Abstract
Purpose
To assess the feasibility and performance of conical k-space trajectory free-breathing ultrashort echo time (UTE) chest MRI versus four-dimensional (4D) flow and effects of 50% data subsampling and soft-gated motion correction.
Materials and Methods
32 consecutive children who underwent both 4D flow and UTE ferumoxytol-enhanced chest MR (mean age: 5.4 years, range: 6 days–15.7 years) in one 3T exam were recruited. From UTE k-space data, three image sets were reconstructed: (i) one with all data, (ii) one using the first 50% of data, and (iii) a final set with soft-gating motion correction leveraging the signal magnitude immediately after each excitation. Two radiologists in blinded fashion independently scored image quality of anatomical landmarks on a 5-point scale. Ratings were compared using Wilcoxon rank-sum, Wilcoxon signed-ranks, and Kruskall-Wallis tests. Interobserver agreement was assessed with the intra-class correlation coefficient (ICC).
Results
For fully-sampled UTE, mean scores for all structures were ≥4 (good-excellent). Full UTE surpassed 4D flow for lungs and airways (P < 0.001), with similar pulmonary artery (PA) quality (P = 0.62). 50% subsampling only slightly degraded all landmarks (P < 0.001), as did motion-correction. Subsegmental PA visualization was possible in >93% scans for all techniques (P = 0.27). Interobserver agreement was excellent for combined scores (ICC = 0.83).
Conclusion
High-quality free-breathing conical UTE chest MR is feasible, surpassing 4D flow for lungs and airways, with equivalent PA visualization. Data subsampling only mildly degraded images, favoring lesser scan times. Soft-gating motion correction overall did not improve image quality.
Keywords: ultrashort echo time (UTE), conical, chest MRI, 4D flow, free-breathing, ferumoxytol
INTRODUCTION
Achieving diagnostic-quality chest MRI has long been an important goal in pediatric imaging. Currently, when chest radiographs are insufficient, patients typically undergo computed tomography (CT) [1]. While CT benefits from rapid acquisition, technical simplicity, and generally adequate visualization, there are several important drawbacks. Most important is the exposure to ionizing radiation, which poses greater risk at younger age [2, 3]. This risk is further compounded by the frequent need for long-term serial follow-up imaging in children with chronic diseases such as cystic fibrosis (CF) [1, 4–5]. In contrast, MRI is ionizing radiation-free. In addition, compared to CT, MRI offers superior inherent soft tissue contrast such as for mediastinal imaging, better anatomical delineation in the absence of intravenous contrast, and a potential for robust functional assessment of lung perfusion and ventilation [1, 6–14]. Moreover, children who need chest imaging not uncommonly require an MRI for other purposes, such as congenital heart disease or abdominal oncology. The process of transferring these patients, often anesthetized, from the MR to the CT scanner can prove challenging and prolong overall exam and anesthesia times.
Nevertheless, pediatric chest MRI has traditionally been hampered by several factors. These include: intrinsic low lung signal with short T2* properties (<2 msec), high cardiac and respiratory rates in children resulting in motion blur, lack of patient cooperation with long acquisition times, breath-holds, and quiet breathing, and potential greater need for sedation [1, 6, 11–12, 15]. Recently, UTE MRI with three-dimensional (3D) radial k-space trajectories has shown promise in overcoming these obstacles. However, experience with 3D radial UTE chest MR is limited to animal models and small human studies, mostly in adults [15–23]. Moreover, 3D radial acquisitions suffer from inherent inefficiencies in fully populating k-space, leading to tradeoffs between longer scan times and data undersampling with lesser resolution [24].
UTE imaging utilizing conical k-space sampling permits greater scan efficiency than do radial acquisitions. At the same time, conical UTE diffuses motion and aliasing artifacts and is thus robust to flow and motion. As a result, this technique has potential to yield diagnostic free-breathing chest MR images, even in children. However, use thus far has been limited to early experimental studies [25, 26].
The purpose of this study is to investigate the feasibility of a golden-angle ordered conical UTE MR technique for pediatric chest MRI.
