Abstract
Objective
Alzheimer's disease (AD) is a neurodegenerative disease, manifesting in clinically observable deficits in memory, thinking, and behavior that disproportionately affects older adults. Susceptibility genes, such as apolipoprotein ε4, have long been associated with an increased risk of AD diagnosis. Studies have shown associations between depression and increased risk of AD development. Furthermore, findings from previous investigations suggest mixed effects in the use of psychotropic medication in older adults. The hypothesis for this study is that antidepressant use modifies the increased hazard of depression or such that a non-significant hazard will result with respect to eventual AD development.
Methods
Utilizing data from the National Alzheimer's Coordinating Center, we examined evaluations of 11,443 cognitively intact participants. Survival analysis was used to explore relationships between depression, apolipoprotein E, AD diagnosis, and antidepressant use.
Results
An analytical sample of 8732 participants with normal cognition was examined. Among users of antidepressant medication, the hazard, in most cases, was no longer statistically significant. One generic medication showed protective benefits for users (p < 0.001). In addition, there was a statistically significant relationship between recent depression (n = 2083; p < 0.001), lifetime depression (n = 2068; p < 0.05), and ε4 carrier status (n = 2470; p < 0.001) and AD development.
Conclusions
The findings suggest that a mechanism related to antidepressant use may reduce the hazard of eventual AD. Furthermore, the findings reinforce the association between depression, apolipoprotein E (APOE) ε4, and AD diagnosis. This study contributes to the emerging literature exploring interventions aimed at decreasing the risk of AD by targeting potentially modifiable psychosocial risk factors such as depression.
Keywords: Alzheimer's disease, antidepressants, apolipoprotein E, depression
Introduction
Affecting more than 46.8 million people worldwide, dementia is an illness with no known cure or treatment. This number is expected to double every 20 years (Alzheimer's Disease International, 2015). AD pathology is characterized by a progressive decrease in neurons, axons, and dendrites in the brain, with progressive impairment in cognitive functioning (Sperling et al., 2011). Most theories targeting AD have focused on the role of β-amyloid deposition and accumulation in the brain, and subsequent clinically observable neurodegeneration (Hardy and Selkoe, 2002). Although the exact role of β-amyloid (Aβ) in the pathogenesis of AD is still debated, Aβ is widely believed to be a neurotoxin, inducing oxidative stress and accumulating as extracellular deposits (plaque) in the brain (Masters et al., 2013). A susceptibility gene, such as apolipoprotein E (APOE), may be influential as a catalyst in the pathophysiological progression to AD (Potter and Wisniewski, 2012). In healthy individuals, APOE is a key component in the regulation and clearance of Aβ. APOE ε3 or ε4 may decrease the rate at which Aβ protein, the precursor to plaques, is cleared from the brain, resulting in elevated levels of Aβ (Jiang et al., 2008). APOE ε4 appears to slow this process more so than other haplotypes (Castellano et al., 2011). The debate on the precise impact of APOE on AD dementia development and progression continues (Brainerd et al., 2013).
In addition to the possible relationship between APOE and AD, evidence indicates an increased risk of AD development in those with depression (Burke et al., 2016a, 2016b; Caraci et al., 2010; Meng and D'Arcy, 2013), although reverse causation concerns remain a challenge in determining whether depression is an early symptom of AD or an actual risk factor, especially given the long prodromal time course of AD. Depression is a neuropsychiatric disorder, generally characterized by melancholic mood, loss of pleasure in preferred activities, weight and appetite fluctuations, sleep disruption, psychomotor changes, and fatigue (American Psychiatric Association, 2013). A history of depression may decrease the age of onset of AD, especially among women (de Oliveira et al., 2014). Empirical resarch has identified an increased risk of dementia in those with the APOE ε3, ε4 genotype (Burke et al., 2016a), cardiovascular health conditions (Whitmer et al., 2005), and depression (Burke et al., 2016a, 2016b; Meng and D'Arcy, 2013). Antidepressants are the third most used medication in the USA and the most commonly used medication for adults between the ages of 18 and 44. Men and women over the age of 40 are significantly more likely to utilize antidepressant medication, and 23% of women between the ages of 40 and 59 take antidepressant medication (Pratt et al., 2011).
Through exploration of serotonin signaling, Cirrito et al. (2011) found that a type of antidepressant medication (selective serotonin reuptake inhibitors (SSRIs)) reduced β-amyloid levels in mouse AD dementia models. Five milligrams of citalopram reduced Aβ found in interstitial fluid by 16%, while 10 mg of the same SSRI reduced β-amyloid by 26%. These reductions occurred soon after administration and were sustained for 12 to 14 hours. As an expansion of this investigation, Cirrito et al. (2011) examined this relationship with cognitively impact (n = 186) who underwent positron-emission tomography Pittsburgh compound B imaging examined β-amyloid plaques. There was a negative relationship between chronic SSRIs users (use of an SSRI in the last 5 years; mean 34.5 months of use) and β-amyloid plaques. In fact, the longer a subject had used an SSRI, the lower their β-amyloid levels. Among those not treated with SSRIs, the increase in β-amyloid plaques was found in the same brain regions affected in AD patients (Cirrito et al., 2011).
On the basis of the empirical literature and previous investigations, the current study explores the role of antidepressants among adults who have experienced depression and the role of antidepressants in decreasing the hazard of AD development among ε4 carriers, with or without depression. We hypothesized that the use of antidepressants, despite the presence of APOE ε4 and/or depression, will either decrease or neutralize the increased hazard of depression and/or ε4, such that a non-significant hazard of AD development will be produced.
