Abstract
Objective
Patients with cancers frequently experience sleep and circadian dysfunction. To date, only a few studies have used both a questionnaire and actigraphy for concomitant evaluation of sleep and circadian function in patients with cancer. We sought to evaluate objective sleep and circadian parameters in metastatic colon cancer (MCC) patients and their associations with symptoms and quality of life (QOL).
Methods
Patients reported subjective sleep problems on the EORTC QLQ-C30. Sleep and circadian parameters were calculated using a wrist-actigraph that patients wore for 72 hours.
Results
237 Patients with MCC (age: 60.4 years; range: 20.7–77.6; Male/Female ratio: 1.66) participated in this cross-sectional study. Subjective sleep problems were reported by 63.4% of patients (S+). No differences in any sleep parameters (sleep efficiency, sleep latency, total sleep time, total time in bed, wake after sleep onset, activity bathyphase) were observed between S+ and S- patients. However, S+ patients displayed a significantly worse circadian function than S-patients (96.4% versus 98.1%; p=.005). Presence of poor subjective sleep and objective circadian dysfunction negatively affected symptoms and QOL domains (p=.038).
Conclusions
Subjective report of sleep problems was not associated with worse objectively-measured sleep parameters in patients with MCC although it was associated with disrupted circadian rest-activity rhythm and poorer QOL. These findings coincide with prior research in cancer patients in that an inconsistent relationship exists between subjective and objective sleep measurements on some sleep domains. This study supports the value of coupled evaluation of self-reported and objective measures of sleep and circadian function in cancer patients.
Keywords: Cancer, Oncology, Sleep, Actigraphy, Metastatic Colorectal Cancer, Circadian
BACKGROUND
Colorectal cancer is the third most commonly diagnosed cancer in the United States, corresponding to the third highest mortality rate.[1] To improve prognosis and well-being of patients with metastatic colorectal cancer, it is important to identify novel biomarkers and therapeutic targets. An area of active research concerns circadian function, constituted by biological rhythms with a period of about 24 hours[2] that temporally control physiological processes, including sleep-wake, physical and mental performance, and appetite. Circadian function research seeks to understand contributors to the regulation and disruption of its processes including sleep, as well as the beneficial and harmful health consequences of circadian function regulation and disruption, respectively. Several studies have indicated that because circadian rhythm physiology is coordinated by a hierarchical system,[3; 4] disruption of the function of the circadian timing system (e.g., induced by shift work or jet-lag) is associated with several systemic complaints, such as asthenia, low vitality, poor performance, mood alterations, sleep disruption, and appetite loss.[5–7] Interestingly, these are also the symptoms most commonly reported by patients with cancer.[8] Moreover, these same systemic symptoms often cluster together in cancer patients, suggesting a common underlying pathogenic mechanism.[9; 10]
Circadian dysregulation and sleep disruption have a reciprocal relationship in that sleep disruption might adversely affect circadian function, and deregulation of circadian function can contribute to the development of sleep disruption.[11] Sleep disruption, insomnia, (difficulty falling and staying asleep or waking up earlier than intended), and non-restorative sleep are highly prevalent issues among cancer patients and cancer survivors throughout the whole disease span.[12–15] Moreover, poor, short sleep and daytime naps have been shown to be associated with an increased risk for the development of colorectal cancer in a recent meta-analysis involving about 1.5 million individuals worldwide.[16] Approximately half of colorectal cancer patients report sleep disruption.[17] Similar figures have been described for circadian disruption in this clinical setting.[18; 19] While the etiology of sleep and circadian disruption in cancer is not entirely understood, several precipitating factors include receipt of chemotherapy, diagnosis-induced stress, and side-effects from treatments.[14]
Actigraphy, a wrist-worn accelerometer, is a widely accepted measure of sleep as well as activity, thus providing an approximation of circadian activity patterns.[20] The rest-activity rhythm, which can be evaluated with an actigraph, is often a preferred biomarker of the circadian timing system compared to evaluation of serum or salivary cortisol because of its non-invasiveness, relative cost, continuity of monitoring throughout day and night, and practical considerations for patients with high disease burden. Analysis of actigraphy recordings provides relevant and robust parameters, such as the dichotomy index I<O, which represents relative differences in activity between time spent in bed versus out of bed.[21] Better circadian function is represented by a higher I<O index, which indicates that patients are spending the nighttime asleep in a restful way and spending the daytime awake in an active way.[22] In previous studies, I<O has been shown to be one of the most clinically relevant circadian parameters in terms of prognosis and correlation with health-related quality of life and symptoms.[18; 23–26]
Thus, there is substantial evidence to support the notion that sleep and circadian disruption are prevalent, bothersome, and clinically relevant in cancer patients. However, limited research exists in patients with metastatic colorectal cancer that has evaluated both sleep and circadian dysfunction concurrently, using both objective and subjective measures.[15] The aim of this study is to fill this knowledge gap by examining the relationship between subjective sleep problems and objective evidence of sleep and circadian disruption, as well as their association with factors related to health-related quality of life in patients with metastatic colorectal cancer.
