactivity (AHR) that was more steroid resistant [25]. When BAL cells of asthmatics were examined, higher frequencies of CD4+ T cells expressing IFN-γ and IL-17 in the airways of severe asthmatics as compared to that in milder asthmatics were reported in independent studies [22, 26]. Using a mouse model of severe asthma that matched the Th1/Th17 profile in humans, a role for IFN-γ but not for IL-17 was observed in promoting AHR [22]. Similarly, a recently published study has identified Th1 and Th17 cells in the BAL of severe asthmatics based on chemokine receptor expression - Th1 cells being CCR6-CCR4- and Th17 cells being CCR6+CCR4+ [27]. In this study, the frequency of Th1 but not of Th17 cells was inversely correlated with lung function (% forced expiratory volume in 1s or %FEV1) [27].
Collectively these studies show that non-type 2 inflammation (in the presence or absence of persistent type 2 inflammation) is likely to be important in inducing specific clinical phenotypes in severe disease. In particular, type 1 responses and IFN-γ appear as most often associated with neutrophilic airway inflammation, IFN-driven nitric oxide production, and poor response to corticosteroids.
Obesity, Smoking, Gastroesophageal Reflux and Airway Neutrophilia
The percentage of neutrophils in the sputum of healthy controls ranges from 0-30% [28, 29], the etiology of which may be shear stress in the mucosa during the induction process, influence of specific resident microbiota or prior exposures to environmental agents. For example, higher sputum neutrophil counts were documented in ex-smokers [28]. However, the percentage of neutrophils in sputa can be much higher in asthma. For example, a recent UBIOPRED hierarchical clustering analysis of sputum transcriptomics identified three clusters in asthma patients [30]. Cluster 1 was associated with sputum eosinophilia and the Th2 genes IL1RL1 and CCR3. Cluster 2 was associated with the highest level of sputum neutrophilia (> 90%) accompanied by a marked increase in the expression of CXCR1/2 and members of the IFN (both type I and type II interferon inducible genes) and TNF families. Pathway analysis revealed an association of cluster 2 with innate lymphoid cell types 1 and 3 as well as with inflammasome signatures, suggesting that type I and II IFNs and the inflammasome may be key drivers of this endotype. Cluster 3 was found to comprise genes associated with metabolic pathways, ubiquitination and mitochondrial function and this cluster also tracked with moderate to high sputum eosinophil counts. Thus, since both type 17 responses and type 1 responses can drive neutrophilia, transcript analysis may improve the specification of neutrophils in sputum as well as disease classification. This idea is also supported by recent single cell sequencing technologies that have revealed the underappreciated heterogeneity of myeloid cell types, even in cells of similar morphology [31]. Moreover, similar to that observed in a prior study [32], cluster 2 was associated with systemic inflammation as demonstrated by elevated CRP and IL-6 levels in blood.
Gastroesophageal reflux disease (GERD) has been found to be a common feature in severe asthmatics in both the SARP [33] and UBIOPRED [15] cohorts. In the latter study, both nasal polyps and symptoms of GERD were found to be more frequent among severe asthmatics. In one cohort, GERD was associated with sputum neutrophilia [34]. Initial studies failed to detect an association between obesity and neutrophilic airway inflammation in asthma, which may have been due to small sample size and neglecting to consider the influence of sex in the analysis [35-37]. In a subsequent study, an association between obesity and neutrophil numbers in the airways was evident in females only. In males, serum levels of saturated fatty acids correlated with airway neutrophil counts [38]. Smoking worsens asthma symptoms and morbidity and promotes neutrophilic asthma [39]. In pre-clinical models of high fat diet consumption, obesity-induced changes in AHR was driven by high IL-17 production from group 3 innate lymphoid cells, which can also mediate airway neutrophilia [40]. Obesity in pediatric subjects with asthma can also exacerbate lung obstruction [40] but the role of airway neutrophilia in this process remains unclear. Subjects with obesity and asthma also have higher occurrences of GERD and obesity was inversely associated with sputum eosinophils and FeNO levels [41]. Thus, co-morbid factors such as obesity and GERD as well as obstructive sleep apnea may influence airway inflammatory responses and are important covariates in sputum analysis.
Altogether, these studies highlight the association of neutrophilia with a particular transcriptomic signature, as well as with specific risk factors, suggesting that neutrophilic asthma could be a bona fide subtype of asthma with a distinct pathological process.
Infections and Neutrophilic Asthma
In the context of airway disease, microbial infection has been best appreciated and examined in chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF). Fungi, viruses, and bacteria have all been associated with neutrophilic corticosteroid-refractory severe asthma. Among fungi, Aspergillus fumigatus has been identified in severe asthma with fungal sensitization (SAFS), and a neutrophilic response is mounted to combat the infection [42]. Infections by respiratory viruses have been associated with both asthma onset and asthma exacerbations [43]. In infants less than 2 years of age, respiratory syncytial virus (RSV)-induced severe bronchiolitis is a risk factor for developing asthma in later life [44, 45]. RSV infection can cause severe wheezing and induce neutrophilic airway inflammation [44, 45]. In addition to RSV, in young children and adults, human rhinovirus (HRV) infection can cause acute severe asthma exacerbations [43]. In one study, HRV inoculation into asthmatics, but not healthy controls, induced severe lower respiratory tract symptoms associated with bronchoconstriction, impairment of lung function and infiltration of both neutrophils and eosinophils into the airways [46]. Early reports of bacterial infections of the asthmatic airway [47, 48] were followed up by additional investigations that suggest a role for bacterial colonization of the airways in stable neutrophilic corticosteroid-refractory severe asthma [49, 50]. Several bacterial species have been detected in the sputum of stable severe asthmatics harboring neutrophilic airway inflammation, which include Chlamydia pneumoniae, Streptococcus pneumoniae, Mycoplasma pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and Staphylococcus aureus [49, 50]. Neutrophilic asthma has been also associated with a reduced diversity of the lung microbiota which is replaced by a higher frequency of the bacteria Tropheryma whipplei and H. influenzae [51]. This suggests that airway dysbiosis may be responsible for some aspects of sputum neutrophilia in asthma. Increased neutrophil numbers in the airways have been also associated with viral infections often in the context of asthma exacerbations [1, 5, 52-54]. It is important to note that association between neutrophils and asthma exacerbations has been also made in the absence of infections [55].