Methods
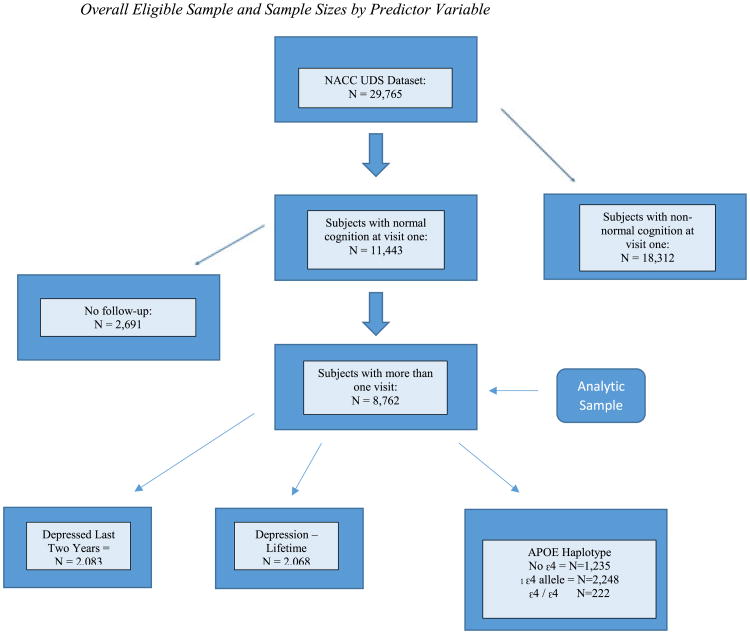
Utilizing data from the National Alzheimer's Coordinating Center (NACC) Uniform Data Set (UDS), the current study examined the impact of antidepressant use on the hazard of eventual AD development among participants with normal cognition at baseline. Demographic information, family history, medications used, and health history are gathered yearly by trained clinicians. Participants undergo a physical, provide responses to psychological and cognitive questionnaires, and may voluntarily provide imaging and laboratory specimens at some of the participating Alzheimer's Disease Centers (ADCs). The number of visits ranged from 1 to 10 (M = 2.12). The overall sample consisted of 29,913 participants from 34 ADCs in the USA who entered their data in the NACC repository between September 2005 and December 2015. Those with normal cognition in their first visit (n = 11,443) were the sample of interest; however, a final analytic sample of 8,732 older adults who underwent at least two visits was selected to fulfill the time course required to perform survival analysis. Time zero was equal to the subject's first observation (visit number 1), and time was measured in days. Figure 1 shows the sampling selection process. Figure 1 shows the sampling selection process.
Figure 1.
Overall eligible sample and sample sizes by predictor variable. NACC, National Alzheimer's Coordinating Center; UDS, Uniform Data Set; APOE, apolipoprotein E. [Colour figure can be viewed at wileyonlinelibrary.com]
The variables utilized for this study were obtained using version 2 of the NACC UDS and include normal cognition, probable AD, self-reported depression in the last 2 years, episodes of depression more than 2 years prior to baseline, reported use of antidepressants, and reported use of specific generic medications targeting depression symptoms. Probable AD is the outcome of interest. The creation of this variable accounted for cognitive testing and etiology (dementia and probable AD) to rule out dementia due to other causes. Normal cognition required a Clinical Dementia Rating score of 0 and cognitive testing within normal limits. On the basis of information obtained at annual observations, diagnoses (including normal cognition and probable AD) were assigned by either a consensus team or the examining physician (Beekly et al., 2004), following UDS criteria originally set forth by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (McKhann et al., 2011).
Depression was measured in two ways. The first variable, which was categorical, included self-reported absence or presence of depression within the last 2 years. This includes depressive disorders for which a clinician was consulted, even if treatment or medication was not received. The second variable, depression: other episodes, was also categorical and includes self-reported absence or presence of episodes prior to the last 2 years. For the purpose of this study, depression: other episodes is considered to be the measure of lifetime depression.
Apolipoprotein E is indicated here by the presence or absence of ε4, denoted by the terms ε4 carrier and non-carrier. An ε4 carrier has the potential to possess one or two ε4 alleles, while a non-carrier possesses other combinations of APOE, none of which contain ε4. APOE genotyping techniques vary by ADC but include either a blood draw or buccal swab and subsequent genotyping.
A general prescription antidepressant category was examined, which included SSRI, tricyclic, monoamine oxidase inhibitor, phenylpiperazine, tetracyclic, and serotonin–norepinephrine reuptake inhibitor antidepressant medications. Within this category, these medications are combined by the NACC repository. As a result, this category allows for a group level analysis, and the medications are not able to be analyzed individually. This is discussed as a limitation. Additionally, specific generic medications for the treatment of depression were analyzed (broad categories without specific trade names but equivalent quality and action), including bupropion, citalopram, escitalopram, fluoxetine, mirtazapine, paroxetine, sertraline, and trazodone. These specific generic medications were likely included in the general antidepressant category, although individual analyses of these medications were included to examine the individual contributions to modification of the hazard of AD development. This study received approval from the Simmons College Institutional Review Board.
Analysis
Utilizing survival analysis, the hypothesis for the current study is that the use of antidepressants, despite the presence of APOE ε4 and depression, either decreases or neutralizes the increased hazard of depression and/or ε4, such that a non-significant hazard will result with respect to eventual AD development. Survival analysis is used to measure the time to an event or outcome of interest and is frequently used to analyze longitudinal data (Kleinbaum and Klein, 2012). An event was defined as the diagnosis of probable AD by a participant's last evaluation. Outcomes are presented as hazard ratios (HRs). Right censoring was utilized to account for the fact that a subject may not receive a diagnosis of AD prior to their last observation, or may leave the study prior to completion. True survival time is unidentified unless a participant develops and is diagnosed with AD by their last observation. All analyses were conducted utilizing STATA, release 13 (StataCorp, 2015), and a p value <0.05 was considered statistically significant.
Univariate analyses calculated frequencies and distributions of predictor variables and covariates. Fisher's exact test determined if there were associations between users of each type of antidepressant medication. Baseline survival function was determined using log-rank tests. The relationship of certain predictor variables was examined relative to the outcome variable using the Cox proportional hazards model (Cox, 1972). Main effects are presented in the first model, while the second model adjusted for sex, age, education, and race. APOE was added as a covariate in the third model, while the use of AD medication was added and controlled in the fourth model, in addition to the demographic covariates previously mentioned. Additive models were explored in the final two tables, through which the combined hazard of ε4 carrier status and either depression condition was stratified across the medication condition, using the models previously described. The additive effect modification model is preferred by many epidemiologists with regard to public health risk analysis (VanderWeele and Knol, 2014). The assumption of proportionality was examined in order to determine whether the Cox proportional hazards assumption was met.
Results
Table 1 provides percentages, means, and standard deviations of participants at baseline by each predictor. The mean number of visits for those with normal cognition was three, with a range of one to nine visits. There were 313 diagnoses of AD by the end of the observation period among 8,732 older adults who underwent at least two visits. The minimum amount of time under observation for participants was 224 days until the first AD diagnosis, and the maximum was 3316 days (M = 1493.94 days). The mean age of subjects with normal cognition at visit 1 was 74.51 years (SD = 10.43; Mdn = 72 years of age). At visit 1, 80.72% of the sample was White, 13.28% were African-American, and 6.01% were from other ethnic groups.