METHODS
The current descriptive study involved cross-sectional comparisons of 2 main groups (those with and without subjective sleep problems) and 4 subgroups (sleep problems with or without concomitant circadian disruption) of patients with metastatic colorectal cancer with available data for both wrist-actigraphy and the sleep item from a health-related quality of life questionnaire). Patients participated in two separate prospective studies: the first one included 145 patients,[24] and the second was a companion study to an international randomized phase III trial [27] that included 92 patients.[23] Both studies were approved by the appropriate ethics review boards at the participating research centers. All patients were adults (> 18 years) who provided written informed consent, completed the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30 v2), and wore the wrist-actigraph for 3 full days and nights before receiving the first course of chemotherapy for histologically-proven metastatic colorectal cancer. Patients with symptomatic brain metastases or uncontrolled endocrine, neurological, or psychiatric comorbidities were excluded.
QUALITY OF LIFE QUESTIONNAIRE AND SUBJECTIVE SLEEP ASSESSMENT
The EORTC QLQ-C30 is the most widely used multidimensional questionnaire for assessing health-related quality of life in cancer patients.[28] Each patient completed the test in her/his first language according to the procedures suggested by the EORTC.[29] The questionnaire includes a single straightforward question regarding sleep difficulty: “[During the past week] Have you had trouble sleeping?”[28] We considered that patients had no subjective sleep complaints if they answered “not at all”. The other possible answers (i.e., “a little”, “quite a bit,” and “very much”) were considered a subjective report of sleep problems. This dichotomization exhibited clinical impact on patient outcomes in a prior study.[17] This item uniquely assesses sleep, and is a standalone question; hence, it is not included in the calculation of any other subscale of the questionnaire. We also calculated health-related quality of life domains and symptoms from the same questionnaire according to the recommended methodology, which involved the transformation of raw scores into a linear scale ranging from 0 to 100.[28] For health-related quality of life domains, higher values reflect better functioning; conversely, for symptoms, higher values indicate more severe complaints. In the general population, median symptom scores are mostly 0 (except for fatigue, which is 22.2), median function scores are 100, and median global quality of life is 75.[30]
WRIST ACTIGRAPHY
Patients wore a Mini-Motionlogger actigraph (Ambulatory Monitoring Inc., USA) for 72 consecutive hours on their non-dominant wrist. This device is a watch-sized piezo-electric linear accelerometer with a memory storage that records the number of limb accelerations that occur per minute.[15; 23; 24] It is widely used in sleep and chronobiology research, particularly for its non-invasive presence in the subject’s own environment.[20] Actigraphy time series were analyzed using dedicated software provided by the manufacturer (Action W, Version 2.7 and Action 4, Version 1.16; Ambulatory Monitoring Inc., USA). These programs provided validated parameters for objectively assessing sleep and circadian function. In particular, using the validated University of California San Diego algorithm to infer sleep and wake,[31] the analysis allowed the computation of average total time scored as sleep (TST), sleep efficiency (SE, percentage of TST within total time spent in bed), sleep latency (SL, minutes to the start of the first 20-minute block with > 19 minutes of sleep), and wake after sleep onset (WASO, duration of time scored as wake during the interval consumed in bed).[15; 32] Furthermore, we calculated the dichotomy index I<O, the most relevant circadian parameter in cancer research,[18; 23; 24] which indicates the percentage of activity counts during time in bed whose values are lower than the median activity out-of-bed with higher values suggesting more robust circadian function.[19; 21] Finally, we computed the clock time of lowest activity (bathyphase) using a cosinor analysis and the average activity counts (accelerations per minute). For these parameters, the best cut-off values to differentiate healthy subjects from cancer patients are 99% for I<O, 7h20m for TST, 92% for SE, 12 minutes for SL, and 25 minutes for WASO. [22]
STATISTICAL ANALYSES
Patients were initially categorized into two subgroups based on their report of having trouble sleeping or not, according to their answer to the question, “[During the past week] Have you had trouble sleeping?” on the EORTC QLQ-C30. The distribution of the aforementioned objective actigraphy sleep parameters, I<O, average activity, and bathyphase were compared between the 2 categories of patients using the nonparametric Mann-Whitney U test. Following the initial results, patients were categorized into 4 subgroups according to subjective sleep problems (yes/no) and objective circadian disruption (“presence of circadian disruption”, dichotomy index >= 97.5%,[18; 19] “no circadian disruption”, dichotomy index <97.5%) Additionally, the distribution of global quality of life measures was compared among the 4 subgroups using the nonparametric Kruskal-Wallis test. Differences deemed significant demonstrated p<0.05. Analyses were performed using PASW software 22 (SPSS, IBM, USA).
RESULTS
CLINICAL FEATURES
The study included a total of 237 patients with metastatic colorectal cancer. The main demographic and medical characteristics of our study’s subjects are summarized in Table 1 and generally reflect similar descriptors to those reported by clinical trials in patients with metastatic colorectal cancer. The concomitant chronic use of drugs—potentially affecting sleep—proved infrequent (0.8–2.5%) in our study population (Table 1). Eighty-two patients (34.6%) described no trouble sleeping, whereas 155 patients (65.4%) complained of sleep problems on the EORTC QLQ-C30 questionnaire.
Table 1.