MATERIALS AND METHODS
With Institutional Review Board (IRB) approval, Health Insurance and Portability and Accountability Act (HIPAA) compliance, informed parent/guardian consent, and informed patient assent (for subjects ages 7–17 years-old with decision-making capacity), 32 consecutive children were recruited to undergo both conical UTE and 4D flow chest MR during one exam between April 2016 and September 2016. All patients were imaged free-breathing on a 3T MRI scanner (MR 750 3T, GE Healthcare, Waukesha, WI, USA) with a 32-channel cardiac coil (Invivo, Gainesville, FL, USA). 4 exams (12.5%) were performed without anesthesia, 10 (31.3%) under light anesthesia with facemask (FM) or nasal cannula (NC) for respiratory support, and the remaining 18 (56.3%) under ventilator-assisted deep anesthesia using a laryngeal mask airway (LMA) or an endotracheal tube (ETT). For cases performed under deep anesthesia, mean ± SD reported maximum tidal volume was 154.1 ± 111.1 mL (range: 25–389 mL), or 9.0 ± 6.4 mL/kg (range: 2.1–28.0 mL/kg). Positive end expiratory pressure (PEEP) was applied in 10/18 (55.6%) deep-anesthetized exams; the amount of PEEP utilized varied during the imaging time and ranged from 0–8 cm H2O. 26 (81.3%) studies were performed for congenital heart disease, 2 (6.3%) for noncompaction cardiomyopathy, 2 (6.3%) for pre-transplant vascular mapping, and 1 (3.1%) for pulmonary hypertension. Mean ± SD patient heart rate during acquisition was 93.1 ± 21.8 beats/min (range: 55–157 beats/min). Despite anticipated variation in body size with subject age, no special corrections to account for potential RF inhomogeneity were needed or performed in this qualitative imaging assessment study (as detailed below).
All scans were enhanced with ferumoxytol (Feraheme, AMAG Pharmaceuticals, Inc., Waltham, MA, USA), injected intravenously before imaging as a slow infusion over 5–15 min using a diluted volume in normal saline (3 mg/kg with 1:5 dilution or 0.1 mL/kg) [26–33]. Vital signs were monitored for 30 min after ferumoxytol administration in the radiology department or an inpatient unit under the supervision of an advanced cardiac life support (ACLS) or pediatric advanced life support (PALS)-certified physician. In order to perform a dynamic perfusion scan, one patient in addition to ferumoxytol received gadobenate dimeglumine (MultiHance, Bracco Diagnostics, Inc, Cranbury, NJ, USA), administered via rapid bolus intravenous (IV) injection during imaging at a dosage of 0.1 mmol/kg (0.2 mL/kg) and followed by a saline flush.
UTE data were acquired using a radiofrequency (RF)-spoiled gradient echo (SPGR) sequence with a 3D conical k-space sampling trajectory (Figure 1) [25–26, 34]. The cone interleaves (one interleaf per readout) were first ordered sequentially by the kz position of the last acquisition point of each readout. That is, the cone interleaves were ordered from the “north” pole to “south” pole. The ordering was then permuted according to the golden-ratio permutation [35, 36]. This re-ordering of the acquisition increases motion robustness and enables retrospective data subsampling. Each cone interleaf began at the k-space center, providing the ability for self-navigation; the first few samples of each cone interleaf were processed to compute motion waveforms [37]. Scan parameters included a flip angle of 15°, minimum repetition time (TR) of 4.70–5.60 ms, minimum TE of 0.032 ms, maximum TE of 2 ms, acquired matrix of 320–416 × 320–416 × 70–200 (z-axis varying with body size), spatial resolution of 0.8 × 0.8 × 1.8 mm3, and bandwidth of 125 kHz. In-plane field-of-view (FOV) was adjusted to maintain a constant spatial resolution and account for the inability to perform readout filtering. On the order of 60,000 cones were utilized, with 600 sample readouts per cone interleaf. There was no acceleration. Mean ± SD scan duration was 4.9 ± 1.0 min (range: 2.1–5.9 min). An anticipated average scan time of 5 minutes (varying with body size) was prescribed based on preliminary phantom testing and supported reduction factors. Because the sequence does not support parallel imaging, further potential reductions in scan time without compromising image quality could only be established through retrospective data subsampling.
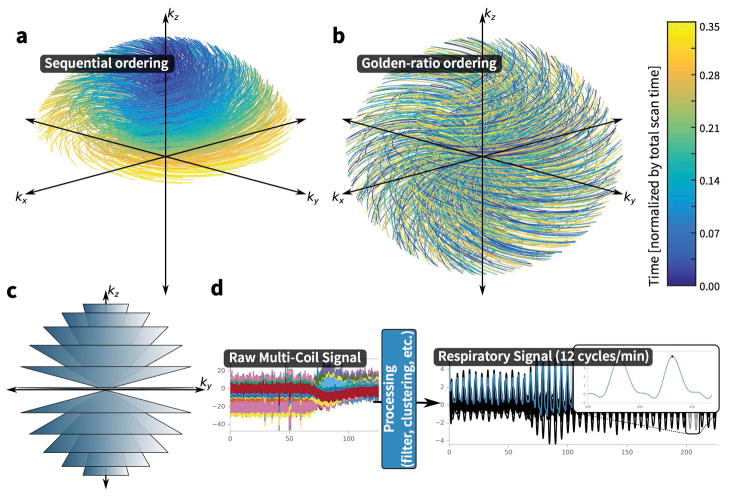
Figure 1.
