Abstract
Introduction
Myofiber type grouping is a histological hallmark of age-related motor unit remodeling. Despite the accepted concept that denervation-reinnervation events lead to myofiber type grouping, the completeness of those conversions remains unknown.
Methods
Type I myofiber grouping was assessed in vastus lateralis biopsies from Young (26 ± 4 yrs, n = 27) and Older (66 ± 4 yrs, n = 91) adults. Grouped and ungrouped type I myofibers were evaluated for phenotypic differences.
Results
Higher type I grouping in Older vs. Young was driven by more myofibers per group (i.e. larger group size) (p < 0.05). In Older only, grouped type I myofibers displayed larger cross-sectional area, more myonuclei, lower capillary supply, and more sarco(endo)plasmic reticulum calcium ATPase I (SERCA I) expression (p < 0.05) than ungrouped type I myofibers.
Discussion
Grouped type I myofibers retain type II characteristics implying that conversion during denervation-reinnervation events is either progressive or incomplete.
Keywords: myofiber type grouping, myofiber switching, denervation, reinnervation, motor unit
Introduction
Human aging is associated with gradual declines in skeletal muscle mass, a process commonly termed sarcopenia1. This aging muscle atrophy is predominantly attributed to a loss of total myofiber number consequent to central and/or peripheral denervation combined with type II myofiber atrophy 2–4. A denervated myofiber follows one of two fates: death, or reinnervation by the branching axon of a viable motor neuron nearby. The latter leads to motor unit reorganization with an increase in motor unit size 5–7, which is revealed histopathologically as abnormal grouping of myofibers expressing the same myosin heavy chain (MHC) isoform 8,9. Such myofiber type grouping, particularly type I grouping within the human vastus lateralis, has become a hallmark of motor unit reorganization during aging and neurogenic atrophy.
Although the notion that myofiber type grouping increases as humans age is widely accepted, there is not a consensus methodology to quantitatively assess the extent of myofiber grouping. Traditional methods to assess myofiber type grouping include runs, enclosed fibers, points-sommets, co-dispersion indices, and distance methods 10; while more recent methods include variations of the aforementioned techniques or arbitrary thresholds for contiguous fibers of the same type 11. Despite the numerous descriptions and derivations of myofiber type grouping, attempts to quantify the degree of grouping within a specimen are largely tests of non-randomness based on the assumption that the spatial distribution of myofiber types in healthy tissue is completely random 10. However, myofiber type distribution clearly influences the spatial arrangement of myofiber types in cross-section. For example, a biopsy specimen containing 60% type I myofibers will display more enclosed and contiguous type I myofibers by random chance than a specimen with only 30% type I myofibers. With this in mind, we developed a modified method to quantify the number and size of abnormal myofiber groups that takes into account myofiber type distribution, with the goal of more effectively comparing widely divergent specimens.
In addition to the current lack of quantitative data regarding the degree of type I myofiber grouping in aged human skeletal muscle, it remains completely unknown whether grouped and ungrouped type I myofibers are phenotypically the same. Although they both express the MHC I isoform, it has long been known that motor neuron impulse patterns determine myofiber MHC expression 12,13. Thus, MHC I expression may only be reflective of their low threshold motor unit innervation status. Since denervated myofibers can undergo reinnervation by both low (innervate type I myofibers) and high (innervate type II myofibers) threshold neighboring motor neurons 14, a portion of grouped MHC type I myofibers will have experienced a switch from a high threshold type II motor unit to a low threshold type I motor unit. In such cases it is currently not known if the denervation-reinnervation process only changes the MHC isoform expression or results in a complete phenotypic myofiber type switch.
Using our new model to identify grouped type I myofibers we quantified the effects of aging on abnormal type I myofiber grouping. Further, we performed a phenotypic analysis of grouped type I myofibers, using ungrouped type I and type II myofibers as comparators. We hypothesized that grouped type I myofibers, specifically in Older adults with pathological grouping, would display a phenotypic resemblance to type II myofibers, indicating incomplete myofiber type switching among grouped type I myofibers which were previously type II.
Materials and Methods
Human Subjects
All myofiber type grouping and phenotyping assessments were accomplished by utilizing available vastus lateralis muscle biopsy specimens collected from 20–35 (Young: 26 ± 4 yrs, n = 27; 12F, 15M) and 60–75 (Older: 66 ± 4 yrs, n = 91; 41F, 50M) year-old adults over two consecutive, 5-year award periods of R01AG017896. Participants were recruited from the Birmingham, Alabama metropolitan area and gave written, informed consent allowing their samples and data to be used for future research as approved by the University of Alabama at Birmingham (UAB) Institutional Review Board. All subjects completed health history and physical activity questionnaires, and older adults underwent a physical exam during screening. The young cohort was recruited only during the first 5-year period, while the older adults were recruited in both periods. Identical inclusion/exclusion criteria were used in recruitment of the older adults across the two periods. Briefly, individuals were excluded for: lidocaine allergy, prescription anticoagulants, exogenous testosterone or other pharmacological interventions thought to influence muscle mass, acute illness or active infection, chronic end-stage disease, uncontrolled hypertension, unstable or exercise-induced angina pectoris or myocardial ischemia, diabetes mellitus, musculoskeletal disorder or any known contraindication to exercise training or testing. Additionally, subjects who were currently adherent to a weight reduction diet, had a body mass index >30, or had performed regular resistance training during the previous three years were excluded.15,16
Skeletal Muscle Biopsy and Tissue Preparation
Muscle tissue samples were previously collected by percutaneous needle biopsy of the right vastus lateralis muscle under local anesthesia (1% lidocaine) using a 5-mm Bergstrom-type biopsy needle with suction. Samples were prepared for immunohistochemistry as previously described 17. Biopsies were performed in the Clinical Research Unit of the UAB Center for Clinical and Translational Science.