Clinical and demographic characteristics of the study population (n=237), and of the two subgroups according to the presence (n=155; 65.4%) or absence (n=82; 34.6%) of subjective sleep trouble.
| Demographic and Medical Variables | Whole population |
Subjective sleep trouble |
||
|---|---|---|---|---|
| N=237 | Yes (N=155) |
No (N=82) |
||
| N(%) | ||||
| Median age (years) | (range) | 60.4 (20.7–77.6) | 59.9 (20.7–75.4) | 60.5 (35.7–77.6) |
| Gender | Women | 89 (37.6) | 58 (37.4) | 31 (37.8) |
| Men | 148 (62.4) | 97 (62.6) | 51 (62.2) | |
| PS (WHO scale) | 0 | 141 (59.5) | 81 (52.3) | 60 (73.2) |
| 1 | 79 (33.3) | 61 (39.4) | 18 (22.0) | |
| 2 | 17 (7.2) | 13 (8.4) | 4 (4.8) | |
| Number of metastatic sites | 1 | 107 (45.1) | 67 (43.2) | 40 (48.8) |
| 2 | 89 (37.6) | 57 (36.8) | 32 (39.0) | |
| >2 | 41 (17.3) | 31 (20.0) | 10 (12.2) | |
| Site of primary tumor | Colon | 171 (72.2) | 113 (72.9) | 58 (70.7) |
| Rectum | 66 (27.8) | 42 (27.1) | 24 (29.3) | |
| Synchronous metastases | No | 86 (36.3) | 54 (34.8) | 32 (39.0) |
| Yes | 145 (61.2) | 97 (62.6) | 48 (58.5) | |
| Unknown | 6 (2.5) | 4 (2.6) | 2 (2.4) | |
| Prior chemotherapy for metastatic disease | No | 154 (65.0) | 105 (67.7) | 49 (59.8) |
| Yes | 83 (35.0) | 50 (32.3) | 33 (40.2) | |
| BMI (Kg/m2) | Normal (18.5–24.9) | 114 (48.1) | 72 (46.5) | 42 (51.2) |
| Underweight (below 18.5) | 11 (4.6) | 8 (5.2) | 3 (3.7) | |
| Overweight-Obese (above 25.0) | 109 (45.9) | 73 (47.0) | 36 (43.9) | |
| Unknown | 3 (1.3) | 2 (1.3) | 1 (1.2) | |
| Concomitant medications | Opioids | 6 (2.5) | 6 (3.9) | 0 |
| Antidepressants | 4 (1.7) | 4 (2.6) | 0 | |
| Betablockers | 3 (1.3) | 1 (0.6) | 2 (2.4) | |
| Corticosteroids | 2 (0.8) | 2 (1.3) | 0 | |
| Hypnotic drugs | 4 (1.7) | 4 (2.6) | 0 | |
| Anemia (Hemoglobin < 12.0 g/dl) | Yes | 97 (40.9) | 60 (38.7) | 37 (45.1) |
| Leukocytosis (white blood cells > 10.0 giga/l) | Yes | 53 (22.4) | 41 (26.5) | 12 (14.6) |
| Observed median overall survival (months) | (95% confidence interval) | 15.1 (13.1–17.2) | 14.4 (12.4–16.3) | 17.7 (10.6–24.8) |
PS-Performance Status, WHO-World Health Organization, BMI-Body Mass Index
ACTIGRAPHY PARAMETERS AND SUBJECTIVE SLEEP
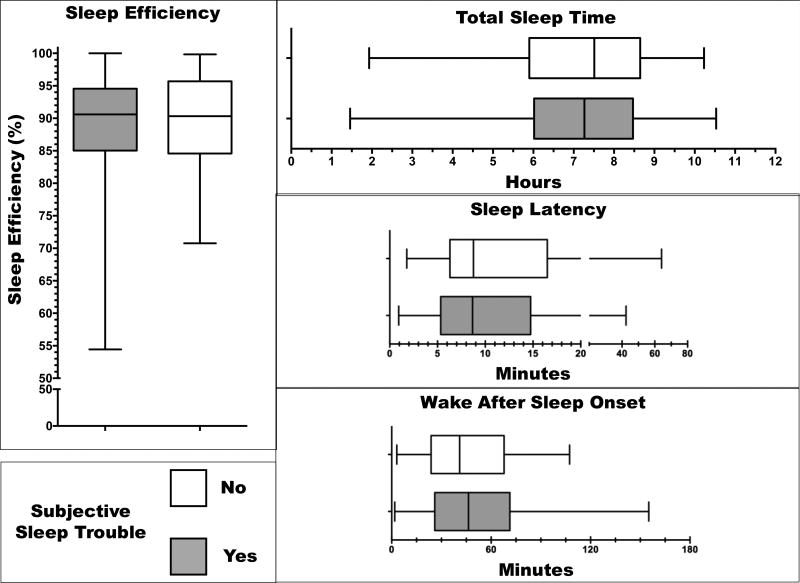
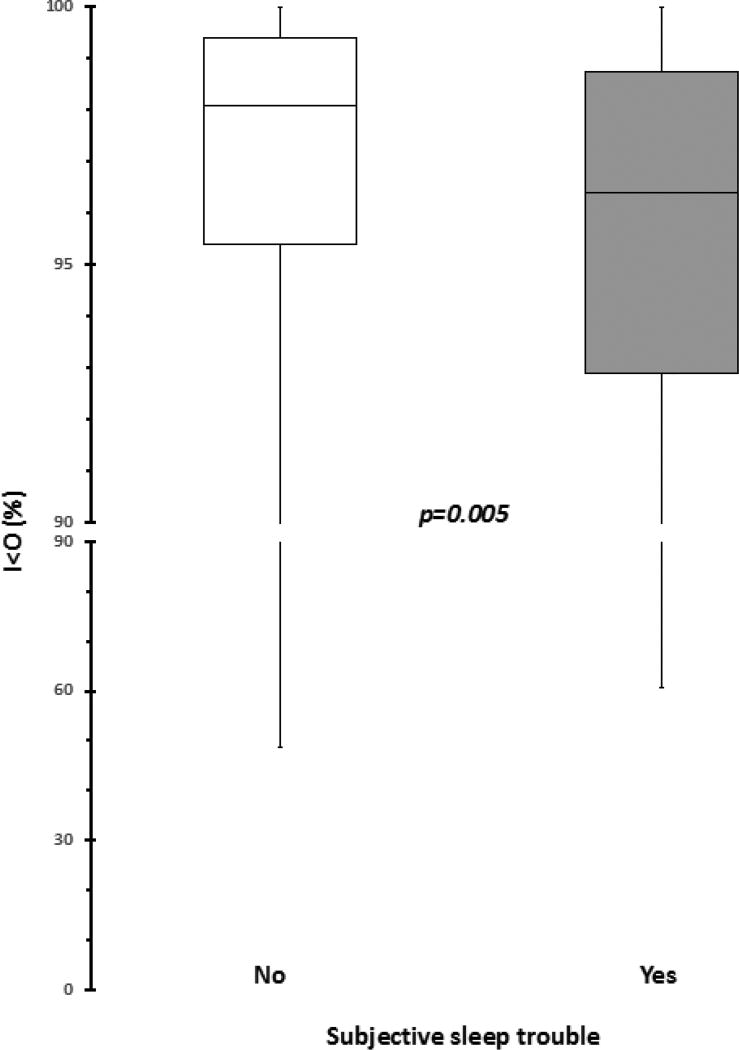