Three-dimensional cones k-space trajectory overview. (A) First 35% of the scan acquisition using sequential ordering. (B) First 35% of the scan acquisition using the golden-ratio permutation ordering. (C) Illustration of the entire cones k-space trajectory in which individual readouts trace the surface of the conical shells. (D) Processing of DC signal from the cones acquisition. To increase motion-robustness, the acquisition of the cones trajectory is ordered using the golden-ratio increment. Since the k-space center is sampled at the start of each cone interleaf, this data sample is extracted and processed as shown, filtered to highlight the respiratory signal and clustered to select the dominant motion waveform, with applied peak finding to determine trigger points. The resulting waveforms can be either used either to resolve motion dynamics or to suppress artifacts from motion with soft-gating.
For each exam, three image sets were reconstructed from UTE k-space data for review: (i) one with all the data, (ii) one using the first 50% of the data, and (iii) a final set with soft-gating motion correction leveraging the phase of the signal after each excitation, in effect utilizing the expiratory half of the data. As detailed in prior work, coil-clustering, a spectral-based clustering algorithm, was used to select the subset of highly correlated coils whose navigators most closely matched a dominant respiratory waveform, in turn facilitating motion suppression [34]. Image set (i) was reconstructed using gridding. Image set (ii) was reconstructed using parallel imaging (PI) and compressed sensing (CS). Lastly, image set (iii) was reconstructed using soft-gated PI and CS [38, 39]. Soft-gating weights were computed using the algorithm detailed in prior work [39]. Weights were applied both based on this prior research and the work of Johnson, et al. [38]. All reconstructions were performed using the Berkeley Advanced Reconstruction Toolbox (BART) [40]. The motivation to perform 50% subsampling was to determine if a 50% shorter scan time would provide diagnostically comparable image quality compared to fully-sampled UTE. The intention of soft-gating was to explore if motion artifacts could be reduced using this reconstruction technique.
4D flow acquisitions used minimum echo time (TE) flow-encoding gradients and a 4-point encoding strategy in a cardiac synchronized 3D Cartesian RF-spoiled gradient echo sequence with pseudo-random k-space undersampling and built-in navigators [28–30]. The 4D flow was cardiac resolved and corrected for respiratory-motion, providing a reference to compare motion effects. Acceleration was performed using both PI and CS. The typical acceleration factor was 12. Scan parameters included a flip angle of 15°, minimum TR of 3.15–4.73 ms, minimum TE of 1.22–2.28 ms, acquired matrix of 224–320 × 100–224 × 80–320 (z-axis varying with body size), spatial resolution of 0.8 × 0.8 × 1.4 mm3, bandwidth of 83–100 kHz, and velocity encoding range (VENC) of 100–350 cm/s.. Mean ± SD scan duration was 12.8 ± 3.4 min (range: 4.8–17.0 min). The scan time (anticipated to average 10 minutes, varying with body size) was prescribed based on an established clinical protocol, locally in use for >4 years.
Two radiologists (first reader, E.J.Z., board-certified, subspecialty-trained pediatric and cardiovascular radiologist with 7.5 years of experience; second reader, A.H., board-eligible MRI radiology fellow with 4.5 years of experience) independently scored image quality of the lungs, pulmonary arteries (PAs), and airways on a 5-point scale (1-nondiagnostic, 3-adequate for diagnosis, 5-excellent) for each UTE reconstruction and 4D flow in random order in blinded fashion. The scoring system, modified from prior studies on UTE chest MR, is shown in Table 1 [15–17]. In addition, readers indicated the smallest visible PA level (main, lobar, segmental, or subsegmental).
Table 1.
Criteria for Scoring Image Quality and Delineation of Anatomic Structures
| Score | Overall quality | Lungs | Pulmonary arteries | Airways |
|---|---|---|---|---|
| 5 | Excellent; high SNR; answers all clinical questions | Clearly distinguishable from air; fissures visible | No appreciable blurring of structures | Lower signal and clearly distinguishable from nearby lung; bronchial wall visible |
| 4 | Good; high SNR; more than sufficient for the majority of clinical questions | Clearly distinguishable from air; fissures not visible | Slight blurring of structures | Lower signal and clearly distinguishable from nearby lung; bronchial wall not visible |
| 3 | Adequate (moderate); sufficient for the most important clinical questions | Distinguishable from air; fissures not visible | Distinguishable with moderate blurring of structures | Lower signal and distinguishable from nearby lung; bronchial wall not visible |
| 2 | Limited (poor); barely sufficient for assessing some clinical questions | Barely distinguishable from air; fissures not visible | Barely distinguishable with extensive blurring of structures | Lower signal but barely distinguishable from nearby lung; bronchial wall not visible |
| 1 | Nondiagnostic; not clinically useful | Indistinguishable from air; fissures not visible | Not distinguishable | Indistinguishable from nearby lung |
SNR = signal-to-noise ratio
For each exam, there were 3 UTE reconstructions (fully-sampled UTE, 50% subsampled UTE, and soft-gated motion-corrected UTE) and the 4D flow images to review. Reading sessions were performed in blinded fashion and conducted in batches with 3 weeks interval between sessions to allow a “washout” period. Each session consisted of admixed datasets containing each type of the 4 reconstructions, presented in random order. UTE images were viewed using OsiriX MD 8.0.2 software (Pixmeo SARL, Geneva, Switzerland). 4D flow images were viewed using the Arterys platform (Arterys, Inc., San Francisco, CA, USA). Both UTE and 4D flow data allow reformation in any plane (Figure 2). However, readers scored images in the axial plane, using other planes only if needed for clarification. Window and level settings could be adjusted at the radiologist’s discretion for both UTE and 4D flow. For 4D flow, readers were allowed to view images from the entire cardiac cycle but only in grayscale mode (magnitude images without flow data).