Immunohistochemistry and Type I Myofiber Grouping
We quantified the prevalence of type I grouping after first determining myofiber type distribution (I, IIa, IIx) via MHC isoform immunofluorescence methods routinely used in our laboratory17,18 with the following antibodies (Ab): Mouse monoclonal primary Ab – anti-MHC I (Leica NCL-MHCs, 1:100 (w/v)), anti-MHC IIa (DSHB A4.74, 1:80 (w/v)), anti-laminin (Sigma L-8271, 1:40 (w/v)) all in 1% goat serum; goat anti-mouse secondary Ab – Alexa Fluor 594 (ThermoFisher A-11005, 1:200), Alexa Fluor 488 (ThermoFisher A-11001, 1:200) both in 1% goat serum, and Hoechst DNA counterstain (AnaSpec Inc, Cat #83219, 1:200,000). 10X microscopic images were captured in a grid format and stitched together using Image-Pro Plus (Media Cybernetics, Inc.) software to render one seamless image of the entire cross-section of the fiber typed specimen. Within each sample, all cross-sectionally oriented myofibers were included in the analyses of myofiber type distribution and type I grouping prevalence. The average numbers of myofibers per sample were: Young (1333 ± 86) and Older (1643 ± 81). To determine the degree of abnormal type I myofiber grouping, we developed the following quantitative method. First, we found the expected mean (M) number of like myofibers touching a given type I myofiber by multiplying the total number (n) of myofibers touching a given myofiber by the type I myofiber percent distribution (p) as a decimal; such that M=np. We then determined the standard deviation (SD) around M, which equaled the square root of npq, where q is 1-p. To qualify as a myofiber group, the number of like myofibers touching a given type I myofiber (X) was required to be equal to or greater than M+SD for at least two contiguous myofibers in the core of a group; such that X1 ≥ M1+SD1 and X2 ≥ M2+SD2. This process ensured that the core myofibers of a group exceed the normal probability of adjacent like myofibers. Once two contiguous core myofibers were identified, all subsequent contiguous type I myofibers were counted as part of the group. Myofibers were not considered contiguous if separated by clearly visible fascicle borders (i.e. perimysium).
By factoring type I distribution into the determination of core fibers, this new approach appropriately makes it more difficult to classify type I myofibers as “abnormally grouped” in samples with a high overall type I distribution; however, it does not completely eliminate the influence of myofiber type distribution. For example, with the approach used here, correlations between type I distribution and the amount of type I grouping are modest but statistically significant (R2 = 0.58 overall, and R2 = 0.35 for the subset of Older with < 50% type I grouping). Thus, across a wide range between subjects, individual percent type I distribution may account for up to 58% of the variance in type I grouping prevalence.
We also performed immunofluorescent detection of myonuclei, satellite cells, capillaries, and sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression. All immunohistochemistry was performed on fresh-frozen 6μm thick serial cryo-sections and each stain included detection of MHC I. Unless otherwise noted, all primary and secondary antibody dilutions were made in 1% goat serum and were incubated at 37°C for 30 min, all blocking steps were performed for 20 min at room temperature prior to primary antibody incubations and consisted of 5% goat serum, and each step was separated by a 3 X 5 min 1X phosphate-buffered saline (PBS) wash. Upon completion of each stain, tissue sections were dried, mounted, cover slipped and stored at −20°C until analysis.
To detect myofiber type-specific satellite cells and myonuclei, sections were first fixed in ice cold acetone for 3 min at room temperature then washed 3 × 3 min in 1X PBS and incubated in 3% H2O2 (in 1X PBS) for 10 min at room temperature. Following another PBS wash, samples were blocked in 2.5% horse serum for 1 hr at room temperature and incubated overnight in paired box 7 (PAX7) primary antibody (1:100 in 2.5% horse serum; Developmental Studies Hybridoma Bank) at 4°C. Samples were then incubated in a biotinylated goat-anti-mouse (GAM) secondary (1:1000 in 2.5% horse serum; Jackson Labs, 115-065-003) for 1 hr at room temperature, washed, and incubated in streptavidin horse radish peroxidase (1:100 in 1X PBS; ThermoFIsher T20932) for 1 hr at room temperature. Lastly, samples were washed, incubated in tyramide conjugated Alexa Fluor 488 (1:200 in amplification buffer; ThermoFisher T20932) for 20 min at room temperature, and an anti-MHC I/laminin immunostain and Hoechst DNA counterstain were performed as described previously 17,19.