Table 2 presents the distribution of the 4 objective sleep parameters and the 3 activity measures in the whole study population. Subjective complaint of sleep trouble was not associated with a difference in the distribution of any actigraphy sleep parameter (Figure 1). Thus, TST duration was approximately the same whether or not the patient complained of sleep trouble (p=0.57). Estimated difficulty falling asleep (SL) proved equally severe, and estimated duration of awakening episodes during the night (WASO) was not significantly longer in patients with subjective sleep complaints than in those who reported no trouble (p=0.25 and p=0.34, respectively). This produced a similar SE in both groups of patients (p=0.61; 1). Neither the timing of lower activity (p=0.27) nor the average activity counts (p=0.53) differed according to reports of sleep problems or a lack thereof (not shown). Conversely, patients with subjective sleep complaints displayed significantly worse circadian function (i.e., lower I<O) as compared to patients without sleep problems (p=0.005; Figure 2). Thus, respective median (IQR) values for I<O in patients with and without subjective sleep complaints were 96.4% (SD=4.0%) and 98.1% (SD=5.8%), a significant difference. Taking 97.5% as the clinically meaningful threshold for I<O,[18; 19] 61.3% of patients with subjective sleep problems, and only 42.7% of patients without, displayed circadian disruption (i.e., I<O lower than the threshold).
Table 2.
Median and interquartile range (IQR) of the distribution of objective sleep parameters derived from wrist-actigraphy in the whole population.
| Actigraphy-derived parameter | Median (IQR) |
|---|---|
| Sleep Variables | |
| Total sleep time (TST; hours-minutes) | 7h22m (2h29m) |
| Sleep efficiency (SE; %) | 90.56 (10.04) |
| Sleep latency (SL; minutes-seconds) | 8m40s (9m27s) |
| Wake after sleep onset (WASO; minutes-seconds) | 46m15s (43m10s) |
| Circadian and Activity Variables | |
| Dichotomy index (I<O; %) | 96.9 (5.5) |
| Bathyphase (clock time; hh:mm) | 02:33 (1:20) |
| Average activity (accelerations/minute) | 99 (39) |
Figure 1.
Distribution of 4 objective actigraphy sleep parameters according to the answer to the question of the quality of life questionnaire about sleep trouble: gray, yes; white, no. Boxes represent 25th and 75th percentiles, whiskers the range, and the middle black line the median. All p values were > 0.05.
Figure 2.