That specific pathogens may promote corticosteroid insensitivity was demonstrated in an interesting study in which infection of BAL macrophages by Haemophilus parainfluenzae but not by commensal bacteria belonging to the genus Prevotella inhibited the expression of genes that confer response to corticosteroids [56]. Pathogens may also enhance neutrophil survival in the airways. Indeed, pattern recognition receptors expressed on the surface of neutrophils serve to prolong neutrophil survival by activation of specific regulators downstream such as NF-κB and mitogen-activated protein kinases (MAPKs) [57]. Clearly, investigations of the role of specific pathogens in disease severity and response to therapy is an emerging area and it will be of particular interest if identification of specific bacteria or their communities can help predict disease severity, nature of the immune response as well as response to treatment.
Like certain forms of asthma, COPD is also characterized by high sputum neutrophils and elevated levels of CXCL8 [58]. Thus, there is an overlap between neutrophilic asthma and certain aspects of COPD. However, there are important differences including a higher prevalence of H. influenzae in the sputum of subjects with COPD [51]. Additionally, FeNO levels have been reported to be higher in subjects with asthma and asthma-COPD overlap syndrome compared to that in subjects with COPD [59]. In an effort to better discriminate between patients with fixed airflow obstruction as caused by asthma versus COPD, recent studies have highlighted differences in the concentrations of urinary metabolites [60] and sagittal-lung CT measurements [61] in the two disease settings. These tools however require replication in additional cohorts of subjects.
Mediators and Their Impact on Neutrophilic Inflammation
A number of soluble mediators have been shown to play a role in regulating lung neutrophil recruitment (Table I) and the adhesion molecules involved in this process have recently been reviewed [62]. An increase in the level of the chemokine CXCL8 (IL-8) was detected in the sputum [2] and nasal secretions of asthmatics [53], CXCL8 being the most potent neutrophil chemoattractant in the lung [63]. Although various cell types including airway epithelial cells, T cells and macrophages can secrete CXCL8, neutrophils themselves have been shown to produce this chemokine, suggesting a feed forward loop that promotes neutrophil recruitment to the airways [2]. Interestingly, neutrophils from asthmatic but not non-asthmatic subjects were shown to express the high-affinity IgE receptor, FcsRI, whose engagement led to release of CXCL8 from the neutrophils [64]. CXCL8 is also induced by IL-17, which can be produced by both γ5 and αβ T cells [65]. The relevance of this induction is highlighted by cross-talk between human neutrophils and Th17 cells, with each favoring the recruitment of the other cell type via release of chemokines including CXCL8 [66]. These studies also showed secretion of the chemokine CXCL10 by neutrophils facilitating recruitment of Th1 cells, the signature cytokine produced by these cells being IFN-γ. IFN-γ has been implicated in the chemotaxis of human neutrophils as well via upregulation of the chemokine receptors CCR1 and CCR3 on the neutrophils [67]. Thus, it is possible that neutrophils, Th1 and Th17 cells establish a communication network in the airways of some severe asthmatics, which as discussed below, is refractory to treatment by inhaled or oral corticosteroids. While the association between IL-17 and neutrophils was found upon sampling of sputum of asthmatics [24, 68], examination of IL-17A and IL-17F levels in biopsies failed to find a relationship between the levels of these cytokines and neutrophilic inflammation [69, 70]. In contrast, the association between IL-17 and neutrophilic inflammation is well-documented in other diseases such as psoriasis, where targeting the IL-17R with Brodalumab reduces neutrophil infiltration of the skin with improvement in disease [71, 72]. These results argue that more studies are needed to establish a cause and effect relationship between IL-17 and neutrophilic airway inflammation in asthma [70]. Studies have also documented an increase in the level of TNF-α in the airways of asthmatics [73, 74]. Exposure of normal human subjects to TNF-α by inhalation induced AHR with increased sputum neutrophil counts [75].
Table 1. Soluble mediators associated with neutrophilic airway inflammation.
| Cytokines | References | |
|---|---|---|
| IL-1α | ↑ | [121] |
| IL-1β | ↑ | [122, 123] |
| IL-1Ra | ↓ | [124] |
| IL-6 | ↑ | [125] |
| IL-10 | ↓ | [126, 127] |
| IL-17 | ↑ | [24, 25, 40] |
| IL-23 | ↑ | [128, 129] |
| IFN-γ | ↑ | [22, 23] |
| TNF-α | ↑ | [130, 131] |
| Lipids | ||
| LTB4 | ↑ | [132] |
| Lipoxin A4 | ↓ | [133, 134] |
| ResolvinD1/E1 | ↓ | [134, 135] |
| PGD2 | ↓ | [136] |
| Chemokines | ||
| CXCL1 | ↑ | [137, 138] |
| CXCL5 | ↑ | [59] |
| CXCL6 | ↑ | [139] |
| CXCL8 | ↑ | [140] |
| Complement/peptides | ||
| C5a | ↑ | [141, 142] |
| FMLP | ↑ | [143, 144] |
Collectively, these studies demonstrate the potential for an active induction of airway neutrophilia by mediators that induce steroid-refractory disease. However, in some patients, neutrophilia could be a passive process due to increased demargination (due to reduced sequestration in the pulmonary vasculature), and the reduction of sputum eosinophils could be due to a consequence of corticosteroid treatment rather than a fundamental difference in physiopathology. Which infiltration mechanism is relevant in any individual patient will likely require assessing these pathways in induced sputum or the development of improved breath and serum biomarkers. These types of approaches may ultimately help further delineate the different pathological mechanisms of different disease subtypes.
Beneficial and Adverse Effects of Neutrophils in the Asthmatic Airway
Neutrophils constitute the first line of defense during pulmonary infection and a host of tools in their armamentarium can target and kill pathogens [76]. Neutrophils can also release chemotactic factors and preformed granule proteins that attract monocytes/macrophages to the site of infection, thus shaping the immune infiltrate [77]. The enzyme NADPH oxidase plays an important role in the anti-microbial activity of neutrophils, through the generation of reactive oxygen species, the activation of granular proteases and the release of neutrophil extracellular traps (NETs) [78]. NETs are composed of neutrophil DNA and coated with anti-microbial factors, including histones and antimicrobial peptides. NETs can therefore functions as physical and biological barriers to pathogen dissemination [79]. NET formation is also facilitated by proinflammatory cytokines, and while NETs participate in pathogen elimination, they can also exacerbate airway disease such as asthma and COPD. Indeed, studies have linked NETs to airway obstruction in both asthma and COPD [80, 81].