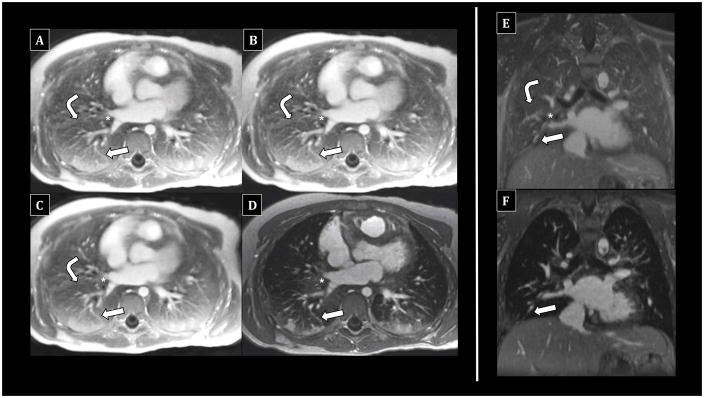
Figure 2.
Comparison of UTE reconstructions vs. 4D flow in an 8-year-old female undergoing chest MRI under anesthesia with endotracheal intubation. Both UTE and 4D flow allow multiplanar reformatting. Fully-sampled UTE images (A, E) allow excellent visualization of peripheral pulmonary vessels (arrows) and bronchi (asterisks), including bronchial wall delineation. Lung signal is distinct from pure air, with fissural delineation (bent arrows). 50% subsampled (B) and soft-gated images (C) are similar. In contrast, 4D flow images (D, F) show dependent atelectasis, but lung signal is not well-differentiated from air; bronchial walls are less visible. Delineation of pulmonary vessels is excellent but essentially equivalent to UTE.
Statistical Analysis
The Wilcoxon rank-sum test was used to evaluate the null hypothesis that fully-sampled UTE image quality scores were equivalent to those of 4D flow. The Wilcoxon signed-rank test was used to evaluate the hypotheses that fully-sampled UTE image quality scores were equivalent to 50% subsampled images as well as soft-gated motion-corrected images. The Kruskall-Wallis test was used to evaluate the null hypothesis that PA delineation was equivalent for the four reconstructions (fully-sampled UTE, 50% subsampled UTE, motion-corrected UTE, and 4D flow). Interobserver agreement was evaluated using the intra-class correlation coefficient (ICC). ICC values were correlated to agreement as follows: poor (<0.40), fair (0.40–0.59), good (0.60–0.74), and excellent (0.75–1.00). 95% confidence intervals (CIs) were also constructed to assess the proportion of cases in which fully-sampled and 50% subsampled UTE image quality scores were ≥3 (diagnostic or better) and PA delineation to the subsegmental level was possible. Statistical significance was set at the 0.05 level.
RESULTS
The final cohort consisted of 21 (65.6%) males and 11 (34.4%) females. Mean ± standard deviation (SD) age was 5.4 ± 4.1 years; ages ranged from 6 days to 15.7 years. Table 2 summarizes mean image quality scores for each reconstruction and anatomic landmark, both for individual and combined readers. For fully-sampled (raw) UTE, combined mean scores for all landmarks were >=4, indicating good to excellent image quality. The proportions (95% confidence interval [CI]) of exams with diagnostic or better image quality (score ≥3) for the lungs, PAs, and airways, respectively, were 96.9% (92.5–100%), 98.4% (95.3–100%), and 96.9% (92.5–100%). The PAs were visible to the subsegmental level in 98.4% (95% CI 95.3–100%) of exams. Overall, 93.8% (95% CI 87.7–99.8%) of exams demonstrated diagnostic or better image quality for all landmarks as well PA visibility to the subsegmental level.
Table 2.