Distribution of the circadian parameter I<O, according to the presence (gray boxes) or absence (white boxes) of subjective sleep complaints derived from the EORTC QLQ-C30. Boxplots represent 1st and 3rd quartiles, whiskers the range, and the middle black line the median. The p value is derived from nonparametric Mann-Whitney U test. The best cut-off value to discriminate between healthy subjects and cancer patients is 99%, [22] while the most clinically relevant cut-off value in advanced colorectal cancer patients, in term of prognosis, is 97.5%. [8; 19]
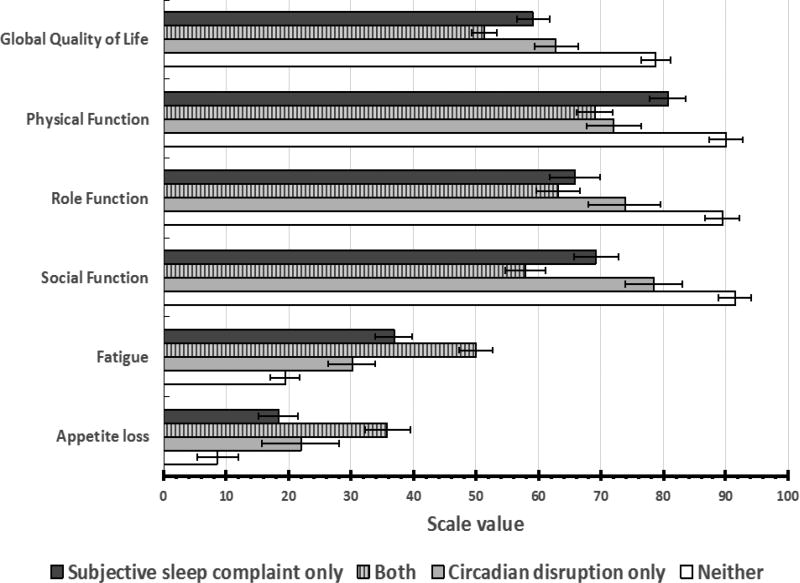
As a result of this finding, we performed further post-hoc analyses to explore the clinical implication of subjective sleep problems and actigraphy-derived circadian disruption. Thus, 40.1% of patients had both circadian (as characterized by a clinical cut-off of 97.5% for I<O) and subjective sleep disruption; 25.3% had subjective sleep problems only; 14.8% had circadian disruption only, and 19.8% of patients had neither. There were significant differences between these groups on the health-related quality of life subscales: Post-hoc analyses indicated that those who had both subjective sleep problems and circadian disruption had the lowest (i.e., worst) overall health-related quality of life scores and the highest (i.e., most severe) systemic symptoms scores (Figure 3).
Figure 3.
Mean (+ SD) values for pertinent quality of life domains or symptoms derived from the EORTC QLQ-C30 questionnaire, according to the presence or absence of subjective sleep complaints and/or objective circadian disruption. P values are derived from nonparametric Kruskal-Wallis H test.
HEALTH-RELATED QUALITY OF LIFE
Thus, global quality of life (p<0.001), physical (p<0.001), social (p=0.023), and role functioning (p=0.044) were significantly different in these 4 subgroups, with patients reporting trouble sleeping and exhibiting disrupted circadian function displaying worse (i.e., higher) scores. Similarly, fatigue (p=0.029) and appetite loss (p=0.038) were significantly different in the 4 subgroups and more severe (i.e., higher scores) in patients with both sleep complaints and circadian disruption (Figure 3).
CONCLUSIONS
To our knowledge, this study is the first one in patients with metastatic colorectal cancer that evaluated both subjective and objective sleep problems, as well as circadian function. Furthermore, this study involves a relatively large sample of patients with colorectal cancer. While the majority (65.4%) of patients reported having subjective sleep problems, their associated actigraphy-measured sleep parameters appeared to be within normal ranges[33] and remarkably comparable to those not reporting subjective sleep problems (Figure 1). However, another study conducted in women with early breast cancer reported a higher number of night awakening episodes than in the general population using wrist-actigraphy.[15] We found that the largest proportion (40%) of patients reported having both subjective sleep problems and circadian disruption, followed by 25% of the sample’s endorsing subjective sleep problems but not meeting criteria for circadian disruption. An additional 15% met criteria for only circadian disruption while another minority of patients (20%) had neither sleep nor circadian disruption problems. While this study observed no difference in actigraphy-measured sleep and activity variables between patients who reported subjective sleep complaints and those who self-rated themselves as good sleepers (Figure 1), we found significant differences in circadian function, as measured by dichotomy index, between these patient groups (Figure 2).
Several studies have found that subjective sleep complaints are generally correlated with actigraphy-measured variables in the general population,[34] yet studies in cancer patients sometimes showed no relationship or an inconsistent relationship between subjective and objective sleep measurements.[15; 35] While our findings are in line with prior research in cancer patients, Moore and colleagues (2015) propose that the discrepancy can be potentially explained by the “importance of sleep perception” in medically ill populations with comorbid sleep disturbance. Patients might overestimate or underestimate their sleep problems,[35] creating lack of agreement between self-reported sleep and actigraphy-measured sleep. Additionally, while actigraphy is accurate in measuring sleep in healthy populations, de Souza and colleagues found that, even in healthy participants, actigraphy overestimates sleep latency (SL), total sleep time (TST), and sleep efficiency (SE), while it underestimates awakenings compared to the gold standard of polysomnography measurement.[36]
Berger and colleagues[37] proposed using both subjective and actigraphy-measured sleep to evaluate various aspects of sleep in women with breast cancer to increase sensitivity and abate missing data. Thus, generally, clinicians and researchers who treat and study sleep disruption rely on both objective measures (actigraphy or PSG) and self-reports (sleep diaries, questionnaires) for assessment of sleep.[12] This suggestion was also endorsed by Dhruva and colleagues,[15] who advocated for a comprehensive and longitudinal evaluation of this symptom trajectory in order to more accurately identify potential targets for intervention.
