Abstract
Background
Alcoholic Liver Disease (ALD) is commonly associated with intestinal permeability. An unanswered question is why only a subset of heavy alcohol drinkers develop endotoxemia. Recent studies suggest that circadian disruption is the susceptibility factor for alcohol-induced gut leakiness to endotoxins. The circadian protein PER2 is increased after exposure to alcohol and siRNA knockdown of PER2 in vitro blocks alcohol-induced intestinal barrier dysfunction. We have shown that blocking CYP2E1 (i.e., important for alcohol metabolism) with siRNA inhibits the alcohol-induced increase in PER2 and suggesting that oxidative stress may mediate alcohol-induced increase in PER 2 in intestinal epithelial cells. The Aim of the current study was to elucidate whether a mechanism incited by alcohol-derived oxidative stress mediates the transcriptional induction of PER2 and subsequent intestinal hyperpermeability.
Methods
Caco-2 cells were exposed to 0.2% alcohol with or without pretreatment with modulators of oxidative stress or PKA activity. Permeability of the Caco-2 monolayer was assessed by transepithelial electrical resistance. Protein expression was measured by Western Blot and mRNA with real-time polymerase chain reaction. Wild-type C57BL/6J mice (WT) mice were fed with alcohol diet (29% of total calories, 4.5% v/v) for 8 weeks. Western Blot was used to analyze PER2 expression in mouse proximal colon tissue.
Results
Alcohol increased oxidative stress, caused Caco-2 cell monolayer dysfunction, and increased levels of the circadian clock proteins Per2 and Clock. These effects were mitigated by pre-treatment of Caco-2 cells with an anti-oxidant scavenger. Alcohol-derived oxidative stress activated CREB via the PKA pathway and increased PER2 mRNA and protein. Inhibiting CREB prevented the increase in PER2 and Caco-2 cell monolayer hyperpermeability.
Conclusions
Taken together, these data suggest that strategies to reduce alcohol-induced oxidative stress may alleviate alcohol mediated circadian disruption and intestinal leakiness, critical drivers of ALD.
INTRODUCTION
Alcoholic liver disease (ALD) encompasses a spectrum of progressive conditions that arise in 30% of chronic alcohol (i.e., ethanol) abusers (Keshavarzian et al., 1999). In these individuals, alcohol causes the abnormal uptake, processing, and metabolism of fatty acids in the liver. This process sets the stage for the injurious retention of fat by hepatocytes (Maher, 2002). The relatively benign pathological process of fat deposition, known as steatosis, can occur after heavy alcohol consumption and is generally accepted as an important first step for further liver injury including steatohepatitis and cirrhosis. Approximately 50% of heavy drinkers with steatohepatitis will develop cirrhosis if alcohol consumption continues (Leevy, 1962; Sørensen et al., 1984). Evidence indicates that clinical ALD is due, in part, to hyperpermeability of the intestinal colon. This increase in permeability of the colonic epithelial barrier allows for the critical introduction of bacterial–derived endotoxins into the systemic circulation (Keshavarzian et al., 1999, 2009; Mutlu et al., 2009; Parlesak et al., 2000; Tang et al., 2008). The unanswered question is why only a subset of heavy alcohol drinkers develop endotoxemia and ALD. Recent in vitro and in vivo rodent studies suggest that disruption of circadian homeostasis may be one susceptibility factor for alcohol-induced gut leakiness to endotoxins (Summa et al., 2013; Swanson et al., 2011).
Circadian rhythms are physiological and behavioral patterns of approximately 24 hours, guided by cell-autonomous molecular pacemakers which can be entrained and coordinated according to environmental cues such as light and food intake (Voigt et al., 2013a). The hierarchy of circadian rhythms coordinates physiological processes between cells, tissues, and organ systems (Bass and Takahashi, 2010). In mammals, the central or “master clock” is found within the suprachiasmatic nucleus (SCN) of the hypothalamus in the brain, which uses photic stimuli to align its protein and RNA production with geological time. The SCN then uses neuronal and hormonal outputs to coordinate the peripheral clocks in cells in other organs such as the intestine (Bellet and Sassone-Corsi, 2010). Circadian rhythms oscillate in clear phases with characteristic amplitudes or peaks during a 24h period. The generation and maintenance of the circadian clock relies on gene expression using a network of transcriptional–translational feedback loops that comprise the core molecular circadian clock (Buijs and Kalsbeek, 2001). Constitutively expressed, the main regulator protein of the circadian rhythm, CLOCK, is an acetyltranferase that dimerizes with BMAL1 and binds to the enhancer box of clock controlled gene regions to form the positive arm of the molecular circadian clock (Ko and Takahashi, 2006). The binding of the CLOCK-BMAL1 heterodimer induces the expression of other circadian genes (e.g., Per1-3 and Cry1-2). Once translated, PER2 and CRY1 proteins are trafficked from the cytoplasm into the nucleus to repress their own production by binding to and inhibiting CLOCK and BMAL1 in completion of a 24h negative feedback loop (Wilkins et al., 2007).