Neutrophils can exert additional adverse effects on the airways including airway narrowing due to airway remodeling, mucus hypersecretion mediated by neutrophil elastase [82], increased airway smooth muscle responsiveness [83] and a rapid decline in lung function [84]. Airway remodeling associated with thickening of the reticular basement membrane, airway smooth muscle hypertrophy and hyperplasia, mucus gland hyperplasia are characteristic features of asthma that contribute to progressive and irreversible loss of lung function [85]. Airway remodeling is generally believed to be a consequence of heightened airway inflammation in asthmatics. Mediators released by both structural cells and inflammatory cells have been implicated in airway remodeling. One such mediator is transforming growth factor (TGF)-β, which can be produced by multiple cell types in the asthmatic airway including neutrophils. TGF-β is a pro-fibrotic cytokine and is the one most associated with airway remodeling in asthma [86]. While peripheral blood neutrophils constitutively express TGF-β in both asthmatics and healthy controls, those present in asthmatics secrete a higher level of TGF-β [87]. Eosinophils are considered a major source of TGF-β in the airways of asthmatics. In addition, airway epithelial cells are also an important source of various mediators including TGF-β and thus these cells can no longer be viewed merely as structural cells [86].
Airway remodeling may be also promoted by neutrophil-generated matrix metalloprotease -9 (MMP-9) [88] and elastase [89]. Neutrophil elastase augments IL-8 production from airway epithelial cells promoting a feed forward loop in neutrophil recruitment to the airways. The level of neutrophil elastase was shown to inversely correlate with lung function (as measured by force expiratory volume in 1 second or FEV1) [90]. Neutrophil elastase also has the ability to inactivate tissue inhibitor of metalloproteinase-1 (TIMP-1), which inhibits MMP-9 [91]. An imbalance in protease (MMP-9)/anti-protease (TIMP-1) levels has been implicated in asthma pathogenesis, that is characterized by the persistence of proteases such as MMP-9 and neutrophil elastase and decreased levels of anti-proteases such as TIMP-1 and secretory leukocyte protease inhibitor (SLPI, an inhibitor of neutrophil elastase) [22, 90, 92]. Although the expression of SLPI can be upregulated by pro-inflammatory cytokines, IFN-γ, which is present in relatively high levels in the airways of many severe asthmatics, inhibits SLPI expression [22, 93, 94]. An imbalance in MMP-9/TIMP-1 has been also associated with persistent wheezing in preschool children [95]. Despite the generally accepted notion of airway inflammation guiding airway remodeling, this association has been disputed based on studies conducted in both children and adults. A study involving pre-school children failed to detect any association between airway inflammation and airway remodeling [96]. In a different study in adults, while allergen exposure induced airway inflammation, bronchoconstriction and airway remodeling, methacholine challenge induced bronchoconstriction and airway remodeling but no airway inflammation [97].
Epithelial barrier function can be also affected by an increased neutrophil burden in the airways. One study showed neutrophils to be a major source of the cytokine Oncostatin M, which affected epithelial barrier function in the airways of patients with severe asthma or in those with chronic rhinosinusitis [98].
Thus, while inflammation and remodeling may not always go hand in hand, it is clear that neutrophils can actively participate in both processes, through the production of chemokines and proteases. The net effect of their activity is in turn likely to be modulated by both lung epithelial cells and other recruited immune cells, highlighting the importance of a thorough evaluation of the micro-environment in different subtypes of asthma patients.
Factors contributing to Neutrophil Persistence in Asthmatic Airways
Inhibition of airway inflammation by inhaled corticosteroids is the cornerstone of asthma therapy. However, severe asthma is poorly responsive to corticosteroids [4, 23, 99, 100]. Multiple mechanisms may underlie the lack of response to corticosteroids in asthma. Severe asthmatics maintained on a high level of inhaled and often systemic corticosteroids display high numbers of sputum neutrophils. While corticosteroids promote the apoptosis of eosinophils, they have been shown to inhibit the apoptosis of neutrophils [101, 102]. In fact, elevation of airway neutrophil numbers has been associated with high dose inhaled [103] and oral corticosteroids [104]. Another factor that may contribute to neutrophil persistence and survival in the airways is ATP, which can be released from dying cells [105-107]. Inflammatory mediators such as LTB4 have been also implicated in human neutrophil survival with reversal observed with LTB4 receptor blockade [108]. As discussed above, pathogen-induced mechanisms can also promote neutrophil survival [57]. It is, however, unclear whether blockade of initiation of apoptosis or removal of dying neutrophils (efferocytosis) or both contributes to persistence of neutrophils in the airways of asthmatics. In any case, it is important to identify all factors that contribute to increased numbers of neutrophils in asthmatics whose symptoms are poorly controlled by conventional therapy.
Potential Treatments for Neutrophilic Asthma
With the inability of corticosteroids to ameliorate neutrophilic asthma, there is considerable interest in alternative strategies to target neutrophils in severe asthma with a high neutrophil burden [23, 100]. Since infections with bacteria such as Chlamydomonas pneumoniae, Mycoplasma pneumoniae and Staphylococcus aureus and viruses such as rhinovirus have been associated with asthma exacerbations [23], macrolides with antibacterial and antiviral properties have been examined in clinical trials as an alternative therapeutic modality. For example, the macrolide clarithromycin was used to treat severe corticosteroid-refractory asthma in a double-blind placebo-controlled study, [109]. The results were encouraging with 8 weeks of clarithromycin treatment causing a significant reduction in airway neutrophil numbers and the level of CXCL8 and improvement in symptoms [109]. Independent meta-analyses have concluded that use of macrolides results in improvements in asthma symptoms and that macrolides are beneficial in patients with non-eosinophilic asthma [110-112]. However, currently, there is insufficient data in support of macrolide use as a standard of care in asthma. Also, there is concern about the emergence of macrolide-resistant organisms and cardiovascular complications resulting from long-term macrolide use [113]. In other approaches, an antagonist to the CXCL8 receptor, CXCR2, was used which caused a significant reduction in airway neutrophil numbers in the airways of the asthmatics [114]. Targeting the IL-17 receptor, however, did not alleviate disease symptoms in a cohort of patients with inadequately controlled moderate to severe asthma [115]. It is also unclear how effective the IL-17 receptor occupancy was and whether this may be a variable in assessing response to drug. Targeting TNF-a has also not improved lung function [116-118] although one of these studies reported a 50% reduction in asthma exacerbations [118]. The lack of efficacy of these studies may be due to the heterogeneity of these patient subgroups with poorly controlled asthma. Collectively, each of these alternate therapeutic approaches may have benefit in alleviating specific outcome measures in sub-groups of asthmatics, and a better understanding of asthma endotypes will help to guide more targeted therapy in the future.