Table 1. Demographic overview of sample and predictor variables.
| Depression last 2 years |
Depression lifetime | General antidepressant use |
Bupropion (Wellbutrin, Zyban) |
Citalopram (Celexa) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Group | At visit 1—baseline | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||
| Normal cognition | 11,433 | 2083 (18.27%) | 9321 (81.73%) | 2068 (18.28%) | 9247 (81.72%) | 1994 (17.43%) | 9449 (82.57%) | 281 (2.46%) | 11,162 (97.54%) | 245 (2.14%) | 11,198 (97.86%) | ||
| Female | 7493 (65.48%) | 1532 (73.55%) | 5932 (63.64%) | 1526 (73.79%) | 5881 (63.60%) | 1474 (73.92%) | 6019 (63.70%) | 193 (68.68%) | 7300 (65.40%) | 186 (75.92%) | 7307 (65.25%) | ||
| Age | X = 74.51 years (SD = 10.43) | X = 68.61 years (SD = 10.75) | X = 68.14 years (SD = 10.52) | X = 68.53 years (SD = 10.12) | X = 65.48 years (SD = 9.91) | X = 67.93 years (SD = 10.87) | |||||||
| Education | X = 15.67 years (SD = 2.98) | X = 15.46 years (SD = 3.05) | X = 15.74 years (SD = 3.03) | X = 15.69 years (SD = 2.90) | X = 16.35 years (SD = 2.67) | X = 15.56 years (SD = 2.74) | |||||||
| White | 9167 (80.72%) | 1797 (86.27%) | 7342 (78.77%) | 1771 (85.64%) | 7229 (78.17%) | 1782 (89.37%) | 7385 (78.16%) | 257 (91.46%) | 8910 (79.82%) | 218 (88.98%) | 8949 (79.92%) | ||
| African-American | 1508 (13.28%) | 162 (7.78%) | 1341 (14.39%) | 173 (8.37%) | 1315 (14.22%) | 127 (6.37%) | 1381 (14.62%) | 13 (4.63%) | 1495 (13.39%) | 15 (6.12%) | 1493 (13.33%) | ||
| Race | Other | 682 (6.01%) | 101 (4.85%) | 576 (6.18%) | 98 (4.74%) | 575 (6.22%) | 74 (3.71%) | 608 (6.43%) | 9 (3.20%) | 673 (6.03%) | 12 (4.90%) | 670 (5.98%) | |
| No ε4 | 5794 (70.11%) | 985 (47.29%) | 4797 (51.64%) | 1012 (48.94%) | 4721 (51.05%) | 75 (3.76%) | 5719 (60.52%) | 12 (44.48%) | 5669 (50.79%) | 108 (44.08%) | 5696 (50.87%) | ||
| 1 ε4 allele | 2248 (27.20%) | 414 (19.88%) | 1831 (19.64%) | 402 (19.44%) | 1827 (19.76%) | 38 (1.91%) | 2210 (23.39%) | 68 (24.20%) | 2180 (19.53%) | 48 (19.59%) | 2200 (19.65%) | ||
| 2 ε4 allele | 222 (2.69%) | 41 (1.97%) | 181 (1.94%) | 43 (2.08%) | 177 (5.45%) | 3 (0.150%) | 219 (2.32%) | 4 (1.42%) | 218 (1.95%) | 9 (3.67%) | 213 (1.90%) | ||
| Group | Escitalopram (Lexapro) | Fluoxetine (Prozac) | Mirtazapine (Remeron) | Paroxetine (Paxil) | Sertraline (Zoloft) | Trazodone (Desyrel) | |||||||
|
| |||||||||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||
|
| |||||||||||||
| Normal cognition | 235 (2.05%) | 11,208 (97.95%) | 245 (2.14%) | 11,198 (97.86%) | 61 (0.53%) | 11,382 (99.47%) | 162 (1.42%) | 11,281 (98.58%) | 296 (2.59%) | 11,147 (97.41%) | 199 (1.74%) | 11,244 (98.26%) | |
| Female | 184 (78.30%) | 7309 (65.21%) | 177 (72.24%) | 7316 (65.33%) | 37 (60.66%) | 7456 (65.51%) | 134 (82.72%) | 7359 (65.23%) | 231 (78.04%) | 7262 (65.15%) | 140 (70.35%) | 7353 (65.39%) | |
| Age | X = 67.57 years (SD = 10.50) | X = 67.21 years (SD = 9.91) | X = 74.64 years (SD = 12.09) | X = 69.99 years (SD = 10.04) | X = 69.32 years (SD = 10.66) | X = 68.36 years (SD = 9.58) | |||||||
| Education | X = 15.47 years (SD = 3.20) | X = 15.68 years (SD = 2.83) | X = 15.92 years (SD = 3.20) | X = 15.19 years (SD = 3.21) | X = 15.56 years (SD = 2.80) | X = 15.5 years (SD = 3.15) | |||||||
| White | 210 (89.36%) | 8957 (79.92%) | 222 (90.61%) | 8945 (79.88%) | 55 (90.16%) | 9112 (80.06%) | 141 (87.04%) | 9026 (80.01%) | 258 (87.16%) | 8909 (79.92%) | 177 (88.94%) | 8990 (79.95%) | |
| African-American | 13 (5.53%) | 1495 (13.39%) | 9 (3.67%) | 1499 (13.39%) | 4 (6.56%) | 1504 (13.21%) | 12 (7.41%) | 1496 (13.26%) | 26 (8.78%) | 1482 (13.30%) | 13 (6.53%) | 1495 (13.30%) | |
| Race | Other | 12 (5.11%) | 670 (5.98%) | 12 (4.90%) | 670 (5.98%) | 1 (1.64%) | 681 (5.98%) | 12 (7.41%) | 8 (0.07%) | 11 (3.72%) | 671 (6.02%) | 7 (3.52%) | 675 (6.00%) |
| No ε4 | 122 (51.91%) | 5672 (50.61%) | 115 (46.94%) | 5679 (50.71%) | 31 (50.82%) | 5763 (50.63%) | 87 (53.70%) | 5707 (50.59%) | 132 (44.59%) | 5662 (50.79%) | 99 (16.58%) | 5695 (50.65%) | |
| 1 ε4 allele | 49 (20.85%) | 2199 (19.62%) | 48 (19.59%) | 2200 (19.65%) | 9 (14.75%) | 2239 (19.67%) | 27 (16.67%) | 2221 (19.69%) | 66 (22.30%) | 2182 (19.57%) | 41 (20.60%) | 2207 (19.63%) | |
| 2 ε4 alleles | 2 (0.851%) | 220 (1.96%) | 6 (2.45%) | 216 (8.18%) | 1 (1.64%) | 221 (1.94%) | 2 (1.23%) | 220 (1.95%) | 6 (2.03%) | 216 (1.94%) | 3 (1.51%) | 219 (1.95%) | |
The log-rank test for equality of survivor functions revealed that there was a statistically significant difference (p < 0.001) in the survival curves of those who did and did not report depression in the last 2 years as well as ε4 carriers and non-carriers. There was a similar difference (p < 0.05) in those reporting lifetime depression versus those who did not. In addition, there was a statistically significant difference (p < 0.001) in the survival curves of those who reported taking citalopram, escitalopram, and mirtazapine, and a statistically significant (p < 0.05) difference in the survival curves of users and non-users of paroxetine, sertraline, and trazodone. This was verified utilizing Fisher's exact test, which was used to determine if statistically significant associations exist between users of each type of antidepressant medication. There was a statistically significant difference between all prescription medication groups (p < 0.05), except for users of trazodone and mirtazapine (p = 0.838), mirtazapine and paroxetine (p = 0.612), and mirtazapine and fluoxetine (p = 0.321).