Genetic or environmental disruption of the delicate forward and feedback loops that form biological clocks can lead to the disruption of circadian homeostasis (Öllinger et al., 2014; Yu and Weaver, 2011). Maintenance of circadian rhythms is critical because disruption of circadian homeostasis creates a pro-inflammatory state and is associated with numerous inflammation-mediated pathologies (Castanon-Cervantes et al., 2010; Narasimamurthy et al., 2012; Summa et al., 2013). One environmental factor that could impact circadian homeostasis is alcohol (Voigt et al., 2013b). Indeed, we have shown that alcohol consumption alters circadian rhythms (e.g., Clock and Per2 in the intestine) in multiple organs including the intestine (Summa et al., 2013, 2015). Alcohol-induced changes in the circadian clock are a key feature associated with alcohol-induced intestinal barrier dysfunction (Swanson et al., 2011). However, the detailed mechanisms by which alcohol disrupts circadian homeostasis and specifically in peripheral organs like the intestine are not known.
Alcohol is predominantly metabolized by alcohol dehydrogenase and aldehyde dehydrogenase (Bullock, 1990); however, under heavy or chronic consumption of alcohol, the two primary metabolic enzymes become saturated and the cytochrome P450 enzyme Cyp2e1 becomes the primary pathway for alcohol metabolism. Cyp2e1 is classified as a ‘leaky’ enzyme that spills harmful free radicals into the cytoplasm during the process of alcohol metabolism (Caro and Cederbaum, 2004,). The harmful ions generated by Cyp2e1 metabolism of alcohol can exceed the capacity of cellular innate antioxidant mechanisms and is one major mechanism by which alcohol can lead to pathology (Forsyth, Voigt and Keshavarzian, 2014,; Haorah et al., 2005; Keshavarzian et al., 2009). Oxidative stress produced as a byproduct of Cyp2e1 alcohol metabolism can influence the circadian clock. Alcohol-induced increase in Clock and Per2 and intestinal barrier dysfunction is dependent on Cyp2e1 (Forsyth, Voigt and Keshavarzian, 2014). In addition, this effect appears to be dependent on oxidative stress since the antioxidant N-acetylcysteine (NAC) prevents alcohol-induced changes in Clock or Per2 expression and barrier dysfunction (Forsyth et al., 2014).
The purpose of the present study was to investigate the mechanism through which alcohol and Cyp2e1-mediated oxidative stress promote the increase in CLOCK and PER2 proteins required for alcohol-induced intestinal hyperpermeability. We hypothesize that production of free radicals by Cyp2e1-mediated alcohol metabolism initiates a cascade of events including activation of PKA, phosphorylation/activation of cAMP response element-binding (CREB), increased PER2, culminating in intestinal barrier dysfunction. The rationale for this hypothesis was based on prior studies showing that CREB activity is affected by alcohol and core circadian clock genes are inducible through the binding of the CREB protein to the camp response element (CRE) site located upstream of the genetic enhancer box (Obrietan et al., 1999). To test our hypothesis, we used Caco-2 cells as well as intestinal tissue from mice chronically fed alcohol to validate our in vitro findings. Our data reveal that CREB may act as a mediator between alcohol-induced oxidative stress and the circadian machinery to promote the development of intestinal barrier dysfunction.
METHODS
Caco-2 cells and alcohol or H2O2 exposure
Caco-2 cells (ATCC no. CRL2101, human colorectal adenocarcinoma; Manassas, VA) (20) were grown to confluence in complete media with an antibiotic and an antifungal agent (37°C, 5% CO2, 10% fetal bovine serum media with 5mM penicillin-streptomycin). Caco-2cells were grown on Type 1 collagen-coated 12mm/0.4μM pore tissue culture plate inserts (Transwell; Corning, Corning, NY) as previously described (Forsyth et al., 2014). Cell viability under these conditions was previously measured and verified using live/dead assay (Invitrogen, Life Technologies, Grand Island, NY) or Trypan blue staining (>95% cell viability for all assays) (Swanson et al., 2011). Caco-2 cell monolayers were treated with alcohol (0.2% vol/vol, 43mM; equivalent to 2–3 alcoholic drinks, a physiologically relevant dose of alcohol at the level of the colon) for the specified time periods with or without N-acetylcysteine (NAC, anti-oxidant) or H89 (PKA/CREB inhibitor) as indicated. 25mM of H2O2 was added to media as an alternative oxidant. Experiments were terminated with the removal of media and the addition of PBS for scraping and mRNA expression analysis, SDS/RIPA buffer for whole cell lysates (Western blot), or Qiagen lysis buffer for mRNA analysis of gene expression (RNeasy kit, Qiagen, Valencia, CA). Intestinal barrier integrity in Caco-2 cells was assessed via transepithelial electrical resistance (TEER) as previously described (Swanson et al., 2011). TEER was assessed using an Epithelial Volt/Ohm Meter (EVOM), a dual electrode system designed for cell culture insert analysis (World Precision Instruments, Sarasota, FL) (Hidalgo et al., 1989). Blank culture inserts were used for baseline values, which were subtracted from all values using inserts with living cells. When indicated, cells were pretreated with either 20mM NAC for up to 48h or 10mM H89 PKA/CREB inhibitor for 1h.
Western blot and slot blot protein analysis
Protein samples were prepared using Laemmli sample buffer with 2-ME (Bio-Rad, Hercules, CA), and total protein via Western blot protein quantification analysis was determined (Bio-Rad). 20μg of protein was loaded into each lane (4/10% stacking acrylamide Tris gel) and electrophoresed at 100V for 2h as previously described (Swanson et al., 2011).