State of Neutrophil Maturation and Activation
Another important consideration in the context of disease complexity is heterogeneity within the neutrophils themselves (Figure 1). Recent literature highlights significant heterogeneity in neutrophil populations both in circulation and in tissues [119]. The neutrophils recovered from different sites in health or in different disease states appear to be at different states of maturity and activation. While the concept of neutrophil heterogeneity is not new [120], there is renewed interest in understanding the effector function of neutrophils as pro-inflammatory or immunosuppressive cells. There is considerable ongoing effort to phenotypically better define inflammatory versus suppressive neutrophils, which will be immensely helpful to stratify patients for specific therapies. Thus, it can be expected that improved characterization of neutrophils in the airways of asthmatics rather than their mere numbers will better inform us whether or not the neutrophils should be targeted to alleviate disease symptoms.
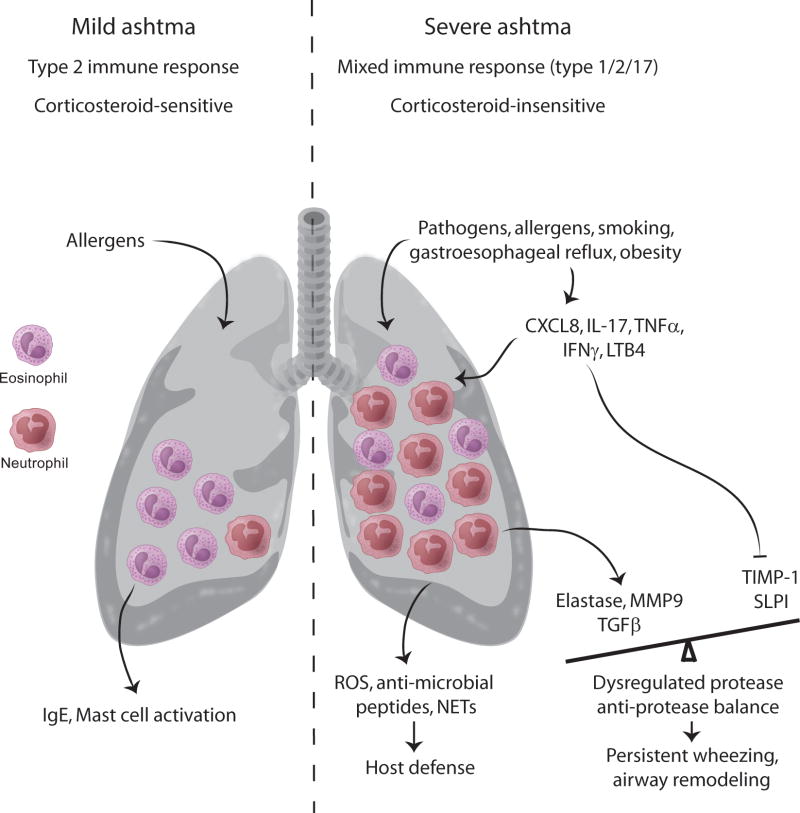
Figure 1. Contrasting features of eosinophilic mild asthma and mixed neutrophilic/eosinophilic severe asthma.
Eosinophilic allergic asthma is characteristic of 50% of mild asthma. Other features of mild allergic asthma include increased type 2 inflammation in the airways accompanied by elevated serum IgE levels, mast cell activation and attenuation of symptoms by low dose inhaled corticosteroids. In mild allergic asthma, eosinophils typically undergo apoptosis in the presence of corticosteroids. In contrast, neutrophils can be detected in the airways of severe asthmatics, often in conjunction with eosinophils. Neutrophils infiltrate the airways of asthmatics during asthma exacerbations, and have been detected in the airways of individuals who died from an asthma attack. Pathogens, smoking and different co-morbidities can trigger neutrophil recruitment to the airways. The cytokines CXCL8, IL-17, tumor necrosis factor (TNF)-α, interferon (IFN)-γ and the leukotriene LTB4 promote neutrophil infiltration of the airways with the neutrophils themselves occasionally being a source of CXCL8 providing a feed-forward loop. While mediators released from neutrophils like reactive oxygen species (ROS) and proteases help in host defense, some of these proteases such as elastase and MMP-9 negatively regulate the levels of TIMP-1 and SLPI creating a protease-antiprotease imbalance, which can promote bronchoconstriction. Increased secretion of TGF-p by neutrophils promotes airway remodeling, a characteristic feature of persistent asthma. Neutrophils are generally resistant to the anti-inflammatory or pro-apoptotic effects of corticosteroids. Resistance to corticosteroid-induced apoptosis is also observed in the case of eosinophils in severe disease. Although neutrophilic airway inflammation is well described in asthmatics, there is insufficient information regarding their phenotype or state of activation or even their inflammatory or suppressive function in different asthmatics, which should be taken into consideration before targeting these cells to alleviate asthma.