Recent depression
There was a statistically significant relationship between recent depression (p < 0.001) and eventual AD development (HR = 2.42 [95% CI = 1.92–3.04]). Among users of antidepressant medication, the association between depression and AD was no longer significant (HR = 1.25 [95% CI = 0.819–19.91]). When individual generic medications were examined, users of the generic drugs with depression experienced a statistically non-significant hazard of AD development as compared with non-users. The main effects for those reporting depression in the last 2 years are displayed in Table 2.
Table 2. Hazard ratios of probable Alzheimer's disease development among participants reporting depression within the last 2 years of baseline.
| Predictor variables | Analytic sample size | Depression—last 2 years Main effects (unadjusted) Hazard ratio (95% CI) | Depression—last 2 years Main effects adjusteda without ε4 carrier status Hazard ratio (95% CI) | Depression last 2 years Main effects adjusted with ε4 carrier status Hazard ratio (95% CI) | Depression last 2 years Main effects adjusted with ε4 carrier status and AD Medication use Hazard ratio (95% CI) |
|---|---|---|---|---|---|
| Depression—last 2 years | 2083 | 2.42 (1.92–3.04)** | 2.60 (2.05–3.30)** | 2.46 (1.90–3.18)** | 1.52 (1.16–1.99)* |
| Use of antidepressant | 1994 | 1.25 (0.819–1.91) | 1.62 (1.03–2.55)* | 1.83 (1.12–3.07)* | 1.15 (0.90–1.92) |
| Did not use antidepressant | 2.25 (1.61–3.15)** | 2.03 (1.43–2.88)** | 1.71 (1.15–2.55)* | 1.46 (0.978–2.18) | |
| Bupropion use | 293 | 2.01 (0.255–15.76) | 2.65 (0.323–21.74) | 2.25 (0.244–20.72) | 1.70 (0.155–18.71) |
| No bupropion use | 2.44 (1.93–3.09)** | 2.53 (1.98–3.23)** | 2.42 (1.86–3.15)** | 1.50 (1.14–1.99)* | |
| Citalopram use | 358 | 0.876 (0.371–2.07) | 1.60 (0.556–4.63) | 3.14 (0.744–13.23) | 1.74 (0.457–6.64) |
| No citalopram use | 2.27 (1.78–2.91)** | 2.38 (1.84–3.07)** | 2.17 (1.64–2.86)** | 1.40 (1.05–1.88)* | |
| Escitalopram use | 279 | 0.533 (0.204–1.40) | 0.721 (0.269–1.93) | 0.761 (0.251–2.12) | 0.532 (0.170–1.66) |
| No escitalopram use | 2.45 (1.93–3.11)** | 2.61 (2.04–3.34)** | 2.43 (1.86–3.18)** | 1.53 (1.15–2.03)* | |
| Fluoxetine use | 236 | — | — | — | — |
| No fluoxetine use | 2.51 (1.99–3.16)** | 2.65 (2.09–3.37)** | 2.51 (1.94–3.25)** | 1.55 (1.18–2.03)* | |
| Mirtazapine use | 113 | 2.96 (0.374–23.38) | 4.16 (0.456–37.96) | 4.10 (0.408–41.24) | 1.29 (0.120–13.89) |
| No mirtazapine use | 2.31 (1.83–2.93)** | 2.52 (1.92–3.21)** | 2.39 (1.83–3.11)** | 1.54 (1.17–2.04)* | |
| Paroxetine use | 152 | 1.78 (0.213–14.90) | 1.43 (0.156–13.01) | 1.21 (0.127–11.59) | 1.01 (0.097–10.43) |
| No paroxetine use | 2.40 (1.90–3.03)** | 2.57 (2.02–3.27)** | 2.41 (1.85–3.13)** | 1.48 (1.12–1.95)* | |
| Sertraline use | 355 | 1.50 (0.417–5.38) | 2.61 (0.551–12.33) | 3.97 (0.461–34.13) | 2.26 (0.254–20.13) |
| No sertraline use | 2.42 (1.91–3.07)** | 2.57 (2.01–3.28)** | 2.50 (1.92–3.25)** | 1.56 (1.18–2.05)* | |
| Trazodone use | 257 | 1.40 (0.483–4.06) | 1.95 (0.579–6.57) | 1.82 (0.517–6.42) | 1.09 (0.282–4.20) |
| No trazodone use | 2.42 (1.91–3.06)** | 2.59 (2.02–3.30)** | 2.47 (1.89–3.31)** | 1.54 (1.17–2.03)* |
indicates sample size too small for analysis.
Adjusted for sex, age, education, and race.
Statistical significance at p < 0.05.
p < 0.001.
Depression occurring more than 2 years prior to baseline
There was a statistically significant relationship between lifetime depression (p < 0.05) and eventual AD development (HR = 1.34 [95% CI = 1.04–1.73]). There was no longer a significant association between lifetime depression and AD accounting for the use of dementia medication (HR = 1.12 [95% CI = 0.843–1.49]). When examining the broad categorization of antidepressant medication, there was not a statistically significant hazard of AD development for either users or non-users. When individual generic medications were examined, users of the generic drugs did not experience a statistically significant hazard of AD development as compared with non-users (p < 0.05), with the exception of citalopram. A notable result emerged in the examination of sertraline use among participants experiencing lifetime depression. When the hazard of lifetime depression was adjusted for demographic factors and again for ε4 carrier status, sertraline exerted a protective effect for users. A statistically significant hazard (p < 0.001) of AD development remained for sertraline non-users. The main effects for those reporting lifetime depression are displayed in Table 3.