Oxidative stress evaluations
N-acetylcysteine
Caco-2 cells were grown to confluence, on 24mm Transwell inserts (Corning) in six-well plates in complete DMEM media with 10% fetal bovine serum. For experiments with NAC, cells in NAC groups were pretreated for 48h with 10mM NAC. During the experiment cells were stimulated with 0.2% alcohol in serum free media for the indicated times. Whole cell lysates were made for Western blot analysis as previously described at the indicated time points of 2 and 4h for analysis of CLOCK and PER2 proteins (Swanson et al., 2011). Free Radical Analysis (H2DCFDA). Caco-2 cells were grown to confluence on glass cover-slips. When appropriate, Caco-2 cells were pretreated for 48h with 10mM NAC in DMEM with 10% FBS with a 2h washout to decrease NAC-related background. All groups were labeled with 10uM cell-permeant 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) in serum free DMEM for 1h (Molecular Probes, Eugene OR) in HANKS media. When appropriate, Caco-2 cells were subsequently exposed to 0.2% alcohol in serum-free DMEM for 4h then fixed in 4% paraformadehyde and fluorescence was measured using fluorescent microscopy and image J analysis software. Caco-2 fluorescence was assessed at 520nm following excitement at 480nm. Cells were incubated at 37°C for 30min and washed twice in PBS then incubated with alcohol or control media with or without NAC. Cover-slips were fixed with paraformaldehyde and images were taken via fluorescent microscopy using a 488nm excitation laser. Fluorescence was measured using ImageJ software. Results are presented as mean ± standard error of the mean (SEM).
Gene expression analysis with qRT-PCR
Analysis of mRNA expression was carried out as previously described (Forsyth et al., 2014). Briefly, RNA was isolated from Caco-2 cells or mouse intestinal tissue (collected 4h after lights off (i.e., ZT16)) using the Qiagen RNeasy kit (Qiagen). RNA was converted to cDNA using the high-capacity cDNA kit (Applied Biosystems, Life Technologies, Carlsbad, CA) and PCR amplified using fast Sybr green master mix (Applied Biosystems) using a 7500 fast real-time PCR system (Applied Biosystems). PCR primer sequences were as follows: for human CLOCK: F-5′-TGCGAGGAACAATAGACCCAA-3′, R-5′-ATGGCCTATGTGTGCGTTGTA-3′ BMAL1: F-5′-AAGGGAAGCTCACAGTCAGAT-3′, R-5′-GGACATTGCGTTGCATGTTGG -3′ PER2: F-5′-GACATGAGACCAACGAAAACTGC-3′, R-5′-AGGCTAAAGGTATCTGGACTCTG-3′ CRY1: F-5′-CTCCTCCAATGTGGGCATCAA-3′, R-5′-CCACGAATCACAAACAGACGG-3 Primers for β-actin were as follows: F-5′-CATGTACGTTGCTATCCAGGC-3′, R-5′-CTCCTTAATGTCACGCACGAT-3′
Experimental diet and animals
To assess alcohol-induced effects in vivo, tissue from a previous study were used (Summa et al., 2013). In brief, male C57BL/6 mice (Jackson Laboratory, Bar harbor, ME) 6–8 weeks at the start of the experiment were used. Mice were housed individually and food and water were available ad libitum. After 12 weeks on a standard chow diet mice were switched to, an alcohol-containing diet (i.e., the Nanji diet, (Nanji et al., 1994)) that is a modified Lieber DiCarli diet whereby fat calories were in the form of fish oil rather than a combination of corn oil, fish oil, and vegetable oil (Summa et al., 2013; Voigt et al., 2014). Final alcohol concentration was 4.5% v/v. Components of the Nanji liquid diet included mineral mix, vitamin mix, choline bitartrate, DL-methionine, lactalbumin, xanthan gum, dextrose (all from Dyets, Bethlehem, PA), fish oil from menhaden, ethanol (both from Sigma, St. Louis, MO), and Hersey’s chocolate syrup for flavor (Hershey, PA). The caloric composition of the diet was 36% protein, 29% dextrose (control) or alcohol, and 35% fat (fish oil). The caloric composition of the control dextrose diet was the same except that alcohol calories were replaced with dextrose. During the first two weeks of the Nanji alcohol diet, dietary alcohol concentration was gradually increased followed by eight weeks at the full alcohol concentration (4.5% v/v, 29% of total daily caloric intake came from alcohol). At the end of the experiment proximal colon tissue was harvested immediately after decapitation which occurred 4h after lights off (i.e., ZT16). Tissue was snap frozen in liquid nitrogen and stored at −80°C until use.
Statistical analysis
Data are presented as mean±SEM. Group means were compared by analysis of variance (ANOVA) and post hoc analysis. Significance was set at α < 0.05. All analyses were conducted using GraphPad Prism (GRAPHPAD, La Jola, Ca, version 6)
RESULTS
Free Radicals Contribute to Alcohol-Induced Intestinal Barrier Dysfunction
Using Caco-2 cells, we first determined whether alcohol causes oxidative stress and if oxidative stress mediated alcohol-induced monolayer barrier dysfunction. Alcohol (0.2%) exposure for 4h significantly increased levels of oxidative stress in Caco-2 cells, an effect that was prevented by the free radical scavenger NAC (Figure 1A,B). There were significant (P<.05) main effects of alcohol, anti-oxidant treatment, as well as an alcohol by anti-oxidant treatment interaction.
Figure 1. Alcohol-induced effects on intestinal barrier integrity and expression of circadian clock proteins is inhibited by the free-radical scavenger N-acetylcysteine (NAC).
