Abstract
Our understanding of cancer progression or response to therapies would benefit from benchtop, tissue-level assays that preserve the biology and anatomy of human tumors ex vivo. We present methodology for maintaining patient tumor samples ex vivo for the purpose of drug testing in the clinical setting. The harvested tumor biopsy, excised from mice or patients, is integrated into a support tissue that includes stroma and vasculature. This support tissue preserves tumor histo-architecture and relevant expression profiles, and tumor tissues cultured using this system display different sensitivities to chemotherapeutics compared to tumor explants with no supporting tissue. The methodology is more rapid than PDX models, easy to implement and amenable to high throughput assays, making it an attractive tool for in vitro drug screening or for the guidance of patient-specific chemotherapies.
1. Introduction
Despite advances in precision medicine and targeted therapies, identifying the most efficacious treatment for an individual patient is still a challenge. In pancreatic ductal adenocarcinoma, patients usually progress through first line treatment, and oncologists must choose a more efficacious adjuvant chemotherapy. There is currently no standard, reliable protocol for maintaining intact tumor tissue ex vivo for drug testing. An extensive description of biomimetic approaches to engineering tumors in vitro is given by Bazou et al., 2016. Briefly, tumor organoid (Huang et al., 2015) and tissue slice (Merz et al., 2013) cultures retain the cell subpopulations, histo-architecture and phenotypic heterogeneity of the primary tumor. However, they still lack an extended, supporting vasculature and often use non-representative matrices such as matrigel for support. The vasculature and its associated stroma control much of tumor biology, including metabolism, invasion and metastasis. To date there are a few vascularized models of human tumors that use microfluidic models (Ehsan et al., 2014) and bioreactors (Ferrarini et al., 2013). Although the microfluidic model achieved tumor vascularization and early tumor intravasation steps were identified, the tumor spheroid consisted of a single cancer cell population, and lacked other components of the tumor microenvironment. Bioreactors have been successful in the culture of tumor explants under dynamic flow but are difficult to adapt for high-throughput drug screening, are not amenable for longitudinal microscopic imaging, and the explant blood vessels are isolated, not integrated into a surrounding network formed in vitro.
Methodology for maintaining a patient’s tumor tissue ex vivo for the purpose of drug testing would greatly improve personalized medicine in oncology. We have established such method by implanting pancreatic tumor explants into a supporting tissue bed that contains stromal cells, matrix and vasculature. The resulting “vascularized tumor explants” (VTEs) maintain their anatomy and viability, and can be used for studying tumor biology, for drug screening, or for testing personalized therapies.
2. Materials and Methods
Detailed methods are available as Supplementary Material.
3. Results
First we formed the support tissue by co-culturing endothelial cells (ECs) and smooth muscle cells (SMCs) in 96-well plates. This co-culture forms a self-organized, multi-layered, vascularized tissue. To characterize the system with mouse tumors, we grew PANC-1 tumors in mice, harvested the tumor and created 0.2 – 0.5 mm fragments. The explants were added to one-day old support tissues. The pre-formed vascular network surrounded and penetrated the tumor explants in many locations (Figure 1a, Supplementary material online, Video S1). The tumor-associated vessels had well-established lumens with diameters as large as 40 μm (Figure 1b). Cultured vessels (Figure 1c, shown in green) migrated and established connections with the endogenous mouse vessels inside the tumor (Figure 1c; shown in red; Supplementary material online, Video S2).
Figure 1. Mouse PANC-1 VTE cultures.
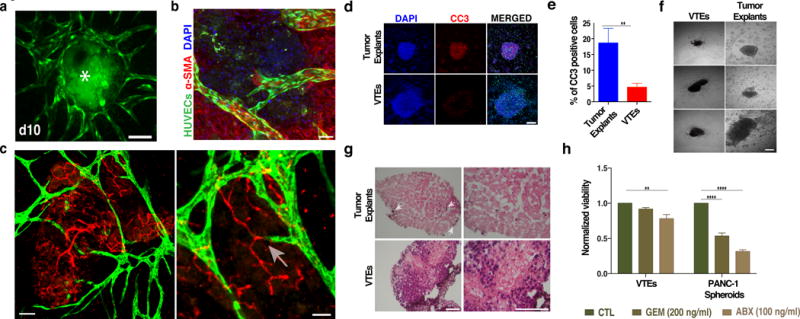
a) Vascularization of PANC-1 VTEs (asterisk). Vessels (green) surround and penetrate the tumor explants (due to auto-fluorescence, also shown in green) over 10 days. Scale bar, 150 μm. b) Confocal micrograph shows a lumenized vessel (green) extending into a tumor explant. Scale bar, 50 μm. Nuclei are shown in DAPI (blue). c) Cultured vessels (green) interact and connect with the mouse explant vessels (red). Scale bar, 50 μm. Boxed area shows two vessels connecting (arrow); scale bar, 25 μm. d, e) Tumor explants without the vessel bed have more cleaved caspase-3 staining (red) compared to VTEs. Nuclei were stained with DAPI (blue). Scale bar, 150 μm. f) PANC-1 VTEs maintain their original structure, while tumor explants (outlined with a red dotted line) cultured without the EC-SMC support disperse within three days and cells migrate away. Three different examples of each are shown. Scale bar, 100 μm. g) H&E sections of PANC-1 tumor explants and VTEs. Few cells remain viable (arrows) in tumor explants after 10 days in culture. Scale bar, 50 μm. h) Normalized viability of PANC-1 VTEs and spheroids in response to a three-day GEM or ABX treatment.