Table 3. Hazard ratios of probable Alzheimer's disease development among participants reporting depression more than 2 years prior “lifetime” but not at baseline.
| Predictor variables | Analytic sample size | Depression—lifetime Main effects (unadjusted) Hazard ratio (95% CI) | Depression—lifetime Main effects adjusteda without ε4 carrier status Hazard ratio (95% CI) | Depression—lifetime Main effects adjusted with ε4 carrier status Hazard ratio (95% CI) | Depression—lifetime Main effects adjusted with ε4 carrier status and AD medication use Hazard ratio (95% CI) |
|---|---|---|---|---|---|
| Lifetime depression | 2068 | 1.34 (1.04–1.73)* | 1.53 (1.18–1.99)** | 1.51 (1.14–2.00)* | 1.12 (0.843–1.49) |
| Use of antidepressant | 0.649 (0.441–0.956) | 0.798 (0.545–1.19) | 0.803 (0.524–1.23) | 0.734 (0.475–1.14) | |
| Did not use antidepressant | 1.26 (0.875–1.81) | 1.32 (0.905–1.92) | 1.29 (0.860–1.95) | 1.24 (0.822–1.86) | |
| Bupropion use | 292 | 1.03 (0.272–3.93) | 0.961 (0.225–4.11) | 1.38 (0.226–8.56) | 1.06 (0.151–7.39) |
| No bupropion use | 1.32 (1.01–1.71)* | 1.46 (1.11–1.92)* | 1.44 (1.08–1.93)* | 1.08 (0.806–1.46) | |
| Citalopram use | 358 | 0.950 (0.452–2.00) | 1.29 (0.573–2.92) | 1.69 (0.682–4.17) | .964 (0.389–2.39) |
| No citalopram use | 1.20 (0.907–1.58) | 1.34 (1.00–1.79)* | 1.29 (0.948–1.77) | 1.04 (0.760–1.42) | |
| Escitalopram use | 279 | 0.275 (0.087–0.868) | 0.270 (0.070–1.04) | 0.296 (0.074–1.18) | 0.483 (0.870–2.68) |
| No escitalopram use | 1.40 (1.08–1.82)* | 1.60 (1.22–2.09)** | 1.58 (1.18–2.10)* | 1.16 (0.867–1.56) | |
| Fluoxetine use | 237 | 1.64 (0.139–19.32) | 5.20 (0.112–240.99) | — | — |
| No fluoxetine use | 1.38 (1.07–1.79)* | 1.56 (1.19–2.03)** | 1.55 (1.17–2.05)* | 1.15 (0.864–1.53) | |
| Mirtazapine use | 112 | 0.221 (0.044–1.10) | 0.243 (0.043–1.38) | 0.322 (0.051–2.02) | 0.554 (0.070–4.39) |
| No mirtazapine use | 1.34 (1.04–1.74)* | 1.56 (1.20–2.04)** | 1.54 (1.16–2.05)* | 1.17 (0.872–1.56) | |
| Paroxetine use | 153 | 0.785 (0.170–3.62) | 0.746 (0.157–3.55) | 1.65 (0.269–10.13) | 1.89 (0.296–12.09) |
| No paroxetine use | 1.33 (1.03–1.72)* | 1.52 (1.16–1.99)* | 1.48 (1.11–1.98)* | 1.09 (0.815–1.46) | |
| Sertraline use | 354 | 0.294 (0.092–0.938) | 0.157 (0.037–0.671)* | 0.174 (0.031–0.972)* | 0.133 (0.016–1.12) |
| No sertraline use | 1.41 (1.09–1.83)* | 1.61 (1.23–2.10)** | 1.62 (1.22–2.16)** | 1.23 (0.921–1.64) | |
| Trazodone use | 256 | 0.650 (0.212–1.99) | 0.810 (0.215–3.06 | 1.23 (0.292–5.16) | 0.423 (0.076–2.36) |
| No trazodone use | 1.35 (1.04–1.76)* | 1.54 (1.18–2.02)** | 1.51 (1.13–2.01)* | 1.14 (0.850–1.52) |
indicates sample size too small for analysis.
Adjusted for sex, age, education, and race.
Statistical significance at p < 0.05.
p < 0.001.
APOE ε4 carrier status
There was a statistically significant relationship between ε4 carrier status (p < 0.001) and eventual AD development, which persisted despite adjustment for demographic factors and dementia medication use. When individual generic medications were examined, there was a non-significant relationship between ε4 carriers and AD development as compared with those of non-users (p < 0.05). This remained true except for users of citalopram and paroxetine, who did not experience the modification of the hazard. Unlike participants experiencing recent and lifetime depression, the use of bupropion, escitalopram, mirtazapine, sertraline, and trazodone remained statistically significant (p < 0.001) for ε4 carriers, even when adjusted for AD medication as a confounder. The main effects by APOE carrier status are displayed in Table 4.
Table 4. Hazard ratios of probable Alzheimer's disease development among ε4 carriers.