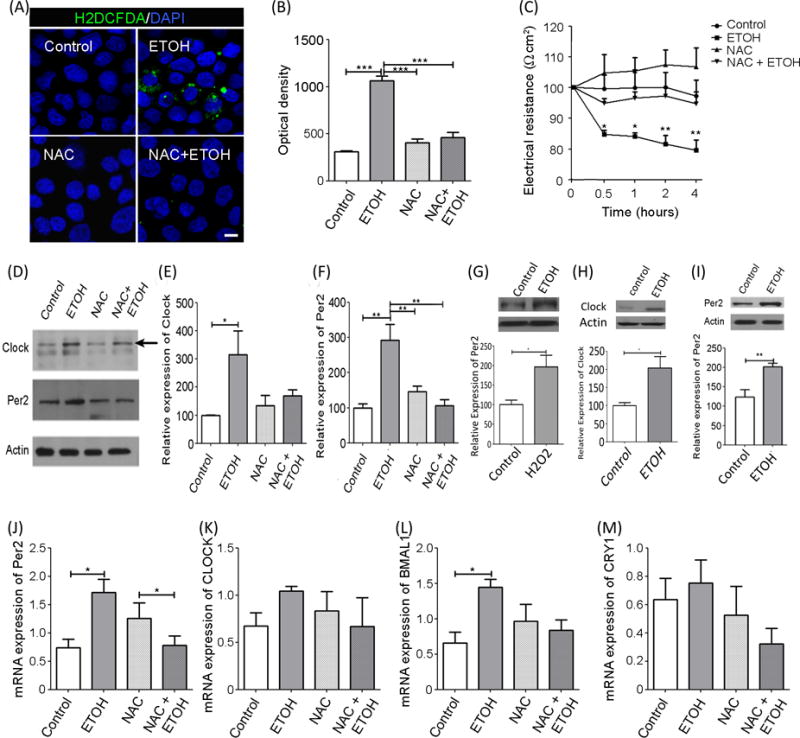
Caco-2 cells were pre-treated with NAC (an antioxidant) and treated with 0.2% alcohol. Cells were stained and imaged, used to assess intestinal barrier integrity, and/or used to assess protein / mRNA levels. Two-way ANOVA with post-hoc Bonferroni Multiple-Comparison test indicated significance of experiments. (A) Cells were stained to visualize oxidative stress using chloromethyl 2′,7′-dichlorofluorescein diacetate (CM-H2DCFDA, green) probe (10μM) and a nuclear stain (DAPI, blue). (B) CM-H2DCFDA florescence was quantified and analysis revealed that alcohol-induced oxidative stress was blocked by NAC. Statistical tests showed significant effect of alcohol (p<0.01), NAC (p<0.01), and time (p<0.01). (C) Transepithelial electrical resistance (TEER) was used to assess barrier integrity 0.5, 1, 2, and 4h after alcohol +/− NAC pretreatment. Repeated measures two-way ANOVA revealed a significant effect of alcohol (p<0.02), NAC (p<0.01), and time (p<0.01). (D) Representative Western blot images of CLOCK and PER2 from alcohol treated (+/−NAC) Caco-2 cells (n=4). (E-F) Protein was normalized to β-actin. (E) Alcohol significantly increased CLOCK protein, an increase that was blocked by NAC Analysis showed a significant effect of alcohol (p<0.02) but no effect of NAC or an alcohol-NAC interaction. (F) Alcohol significantly increased PER2, an effect that was inhibited by NAC. There was a significant effect of alcohol (p<0.01), NAC (p<0.02), and alcohol-antioxidant interaction (p<0.01). (G) H2O2 oxidant lead to significant increase in Per2 (t-test, p<0.05) in Caco-2 cells over 4 hours (N=4) (H-I) Five mice were assessed per treatment group. Protein was normalized to β-actin. CLOCK and PER2 is increased in colon tissue from alcohol-fed mice (t-test, p<0.05, p<0.01 respectively). (J-M). RT-PCR analysis of Caco-2 circadian clock genes (n=4/group). mRNA was normalized to β-actin. Alcohol increased Per2 gene expression significantly compared to control. Comparison tests showed no significant effects of alcohol, antioxidant, or an interaction. (I) Clock gene expression was unchanged with treatment in Caco-2 cells with no significant effects of alcohol, antioxidant or interaction. (J) Bmal1 gene expression was increased by alcohol, an effect that was blocked by NAC. No significant effects of alcohol, antioxidant, or interaction were found. (K) Cry1 mRNA was unaffected by alcohol, antioxidant, or an interaction. Each bar graph represents the mean values ± SEM. A priori pairwise comparisons were conducted for all groups (i.e., Bonferroni-Multiple comparison) and these are indicated on each individual graph. *P<0.05, **P<0.01.
This inhibition of oxidative stress by NAC appears to have biological relevance since treatment of Caco-2 cells with NAC prevented alcohol-induced monolayer barrier dysfunction (Figure 1C). Caco-2 cells were pretreated with the antioxidant NAC (10mM) or vehicle for 48h prior to exposure to alcohol. As expected, alcohol decreased trans-epithelial electrical resistance (TEER) (i.e., 20% reduction) consistent with Caco-2 monolayer barrier dysfunction. Importantly, NAC pretreatment prevented the alcohol-induced barrier dysfunction. These data suggest that alcohol-induced oxidative stress promotes intestinal epithelial hyperpermeability.