Myofiber type-specific capillaries were visualized by incubating cryo-sections in FITC-conjugated Ulex europaeus agglutinin (UEA-I; 1:100 in 1X PBS; Sigma-Adrich L9006) at room temperature for 45 min without a previous fixative step. Next, the sections were blocked and subsequently incubated in anti-MHC I primary (1:100; Leica NCL-MHCs) and Alexa Fluor 594-GAM secondary (1:200; Life Technologies A-11005) for visualization of MHC I+ myofibers. Following another block, the sections were incubated in anti-laminin primary (1:40; Sigma-Aldrich L8271), and then Alexa Fluor 594-GAM secondary (1:200) for visualization of myofiber membrane borders.
MHC I and SERCA I co-expression was detected by fixing sections in 3% formalin for 30 min at room temperature, washing, blocking, and then incubating sequentially in anti-MHC I primary (1:100), Alexa Fluor 594-GAM secondary (1:200), anti-laminin primary (1:40), Alexa Fluor 594-GAM (1:200), anti-SERCA 1 primary (1:250; Santa Cruz sc-58287), and Alexa Fluor 488-GAM (1:200; Thermo Fisher A-11001).
Statistical Analysis
All biopsy specimens were fiber typed, however, since the majority of specimens (Young: n = 27/27; Older: n =66/91) were obtained from previously completed clinical trials, sample sizes varied for each assessment based on sample availability. Sample sizes are as follows: myofiber cross-sectional area (CSA) (Young: n = 27 [12F, 15M]; Older: n = 66 [31F, 35M]), myonuclear counting/domain (Young: n = 16 [8F, 8M]; Older: n = 16 [8F, 8M]), PAX7+ satellite cell analysis (Young: n = 14 [7F, 7M]; Older: n = 16 [8F, 8M]), capillary counting (Young: n = 21 [8F, 13M]; Older: n = 36 [20F, 16M]), MHC I / SERCA 1 co-expression (Young: n = 16 [8F, 8M]; Older: n = 16 [8F, 8M]). Immunohistological differences between Young and Older subjects were compared via independent two-tailed t-tests. Within group myofiber population (grouped type I, ungrouped type I, and type II) differences were determined using one-way ANOVAs. Significant main effects were followed up by Fisher’s least significant difference tests post hoc. Ages of human subjects are reported as means ± SD; all other data are reported as means ± SE. For all tests, p ≤ 0.05 (two-tailed) was considered statistically significant.
Results
Age-related type I myofiber grouping
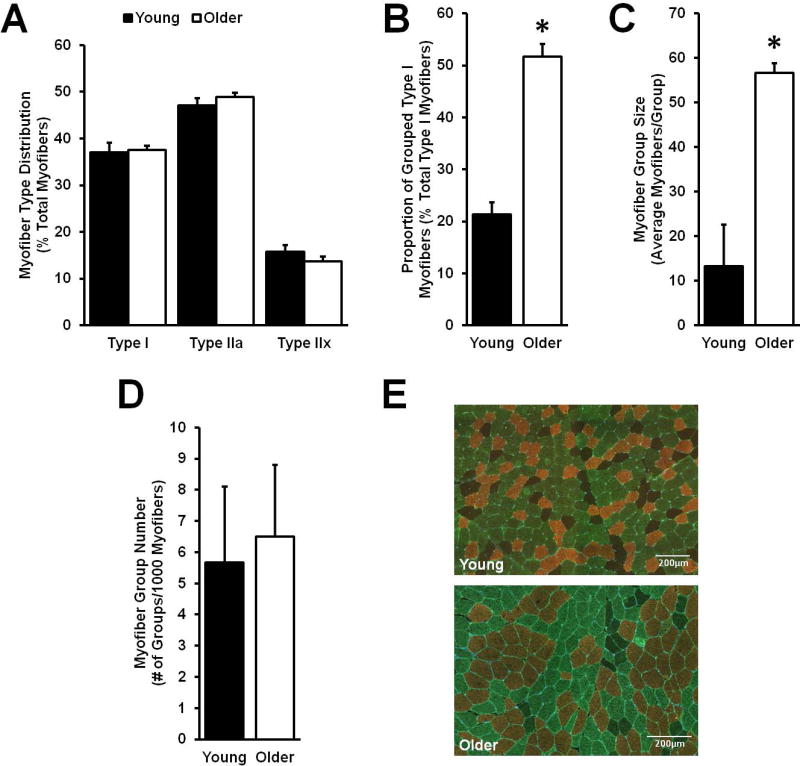
Normal human aging through the seventh decade of life does not appear to significantly alter myofiber type distribution (Fig. 1A). On the other hand, there is a spatial change in myofiber distribution with aging, resulting in heightened type I myofiber grouping (Fig. 1B). The age associated increase in type I myofiber grouping stems from an enlargement of the number of type I myofibers per group (i.e. group size) (Fig. 1C) rather than the number of abnormal myofiber groups (Fig. 1D).