Summary of Reader Image Quality Scores by Anatomy and Technique
| Anatomy | Reader 1 Mean Score (SD) |
Reader 2 Mean Score (SD) |
Combined Mean Score (SD) |
|---|---|---|---|
| Lungs | |||
| Full UTE | 4.0 (0.7) | 4.0 (0.8) | 4.0 (0.8) |
| 50% UTE | 3.5 (0.7) | 3.7 (0.9) | 3.6 (0.8) |
| Soft-Gated | 3.5 (0.7) | 3.3 (0.9) | 3.4 (0.8) |
| 4D Flow | 2.0 (0.2) | 2.4 (0.8) | 2.2 (0.6) |
|
| |||
| Pulmonary Arteries | |||
| Full UTE | 4.2 (0.8) | 3.9 (0.9) | 4.1 (0.8) |
| 50% UTE | 3.7 (0.5) | 3.6 (0.8) | 3.7 (0.6) |
| Soft-Gated | 4.3 (0.7) | 3.5 (0.8) | 3.9 (0.8) |
| 4D Flow | 4.4 (0.7) | 3.8 (0.8) | 4.1 (0.8) |
|
| |||
| Airways | |||
| Full UTE | 4.3 (0.9) | 4.1 (0.8) | 4.2 (0.9) |
| 50% UTE | 3.9 (0.8) | 3.7 (0.9) | 3.8 (0.8) |
| Soft-Gated | 4.3 (0.9) | 3.6 (0.7) | 4.0 (0.8) |
| 4D Flow | 2.9 (0.5) | 2.1 (0.7) | 2.5 (0.8) |
SD = standard deviation; UTE = ultrashort echo time; 4D = four-dimensional
Compared to 4D flow, fully-sampled UTE demonstrated better lung and airway image quality (P < 0.001 for both) and similar PA quality (P = 0.62). Compared to raw UTE, 50% subsampling degraded image quality for all landmarks (P < 0.001). Nevertheless, the mean score difference was only 0.4 for each structure. The proportions (95% CI) of 50% subsampled reconstructions with diagnostic or better image quality for the lungs, PAs, and airways, respectively, were 89.1% (81.2–96.9%), 96.9% (92.5–100%), and 93.8% (87.7–99.8%). Moreover, the PAs were visible to the subsegmental level in 93.8% (87.7–99.8%). Overall, 85.9% (77.2–94.7%) of 50% subsampled reconstructions demonstrated at least diagnostic image quality for all landmarks in addition to PA visibility to the subsegmental level. The proportion of diagnostic or better exams was statistically significantly lower in the 50% subsampled group compared to the fully-sampled UTE group (P = 0.03), although the absolute difference was small (7.8%).
Overall, motion correction degraded lung (P < 0.001) and airway (P = 0.009) image quality, while PA quality trended downward (P = 0.09). For reader 1, mean PA image quality increased from 4.2/5 with fully-sampled UTE to 4.3/5 with soft-gating. However, the difference was not statistically significant (P = 0.61). The PAs were visible to the subsegmental level in >93% scans for all techniques (P = 0.27). For all 3 sedation states (awake, light anesthesia, and deep anesthesia), soft-gating degraded or produced no statistically significant change in image quality of all landmarks, as detailed in Table 3.
Table 3.
Effects of Soft-Gating Motion Correction on Image Quality by Sedation State
| Anatomy Sedation State |
Full UTE Mean Score (SD) |
Soft-Gated UTE Mean Score (SD) |
P-Value |
|---|---|---|---|
| Lungs | |||
| Awake (n = 4) | 3.6 (0.7) | 2.8 (0.7) | P = 0.008** |
| Light Anesthesia (n = 10) | 4.0 (0.9) | 3.2 (0.6) | P = 0.002** |
| Deep Anesthesia (n = 18) | 4.1 (0.7) | 3.6 (0.8) | P = 0.006* |
|
| |||
| Pulmonary Arteries | |||
| Awake (n = 4) | 3.9 (0.8) | 3.5 (0.9) | P = 0.18 |
| Light Anesthesia (n = 10) | 4.1 (0.8) | 3.9 (0.7) | P = 0.26 |
| Deep Anesthesia (n = 18) | 4.1 (0.8) | 4.0 (0.9) | P = 0.45 |
|
| |||
| Airways | |||
| Awake (n = 4) | 3.8 (0.7) | 3.5 (0.5) | P = 0.16 |
| Light Anesthesia (n = 10) | 4.5 (0.8) | 4.1 (0.9) | P = 0.033* |
| Deep Anesthesia (n = 18) | 4.2 (0.9) | 4.0 (0.9) | P = 0.18 |
Note: Mean score refers to the combined mean image quality score from both readers
UTE = ultrashort echo time; SD = standard deviation
P < 0.05;
P < 0.001
Interobserver agreement was excellent for image quality assessment of lungs (ICC = 0.75), good for the airways (ICC = 0.67), and fair for the PAs (ICC = 0.47). Interobserver agreement was also fair for delineation of the smallest visible PA level (ICC = 0.43). For combined ratings of image quality and PA delineation, interobserver agreement was excellent (ICC = 0.83).