Protein levels of CLOCK and PER2 were assessed following alcohol exposure. Alcohol increased the levels of clock gene proteins CLOCK and PER2 (Figure 1D–F). A significant Increase in PER2 protein was also seen with the alternative oxidant H2O2, which was similar to the rise seen with alcohol exposure. (Figure 1G). Indeed, CLOCK and PER2 protein were increased in colonic mucosa of alcohol-fed mice (Figure 1H–I) and these mice also demonstrated intestinal hyperpermeability (Summa et al., 2013). Importantly, the alcohol-induced increase in CLOCK and PER2 protein was prevented by the NAC treatment that also prevented alcohol-induced oxidative stress and monolayer hyperpermeability.
To determine if the changes in protein were due to changes in mRNA (i.e., transcriptionally regulated), alcohol-induced changes in circadian gene expression were examined in Caco-2 cells. Alcohol increased Per2 expression (Figure 1J) but had differential effects on the core drivers of circadian expression with Clock (Figure 1K) showing no change and a significant increase in alcohol-induced in Bmal1 expression (Figure 1L) being blocked by antioxidant. Treatment with either alcohol or anti-oxidant NAC didn’t reveal any statistically significant change in Cry1 (Figure 1M) mRNA. These data suggest that alcohol-induced effects on PER2 protein may be transcriptionally mediated whereas alcohol-induced increase in Clock protein level is not transcriptional and could be due to a different mechanism such as decreased in CLOCK protein degradation.
CREB activation mediates Alcohol-Induced Intestinal Barrier Dysfunction
We hypothesized that free radical production by Cyp2e1-mediated alcohol metabolism activates PKA which results in CREB phosphorylation that in turn increases CLOCK/PER2 proteins thus resulting in intestinal hyperpermeability. Accordingly, we measured levels of phosphorylated CREB (i.e., activated CREB) and total CREB (Figure 2A). Alcohol (0.2%, 2h) significantly increased CREB phosphorylation at serine 133, an effect that was prevented by pretreatment with the free radical scavenger NAC (Figure 2B,C,D). In contrast, alcohol did not statistically impact total CREB expression. These data are consistent with a role for alcohol-induced oxidative stress in activation of CREB.
Figure 2. Alcohol-mediated oxidative stress-induced CREB activation leads to change in circadian PER2 expression and increased intestinal permeability.
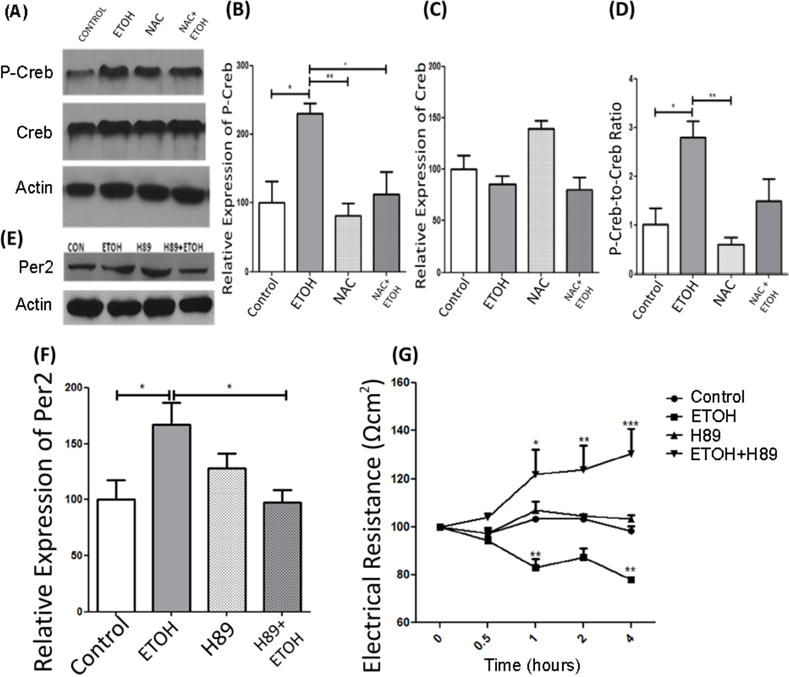
Caco-2 cells were pre-treated with NAC (an antioxidant) and treated with 0.2% alcohol. Cells were used to assess protein levels and assess intestinal barrier integrity. Two-way ANOVA with post-hoc Bonferroni Multiple-Comparison test indicated significance of experiments. (A) Representative Western blots of P-CREB and CREB from alcohol treated (+/−NAC) Caco-2 cells (n=4). (B-D) Protein was normalized to β-actin. (B) p-CREB expression was increased by alcohol and this increase was inhibited by NAC. Comparison tests revealed significant effects of alcohol (p<0.01), and NAC (p<0.001) but no significant alcohol-NAC interaction. (C) Total CREB protein was unchanged by alcohol or NAC with two-way ANOVA showing no significant effects of alcohol, antioxidant, or an interaction. (D) The ratio of pCREB to CREB is significantly increased in CACO-2 cells with exposure to ETOH an effect that is blunted with NAC. Statistical tests showed significant effects of alcohol (p<0.02), and NAC (p<0.002) with no significant alcohol-NAC interaction. (E) Representative Western blots of PER2 from alcohol-treated (+/−NAC) CACO-2 cells. (F) PER2 was increased by alcohol an effect that was prevented by pretreatment with H89 PKA/CREB inhibitor. Analysis revealed significant effects of alcohol (p<0.02), H89 (p<0.02), and an alcohol-H89 interaction (p<0.02). (G) Alcohol-induced intestinal barrier dysfunction beginning at the 1h time-point compared to controls which was blocked by pretreatment with H89. Repeated measures two-way ANOVA with post-hoc Bonferroni Multiple-Comparison test revealed, treatment p<0.01, time p<0.03, interaction p<0.01. Each bar graph represents the normalized mean values ± SEM. A priori pairwise comparisons were conducted for all groups (i.e., Bonferroni-Multiple comparison) and these are indicated on each individual graph. *P<0.05, **P<0.01.