While we did not find agreement between our subjective and objective sleep measurements, we observed that patients with poor subjective sleep also had a significantly more disrupted circadian rhythm, as measured by lower I<O index (Figure 2). It is possible that the dichotomy index is a more sensitive marker of perceived sleep function because it takes both daytime and nighttime activity into account.[21; 22; 26] Indeed, patients who complained of poor sleep allegedly suffered from consequences of unrestful sleep during daytime, and the circadian rest-activity rhythm was reported as a correlate to fatigue,[8; 25] an expected repercussion of altered nighttime sleep.[38]
Interestingly, in our study, patients who reported subjective sleep complaints and had circadian disruption demonstrated significantly worse outcomes on pertinent health-related quality of life subscales than those with only one disruption or none, suggesting that there might be a cumulative, negative impact on patients’ well-being from experiencing poor subjective sleep and disrupted circadian function (Figure 3).
This study is relevant within the larger context of cancer patient well-being. Indeed, patient-reported outcomes are being increasingly recognized as valid and reliable measurements, with important clinical relevance.[39; 40] This recognition given to patient-reported outcomes seems particularly appropriate for sleep quality, a common, bothersome, yet overlooked problem in cancer patients.[13] Similarly, circadian function is rarely evaluated in cancer patients, despite its clinical impact.[3; 8] While our findings further support the concomitant evaluation of subjectively-reported symptoms and of objectively-measured circadian function in future studies in cancer patients, they also highlight the need for more sensitive, non-invasive measures of sleep and circadian disruption.
The cumulative and negative clinical impact of altered sleep and circadian functions further supports the development of research strategies for novel, targeted interventions to normalize circadian homeostasis. Given the reported prognostic impact of poor sleep[17; 32] and of circadian disruption[3; 18; 19] in cancer patients, improving circadian function with associated improvements in sleep might potentially improve overall survival.
We acknowledge the main limitation in our study, which is the lack of use of a benchmark sleep questionnaire in cancer patients.[12] Instead, we used a single-item question, although face-valid, to screen for sleep complaints, precluding us from evaluating self-reported sleep duration, sleep maintenance, or relevant sleep-related daytime dysfunction, which could have been examined in relation to actigraphic data. These additional data would have been informative given the absence of differences in objective sleep duration/sleep continuity measures between subgroups of patients with or without sleep complaints. Using a one-item sleep question, in comparison with a validated measure (e.g., Pittsburgh Sleep Quality Index or Insomnia Severity Index), provides less information and has different sensitivity and specificity compared to a more detailed evaluation of sleep.
Additional subjective sleep disruption questions might have helped elucidate our findings, however, a self-report measure of sleep disruption involving multiple questions may still not have more fully captured a patient’s distinct forms of sleep disruption due to the possibility that these distinct forms are unrecognized by the patient. Furthermore, the aim of our study was to identify objective measures of subjective sleep complaints and not to evaluate the incidence of specific facets of sleep disruption. The single-item question used in the current study was included in a validated, broadly-used questionnaire in cancer patients,[28] had demonstrated clinical relevance in patients with metastatic colorectal cancer,[17] and did not require the complicated retrospective recall of sleep experiences, unlike other multi-item sleep questionnaires. This one-item question was valid in discriminating patients with and without circadian disruption. In addition, using a one-item measure for sleep to screen patients with sleep problems in a busy oncology clinic might be more acceptable and feasible.
Our findings provide evidence for a rather weak association between wrist-actigraphy conventional estimates of poor sleep and patients’ own impressions of having trouble sleeping, but our findings are not suitable to precisely gauge the incidence and type of sleep pathologies or the entire range of their daytime consequences on the patients. Additional studies incorporating multi-dimensional evaluations of sleep, ideally on an individual night basis, are warranted to further address these issues.
Another limitation of this study is the use of wrist-actigraphy measurement for assessment of circadian disruption. Actigraphy only approximates circadian rhythms; to accurately measure circadian disruption would require patients to be observed in the laboratory under stable light, food, and activity conditions while undergoing continuous biomarker measurement, which is not realistic given the clinical sample. Nevertheless, this study has many strengths, such as a large sample size of patients with metastatic disease, an objective evaluation of sleep and circadian function for at least 72 hours performed in a clinical setting, and the availability of a multi-dimensional array of patient-reported outcomes.
The goal of our study was to evaluate subjective and objective evidence of sleep and circadian disruption in patients with metastatic colorectal cancer and to examine the effect of sleep and circadian dysfunction on health-related quality of life. Interestingly, we found that subjective sleep difficulty correlated positively with objective circadian disruption (Figure 2). In our sample, we found no relationship between subjective sleep disruption and objective sleep disruption as measured by actigraphy (Figure 1). These novel findings add to the paucity of literature regarding sleep problems and circadian disruption in patients with metastatic colorectal cancer. Sleep problems are common but are undertreated and underrecognized in cancer patients, particularly in those diagnosed with metastatic disease. Evaluating and treating sleep and circadian problems can significantly improve quality of life in patients diagnosed with metastatic cancer. Future research should examine the impact of behavioral and/or pharmacological interventions with the aim to normalize both circadian and sleep disruption to understand whether such interventions could improve quality of life and potentially overall survival.