Figure 1.
Quantitative and spatial distribution of aged type I myofibers. Although overall myofiber type distribution did not change with aging (A), the spatial distribution changed from a random assortment in Young to myofiber type I grouping in Older (B), driven by an increase in type I myofiber group size among Older (C). The number of type I myofiber groups was no different between Older and Young (D). Representative immunohistological images display myosin heavy chain (MHC) type I (copper/red), MHC IIa (green), and MHC IIx (black/negative) myofibers (E). Panels A-D (Older: n = 91; Young: n = 27). *Different from Young, p < 0.05. Values are means + SE.
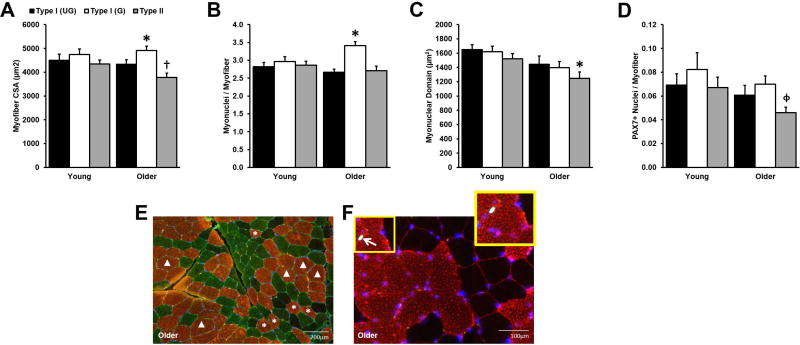
Grouped type I myofiber CSA, nuclei, and satellite cells
As expected, Older displayed type II myofiber atrophy (Fig. 2A); however, surprisingly Older grouped type I myofibers were on average 576 µm2 larger than their ungrouped counterparts (Fig. 2A). The enlarged CSA of Older grouped type I myofibers was apparently supported by myonuclear addition (Fig. 2B), which maintained a constant myonuclear domain among Older type I myofibers (Fig. 2C). Older type II myonuclear domain, however, was lower than Young, concomitant with the type II myofiber atrophy since type II myonuclear content did not differ by age. There were also no age-related differences in PAX7+ nuclei associated with any of the myofiber types, implying that the resident muscle stem cell population is preserved with age (Fig. 2D) and therefore capable of supporting myonuclear addition. There were no CSA, myonuclear, or satellite cell differences among any of the myofiber types in Young.
Figure 2.
Cross-sectional area (CSA) and myonuclear number among grouped (G) and ungrouped (UG) myosin heavy chain (MHC) type I myofibers. No differences between G and UG MHC I myofibers in Young. Older G myofibers were larger (A) and had more myonuclei (B) than UG. No differences in myonuclear domain (C) or PAX7+ satellite cell (SC) number (D) between G and UG myofibers. Type II myonuclear domain (C) was lower in Older vs. Young as expected with age-related type II atrophy and, in Older, more SCs surrounded G MHC I myofibers than type II (D). (E) Representative immunohistochemical image displays CSA differences in G (Δ) vs. UG (*) (MHC I, copper; MHC IIa, green; MHC IIx, black; laminin, green; myonuclei, blue). (F) Representative immunohistochemical image displays myonuclei (blue), SC (arrow), MHC I myofibers (red), and laminin (red). Sample sizes: A, Older: n = 64; Young: n = 20; B/C, Older: n = 16; Young: n = 16; D, Older: n = 16; Young: n = 14. *Different from within subjects UG, p < 0.05. †Different from Young type II, p < 0.05. ϕDifferent from within subjects G, p < 0.05. Values are means + SE.
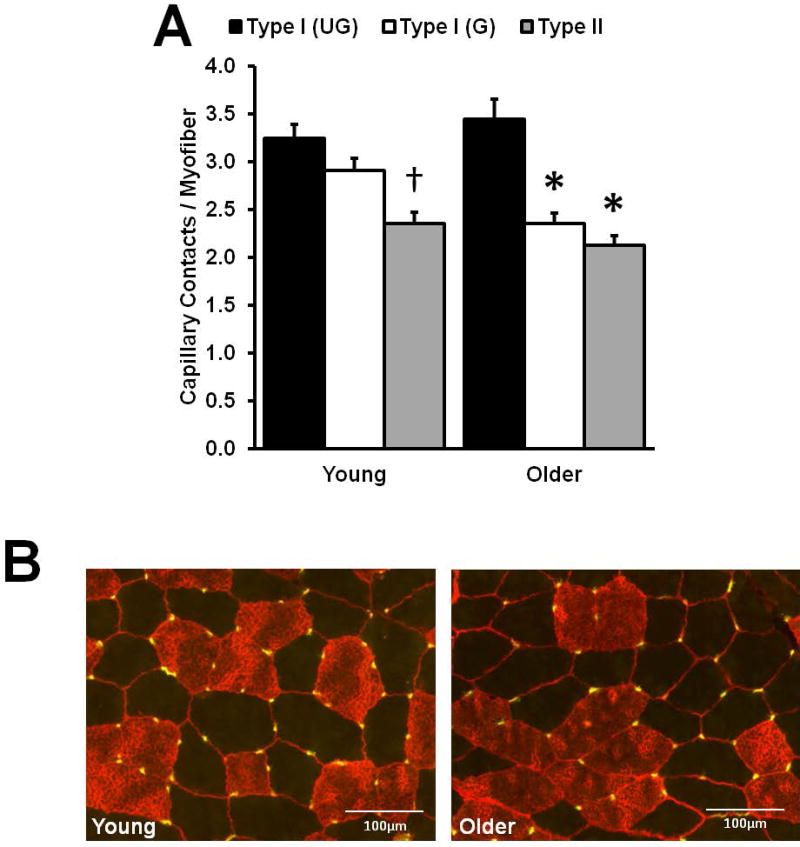
Grouped type I myofiber capillary supply
In Fig. 3A, Young displays the well-documented higher capillary supply of oxidative, fatigue-resistant type I myofibers compared to the lower capillary supply of type II myofibers. Grouped and ungrouped type I myofibers showed the same capillary supply in Young. On the other hand, among Older, the capillary supply of grouped type I myofibers was substantially lower than in ungrouped type I fibers, and resembled type II myofiber capillary supply.