In culture, tumor explants retained important stromal and immune components such as collagen I (Supplementary material online, Figure S1a) and macrophages (Supplementary material online, Figure S1b). The explants were also angiogenic and contained many lumenized vessels (Supplementary material online, Figure S1a, b). PANC-1 VTEs also released growth factors involved in vascularization (Supplementary material online, Figure S1c). These PANC-1 VTEs grew slowly in culture (Supplementary material online, Figure S1d), consistent with their growth in vivo (Nakamura et al., 2013). Thus, the tumor explants maintain important structural and biological properties when cultured in this system. To assess how the vascular bed influences the tumor explants, we cultured PANC-1 tumor explants alone or VTEs for 10 days and then quantified cell death. Tumor explants without the vascular bed support showed extensive cell death as revealed by cleaved caspase-3 (CC-3) (Figure 1d, top row and Figure 1e); in contrast, cell death was minimal in VTEs (Figure 1d, bottom row and Figure 1e). The vascular bed is also essential for the maintenance of the integrity of the tumor explants: without a support bed, tumors quickly dispersed as cells migrated away (Figure 1f). We then analyzed the long-term viability and histo-architecture of the VTEs (Figure 1g). Significant apoptosis was observed for PANC-1 tumor explants alone as very few cancer cells (arrows) remained viable after 10 days in culture. In contrast, VTEs retained higher viability. We note that we have been able to maintain VTEs in culture for up to 3 weeks without any detectable loss of their original size.
To investigate the responses to chemotherapeutic drugs, we cultured PANC-1 tumor explants and VTEs and treated each with gemcitibine (GEM) or abraxane (ABX) (Russo et al., 2016). We also tested PANC-1 cell spheroids, as this is a commonly-used model to study tumors in vitro. VTEs were resistant to GEM (200 ng/ml), while approximately 20% cell death was observed with ABX (100 ng/ml) (Figure 1h), in accordance with the responses in mouse models (Awasthi et al., 2013) and patients (Von Hoff et al., 2013). For the spheroids, 100 ng/ml ABX or GEM reduced the viability to 30% and 50% respectively (Figure 1h), while the cores of the spheroids become necrotic (Supplementary material online, Figure S2a). Tumor explants without a vascular bed dispersed extensively in response to these drugs, making the core of the tumor explant unidentifiable (Supplementary material online, Figure S2b) and no longer amenable to analysis. These effects suggest that the cells in the tumor tissue and EC/SMC co-cultures are co-dependent, likely through both physical and biochemical interactions. This is supported by studies showing that endothelial-derived (“angiocrine”) factors support tumor growth in vivo (Brantley-Sieders et al., 2011). Importantly, it is known that these angiocrine factors can also alter responses to chemotherapy (Cao et al., 2014).
We next tested our system with human tumor samples. Patient-derived pancreatic tumor explants were harvested after distal pancreatectomy surgery and immediately added to one-day old vascular beds. Significant vessel infiltration was observed within 7 days (Figure 2a, b). No significant size increase was observed over a 7-day culture period (Supplementary material online, Figure S1e). This is consistent with the growth of these tumors in vivo, as they can take up to 29 months to reach 1 cm size (Nakamura et al., 2013). During 10-days of culture, the VTEs released growth factors associated with vasculogenesis (Figure 2c, d). VTEs from both patients released significantly more s-FLT1, PLGF and VEGF than tumor explants alone throughout the 10-day culture period, while tumor explants alone released significantly more bFGF than VTEs particularly in the first 3 days in culture. No difference could be detected between the growth factor profiles of the two patients with the exception of VEGF, with patient A having a more angiogenic profile.