Next we investigated the role of phosphorylated CREB (p-CREB, activated CREB) in alcohol-induced barrier dysfunction by manipulating the function of PKA, which contributes to CREB phosphorylation. Caco-2 cells were treated with the PKA inhibitor H89 to prevent phosphorylation and activation of CREB. If p-CREB is critical for alcohol-induced increase in PER2 protein and intestinal barrier function, then administration of H89 should be protective (Figure 2B,C,D). As would be expected, exposure of Caco-2 cells to alcohol resulted in a time-dependent decrease in Caco-2 monolayer barrier resistance (i.e., indicative of monolayer hyperpermeability). In agreement with our hypothesis, H89 prevented the alcohol-induced increase in PER2 protein (Figure 2F) and intestinal barrier function (Figure 2G).
DISCUSSION
The current study hypothesized that production of free radicals due to alcohol metabolism by Cyp2e1 activates PKA causing CREB phosphorylation, increases PER2 protein, resulting in intestinal hyperpermeability. This hypothesis was based on a growing body of literature demonstrating that alcohol-induced oxidative stress is associated with increased permeability of intestinal epithelial cells (Banan et al., 1999, 2000; Haorah et al., 2005; Keshavarzian et al., 2009; Roskams et al., 2003). The data presented in the current study support the hypothesis: (1) the free radical scavenger NAC prevented alcohol-induced intestinal barrier dysfunction, (2) inhibition of PKA with H89 prevented alcohol-induced intestinal barrier dysfunction, (3) CREB phosphorylation was impacted by alcohol and the amount of p-CREB was influenced by NAC, (4) Per2 protein increased with the alternative oxidant H2O2 in a similar manner to that of alcohol in Caco-2 cells and 5) both NAC and H89 prevented the alcohol-induced increase in the circadian protein PER2. Taken together, these pieces of evidence provide a plausible platform supporting the proposed hypothesis.
Our group previously showed that siRNA inhibition of CLOCK or PER2 proteins prevents alcohol-induced intestinal permeability in Caco-2 cell monolayers (Swanson et al., 2011). In the current study, we expand on these observations, and identify oxidative stress and PKA activity as critical factors that contribute to alcohol-induced effects on intestinal barrier integrity. Our data support one mechanism whereby oxidative stress impacts circadian clock gene expression and protein levels, specifically Per2 and Bmal1 mRNA expression and CLOCK and PER2 protein. There are, however, alternative mechanisms by which oxidative stress can influence intestinal barrier integrity. It is possible for alcohol to elicit changes in the levels of circadian proteins independent of transcriptional mechanisms. Alcohol-induced increase in PER2 may be due to alterations in PER2 degradation occurring subsequent to changes in CRY1 inhibition of casein kinase 1. In addition, BMAL1 is a regulator of translation and alcohol-induced activation of BMAL1 may stimulate increased protein synthesis with little change in gene expression (Horst et al., 1999; Lipton et al., 2015). Finally, the transcriptional–translational feedback loops that comprise the core molecular circadian clock could be perturbed due to impaired shuttling of PER2 and CRY1 proteins into the nucleus, effectively blunting the negative feedback portion of the molecular clock. In summary, the alcohol mediated rise in PER2 protein could be due to a change in transcription, translation, degradation or another yet unknown mechanism. Another alternative explanation for the data generated in the study may have to do with a more broad sensitivity of the circadian clock to redox conditions. Many circadian clock protein components, such as PAS (Per-ARNT-Sim) Domain containing proteins (which include PER2), and certain regulators of the circadian rhythm are sensitive to changes in redox potential and can change conformation or function in response to increased oxidative stress burden (Bass and Takahashi, 2011; Caito et al., 2010; Edgar et al., 2012). The contributions of these alternative mechanisms need to be considered.
Our data suggest that CREB may act as a mediator between alcohol-induced oxidative stress and the circadian machinery to promote the development of intestinal hyperpermeability. We found an alcohol-mediated increase in p-CREB but with no change in total CREB protein. This increase in CREB phosphorylation was not observed when cells were pre-treated with NAC linking oxidative stress-induced effects of alcohol to CREB phosphorylation. Further and more comprehensive characterization of CREB-mediated effects of alcohol could be explored in the future but these data support that CREB is activated (via PKA) by oxidative stress (Bai et al., 2005; Gerhart-Hines et al., 2011). CREB is instrumental in light-induced clock resetting and circadian phase-shift in the SCN eliciting changes through CRE-mediated transcription (von Gall et al., 1998; Ginty et al., 1993; Obrietan et al., 1999). We sought to elucidate the role that PKA-induced CREB activation may play in alcohol-mediated intestinal barrier dysfunction. If PKA-induced CREB activation is mediating alcohol-induced effects on the circadian clock and the intestinal barrier, then inhibition of PKA should block alcohol-mediated PER2 protein expression and barrier disruption. Indeed, pretreatment of Caco-2 cells with H89 a chemical inhibitor of PKA blocked the alcohol-mediated increase in PER2 and disruption of the Caco-2 monolayer barrier integrity. A number of kinases promote CREB phosphorylation at Ser-133 including (Yu et al., 2001) Ca+ calmodulin kinases, AKT, ERK, MAPK, MAPKAP-Kinase 2, PKC, and PKA (Du and Montminy, 1998; Impey et al., 1998; Tan et al., 1996; Xing et al., 1996). However, our data suggests that PKA is the important regulator of alcohol-induced CREB activation. Our previous data show a significant effect of alcohol on PKA activation linking CREB regulation in the colon to alcohol intake (Forsyth et al., 2010) and the current data builds upon this previous work to support a link between alcohol-induction of the PKA-CREB pathway and barrier dysfunction. We recognize the limitations of this study and acknowledge, for example, that in vitro results may not necessarily reflect those obtained in vivo; however, we believe that the novel acute mechanisms revealed in this study should be investigated further.