Figure 3.
Capillary supply differences between grouped (G) and ungrouped (UG) myosin heavy chain (MHC) type I myofibers. (A) As expected, Young type II myofibers displayed a lower capillary supply compared to both G and UG MHC I myofibers. Among Older, G myofibers had a lower capillary supply vs. UG, and a supply similar to type II myofibers (Older: n = 36; Young: n = 21). (B) Representative immunohistological images display MHC I (copper/red) and MHC II (black/negative) myofibers, laminin (red), and capillaries (yellow). *Different from within subjects ungrouped type I, p < 0.05. †Different from Young grouped and ungrouped type I, p < 0.05. Values are means + SE.
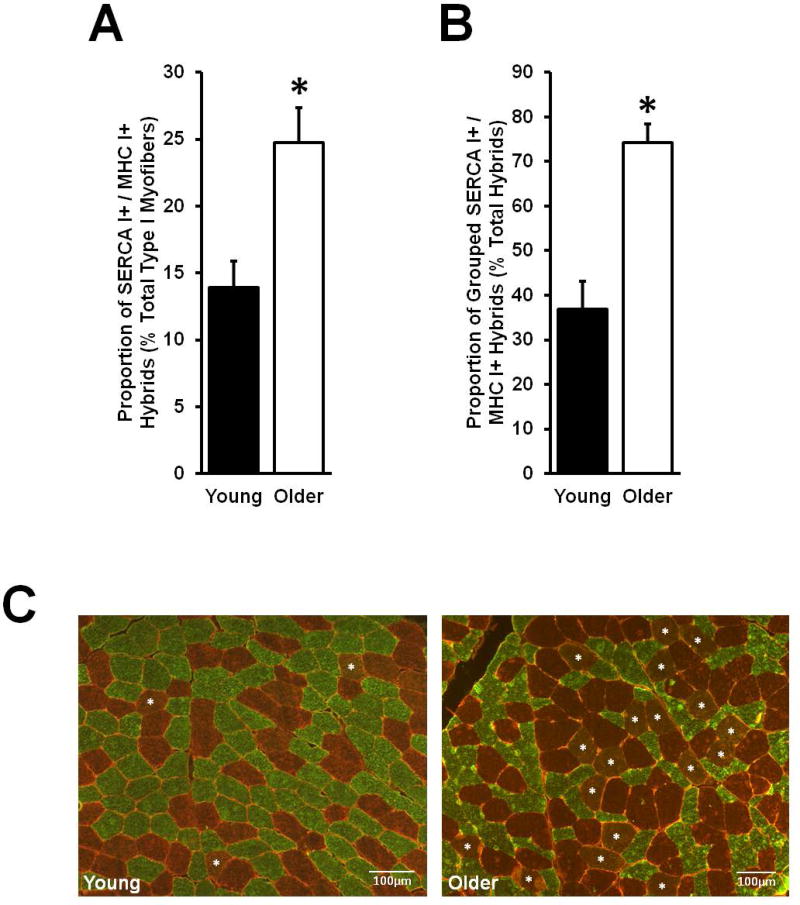
Grouped type I myofiber SERCA I expression
The proportion of hybrid myofibers expressing both SERCA I and MHC I was higher in Older compared to Young (Fig. 4A). The aging effect was driven by greater co-expression among grouped (Older: 19 ± 2 vs. Young: 6 ± 1%; p< 0.05) rather than ungrouped (Older: 5 ± 1% vs. Young: 8 ± 1%) MHC type I myofibers when normalized to total number of type I myofibers. Within the total pool of SERCA I+ / MHC I+ myofibers, nearly 75% of Older hybrids were located among grouped type I myofibers compared to just 37% in Young (Fig. 4B).
Figure 4.