Concluding Remarks
Airway neutrophils in asthma have been associated with disease severity and acute asthma exacerbations (Figure 1). Recent reports show association of neutrophilic airway inflammation with severe asthma in the context of non-type 2 inflammation, which may or may not be accompanied by type 2 inflammation and airway eosinophils. Several mediators have been implicated in neutrophil recruitment to the airways, although a direct cause and effect relationship in each case is not sufficiently established. Infection of the respiratory tract by pathogens induces a neutrophilic response as a measure of host defense, and could represent a driver for the disease. While neutrophils play an important role in pathogen elimination, persistent neutrophilia and the associated secretion of proteases has detrimental consequences in the airways, including airway injury and obstruction, mucus hypersecretion and airway remodeling. Corticosteroids, which are the mainstay of asthma therapy, fail to suppress neutrophilic inflammation and may even promote neutrophil survival. While alternate therapeutic agents such as macrolides, that target pathogens, have shown promise in reducing sputum neutrophil numbers with improvement in symptoms, this treatment modality is not considered a viable long-term option because of the potential for inducing macrolide-resistant organisms as also adverse cardiovascular outcomes. Molecules associated with high neutrophil burden such as the chemokine receptor CXCR2 have been also been targeted in clinical trials with some efficacy but it is doubtful that a single approach will benefit all subjects because of differences in the underlying pathophysiologies in different asthmatics. An additional issue at stake is heterogeneity among neutrophil populations both with respect to state of maturity and activation. Notwithstanding these handicaps, neutrophils remain an important biomarker of disease. However, the mere presence of neutrophils in sputum or BAL fluid lacks precision to serve as an independent biomarker because of the multiple confounders that can influence their presence and function including obesity, GERD, smoking and medications. Controlling for these confounders combined with assessment of proximal mediators that can regulate active neutrophil recruitment and function such as leukotrienes, IL-17, IFN-γ and TNF-α and increased use of single cell transcriptomics may better guide future clinical trials for patients with corticosteroid-refractory neutrophilic asthma. This, in turn, would allow better precision in utilizing neutrophils to define asthma endotypes (see Outstanding questions).
Outstanding questions.
What are the immune pathways (type I, type 17, others) that result in airway neutrophilia?
How do disease exacerbations influence this response and are neutrophils a useful biomarker in stable versus unstable disease?
Are all neutrophils the same or does neutrophil heterogeneity contribute to asthma severity?
Do neutrophils play a role in resolution of inflammation in the asthmatic airway?
Box.
Airway neutrophilia has been associated with asthma severity and asthma exacerbations, however, neutrophils can also be detected in the airways of both healthy subjects and mild asthmatics.
Multiple mediators including chemokines, cytokines and lipids can promote neutrophil recruitment to the airways, and smoking and comorbidities such as gastroesophageal reflux and obesity have shown positive relationships with sputum neutrophil counts.
Neutrophils can combat infections and kill pathogens but can also have adverse effects on the airways, through the effects of proteases and reactive oxygen species.
An improved understanding of neutrophil populations would allow better precision in using neutrophils to define asthma phenotypes.
Acknowledgments
The authors would like to acknowledge support for this work form the following NIH grants: AI106684 (AR and JKK) and HL113956 (AR)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sur S, et al. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis. 1993;148(3):713–9. doi: 10.1164/ajrccm/148.3.713. [DOI] [PubMed] [Google Scholar]
- 2.Gibson PG, et al. Heterogeneity of airway inflammation in persistent asthma : evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119(5):1329–36. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 3.Wenzel SE, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160(3):1001–8. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 4.Jatakanon A, et al. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1532–9. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 5.Norzila MZ, et al. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am J Respir Crit Care Med. 2000;161(3 Pt 1):769–74. doi: 10.1164/ajrccm.161.3.9809071. [DOI] [PubMed] [Google Scholar]
- 6.Green RH, et al. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57(10):875–9. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore WC, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133(6):1557–63 e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miranda C, et al. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–8. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 9.Woodruff PG, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fajt ML, et al. Prostaglandin D(2) pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131(6):1504–12. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modena BD, et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am J Respir Crit Care Med. 2014;190(12):1363–72. doi: 10.1164/rccm.201406-1099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu W, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014;133(5):1280–8. doi: 10.1016/j.jaci.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J. 2003;22(3):470–7. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 14.Moore WC, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw DE, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46(5):1308–21. doi: 10.1183/13993003.00779-2015. [DOI] [PubMed] [Google Scholar]
- 16.Haldar P, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–24. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore WC, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–23. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stirling RG, et al. Increase in exhaled nitric oxide levels in patients with difficult asthma and correlation with symptoms and disease severity despite treatment with oral and inhaled corticosteroids. Asthma and Allergy Group. Thorax. 1998;53(12):1030–4. doi: 10.1136/thx.53.12.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wysocki K, et al. Characterization of factors associated with systemic corticosteroid use in severe asthma: data from the Severe Asthma Research Program. J Allergy Clin Immunol. 2014;133(3):915–8. doi: 10.1016/j.jaci.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muijsers RB, et al. L-Arginine is not the limiting factor for nitric oxide synthesis by human alveolar macrophages in vitro. Eur Respir J. 2001;18(4):667–71. doi: 10.1183/09031936.01.00101301. [DOI] [PubMed] [Google Scholar]