In summary, our data expands on our previous findings to reveal a pathway in which oxidative stress affects the molecular circadian clock and intestinal barrier integrity. These studies reveal new links between alcohol-mediated oxidative stress and the elements of the circadian rhythm and intestinal hyperpermeability that is critical to the development of alcoholic liver disease. Utilizing these studies it may be possible to formulate new therapies that address oxidative stress mediated circadian disruption via gene expression possibly intervening in the pathway of alcohol induced intestinal hyperpermeability and the subsequent endotoxemia and progression towards alcoholic liver disease.
Supplementary Material
Acknowledgments
The studies were supported by NIH grants: AA020216S1 (AK, BD), AA023417S2 (AK, BD) AA020216 (AK, CBF, RV), AA023417 (AK)
Footnotes
DR. BOOKER T DAVIS (Orcid ID : 0000-0003-0029-7900)
Contributor Information
Booker T Davis, IV, Rush University Medical Center, 1725 W Harrison St, Suite 206, Chicago IL 60612, 312-942-0721.
Robin M. Voigt, Rush University Medical Center, 1725 W Harrison St, Suite 206, Chicago IL 60612, 312-942-8973
Maliha Shaikh, Rush University Medical Center, 1725 W Harrison St, Suite 206, Chicago IL 60612, 312-942-8798
Christopher B. Forsyth, Rush University Medical Center, 1725 W Harrison St, Suite 206, Chicago IL 60612, 312-942-9009
Ali Keshavarzian, Josephine M. Dyrenforth Chair of Gastroenterology, Director, Division of Digestive Disease and Nutrition, Rush University Medical Center, Department of Internal Medicine, Section of Gastroenterology, 1725 W Harrison St, Suite 206, Chicago IL 60612, Phone: 312-563-3890 (Direct Line), Phone: 312-563-4175 (Admin. Ms. Denise Labedz), Fax: 312-563-3883.
References
- Bai X, Lu D, Liu A, Zhang Z, Li X, Zou Z, Zeng W, Cheng B, Luo S. Reactive Oxygen Species Stimulates Receptor Activator of NF-κB Ligand Expression in Osteoblast. J Biol Chem. 2005;280:17497–17506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-Induced Barrier Dysfunction and Its Prevention by Growth Factors in Human Intestinal Monolayers: Evidence for Oxidative and Cytoskeletal Mechanisms. J Pharmacol Exp Ther. 1999;291:1075–1085. [PubMed] [Google Scholar]
- Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric Oxide and Its Metabolites Mediate Ethanol-Induced Microtubule Disruption and Intestinal Barrier Dysfunction. J Pharmacol Exp Ther. 2000;294:997–1008. [PubMed] [Google Scholar]
- Banan A, Keshavarzian A, Zhang L, Shaikh M, Forsyth CB, Tang Y, Fields JZ. NF-κB activation as a key mechanism in ethanol-induced disruption of the F-actin cytoskeleton and monolayer barrier integrity in intestinal epithelium. Alcohol. 2007;41:447–460. doi: 10.1016/j.alcohol.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian rhythms: Redox redux. Nature. 2011;469:476–478. doi: 10.1038/469476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet MM, Sassone-Corsi P. Mammalian circadian clock and metabolism – the epigenetic link. J Cell Sci. 2010;123:3837–3848. doi: 10.1242/jcs.051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- Bullock C. The biochemistry of alcohol metabolism — A brief review. Biochem Educ. 1990;18:62–66. [Google Scholar]
- Caito S, Rajendrasozhan S, Cook S, Chung S, Yao H, Friedman AE, Brookes PS, Rahman I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J Off Publ Fed Am Soc Exp Biol. 2010;24:3145–3159. doi: 10.1096/fj.09-151308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Oxidative Stress, Toxicology, and Pharmacology of Cyp2e1*. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A. Alcohol stimulates activation of Snail, epidermal growth factor receptor signaling, and biomarkers of epithelial-mesenchymal transition in colon and breast cancer cells. Alcohol Clin Exp Res. 2010;34:19–31. doi: 10.1111/j.1530-0277.2009.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Voigt RM, Shaikh M, Tang Y, Cederbaum AI, Turek FW, Keshavarzian A. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am J Physiol-Gastrointest Liver Physiol. 2013;305:G185–G195. doi: 10.1152/ajpgi.00354.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Voigt RM, Keshavarzian A. Intestinal CYP2E1: A mediator of alcohol-induced gut leakiness. Redox Biol. 2014;3:40–46. doi: 10.1016/j.redox.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gall C, Duffield GE, Hastings MH, Kopp MD, Dehghani F, Korf HW, Stehle JH. CREB in the mouse SCN: a molecular interface coding the phase-adjusting stimuli light, glutamate, PACAP, and melatonin for clockwork access. J Neurosci Off J Soc Neurosci. 1998;18:10389–10397. doi: 10.1523/JNEUROSCI.18-24-10389.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Dominy JE, Jr, Blättler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, Puigserver P. The cAMP/PKA Pathway Rapidly Activates SIRT1 to Promote Fatty Acid Oxidation Independently of Changes in NAD+ Mol Cell. 2011;44:851–863. doi: 10.1016/j.molcel.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- Horst GTJ van der, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, Wit J de, Verkerk A, Eker APM, Leenen D van, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Kratsovnik E, Bromberg Y, Sperling O, Zoref-Shani E. Oxidative stress activates transcription factor NF-kB-mediated protective signaling in primary rat neuronal cultures. J Mol Neurosci MN. 2005;26:27–32. doi: 10.1385/jmn:26:1:027. [DOI] [PubMed] [Google Scholar]