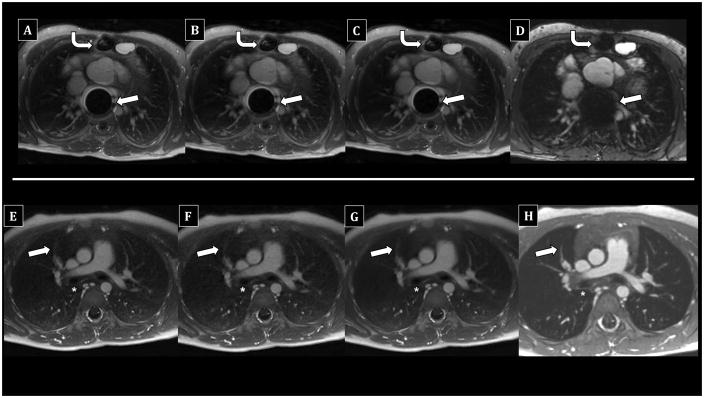
Figure 3 shows representative examples of good to excellent UTE image quality in paired patients who underwent chest MRI after deep sedation with endotracheal intubation and laryngeal mask airway support, respectively. Figure 4 demonstrates good to excellent image quality in paired patients who underwent MRI awake without anesthesia. Figure 5 shows examples of degraded image quality due to susceptibility and motion artifacts. Corresponding 50% subsampled UTE, soft-gated UTE, and 4D flow images are presented alongside the fully-sampled UTE images for visual comparison.
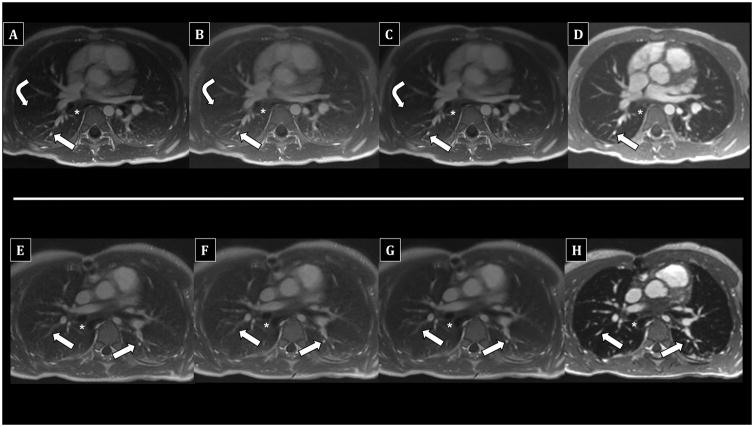
Figure 3.
Comparison of UTE reconstructions vs. 4D flow in children undergoing chest MRI with anesthesia using laryngeal mask airway. Top row (A–D) - 5-year-old male; bottom row (E–H) - 10-year-old male. Fully-sampled UTE images (A, E) show diagnostic delineation of subsegmental PAs (arrows) and airways (asterisks), including bronchial walls. 50% subsampled (B, F) and soft-gated motion-corrected (C, G) UTE images are similar. The right major fissure is faintly visible on the top UTE images (A–C). In contrast, 4D flow (E, H) offers diagnostic PA assessment, but airway walls are less distinct. Lung signal is similar to pure air, and fissures are not visible.
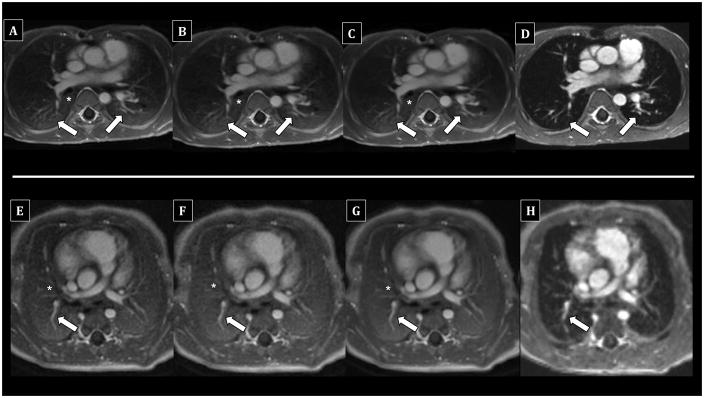
Figure 4.
Comparison of UTE reconstructions vs. 4D flow in awake children undergoing chest MRI without anesthesia. Top row (A–D) - 6-year-old male; bottom row (E–H) - 6-day-old female. Fully-sampled UTE images (A, E) show diagnostic delineation of the PAs (arrows) to the subsegmental level as well as the airways (asterisks), including bronchial wall visualization. Image quality of 50% subsampled (B, F) and soft-gated motion-corrected (C, G) UTE images is very similar, although soft-gated images (C, G) appear slightly “smoother” with less detailed internal architecture. In contrast, 4D flow (D, H) offers diagnostic PA assessment, but airway walls are less distinct, and lung signal is similar to pure air.
Figure 5.