| Predictor variables | Analytic sample size | ε4 carrier Main effects (unadjusted) Hazard ratio (95% CI) | ε4 carrier Main effects adjusteda Hazard ratio (95% CI) | ε4 carrier Main effects adjusted with AD medication use Hazard ratio (95% CI) |
|---|---|---|---|---|
| ε4 carrier | 2470 | 2.07 (1.63–2.64)** | 2.77 (2.16–3.56)** | 2.19 (1.70–2.82)** |
| Use of an antidepressant | 1713 | 2.15 (1.43–3.24)** | 2.77 (1.80–4.25)** | 1.99 (1.29–3.08)* |
| Did not use antidepressant | 1.93 (1.43–2.61)** | 2.55 (1.86–3.48)** | 2.24 (1.64–3.07)** | |
| Used bupropion | 241 | 1.69 (0.453–6.33) | 2.41 (0.465–12.50) | 2.63 (0.394–17.51) |
| Did not use bupropion | 2.08 (1.63–2.66)** | 2.77 (2.15–3.58)** | 2.22 (1.71–2.87)** | |
| Used citalopram | 304 | 3.89 (1.76–8.60)** | 5.10 (2.21–11.80)** | 2.79 (1.14–6.80)* |
| Did not use citalopram | 1.94 1.50–2.50)** | 2.62 (2.01–3.41)** | 2.13 (1.63–2.78)** | |
| Used escitalopram | 233 | 1.44 (0.537–3.84) | 1.93 (0.669–5.58) | 0.631 (0.194–2.05) |
| Did not use escitalopram | 2.10 (1.64–2.70)** | 2.79 (2.16–3.62)** | 2.26 (1.74–2.93)** | |
| Used fluoxetine | 189 | 1.55 (0.097–24.78) | — | — |
| Did not use fluoxetine | 2.08 (1.63–2.65)** | 2.78 (2.16–3.58)** | 2.19 (1.70–2.82)** | |
| Used mirtazapine | 98 | 0.576 (0.136–2.43) | 0.877 (0.122–6.30) | 6.09 (0.331–112.26) |
| Did not use mirtazapine | 2.12 (1.66–2.71)** | 2.81 (2.1–3.63)** | 2.17 (1.68–2.81)** | |
| Used paroxetine | 125 | 4.76 (1.04–21.83)* | 5.66 (1.10–29.14)* | 6.21 (1.14–33.91)* |
| Did not use paroxetine | 2.04 (1.60–2.60)** | 2.73 (2.12–3.53)** | 2.14 (1.66–2.77)** | |
| Used sertraline | 296 | 0.694 (0.183–2.62) | 0.886 (0.196–4.00) | 0.961 (0.194–4.75) |
| Did not use sertraline | 2.16 (1.69–2.77)** | 2.88 (2.23–3.72)** | 2.34 (1.80–3.02)** | |
| Used trazodone | 217 | 2.30 (0.737–7.16) | 3.98 (1.03–15.33)* | 3.29 (0.694–15.59) |
| Did not use trazodone | 2.06 (1.61–2.64)** | 2.72 (2.11–3.52)** | 2.18 (1.68–2.82)** |
indicates sample size too small for analysis.
Adjusted for sex, age, education, and race.
Statistical significance at p < 0.05.
p < 0.001.
Additive effects
The hazard of AD development among ε4 carriers reporting recent depression was substantially non-significant for users of bupropion, escitalopram, paroxetine, sertraline, and trazodone. Notably, non-users of these medications with the same carrier status and depression symptoms experienced a statistically significant (p < 0.001) association with subsequent AD development. This association continued despite adjustment for the use of AD medication. The additive effects for ε4 carriers reporting depression in the last 2 years are displayed in Table 5.
Table 5. Additive hazard ratios of probable Alzheimer's disease development among ε4 carriers reporting depression within the last 2 years of baseline.
| Predictor variables | Sample size with AD endpoint | Depression—last 2 years × ε4 Main effects (unadjusted) Hazard ratio (95% CI) | Depression—last 2 years × ε4 Main effects adjusteda Hazard ratio (95% CI) | Depression last 2 years Main effects adjusted with AD medication use Hazard ratio (95% CI) |
|---|---|---|---|---|
| Depression—last 2 years × ε4 carrier | 455 | 4.65 (3.24–6.68)** | 6.41 (4.43–9.29)** | 3.03 (2.06–4.47)** |
| Use of antidepressant | 90 | 4.24 (1.95–9.23)** | 6.90 (3.02–15.80)** | 3.19 (1.37–7.43)* |
| Did not use antidepressant | 3.26 (1.81–5.86)** | 3.93 (2.14–7.24)** | 2.85 (1.54–5.29)** | |
| Bupropion use | 9 | 1.68 (0.186–15.16) | 4.21 (0.368–48.12) | 3.76 (0.233–60.71) |
| No bupropion use | 4.70 (3.23–6.83)** | 6.30 (4.29–9.24)** | 3.05 (2.04–4.56)** | |
| Citalopram use | 25 | 8.77 (1.14–67.28)* | 14.78 (1.87–116.63)* | 6.84 (0.806–58.08) |
| No citalopram use | 3.67 (2.44–5.54)** | 5.02 (3.30–7.65)** | 2.57 (1.66–3.98)** | |
| Escitalopram use | 17 | 0.841 (0.166–4.27) | 1.51 (0.290–7.87) | 0.339 (0.050–2.28) |
| No escitalopram use | 4.79 (3.30–6.96)** | 6.52 (4.45–9.55)** | 3.22 (2.16–4.79)** | |
| Fluoxetine use | 2 | — | — | — |
| No fluoxetine use | 4.84 (3.36–6.97)** | 6.55 (4.51–9.51)** | 3.08 (2.08–4.55)** | |
| Mirtazapine use | 9 | — | — | — |
| No mirtazapine use | 4.63 (3.21–6.69)** | 6.40 (4.39–9.33)** | 3.01 (2.03–4.46)** | |
| Paroxetine use | 7 | 4.44 (0.480–41.01) | 3.68 (0.316–42.83) | 2.99 (0.245–36.32) |
| No paroxetine use | 4.38 (3.01–6.37)** | 6.08 (4.14–8.97)** | 2.83 (1.90–4.22)** | |
| Sertraline use | 10 | 1.58 (0.143–17.49) | — | — |
| No sertraline use | 4.98 (3.44–7.20)** | 6.97 (4.78–10.17)** | 3.42 (2.31–5.07)** | |
| Trazodone use | 12 | 5.05 (0.457–55.90) | 9.63 (0.819–113.21) | 4.62 (0.343–62.18) |
| No trazodone use | 4.66 (3.22–6.73)** | 6.39 (4.38–9.32)** | 3.07 (2.07–4.56)** |
indicates sample size too small for analysis.
Adjusted for sex, age, education, and race.
Statistical significance at p < 0.05.
p < 0.001.
The additive effects model for lifetime depression by ε4 carrier status did not sustain the protective effect for sertraline. The hazard of AD development among ε4 carriers reporting lifetime depression was no longer statistically significant for the general antidepressant use category as well as bupropion, fluoxetine, mirtazapine, paroxetine, and trazodone. Notably, non-users of these medications with the same carrier status and depression symptoms experienced a statistically significant (p < 0.05) association with eventual AD development. The additive effects for ε4 carriers reporting lifetime depression are displayed in Table 6.