Myosin heavy chain (MHC) I and sarco-endoplasmic reticulum calcium ATPase (SERCA) I hybrid co-expression. (A) The relative number of total MHC I+ / SERCA I+ hybrids was higher in Older compared to Young. (B) The proportion of hybrids which were located in MHC type I myofiber groups was far greater in Older than Young. (C) Representative immunohistological images display MHC I+ (copper/red) and SERCA I+ (green) myofibers, and laminin (red). Sample co-expressing, hybrid myofibers are tagged with a white asterisk. Sample size: Older: n = 16; Young: n = 16. *Different from Young, p < 0.05. Values are means + SE.
Grouped vs. ungrouped type I myofiber phenotype is associated with grouping prevalence
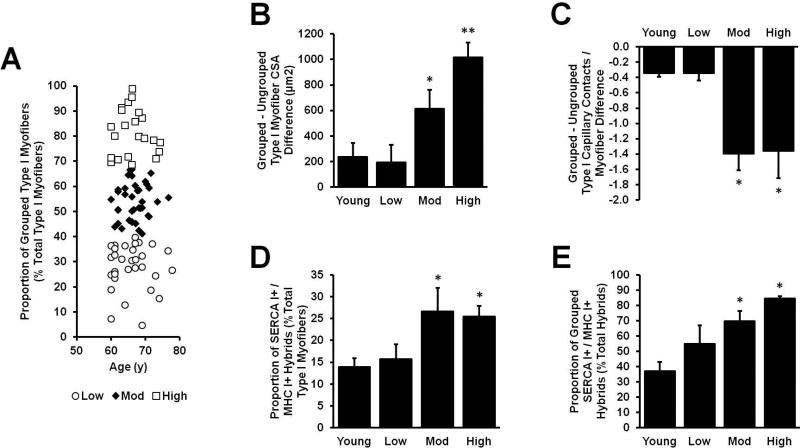
Despite the well-accepted notion that aging results in myofiber type grouping, the inter-individual heterogeneity in the proportion of type I myofibers grouped was quite large among Older individuals in this study. In an effort to explore phenotypic differences along the grouping prevalence spectrum, we applied a K-means cluster analysis to the Older data to derive three distinct clusters of individuals which displayed low (Low: n = 31; 14F, 17M), moderate (Mod: n = 38; 16F, 22M), and high (High: n = 22; 11F, 11M) levels of type I myofiber grouping (Fig. 5A). Using Young as a healthy reference, the Older type I grouped vs. ungrouped differences in CSA (Fig. 5B), capillary supply (Fig. 5C), and MHC I+ / SERCA I+ co-expression (Fig. 5D/E) were assessed. Interestingly, Low was no different than Young on any of the measures, whereas Mod and High grouped type I myofibers displayed similarly lower capillary supply and higher numbers of SERCA I+ / MHC I+ hybrids compared to both Young and Low. Grouped vs. ungrouped type I myofiber CSA disparities presented in a step-wise pattern with Mod displaying a greater difference than Young and Low, while High displayed the largest difference which was greater than Young, Low, and Mod.
Figure 5.
K-means cluster analysis of grouped (G) vs. ungrouped (UG) myosin heavy chain (MHC) type I myofiber differences in Older subjects. Since there was a large range of inter-individual variability, we separated Older into three clusters (Low, Mod, High) based on MHC type I myofiber grouping prevalence (A). The cross-sectional area (B) and capillary supply (C) differentials increased progressively as MHC type I myofiber grouping prevalence increased. Furthermore, there were more MHC I+ / SERCA I+ hybrid myofibers in Mod and High compared to Low and Young (D), and a higher proportion of those hybrids were found in type I myofiber groups (E). There were no differences between Low and Young. Sample sizes: A, Older: n = 91; B, Low: n = 22; Mod: n = 25; High: n = 17; Young: n = 20; C, Low: n = 10; Mod: n = 13; High: n = 13; Young: n = 21; D/E, Low: n = 2; Mod: n = 7; High: n = 7; Young: n = 16. *Different from Young/Low, p < 0.05. **Different from Young/Low/Mod, p < 0.05. Values are means + SE.
Discussion
The current work provides a quantitative assessment of type I myofiber grouping which confirms the expected aging-associated increase in grouping. Additionally, we have conducted a detailed phenotypic characterization of grouped type I myofibers in aged humans. The data indicate that grouped type I myofibers possess features of type II myofibers including recruitment-dependent hypertrophy and myonuclear addition, lower capillary supply, and SERCA I expression. Furthermore, the phenotypic differences between grouped and ungrouped type I myofibers increase as grouping prevalence increases. These findings strongly suggest myofiber type switching resulting from denervation-reinnervation is not complete, as several characteristics of the original myofiber type (II) appear to be retained.