Acknowledgments
Funding: Funded by: NCI R01CA181659, NCI R21CA185678
Footnotes
Conflict of Interest: All authors declared no conflicts of interest
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
This work was presented in part at the MASCC 2015 Annual Conference: Copenhagen, Denmark, June 25–27, 2015.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Mazzoccoli G, Vinciguerra M, Papa G, Piepoli A. Circadian clock circuitry in colorectal cancer. World J Gastroenterol. 2014;20(15):4197–4207. doi: 10.3748/wjg.v20.i15.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Innominato PF, Roche VP, Palesh OG, Ulusakarya A, Spiegel D, Levi FA. The circadian timing system in clinical oncology. Ann Med. 2014;46(4):191–207. doi: 10.3109/07853890.2014.916990. [DOI] [PubMed] [Google Scholar]
- 4.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 5.Foster RG, Wulff K. The rhythm of rest and excess. Nat Rev Neurosci. 2005;6(5):407–414. doi: 10.1038/nrn1670. [DOI] [PubMed] [Google Scholar]
- 6.Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet. 2001;358(9286):999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- 7.Sack RL. Clinical practice. Jet lag. N Engl J Med. 2010;362(5):440–447. doi: 10.1056/NEJMcp0909838. [DOI] [PubMed] [Google Scholar]
- 8.Innominato PF, Mormont MC, Rich TA, Waterhouse J, Levi FA, Bjarnason GA. Circadian disruption, fatigue, and anorexia clustering in advanced cancer patients: implications for innovative therapeutic approaches. Integr Cancer Ther. 2009;8(4):361–370. doi: 10.1177/1534735409355293. [DOI] [PubMed] [Google Scholar]
- 9.Molassiotis A, Wengstrom Y, Kearney N. Symptom cluster patterns during the first year after diagnosis with cancer. J Pain Symptom Manage. 2010;39(5):847–858. doi: 10.1016/j.jpainsymman.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, Brown T, Chesson AL, Jr, Kapur V, Maganti R, Owens J, Pancer J, Swick TJ, Zak R Standards of Practice Committee of the American Academy of Sleep, M. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30(11):1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palesh O, Aldridge-Gerry A, Ulusakarya A, Ortiz-Tudela E, Capuron L, Innominato PF. Sleep disruption in breast cancer patients and survivors. J Natl Compr Canc Netw. 2013;11(12):1523–1530. doi: 10.6004/jnccn.2013.0179. [DOI] [PubMed] [Google Scholar]
- 13.Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, Heckler C, Purnell JQ, Janelsins MC, Morrow GR. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palesh O, Peppone L, Innominato PF, Janelsins M, Jeong M, Sprod L, Savard J, Rotatori M, Kesler S, Telli M, Mustian K. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–162. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhruva A, Paul SM, Cooper BA, Lee K, West C, Aouizerat BE, Dunn LB, Swift PS, Wara W, Miaskowski C. A longitudinal study of measures of objective and subjective sleep disturbance in patients with breast cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2012;44(2):215–228. doi: 10.1016/j.jpainsymman.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erren TC, Morfeld P, Foster RG, Reiter RJ, Gross JV, Westermann IK. Sleep and cancer: Synthesis of experimental data and meta-analyses of cancer incidence among some 1 500 000 study individuals in 13 countries. Chronobiol Int. 2016:1–26. doi: 10.3109/07420528.2016.1149486. [DOI] [PubMed] [Google Scholar]
- 17.Innominato PF, Spiegel D, Ulusakarya A, Giacchetti S, Bjarnason GA, Lévi F, Palesh O. Subjective sleep and overall survival in chemotherapy-naïve patients with metastatic colorectal cancer. Sleep Med. 2015 Mar;16(3):391–8. doi: 10.1016/j.sleep.2014.10.022. Epub 2015 Jan 22. [DOI] [PubMed] [Google Scholar]
- 18.Innominato PF, Giacchetti S, Bjarnason GA, Focan C, Garufi C, Coudert B, Iacobelli S, Tampellini M, Durando X, Mormont MC, Waterhouse J, Levi FA. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int J Cancer. 2012;131(11):2684–2692. doi: 10.1002/ijc.27574. [DOI] [PubMed] [Google Scholar]
- 19.Levi F, Dugue PA, Innominato P, Karaboue A, Dispersyn G, Parganiha A, Giacchetti S, Moreau T, Focan C, Waterhouse J, Spiegel D, Group AC. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. Chronobiol Int. 2014;31(8):891–900. doi: 10.3109/07420528.2014.924523. [DOI] [PubMed] [Google Scholar]
- 20.Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, Sadeh A, Spira AP, Taylor DJ. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behav Sleep Med. 2015;(13 Suppl 1):S4–S38. doi: 10.1080/15402002.2015.1046356. [DOI] [PubMed] [Google Scholar]
- 21.Minors D, Akerstedt T, Atkinson G, Dahlitz M, Folkard S, Levi F, Mormont C, Parkes D, Waterhouse J. The difference between activity when in bed and out of bed. I. Healthy subjects and selected patients. Chronobiol Int. 1996;13(1):27–34. doi: 10.3109/07420529609040839. [DOI] [PubMed] [Google Scholar]