Examples of degraded UTE and 4D flow image quality. Images from left to right in each row are fully-sampled UTE (A, E), 50% subsampled UTE (B, F), soft-gated motion-corrected UTE (C, G), and 4D flow (D, H), respectively. Top row (A–D) - 12-year-old male under deep anesthesia with laryngeal mask airway. Extensive susceptibility artifact related to embolic coils (arrows) and to a lesser extent sternotomy (bent arrows) markedly limits evaluation on all reconstructions. Portions of the lung and peripheral airways and PAs are still evaluable. Bottom row (E–H) - 5-year-old female under light anesthesia with facemask. On UTE images (E–G), motion artifact partially limits evaluation of the PAs (arrows) and airways (asterisks). While still distinct from pure air, lung signal is low. 4D flow (H) shows less perceptible motion artifact. However, lung signal is very low and nearly equivalent to pure air.
DISCUSSION
In this study, we have demonstrated the feasibility and high image quality of free-breathing conical UTE chest MRI in children. This was achieved in a diverse cohort of pediatric patients ranging from neonates to teenagers with sedation status ranging from deep anesthesia to completely awake without any sedation. Image quality of the lungs, airways, and PAs to the subsegmental level was considered diagnostic or better in >93% of exams, without noticeable impact from off-resonance blurring. In addition, conical UTE was superior to 4D flow for assessment of the lungs and airways, most likely due to the markedly shorter TE, while PA delineation was similar. 50% subsampling degraded UTE images and resulted in a smaller proportion of diagnostic or better exams, but absolute differences compared to fully-sampled UTE were small. Finally, the use of soft-gating motion correction overall did not improve and in fact degraded image quality. This held true regardless of sedation state (awake, light anesthesia, or deep anesthesia).
Most of the exams in our cohort were primarily performed for evaluation of congenital heart disease. Our results support the routine addition of conical UTE to 4D flow in such patients when the lungs or airways are of diagnostic concern. In addition, we note that, in general, 4D flow acquisitions are 2–3 times longer than fully-sampled UTE acquisitions. Yet, the shorter conical UTE sequence alone could suffice in place of 4D flow if the PAs are of primary interest. While there was variability in both UTE and 4D flow scan time, likely related to differences in z-axis coverage according to body size, acquisitions remained overall shorter for UTE compared to 4D flow.
Overall, our results also favor the use of a decreased scan time without substantially compromising image quality. With full UTE acquisitions on the order of 5 minutes, sampling only 50% of k-space data has the potential to reduce scan times to just 150 seconds. In turn, this may permit even lesser sedation requirements (including more “awake” scans), greater MR utilization and throughput, and improved experiences for patients and their parents. Lesser degrees of subsampling could also be performed to find an optimal balance between image quality and scan time.
The overall degradation in image quality with soft-gating was contrary to our expectations. We hypothesize that a primary contributing factor to these results was lack of optimization of parallel imaging and compressed sensing reconstruction for the conical UTE sequence. Soft-gating relies on the ability to perform parallel imaging and compressed sensing image reconstruction. With large volumetric datasets, such reconstruction for conical UTE typically takes on the order of hours. Some approximations were performed to allow reconstruction in under one hour, including limiting the number of iterations and compressing the number of channels. Second, the method of respiratory waveform extraction from the DC signal may not have functioned as anticipated based on such factors as variable distance to the coil receiver array. Third, it is conceivable that variations in respiratory rate could have affected the performance of the motion correction algorithm. This possibility cannot be directly ascertained, as respiratory rates documented in anesthesia records were highly heterogeneous throughout exams and imperfectly charted. However, we note that the soft-gating algorithm is applied to an individualized respiratory signal waveform extracted from raw UTE data for each patient. Thus, as long as the respiratory signal demonstrates a regular and recognizable waveform, we would not anticipate the breathing rate would substantially affect motion correction performance. Finally, the soft-gated images qualitatively tended to have a “smoother” appearance with less detailed internal architecture, which could have affected scoring. Still, we stress the excellent image quality of fully-sampled UTE images in the absence of motion correction, highlighting the motion robustness of the conical golden-angle ordered trajectory. At the same time, we are in the process of reevaluating the soft-gating algorithm to determine if further improvements can be made.
It is noteworthy that all scans in our cohort were enhanced with ferumoxytol. This ultrasmall superparamagnetic iron oxide medication was developed to treat anemia but has shown promise as a contrast agent although is not approved by the United States (U.S.) Food and Drug Administration for such use. Ferumoxytol had been administered per established, albeit off-label, institutional protocol in patients undergoing 4D flow for cardiovascular indications and is not required for our conical UTE acquisition [27–32]. However, we suspect it may further enhance the signal-to-noise ratio (SNR) and overall image quality. Previous work has shown ferumoxytol to be a superior agent for arterial and venous imaging [33]. Thus, ferumoxytol likely at least improved PA delineation in our cohort. With its long blood pool residence time (circulating half-life 14–15 hours) and long relaxivity, ferumoxytol permits longer scan times, while preserving vascular signal [28, 33]. As such, contrast enhancement remained robust despite the average 5-minute length of our UTE acquisition. The long half-life also permits a uniform comparison across 4D flow and UTE acquisitions, with preserved vascular enhancement throughout both scans.