- 21.Guo FH, et al. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J Clin Invest. 1997;100(4):829–38. doi: 10.1172/JCI119598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raundhal M, et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125(8):3037–50. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray A, et al. Current concepts of severe asthma. J Clin Invest. 2016;126(7):2394–403. doi: 10.1172/JCI84144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bullens DM, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinley L, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181(6):4089–97. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chambers ES, et al. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-gamma(high) immunophenotypes: Potential benefits of calcitriol. J Allergy Clin Immunol. 2015;136(3):628–637 e4. doi: 10.1016/j.jaci.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duvall MG, Barnig C. Natural killer cell–mediated inflammation resolution is disabled in severe asthma. Science Immunol. 2017;2:eaam5446. doi: 10.1126/sciimmunol.aam5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen Y, et al. Assessment of airway inflammation using sputum, BAL, and endobronchial biopsies in current and ex-smokers with established COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:327–34. doi: 10.2147/COPD.S11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirogov AB, et al. Inflammatory Pattern of the Bronchial Mucosa in Patients with Asthma with Airway Hyperresponsiveness to Hypoosmotic Stimulus. Bull Exp Biol Med. 2016;161(4):550–3. doi: 10.1007/s10517-016-3458-3. [DOI] [PubMed] [Google Scholar]
- 30.Kuo CS, et al. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur Respir J. 2017;49(2) doi: 10.1183/13993003.02135-2016. [DOI] [PubMed] [Google Scholar]
- 31.Villani AC, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356(6335) doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood LG, et al. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. 2012;142(1):86–93. doi: 10.1378/chest.11-1838. [DOI] [PubMed] [Google Scholar]
- 33.Moore WC, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson JL, et al. Neutrophilic asthma is characterised by increased rhinosinusitis with sleep disturbance and GERD. Asian Pac J Allergy Immunol. 2014;32(1):66–74. doi: 10.12932/AP0322.32.1.2014. [DOI] [PubMed] [Google Scholar]
- 35.Lessard A, et al. Obesity and asthma: a specific phenotype? Chest. 2008;134(2):317–323. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 36.Sutherland TJ, et al. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178(5):469–75. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 37.Todd DC, et al. Effect of obesity on airway inflammation: a cross-sectionalanalysis of body mass index and sputum cell counts. Clin Exp Allergy. 2007;37(7):1049–54. doi: 10.1111/j.1365-2222.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 38.Scott HA, et al. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J. 2011;38(3):594–602. doi: 10.1183/09031936.00139810. [DOI] [PubMed] [Google Scholar]
- 39.Polosa R, Thomson NC. Smoking and asthma: dangerous liaisons. Eur Respir J. 2013;41(3):716–26. doi: 10.1183/09031936.00073312. [DOI] [PubMed] [Google Scholar]
- 40.Kim HY, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20(1):54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Veen IH, et al. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63(5):570–4. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 42.Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011;29:1–21. doi: 10.1146/annurev-immunol-030409-101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Busse WW, et al. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376(9743):826–34. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldman AS, et al. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med. 2015;191(1):34–44. doi: 10.1164/rccm.201405-0901PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Openshaw PJ, et al. Protective and Harmful Immunity to RSV Infection. Annu Rev Immunol. 2017 doi: 10.1146/annurev-immunol-051116-052206. [DOI] [PubMed] [Google Scholar]
- 46.Message SD, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105(36):13562–7. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hilty M, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korppi M. Bacterial infections and pediatric asthma. Immunol Allergy Clin North Am. 2010;30(4):565–74. vii. doi: 10.1016/j.iac.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Green BJ, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014;9(6):e100645. doi: 10.1371/journal.pone.0100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Q, et al. Bacteria in sputum of stable severe asthma and increased airway wall thickness. Respir Res. 2012;13:35. doi: 10.1186/1465-9921-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simpson JL, et al. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J. 2016;47(3):792–800. doi: 10.1183/13993003.00405-2015. [DOI] [PubMed] [Google Scholar]
- 52.Fahy JV, et al. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95(4):843–52. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 53.Teran LM, et al. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus-induced asthma. Am J Respir Crit Care Med. 1997;155(4):1362–6. doi: 10.1164/ajrccm.155.4.9105080. [DOI] [PubMed] [Google Scholar]
- 54.Twaddell SH, et al. Assessment of airway inflammation in children with acuteasthma using induced sputum. Eur Respir J. 1996;9(10):2104–8. doi: 10.1183/09031936.96.09102104. [DOI] [PubMed] [Google Scholar]
- 55.Lamblin C, et al. Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am J Respir Crit Care Med. 1998;157(2):394–402. doi: 10.1164/ajrccm.157.2.97-02099. [DOI] [PubMed] [Google Scholar]
- 56.Goleva E, et al. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med. 2013;188(10):1193–201. doi: 10.1164/rccm.201304-0775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013;34(7):317–28. doi: 10.1016/j.it.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Paplinska-Goryca M, et al. Expression of Inflammatory Mediators in Induced Sputum: Comparative Study in Asthma and COPD. Adv Exp Med Biol. 2016 doi: 10.1007/5584_2016_165. [DOI] [PubMed] [Google Scholar]
- 59.Chen K, et al. IL-17 Receptor Signaling in the Lung Epithelium Is Required for Mucosal Chemokine Gradients and Pulmonary Host Defense against K. pneumoniae. Cell Host Microbe. 2016;20(5):596–605. doi: 10.1016/j.chom.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adamko DJ, et al. Metabolomic profiling of asthma and chronic obstructive pulmonary disease: A pilot study differentiating diseases. J Allergy Clin Immunol. 2015;136(3):571–580 e3. doi: 10.1016/j.jaci.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 61.Qu Y, et al. Sagittal-lung CT measurements in the evaluation of asthma-COPD overlap syndrome: a distinctive phenotype from COPD alone. Radiol Med. 2017 doi: 10.1007/s11547-017-0743-9. [DOI] [PubMed] [Google Scholar]
- 62.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 63.Kunkel SL, et al. Interleukin-8 (IL-8): the major neutrophil chemotactic factorin the lung. Exp Lung Res. 1991;17(1):17–23. doi: 10.3109/01902149109063278. [DOI] [PubMed] [Google Scholar]
- 64.Gounni AS, et al. Human neutrophils express the high-affinity receptor for immunoglobulin E (Fc epsilon RI): role in asthma. FASEB J. 2001;15(6):940–9. doi: 10.1096/fj.00-0378com. [DOI] [PubMed] [Google Scholar]