Table 6. Hazard ratios of probable Alzheimer's disease development among ε4 carriers reporting depression more than 2 years prior “lifetime” but without symptoms at baseline.
| Predictor variables | Sample size with AD endpoint | Depression—lifetime × ε4 Main effects (unadjusted) Hazard ratio (95% CI) | Depression—lifetime × ε4 Main effects adjusteda Hazard ratio (95% CI) | Depression—lifetime × ε4 Main effects adjusted with AD medication use Hazard ratio (95% CI) |
|---|---|---|---|---|
| Lifetime depression × ε4 | 445 | 2.35 (1.52–3.61)** | 3.65 (2.35–5.66)** | 2.03 (1.30–3.16)* |
| Use of antidepressant | 92 | 1.41 (0.740–2.67) | 2.17 (1.12–4.21)* | 1.39 (0.715–2.70) |
| Did not use antidepressant | 1.76 (0.884–3.52) | 2.70 (1.34–5.41)* | 2.23 (1.11–4.48)* | |
| Bupropion use | 9 | 2.27 (0.235–21.94) | 3.46 (0.252–47.56) | 2.70 (0.148–49.44) |
| No bupropion use | 2.28 (1.44–3.59)** | 3.48 (2.19–5.52)** | 1.99 (1.25–3.18)* | |
| Citalopram use | 26 | 4.75 (1.28–17.62)* | 9.31 (2.33–37.24)* | 3.44 (0.794–14.92) |
| No citalopram use | 1.70 (1.01–2.84)* | 2.63 (1.56–4.43)** | 1.66 (0.980–2.82) | |
| Escitalopram use | 17 | — | — | — |
| No escitalopram use | 2.60 (1.68–4.02)** | 4.03 (2.59–6.27)** | 2.67 (1.45–3.55)** | |
| Fluoxetine use | 2 | 2.00 (0.125–31.98) | — | — |
| No fluoxetine use | 2.40 (1.54–3.72)** | 3.69 (2.36–5.76)** | 2.05 (1.30–3.22)* | |
| Mirtazapine use | 9 | 0.106 (0.007–1.59) | 0.321 (0.019–5.40) | 3.33 (0.062–179.51) |
| No mirtazapine use | 2.41 (1.55–3.75)** | 3.80 (2.43–5.95)** | 2.06 (1.31–3.25)* | |
| Paroxetine use | 7 | 6.11 (0.494–75.64) | 10.18 (0.675–153.47) | 12.09 (0.865–169.02) |
| No paroxetine use | 2.21 (1.41–3.46)** | 3.46 (2.20–5.45)** | 1.88 (1.19–2.98)* | |
| Sertraline use | 9 | — | — | — |
| No sertraline use | 2.66 (1.72–4.11)** | 4.27 (2.74–6.66)** | 2.50 (1.60–3.91)** | |
| Trazodone use | 12 | 1.51 (0.135–16.76) | 4.30 (0.266–69.45) | 1.11 (0.073–16.71) |
| No trazodone use | 2.36 (1.52–3.66)** | 3.61 (2.31–5.65)** | 2.03 (1.29–3.20)* |
indicates sample size too small for analysis.
Adjusted for sex, age, education, and race.
Statistical significance at p < 0.05.
p < 0.001.
Discussion
A number of studies examine the effect of antidepressant medications for depression secondary to AD (Peters et al., 2011; Rosenberg et al., 2012). The current study is unique in its focus on a baseline, cognitively intact sample with depression, APOE ε4, or both and sought to examine the hazard of eventual AD development for users and non-users of antidepressants.
Existing research provides mixed findings to better clarify the association between depression and AD. APOE ε4 carrier status has been linked to depression symptomology in a geriatric population with more severe depression symptoms (Skoog et al., 2015). Further, studies suggest that APOE ε4 may play a role in higher cortisol levels for some subjects (Peavy et al., 2007). Injury to the hippocampus is a known consequence of high cortisol levels, and this injury could occur as the outcome of recurrent stress and untreated depression, known to result in impairment to the hypothalamic–pituitary–adrenal (HPA) axis (Sheline et al., 2003). A consequence of such damage is decreased hippocampal volume (Arnone et al., 2013). The potential interaction between increased stress levels due to depression, and heightened cortisol levels due to APOE (and for some participants due to depression as well), may impact the HPA axis, which may lead to a higher hazard of AD. While this link between the HPA and APOE is under investigation, it offers a theoretical framework for understanding the potential mechanistic connection by which depression may lead to AD. Furthermore, ongoing investigations are examining whether disturbance in the characteristic regulation of the HPA axis is a contributory factor in the development of AD or a consequence of the underlying pathophysiological AD progression (Gil-Bea et al., 2010), which may better elucidate the role of depression as a risk factor for AD or as a prodromal symptom of AD.
Our findings reinforce the association between depression, APOE ε4, and AD development among a group of cognitively asymptomatic participants at baseline. These findings suggest that a mechanism related to antidepressant use may reduce or neutralize the hazard of eventual AD outcomes, given that this hazard is no longer significant among antidepressant users.
Several strengths to this study exist, including the examination of individual generic antidepressant medication. These medications were most often SSRIs, although an atypical of the aminoketone class was included (bupropion), as well as a tetracyclic of the serotonin antagonist and reuptake inhibitor class (trazodone).
A limitation of this study is that the UDS is not a nationally representative sample of the US population; participation is voluntary. Participants may be required by individual ADCs to agree to post-mortem analysis before acceptance into the study. Participation may be affected by this requirement. Additionally, forward causal inferences cannot be made in the NACC dataset. Reverse causation is a challenge in terms of determining whether a predictor is an early symptom of AD or an actual risk factor. For example, psychiatric symptoms may be an early sign of underlying pathophysiological degeneration or an existing mental health concern that is a risk factor for development of AD. Owing to the nature of secondary analysis, there are potentially confounding factors that were not measured in the initial data collection and cannot be characterized herein. For instance, we used the variable “depression in the past 2 years” to signify current depression episodes, but we used more “depression more than 2 years ago” to provide some historical aspect to the depression measures, though this is not a precise proxy. The use of these two categorical variables has limitations. For instance, a participant may have experienced depressive episodes in the past 10 years, but not in the past 2 years. The proper classification would be a remission, but this type of categorization was not possible in this dataset. A remission would indicate a different level of intensity or persistence in the depressive condition, which is deserving of study. Some participants may experience recurrent symptoms with multiple remitting episodes. It is difficult to distinguish lifetime depressive episode cases from those individuals with symptoms occurring in the past two years in this dataset. It is even more difficult to distinguish, if not impossible to identify those those with relapsing symptoms. This is an issue that can only be resolved through revision of the data collection instruments to collect more detailed depression history information. Participant adherence to antidepressant medication regiments was not measured and cannot be characterized in this cohort. Participants were assumed to be prescribed with and taking antidepressants for the treatment of depression. Other uses for medications besides their intended use were not measured. Similarly, other comorbidities may exist beyond the duality of depression and dementia, but we chose to limit our study to these two conditions. The history of depression symptoms or antidepressant use may play a role in the likelihood of dementia, but we are unable to assess this causal association without additional historical information.