Aging has long been associated with the appearance of myofiber type grouping in histopathological analysis 8,9,20,21. Based on our statistical model a small amount of type I grouping (~20%) is detected in Young healthy muscle and may be considered “normal”, particularly since there are no phenotypic differences between grouped and ungrouped type I myofibers in Young (i.e. not indicative of pathology). Therefore, results in Young here may serve as a valuable reference point in future studies of aging and disease. It is noteworthy that the age-related type I grouping found here was not associated with an aging difference in myofiber type distribution. Based on previously published data, similar type distribution between young adults and 60–75 year old adults is common.3,4,22,23 This implies that denervation-reinnervation and myofiber type conversion events are bidirectional (MHC type II to type I, and vice versa) and occur at similar rates. We focused on quantifying and characterizing type I grouping in part because type II fibers comprise ~65% of the total distribution in human vastus lateralis, which would make an analysis of “abnormal grouping” quite challenging.
This work characterizes in detail grouped vs. ungrouped type I myofibers, revealing type II-like features among grouped type I fibers in the Older cohort. The larger CSA among grouped type I myofibers in Older suggests that despite belonging to similar low threshold motor units, grouped and ungrouped type I myofibers displayed divergent hypertrophic responses to recruitment. We 15 and others 24 have shown a similar divergence in hypertrophic response to recruitment (i.e. resistance exercise training [RT]) between type II and type I myofibers in healthy adults. In our hands, type II myofiber hypertrophy following high-intensity RT is approximately twice that of type I myofibers regardless of age15. Furthermore, in instances where myofiber hypertrophy is substantial following RT, particularly in type II myofibers, hypertrophy is accompanied by satellite cell-mediated myonuclear addition presumably to limit expansion of myonuclear domain size 16,24–26. Here we show that grouped type I myofibers appear to support their large CSA via myonuclear addition in a fashion similar to resistance trained type II myofibers. We therefore speculate that type II myofibers which have converted to grouped type I myofibers via denervation-reinnervation retain their inherently greater hypertrophic potential even after MHC I conversion has taken place. Upon reinnervation-induced conversion, former type II myofibers would experience far more frequent recruitment (now as part of low threshold motor units) which may serve as the growth stimulus resulting in hypertrophy and myonuclear addition. Interestingly, the number of capillary contacts per myofiber is another indicator of a type II-like phenotype among grouped type I myofibers in Older adults.
Regarding SERCA isoform expression, not only did Older have more MHC I+ / SERCA 1+ hybrids than Young, the overwhelming majority of Older hybrid myofibers were found among grouped type I myofibers. The expression pattern of SERCA in grouped type I myofibers suggests that MHC and SERCA isoform expression are not coupled/proportionate during denervation-reinnervation myofiber type switching. In fact, there is evidence supporting this concept in the context of denervation as Talmadge et al. 27 have shown MHC and SERCA transitions occur independently after human spinal cord injury. Because myofiber type conversions are likely bidirectional during the dynamic processes of denervation-reinnervation, one might also expect some MHC type II myofibers to express SERCA 2a, suggesting they were once pure type I myofibers. We did not test this possibility here because of the aforementioned difficulty of quantifying abnormal type II myofiber grouping in a predominantly type II muscle.
We noted a considerable amount of inter-individual heterogeneity in type I myofiber grouping prevalence (range: 5–99%) among Older individuals, which led to the use of K-means cluster analysis. In doing so, we identified a Low cluster among Older adults that did not differ from young on any of the grouped vs. ungrouped type I myofiber analyses. On the other hand, the Mod and High clusters of Older adults displayed a progressive expansion of differences between grouped and ungrouped type I myofibers, suggestive of a higher presence of partially converted type II myofibers among the grouped type I myofibers.
Although type I myofiber grouping is accepted as a biomarker of denervation-reinnervation events, whether type I myofiber grouping prevalence is degenerative or advantageous is unclear. For example, if the rate of denervation is constant with advancing age, then higher type I myofiber grouping prevalence is indicative of higher reinnervative capacity/efficiency (i.e. advantageous). On the other hand, if reinnervation rate is fairly constant, the higher type I myofiber grouping prevalence would be indicative of higher levels of denervation (i.e. degenerative). Therefore, our data imply that either Mod and High experience similar denervation to Low but reinnervate with respectively higher efficiency (preserving more myofibers), or that Mod and High display respectively higher rates of denervation-reinnervation events (ultimately losing more myofibers). Either scenario apparently leads to more type II myofibers in the grouped type I myofiber pool, with type I MHC expression being the only characteristic of fiber type conversion identified here.
Conclusions
Altogether our findings indicate that heightened type I myofiber grouping in Older is driven by an increased number of myofibers per abnormal group and that, as grouping prevalence increases among Older individuals, the presence of type I myofibers displaying type II phenotypic characteristics increases within those groups. Therefore, reinnervation-induced type II myofiber conversion to type I within myofiber groups does not appear to be immediate/complete. In future studies, functional characterizations of older individuals displaying varying amounts of type I myofiber grouping may be helpful in determining whether myofiber grouping is advantageous or deleterious.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grants R01AG017896, T32HD071866, P2CHD086851, R01AG046920, R01HD084124, and UL1TR001417. We are indebted to the research participants for their effort and dedication.
Abbreviations
- CSA
cross-sectional area
- GAM
goat-anti-mouse
- MHC
myosin heavy chain
- PAX7
paired box 7
- SERCA
Sarco(endo)plasmic reticulum calcium ATPase
- UEA-I
Ulex europaeus agglutinin
- PBS
phosphate-buffered saline
- RT
resistance exercise training
Footnotes
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflicts of Interest: None of the authors has any conflict of interest to disclose.