- Leevy CM. Fatty liver: a study of 270 patients with biopsy proven fatty liver and review of the literature. Medicine (Baltimore) 1962;41:249–276. doi: 10.1097/00005792-196209000-00003. [DOI] [PubMed] [Google Scholar]
- Lipton JO, Yuan ED, Boyle LM, Ebrahimi-Fakhari D, Kwiatkowski E, Nathan A, Güttler T, Davis F, Asara JM, Sahin M. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell. 2015;161:1138–1151. doi: 10.1016/j.cell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev. 2006;24:261–274. doi: 10.1111/j.1527-3466.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- Maher JJ. Alcoholic steatosis and steatohepatitis. Semin Gastrointest Dis. 2002;13:31–39. [PubMed] [Google Scholar]
- Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal Dysbiosis: A Possible Mechanism of Alcohol-Induced Endotoxemia and Alcoholic Steatohepatitis in Rats. Alcohol Clin Exp Res. 2009;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res. 1994;18:1280–1285. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Smith D, Athos J, Storm DR. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J Biol Chem. 1999;274:17748–17756. doi: 10.1074/jbc.274.25.17748. [DOI] [PubMed] [Google Scholar]
- Öllinger R, Korge S, Korte T, Koller B, Herrmann A, Kramer A. Dynamics of the circadian clock protein PERIOD2 in living cells. J Cell Sci. 2014;127:4322–4328. doi: 10.1242/jcs.156612. [DOI] [PubMed] [Google Scholar]
- Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease∗. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, Achten R, Verslype C, Diehl AM. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen TI, Orholm M, Bentsen KD, Høybye G, Eghøje K, Christoffersen P. Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of development of cirrhosis. Lancet Lond Engl. 1984;2:241–244. doi: 10.1016/s0140-6736(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, Vitaterna MH, Song S, Turek FW, Keshavarzian A. Disruption of the Circadian Clock in Mice Increases Intestinal Permeability and Promotes Alcohol-Induced Hepatic Pathology and Inflammation. PloS One. 2013;8:e67102. doi: 10.1371/journal.pone.0067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summa KC, Jiang P, Fitzpatrick K, Voigt RM, Bowers SJ, Forsyth CB, Vitaterna MH, Keshavarzian A, Turek FW. Chronic Alcohol Exposure and the Circadian Clock Mutation Exert Tissue-Specific Effects on Gene Expression in Mouse Hippocampus, Liver, and Proximal Colon. Alcohol Clin Exp Res. 2015;39:1917–1929. doi: 10.1111/acer.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson G, Forsyth CB, Tang Y, Shaikh M, Zhang L, Turek FW, Keshavarzian A. Role of intestinal circadian genes in alcohol-induced gut leakiness. Alcohol Clin Exp Res. 2011;35:1305–1314. doi: 10.1111/j.1530-0277.2011.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb MJ. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 1996;15:4629–4642. [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of Alcohol on miR-212 Expression in Intestinal Epithelial Cells and Its Potential Role in Alcoholic Liver Disease. Alcohol Clin Exp Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Voigt RM, Forsyth CB, Keshavarzian A. Circadian disruption: potential implications in inflammatory and metabolic diseases associated with alcohol. Alcohol Res Curr Rev. 2013a;35:87–96. [PMC free article] [PubMed] [Google Scholar]
- Voigt RM, Forsyth CB, Keshavarzian A. Circadian Disruption. Alcohol Res Curr Rev. 2013b;35:87–96. [PMC free article] [PubMed] [Google Scholar]
- Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH, Turek FW, Keshavarzian A. Circadian Disorganization Alters Intestinal Microbiota. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins AK, Barton PI, Tidor B. The Per2 Negative Feedback Loop Sets the Period in the Mammalian Circadian Clock Mechanism. PLOS Comput Biol. 2007;3:e242. doi: 10.1371/journal.pcbi.0030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. 2011 doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CT, Shih H, Lai MZ. Multiple Signals Required for Cyclic AMP-Responsive Element Binding Protein (CREB) Binding Protein Interaction Induced by CD3/CD28 Costimulation. J Immunol. 2001;166:284–292. doi: 10.4049/jimmunol.166.1.284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