Although further study is needed, this exploratory investigation reinforces the notion that a delay in AD diagnosis by remediation of a risk factor may be one way to confront the startling rate at which this disease progresses. Further research should be initiated regarding the role of antidepressant medication in possible delay in AD dementia development and clarify the ability of antidepressants to delay time to dementia diagnosis among participants at different points in the cognitive decline trajectory. This study contributes to the emerging literature by exploring interventions aimed at decreasing the risk of AD by using potentially modifiable psychosocial risk factors such as depression.
Key points.
The current study is unique in its focus on a baseline, cognitively intact sample with depression, APOE ε4, or both, and sought to examine the hazard of eventual AD development for users and non-users of antidepressants.
Our findings reinforce the association between depression, APOE ε4, and AD development among a group of cognitively asymptomatic participants at baseline. These findings suggest that a mechanism related to antidepressant use may reduce or neutralize the hazard of eventual AD outcomes, given that this hazard is no longer significant among antidepressant users.
Acknowledgments
The Simmons College Student Research Fund provided a portion of funding for this project.
The NACC database is funded by NIA/NIH grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (principal investigator [PI] Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI Marie-Francoise Chesselet, MD, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Footnotes
Conflict of interest: None declared.
References
- Alzheimer's Disease International. 2015 World Alzheimer Report. 2015 https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Arnone D, McKie S, Elliott R, et al. State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry. 2013;18:1265–1272. doi: 10.1038/mp.2012.150. https://doi.org/10.1038/mp.2012.150. [DOI] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer's Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Disease & Associated Disorders. 2004;18:270–277. [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF, Petersen RC, et al. The apolipoprotein E genotype predicts longitudinal transitions to mild cognitive impairment but not to Alzheimer's dementia: findings from a nationally representative study. Neuropsychology. 2013;27:86–94. doi: 10.1037/a0030855. https://doi.org/10.1037/a0030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SL, Maramaldi P, Cadet T, Kukull W. Associations between depression, sleep disturbance, and apolipoprotein E in the development of Alzheimer's disease: dementia. Int Psychogeriatr. 2016a;28:1409–1424. doi: 10.1017/S1041610216000405. https://doi.org/10.1017/S1041610216000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SL, Maramaldi P, Cadet T, Kukull W. Neuropsychiatric symptoms and apolipoprotein E: associations with eventual Alzheimer's disease development. Arch Gerontol Geriatr. 2016b;65:231–238. doi: 10.1016/j.archger.2016.04.006. https://doi.org/10.1016/j.archger.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraci F, Copani A, Nicoletti F, Drago F. Depression and Alzheimer's disease: neurobiological links and common pharmacological targets. European Journal of Pharmacology, Cognition Disturbances in Psychiatry: Neurobiology and Treatment. 2010;626:64–71. doi: 10.1016/j.ejphar.2009.10.022. https://doi.org/10.1016/j.ejphar.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3:89r57–89ra57. doi: 10.1126/scitranslmed.3002156. https://doi.org/10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Disabato BM, Restivo JL, et al. Serotonin signaling is associated with lower amyloid- levels and plaques in transgenic mice and humans. Proc Natl Acad Sci. 2011;108:14968–14973. doi: 10.1073/pnas.1107411108. https://doi.org/10.1073/pnas.1107411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. J R Stat Soc B Methodol. 1972;34:187–220. [Google Scholar]
- de Oliveira FF, Bertolucci PHF, Chen ES, Smith MC. Assessment of risk factors for earlier onset of sporadic Alzheimer's disease dementia. Neurol India. 2014;62:625–630. doi: 10.4103/0028-3886.149384. https://doi.org/10.4103/0028-3886.149384. [DOI] [PubMed] [Google Scholar]
- Gil-Bea FJ, Aisa B, Solomon A, et al. HPA axis dysregulation associated to apolipoprotein E4 genotype in Alzheimer's disease. J Alzheimers Dis. 2010;22:829–838. doi: 10.3233/JAD-2010-100663. https://doi.org/10.3233/JAD-2010-100663. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. https://doi.org/10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Lee CD, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Aβ. Neuron. 2008;58(5):681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbaum D, Klein M. Survival Analysis—A Self-learning Text. Third. Springer; New York, NY: 2012. [Google Scholar]
- Masters CL, Beyreuther K, Trillet M. Amyloid Protein Precursor in Development, Aging and Alzheimer's Disease. Springer Science & Business Media; Berlin: 2013. [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. https://doi.org/10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, D'Arcy C. Apolipoprotein E gene, environmental risk factors, and their interactions in dementia among seniors. Int J Geriatr Psychiatry. 2013;28:1005–1014. doi: 10.1002/gps.3918. https://doi.org/10.1002/gps.3918. [DOI] [PubMed] [Google Scholar]
- Peavy GM, Lange KL, Salmon DP, et al. The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biol Psychiatry. 2007;62:472–478. doi: 10.1016/j.biopsych.2007.03.013. https://doi.org/10.1016/j.biopsych.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters ME, Vaidya V, Drye LT, et al. DIADS-2 Research Group. Sertraline for the treatment of depression in Alzheimer disease: genetic influences. J Geriatr Psychiatry Neurol. 2011;24:222–228. doi: 10.1177/0891988711422527. https://doi.org/10.1177/0891988711422527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H, Wisniewski T. Apolipoprotein E: essential catalyst of the Alzheimer amyloid cascade. International Journal of Alzheimer's Disease. 2012;2012 doi: 10.1155/2012/489428. https://doi.org/10.1155/2012/489428.e489428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L, Brody D, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005–2008. Centers for Disease Control and Prevention National Center for Health Statistics; Atlanta, GA: 2011. Data Brief No. 76. [Google Scholar]
- Rosenberg PB, Mielke MM, Han D, et al. The association of psychotropic medication use with the cognitive, functional, and neuropsychiatric trajectory of Alzheimer's disease. Int J Geriatr Psychiatry. 2012;27:1248–1257. doi: 10.1002/gps.3769. https://doi.org/10.1002/gps.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. https://doi.org/10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Skoog I, Waern M, Duberstein P, et al. A 9-year prospective population-based study on the association between the APOE*E4 allele and late-life depression in Sweden. Biol Psychiatry. 2015;78:730–736. doi: 10.1016/j.biopsych.2015.01.006. https://doi.org/10.1016/j.biopsych.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. https://doi.org/10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 14. StataCorp; College Station, TX: 2015. [Google Scholar]
- VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiologic Methods. 2014;3 https://doi.org/10.1515/em-2013-0005. [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. https://doi.org/10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. https://doi.org/10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]