Figure 2. Patient-derived VTEs.
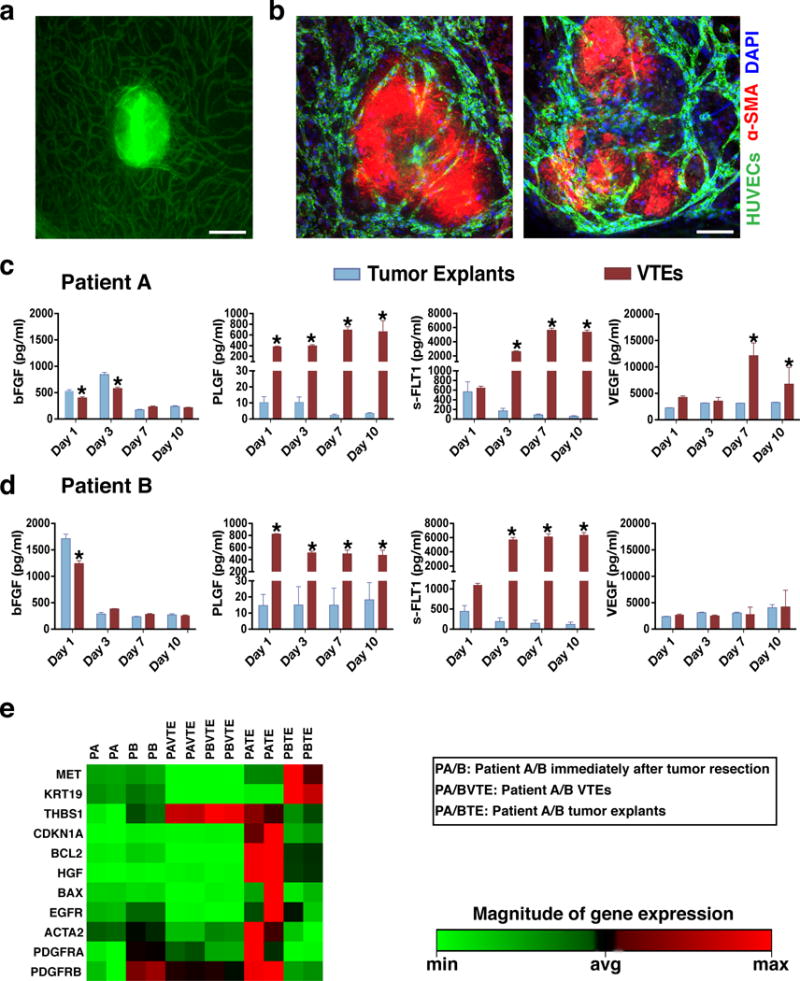
a) A vascularized patient-derived pancreatic VTE on Day 7 in culture. Vessels are shown in green. Scale bar, 150 μm. b) Patient-derived VTEs (which appear red due to high levels of αSMA) exhibit significant vessel infiltration within 7 days in culture; two different explants are shown. Vessels are shown in green and nuclei in blue (DAPI). Scale bar, 150 μm. c, d) VTEs from both patients released significantly more s-FLT1, PLGF and VEGF than tumor explants throughout the 10-day culture period, while tumor explants released significantly more bFGF than VTEs particularly in the first 3 days in culture. No difference could be detected between the growth factor profiles of the two patients with the exception of VEGF, with patient A having a more angiogenic profile. e) Clustergram of qRT-PCR array analysis of Patient A and B-derived fresh tumor samples, VTEs and tumor explants alone grown for 7 days (data normalized to patient A). Each pair of columns represents duplicate samples from the same patient.
To further examine whether VTEs retain the gene expression profile of the primary tumor we performed a gene array comparing pancreatic cancer markers and apoptosis genes in VTEs and tumor explants alone at day 7 as well as samples from the primary tumor immediately after resection (Figure 2e). Patient samples immediately after resection (PA and PB) had similar expression profiles of cancer cell marker cytokeratin 19, stromal markers (alpha smooth muscle actin and thrombospondin 1) and growth factor receptors (MET, EGFR and PDGFRα), while they differed in the expression of PDGFRβ. Expression in tumor explants with no vessel bed (PATE and PBTE) changed in unpredictable patterns as the explants deteriorated. VTEs (PAVTE and PBVTE), in contrast, maintained a similar mRNA profile. Of the genes tested, we only observed significant increases in the expression of thrombospondin 1 and PDGF receptors, which were due to the presence of the support bed (Supplementary material online, Figure S2c). The cancer-specific genes showed no changes when tumors were maintained as VTEs. These results show that by maintaining the tumor integrity, the presence of the vascular bed may allow reliable genetic profiling in tumor explants.