- 65.Laan M, et al. IL-17-induced cytokine release in human bronchial epithelial cellsin vitro: role of mitogen-activated protein (MAP) kinases. Br J Pharmacol. 2001;133(1):200–6. doi: 10.1038/sj.bjp.0704063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pelletier M, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115(2):335–43. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 67.Bonecchi R, et al. Up-regulation of CCR1 and CCR3 and induction of chemotaxis to CC chemokines by IFN-gamma in human neutrophils. J Immunol. 1999;162(1):474–9. [PubMed] [Google Scholar]
- 68.Agache I, et al. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010;104(8):1131–7. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 69.Doe C, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;138(5):1140–7. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chesne J, et al. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190(10):1094–101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 71.Krueger JG, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130(1):145–54 e9. doi: 10.1016/j.jaci.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papp KA, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–9. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 73.Bradding P, et al. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10(5):471–80. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 74.Ying S, et al. TNF alpha mRNA expression in allergic inflammation. Clin Exp Allergy. 1991;21(6):745–50. doi: 10.1111/j.1365-2222.1991.tb03205.x. [DOI] [PubMed] [Google Scholar]
- 75.Thomas PS, et al. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med. 1995;152(1):76–80. doi: 10.1164/ajrccm.152.1.7599866. [DOI] [PubMed] [Google Scholar]
- 76.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15(7):602–11. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 77.Soehnlein O, et al. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009;30(11):538–46. doi: 10.1016/j.it.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 78.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5(8):577–82. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 79.Balloy V, Chignard M. The innate immune response to Aspergillus fumigatus. Microbes Infect. 2009;11(12):919–27. doi: 10.1016/j.micinf.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 80.Dworski R, et al. Eosinophil and neutrophil extracellular DNA traps in humanallergic asthmatic airways. J Allergy Clin Immunol. 2011;127(5):1260–6. doi: 10.1016/j.jaci.2010.12.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grabcanovic-Musija F, et al. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir Res. 2015;16:59. doi: 10.1186/s12931-015-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nadel JA. Role of neutrophil elastase in hypersecretion during COPD exacerbations, and proposed therapies. Chest. 2000;117(5 Suppl 2):386S–9S. doi: 10.1378/chest.117.5_suppl_2.386s. [DOI] [PubMed] [Google Scholar]
- 83.Anticevich SZ, et al. Induction of hyperresponsiveness in human airway tissue by neutrophils--mechanism of action. Clin Exp Allergy. 1996;26(5):549–56. [PubMed] [Google Scholar]
- 84.Stanescu D, et al. Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax. 1996;51(3):267–71. doi: 10.1136/thx.51.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest. 2013;144(3):1026–32. doi: 10.1378/chest.12-3073. [DOI] [PubMed] [Google Scholar]
- 86.Halwani R, et al. Role of transforming growth factor-beta in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44(2):127–33. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 87.Chu HW, et al. Peripheral blood and airway tissue expression of transforming growth factor beta by neutrophils in asthmatic subjects and normal control subjects. J Allergy Clin Immunol. 2000;106(6):1115–23. doi: 10.1067/mai.2000.110556. [DOI] [PubMed] [Google Scholar]
- 88.Cundall M, et al. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J Allergy Clin Immunol. 2003;112(6):1064–71. doi: 10.1016/j.jaci.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 89.Nadel JA. Role of enzymes from inflammatory cells on airway submucosal gland secretion. Respiration. 1991;58(1):3–5. doi: 10.1159/000195961. [DOI] [PubMed] [Google Scholar]
- 90.Vignola AM, et al. Increased levels of elastase and alpha1-antitrypsin in sputum of asthmatic patients. Am J Respir Crit Care Med. 1998;157(2):505–11. doi: 10.1164/ajrccm.157.2.9703070. [DOI] [PubMed] [Google Scholar]
- 91.Simpson JL, et al. Differential proteolytic enzyme activity in eosinophilic and neutrophilic asthma. Am J Respir Crit Care Med. 2005;172(5):559–65. doi: 10.1164/rccm.200503-369OC. [DOI] [PubMed] [Google Scholar]
- 92.Mautino G, et al. Balance in asthma between matrix metalloproteinases and their inhibitors. J Allergy Clin Immunol. 1999;104(3 Pt 1):530–3. doi: 10.1016/s0091-6749(99)70319-2. [DOI] [PubMed] [Google Scholar]
- 93.Jin FY, et al. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88(3):417–26. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 94.Wang Z, et al. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med. 2000;192(11):1587–600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Erlewyn-Lajeunesse MD, et al. Bronchoalveolar lavage MMP-9 and TIMP-1 in preschool wheezers and their relationship to persistent wheeze. Pediatr Res. 2008;64(2):194–9. doi: 10.1203/PDR.0b013e318175dd2d. [DOI] [PubMed] [Google Scholar]
- 96.Lezmi G, et al. Airway Remodeling in Preschool Children with Severe Recurrent Wheeze. Am J Respir Crit Care Med. 2015;192(2):164–71. doi: 10.1164/rccm.201411-1958OC. [DOI] [PubMed] [Google Scholar]
- 97.Grainge CL, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364(21):2006–15. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 98.Pothoven KL, et al. Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–13. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 100.Nanzer AM, Menzies-Gow A. Defining severe asthma - an approach to find new therapies. Eur Clin Respir J. 2014;1 doi: 10.3402/ecrj.v1.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995;154(9):4719–25. [PubMed] [Google Scholar]
- 102.Nguyen LT, et al. Increase in airway neutrophils after oral but not inhaled corticosteroid therapy in mild asthma. Respir Med. 2005;99(2):200–7. doi: 10.1016/j.rmed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 103.Louis R, et al. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161(1):9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- 104.Fukakusa M, et al. Oral corticosteroids decrease eosinophil and CC chemokine expression but increase neutrophil, IL-8, and IFN-gamma-inducible protein 10 expression in asthmatic airway mucosa. J Allergy Clin Immunol. 2005;115(2):280–6. doi: 10.1016/j.jaci.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 105.Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265(3 Pt 1):C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 106.Uddin M, et al. Prosurvival activity for airway neutrophils in severe asthma. Thorax. 2010;65(8):684–9. doi: 10.1136/thx.2009.120741. [DOI] [PubMed] [Google Scholar]
- 107.Vaughan KR, et al. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol. 2007;179(12):8544–53. doi: 10.4049/jimmunol.179.12.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee E, et al. Reversal of human neutrophil survival by leukotriene B(4) receptor blockade and 5-lipoxygenase and 5-lipoxygenase activating protein inhibitors. Am J Respir Crit Care Med. 1999;160(6):2079–85. doi: 10.1164/ajrccm.160.6.9903136. [DOI] [PubMed] [Google Scholar]