- 22.Natale V, Innominato PF, Boreggiani M, Tonetti L, Filardi M, Parganiha A, Fabbri M, Martoni M, Lévi F. The difference between in bed and out of bed activity as a behavioral marker of cancer patients: A comparative actigraphic study. Chronobiol Int. 2015;32(7):925–33. doi: 10.3109/07420528.2015.1053909. [DOI] [PubMed] [Google Scholar]
- 23.Innominato PF, Focan C, Gorlia T, Moreau T, Garufi C, Waterhouse J, Giacchetti S, Coudert B, Iacobelli S, Genet D, Tampellini M, Chollet P, Lentz MA, Mormont MC, Levi F, Bjarnason GA Chronotherapy Group of the European Organization for, R., & Treament of, C. Circadian rhythm in rest and activity: a biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res. 2009;69(11):4700–4707. doi: 10.1158/0008-5472.CAN-08-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, Lellouch J, Misset JL, Touitou Y, Levi F. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6(8):3038–3045. [PubMed] [Google Scholar]
- 25.Mormont MC, Waterhouse J. Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiol Int. 2002;19(1):313–323. doi: 10.1081/cbi-120002606. [DOI] [PubMed] [Google Scholar]
- 26.Chen HM, Wu YC, Tsai CM, Tzeng JI, Lin CC. Relationships of Circadian Rhythms and Physical Activity With Objective Sleep Parameters in Lung Cancer Patients. Cancer Nurs. 2015;38(3):215–223. doi: 10.1097/NCC.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 27.Giacchetti S, Bjarnason G, Garufi C, Genet D, Iacobelli S, Tampellini M, Smaaland R, Focan C, Coudert B, Humblet Y, Canon JL, Adenis A, Lo Re G, Carvalho C, Schueller J, Anciaux N, Lentz MA, Baron B, Gorlia T, Levi F European Organisation for, R., & Treatment of Cancer Chronotherapy, G. Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol. 2006;24(22):3562–3569. doi: 10.1200/JCO.2006.06.1440. [DOI] [PubMed] [Google Scholar]
- 28.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 29.Efficace F, Innominato PF, Bjarnason G, Coens C, Humblet Y, Tumolo S, Genet D, Tampellini M, Bottomley A, Garufi C, Focan C, Giacchetti S, Levi F Chronotherapy Group of the European Organisation for, R., & Treatment of, C. Validation of patient’s self-reported social functioning as an independent prognostic factor for survival in metastatic colorectal cancer patients: results of an international study by the Chronotherapy Group of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2008;26(12):2020–2026. doi: 10.1200/JCO.2007.12.3117. [DOI] [PubMed] [Google Scholar]
- 30.Fayers P, Bottomley A, Group EQo L, Quality of Life U. Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer. 2002;(38 Suppl 4):S125–133. doi: 10.1016/s0959-8049(01)00448-8. [DOI] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 32.Palesh O, Aldridge-Gerry A, Zeitzer JM, Koopman C, Neri E, Giese-Davis J, Jo B, Kraemer H, Nouriani B, Spiegel D. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37(5):837–842. doi: 10.5665/sleep.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medicine AAoS. International classification of sleep disorders: Diagnostic and coding manual. 2. Westchester, Illinois: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 34.Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, Hirshkowitz M, Kapen S, Kramer M, Loube D, Wise M, Johnson SF Standards of Practice Committee of the American Academy of Sleep, M. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26(3):337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 35.Moore CM, Schmiege SJ, Matthews EE. Actigraphy and Sleep Diary Measurements in Breast Cancer Survivors: Discrepancy in Selected Sleep Parameters. Behav Sleep Med. 2015;13(6):472–490. doi: 10.1080/15402002.2014.940108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26(1):81–85. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 37.Berger AM, Farr LA, Kuhn BR, Fischer P, Agrawal S. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manage. 2007;33(4):398–409. doi: 10.1016/j.jpainsymman.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang NK, Sanborn AN. Better quality sleep promotes daytime physical activity in patients with chronic pain? A multilevel analysis of the within-person relationship. PLoS One. 2014;9(3):e92158. doi: 10.1371/journal.pone.0092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Maio M, Basch E, Bryce J, Perrone F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016 doi: 10.1038/nrclinonc.2015.222. [DOI] [PubMed] [Google Scholar]
- 40.Kyte D, Reeve BB, Efficace F, Haywood K, Mercieca-Bebber R, King MT, Norquist JM, Lenderking WR, Snyder C, Ring L, Velikova G, Calvert M. International Society for Quality of Life Research commentary on the draft European Medicines Agency reflection paper on the use of patient-reported outcome (PRO) measures in oncology studies. Qual Life Res. 2016;25(2):359–362. doi: 10.1007/s11136-015-1099-z. [DOI] [PubMed] [Google Scholar]