4. Discussion
By providing a vascular bed, we were able to culture pancreatic tumor explants excised from mice and patient samples in vitro. The VTEs can be maintained for ex vivo studies, drug screening, or to guide personalized treatment regimens (Carneiro et al., 2016). Advantages of this system include: i) because the tumor explants carry their own stroma, it preserves the natural microenvironment of the tumor; ii) the histo-architecture and genomic status of the primary tumor are retained in culture; iii) the culture can be performed in microtiter plates, so it is adaptable to high throughput screening; iv) many tumor explants can be generated for testing from a single mouse or human tumor sample, v) the system is more rapid than PDX models, able to perform drug testing within 1–2 weeks.
Supplementary Material
Acknowledgments
We thank Julia Kahn for her help with tumor implantations and Shan Min Chin for her assistance with the qRT-PRC array. Grant Support: National Institutes of Health (R01HL106584).
Grant Support: National Institutes of Health (R01HL106584).
Footnotes
Author Contributions
DB performed all experiments and data analysis and wrote the manuscript. NM performed experiments, analyzed data and wrote the manuscript. HG, GG and CLE performed experiments and analyzed data. HL collected patient derived samples. GS performed image analysis. LLM guided the research and wrote the manuscript.
References
- Awasthi N, Zhang C, Schwarz AM, Hinz S, Wang C, Williams NS, Schwarz MA, Schwarz RE. Comparative benefits of Nab-paclitaxel over gemcitabine or polysorbate-based docetaxel in experimental pancreatic cancer. Carcinogenesis. 2013;34:2361–9. doi: 10.1093/carcin/bgt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazou D, Maimon N, Gruionu G, Munn LL. Self-assembly of vascularized tissue to support tumor explants in vitro. Integr Biol (Camb) 2016;8:1301–11. doi: 10.1039/c6ib00108d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Dunaway CM, Rao M, Short S, Hwang Y, Gao Y, Li D, Jiang A, Shyr Y, Wu JY, Chen J. Angiocrine factors modulate tumor proliferation and motility through EphA2 repression of Slit2 tumor suppressor function in endothelium. Cancer Res. 2011;71:976–87. doi: 10.1158/0008-5472.CAN-10-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Ding BS, Guo P, Lee SB, Butler JM, Casey SC, Simons M, Tam W, Felsher DW, Shido K, Rafii A, Scandura JM, Rafii S. Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell. 2014;25:350–65. doi: 10.1016/j.ccr.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro BA, Costa R, Taxter T, Chandra S, Chae YK, Cristofanilli M, Giles FJ. Is Personalized Medicine Here? Oncology. 2016;30:293–303. 307. [PubMed] [Google Scholar]
- Ehsan SM, Welch-Reardon KM, Waterman ML, Hughes CC, George SC. A three-dimensional in vitro model of tumor cell intravasation. Integr Biol (Camb) 2014;6:603–610. doi: 10.1039/c3ib40170g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini M, Steinberg N, Ponzoni M, Belloni D, Berenzi A, Girlanda S, Caligaris-Cappio F, Mazzoleni G, Ferrero E. Ex-vivo dynamic 3-D culture of human tissues in the RCCS™ bioreactor allows the study of Multiple Myeloma biology and response to therapy. PLoS One. 2013;8:e71613. doi: 10.1371/journal.pone.0071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, Nostro C1, Wang R3, Muthuswamy LB, Crawford HC, Arrowsmith C, Kalloger SE, Renouf DJ, Connor AA, Cleary S, Schaeffer DF, Roehrl M, Tsao MS, Gallinger S, Keller G, Muthuswamy SK. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015;21:1364–71. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz F, Gaunitz F, Dehghani F, Renner C, Meixensberger J, Gutenberg A, Giese A, Schopow K, Hellwig C, Schafer M, Bauer M, Stocker H, Taucher-Scholz G, Durante M, Bechmann I. Organotypic slice cultures of human glioblastoma reveal different susceptibilities to treatments. Neuro Oncol. 2013;15:670–81. doi: 10.1093/neuonc/not003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Masuda K, Harada S, Akioka K, Sako H. Pancreatic cancer: Slow progression in the early stages. Int J Surg Case Rep. 2013;4:693–6. doi: 10.1016/j.ijscr.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S, Ammori J, Eads J, Dorth J. The role of neoadjuvant therapy in pancreatic cancer: a review. Future Oncol. 2016;12:669–85. doi: 10.2217/fon.15.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