- 109.Simpson JL, et al. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177(2):148–55. doi: 10.1164/rccm.200707-1134OC. [DOI] [PubMed] [Google Scholar]
- 110.Kostadima E, et al. Clarithromycin reduces the severity of bronchialhyper responsiveness in patients with asthma. Eur Respir J. 2004;23(5):714–7. doi: 10.1183/09031936.04.00118404. [DOI] [PubMed] [Google Scholar]
- 111.Richeldi L, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2005;(3):CD002997. doi: 10.1002/14651858.CD002997.pub3. [DOI] [PubMed] [Google Scholar]
- 112.Kew KM, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015;(9):CD002997. doi: 10.1002/14651858.CD002997.pub4. [DOI] [PubMed] [Google Scholar]
- 113.Wong EH, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2(8):657–70. doi: 10.1016/S2213-2600(14)70107-9. [DOI] [PubMed] [Google Scholar]
- 114.Nair P, et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2012;42(7):1097–103. doi: 10.1111/j.1365-2222.2012.04014.x. [DOI] [PubMed] [Google Scholar]
- 115.Busse WW, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188(11):1294–302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 116.Holgate ST, et al. Efficacy and safety of etanercept in moderate-to-severe asthma: a randomised, controlled trial. Eur Respir J. 2011;37(6):1352–9. doi: 10.1183/09031936.00063510. [DOI] [PubMed] [Google Scholar]
- 117.Wenzel SE, et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009;179(7):549–58. doi: 10.1164/rccm.200809-1512OC. [DOI] [PubMed] [Google Scholar]
- 118.Erin EM, et al. The effects of a monoclonal antibody directed against tumor necrosis factor-alpha in asthma. Am J Respir Crit Care Med. 2006;174(7):753–62. doi: 10.1164/rccm.200601-072OC. [DOI] [PubMed] [Google Scholar]
- 119.Scapini P, et al. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev. 2016;273(1):48–60. doi: 10.1111/imr.12448. [DOI] [PubMed] [Google Scholar]
- 120.Gallin JI. Human neutrophil heterogeneity exists, but is it meaningful? Blood. 1984;63(5):977–83. [PubMed] [Google Scholar]
- 121.Dagvadorj J, et al. Lipopolysaccharide Induces Alveolar Macrophage Necrosis via CD14 and the P2X7 Receptor Leading to Interleukin-1alpha Release. Immunity. 2015;42(4):640–53. doi: 10.1016/j.immuni.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wei-Xu H, et al. Anti-Interleukin-1 Beta/Tumor Necrosis Factor-Alpha IgY Antibodies Reduce Pathological Allergic Responses in Guinea Pigs with Allergic Rhinitis. Mediators Inflamm. 2016;2016:3128182. doi: 10.1155/2016/3128182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cai S, et al. NLRP12 modulates host defense through IL-17A-CXCL1 axis. Mucosal Immunol. 2016;9(2):503–14. doi: 10.1038/mi.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hernandez ML, et al. IL-1 receptor antagonist reduces endotoxin-induced airway inflammation in healthy volunteers. J Allergy Clin Immunol. 2015;135(2):379–85. doi: 10.1016/j.jaci.2014.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hubeau C, et al. Interleukin-6 neutralization alleviates pulmonary inflammation in mice exposed to cigarette smoke and poly(I:C) Clin Sci (Lond) 2013;125(10):483–93. doi: 10.1042/CS20130110. [DOI] [PubMed] [Google Scholar]
- 126.Li HD, et al. Exogenous interleukin-10 attenuates hyperoxia-induced acute lung injury in mice. Exp Physiol. 2015;100(3):331–40. doi: 10.1113/expphysiol.2014.083337. [DOI] [PubMed] [Google Scholar]
- 127.Hsu CY, et al. Synergistic therapeutic effects of combined adenovirus-mediated interleukin-10 and interleukin-12 gene therapy on airway inflammation in asthmatic mice. J Gene Med. 2010;12(1):11–21. doi: 10.1002/jgm.1408. [DOI] [PubMed] [Google Scholar]
- 128.Wakashin H, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178(10):1023–32. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 129.Dubin PJ, et al. Interleukin-23-mediated inflammation in Pseudomonas aeruginosa pulmonary infection. Infect Immun. 2012;80(1):398–409. doi: 10.1128/IAI.05821-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nguyen TH, et al. TNF-alpha and Macrophages Are Critical for Respiratory Syncytial Virus-Induced Exacerbations in a Mouse Model of Allergic Airways Disease. J Immunol. 2016;196(9):3547–58. doi: 10.4049/jimmunol.1502339. [DOI] [PubMed] [Google Scholar]
- 131.Fei M, et al. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc Natl Acad Sci U S A. 2011;108(13):5360–5. doi: 10.1073/pnas.1015476108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hicks A, et al. Effects of LTB4 receptor antagonism on pulmonaryinflammation in rodents and non-human primates. Prostaglandins Other Lipid Mediat. 2010;92(1-4):33–43. doi: 10.1016/j.prostaglandins.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 133.Karp CL, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5(4):388–92. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 134.Serhan CN, et al. Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol. 2014;7(2):a016311. doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haworth O, et al. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9(8):873–9. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jandl K, et al. Activated prostaglandin D2 receptors on macrophages enhance neutrophil recruitment into the lung. J Allergy Clin Immunol. 2016;137(3):833–43. doi: 10.1016/j.jaci.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lombard R, et al. IL-17RA in Non-Hematopoietic Cells Controls CXCL-1 and 5 Critical to Recruit Neutrophils to the Lung of Mycobacteria-Infected Mice during the Adaptive Immune Response. PLoS One. 2016;11(2):e0149455. doi: 10.1371/journal.pone.0149455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Trevejo-Nunez G, et al. Ethanol impairs mucosal immunity against Streptococcus pneumoniae infection by disrupting interleukin 17 gene expression. Infect Immun. 2015;83(5):2082–8. doi: 10.1128/IAI.02869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Prause O, et al. Pharmacological modulation of interleukin-17-induced GCP-2-, GRO-alpha- and interleukin-8 release in human bronchial epithelial cells. Eur J Pharmacol. 2003;462(1-3):193–8. doi: 10.1016/s0014-2999(03)01341-4. [DOI] [PubMed] [Google Scholar]
- 140.Rohde G, et al. CXC chemokines and antimicrobial peptides in rhinovirus-induced experimental asthma exacerbations. Clin Exp Allergy. 2014;44(7):930–9. doi: 10.1111/cea.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Staab EB, et al. Treatment with the C5a receptor/CD88 antagonist PMX205 reduces inflammation in a murine model of allergic asthma. Int Immunopharmacol. 2014;21(2):293–300. doi: 10.1016/j.intimp.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun L, et al. Attenuation of IgG immune complex-induced acute lung injury by silencing C5aR in lung epithelial cells. FASEB J. 2009;23(11):3808–18. doi: 10.1096/fj.09-133694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tjabringa GS, et al. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol. 2006;140(2):103–12. doi: 10.1159/000092305. [DOI] [PubMed] [Google Scholar]
- 144.Myou S, et al. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med. 2003;198(10):1573–82. doi: 10.1084/jem.20030298. [DOI] [PMC free article] [PubMed] [Google Scholar]