References
- 1.Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. Journal of bone metabolism. 2013 May;20(1):1–10. doi: 10.11005/jbm.2013.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. Spec No. [DOI] [PubMed] [Google Scholar]
- 3.Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983 Oct;6(8):588–595. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- 4.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. Journal of the neurological sciences. 1988 Apr;84(2–3):275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 5.Gawel M, Kostera-Pruszczyk A. Effect of age and gender on the number of motor units in healthy subjects estimated by the multipoint incremental MUNE method. J Clin Neurophysiol. 2014 Jun;31(3):272–278. doi: 10.1097/WNP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 6.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle & nerve. 2005 Apr;31(4):461–467. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- 7.Power GA, Dalton BH, Behm DG, Vandervoort AA, Doherty TJ, Rice CL. Motor unit number estimates in masters runners: use it or lose it? Medicine and science in sports and exercise. 2010;42(9):1644–1650. doi: 10.1249/MSS.0b013e3181d6f9e9. 20100820 DCOM- 20101213. [DOI] [PubMed] [Google Scholar]
- 8.Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol. 1991;81(4):377–381. doi: 10.1007/BF00293457. 19910607 DCOM- 19910607. [DOI] [PubMed] [Google Scholar]
- 9.Aare S, Spendiff S, Vuda M, et al. Failed reinnervation in aging skeletal muscle. Skeletal muscle. 2016;6(1):29. doi: 10.1186/s13395-016-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lexell J, Downham D, Sjostrom M. Morphological detection of neurogenic muscle disorders: how can statistical methods aid diagnosis? Acta neuropathologica. 1987;75(2):109–115. doi: 10.1007/BF00687070. [DOI] [PubMed] [Google Scholar]
- 11.Fink B, Egl M, Singer J, Fuerst M, Bubenheim M, Neuen-Jacob E. Morphologic changes in the vastus medialis muscle in patients with osteoarthritis of the knee. Arthritis and rheumatism. 2007 Nov;56(11):3626–3633. doi: 10.1002/art.22960. [DOI] [PubMed] [Google Scholar]
- 12.Buller AJ, Eccles JC, Eccles RM. Differentiation of fast and slow muscles in the cat hind limb. The Journal of physiology. 1960 Feb;150:399–416. doi: 10.1113/jphysiol.1960.sp006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. The Journal of physiology. 1960 Feb;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaauw B, Schiaffino S, Reggiani C. Mechanisms modulating skeletal muscle phenotype. Comprehensive Physiology. 2013 Oct;3(4):1645–1687. doi: 10.1002/cphy.c130009. [DOI] [PubMed] [Google Scholar]
- 15.Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Medicine and science in sports and exercise. 2011 Jul;43(7):1177–1187. doi: 10.1249/MSS.0b013e318207c15d. [DOI] [PubMed] [Google Scholar]
- 16.Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. American journal of physiology. Endocrinology and metabolism. 2016 Feb 9; doi: 10.1152/ajpendo.00486.2015. ajpendo 00486 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. Journal of applied physiology. 2006 Aug;101(2):531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kelly NA, Ford MP, Standaert DG, et al. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson's disease. Journal of applied physiology. 2014 Mar 1;116(5):582–592. doi: 10.1152/japplphysiol.01277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab. 2005 Jun;288(6):E1110–1119. doi: 10.1152/ajpendo.00464.2004. [DOI] [PubMed] [Google Scholar]
- 20.Kanda K, Hashizume K. Changes in properties of the medial gastrocnemius motor units in aging rats. Journal of neurophysiology. 1989 Apr;61(4):737–746. doi: 10.1152/jn.1989.61.4.737. [DOI] [PubMed] [Google Scholar]
- 21.Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. The Journal of physiology. 2016 Apr 15;594(8):1965–1978. doi: 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc. 2011 Jul;43(7):1177–1187. doi: 10.1249/MSS.0b013e318207c15d. [DOI] [PubMed] [Google Scholar]
- 23.Merritt EK, Stec MJ, Thalacker-Mercer A, et al. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. Journal of applied physiology. 2013 Sep;115(6):937–948. doi: 10.1152/japplphysiol.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams GR, Bamman MM. Characterization and regulation of mechanical loading-induced compensatory muscle hypertrophy. Comprehensive Physiology. 2012 Oct;2(4):2829–2870. doi: 10.1002/cphy.c110066. [DOI] [PubMed] [Google Scholar]
- 25.Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. American journal of physiology. Endocrinology and metabolism. 2006 Nov;291(5):E937–946. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- 26.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. Journal of applied physiology. 2008 Jun;104(6):1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 27.Talmadge RJ, Castro MJ, Apple DF, Jr, Dudley GA. Phenotypic adaptations in human muscle fibers 6 and 24 wk after spinal cord injury. Journal of applied physiology. 2002 Jan;92(1):147–154. doi: 10.1152/japplphysiol.000247.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.