Because of its favorable signal properties, ferumoxytol may also facilitate free-breathing acquisition (using multiple signal averages) and lesser anesthesia requirements [28]. Moreover, unlike gadolinium-based agents, ferumoxytol does not precipitate nephrogenic systemic fibrosis (NSF) and can be administered to patients with renal failure [27]. Nevertheless, although ferumoxytol has an overall strong safety profile, the rate of serious acute adverse events, such as anaphylaxis and hypotension, is likely greater than that of gadolinium-based agents [27–33]. Such risks must be balanced with potential benefits such as improved image quality and reduced anesthesia time and depth [27, 28].
Our study has several limitations. First, its small sample size over a variety of sedation states may limit extrapolation to a larger, prospective cohort with a particular sedation state. Nevertheless, we demonstrate the feasibility of our conical UTE technique in pediatric patients across the age spectrum with variable sedation states. Second, interobserver agreement was only fair for some landmarks, which may reflect heterogeneous interpretation of the scoring systems. However, overall the majority of UTE acquisitions were considered at least diagnostic for all structures, while specific scores may have differed.
Third, 4D flow was used as an internal comparison for the UTE sequence, as it was available in all cases. We recognize this is not a traditional or universally available MRA surrogate. However, 4D flow, in use for >4 years at our institution, has become our local standard of care for the indications represented in the patient cohort (that is, primarily congenital heart disease and vascular mapping). This single, comprehensive sequence requires minimal user training yet provides reliably diagnostic cardiovascular evaluation in fractions of the time required by traditional methods. Thus, it is now generally performed as a standalone acquisition without the need for a separate MRA that would only prolong scan time in often anesthetized or uncooperative pediatric patients. Moreover, in prior work we demonstrated 4D flow to be at least equivalent to standard RF-SPGR MRA techniques for delineation of cardiovascular structures [28]. This prior study did permit the use of flow data in visual assessment (unlike the current work). However, we would not expect the addition of flow data to substantially alter anatomic image quality of the PAs or evaluation of the lungs and airways (which do not generate flow). While contrast-enhanced chest CT might be the most pertinent comparison, such exams were generally not available due to lack of clinical necessity and risks of ionizing radiation.
Fourth, as noted previously, all scans were enhanced with ferumoxytol, and thus results cannot be directly generalized to noncontrast or gadolinium-enhanced UTE acquisitions; head-to-head comparisons are an area of anticipated future investigation. Fifth, while conical UTE demonstrated excellent performance in delineating selected anatomic landmarks, its utility in depicting specific thoracic pathologies (e.g., consolidation, bronchiectasis) and disease states (e.g., CF, malignancy) remain areas of active investigation. In particular, we note that the fully-sampled and 50% subsampled UTE reconstructions averaged inspiratory and expiratory data, while the soft-gated images were weighted toward end-expiration. In contrast, chest CT is generally ideally (although not exclusively) performed in end-inspiration; these differences could affect image quality. Nevertheless, retrospective UTE weighting toward end-inspiration or utilization of respiratory-resolved techniques could facilitate reconstructions comparable to end-inspiratory CT. Finally, we cannot directly compare the conical UTE sequence to radial UTE, which is not implemented at our institution. However, we would still anticipate improved readout efficiency of conical UTE compared to radial UTE, despite the very short T2* properties of lung tissue at 3T.
In conclusion, free-breathing conical k-space trajectory UTE chest MRI is feasible in children, with consistently diagnostic image quality surpassing 4D flow for the lungs and airways and equivalent for PAs. Data subsampling will allow even shorter scan times. While motion correction did not enhance image quality in our cohort, further optimization of the soft-gating algorithm may potentially allow improved anatomic delineation in select cases.
Acknowledgments
Grant Support: Contract grant sponsor: NIH; contract grant numbers: R01EB009690 and R019241; Contract grant sponsor: GE Healthcare; Contract grant sponsor: Tashia and John Morgridge Faculty Scholar Fund.
Footnotes
Disclosures:
E.J.Z., A.H.: No conflicts of interest to disclose.
J.Y.C.: Research support from GE Healthcare.
M.C.: Employed by GE Healthcare.
S.S.V.: Research support from GE Healthcare; Co-founder and consultant for Arterys.
References
- 1.Ciet P, Tiddens HA, Wielopolski PA, et al. Magnetic resonance imaging in children: common problems and possible solutions for lung and airways imaging. Pediatr Radiol. 2015;45(13):1901–1915. doi: 10.1007/s00247-015-3420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macdougall RD, Strauss KJ, Lee EY. Managing radiation dose from thoracic multidetector computed tomography in pediatric patients: background, current issues, and recommendations. Radiol Clin North Am. 2013;51(4):743–760. doi: 10.1016/j.rcl.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jong PA, Mayo JR, Golmohammadi K, et al. Estimation of cancer mortality associated with repetitive computed tomography scanning. Am J Respir Crit Care Med. 2006;173(2):199–203. doi: 10.1164/rccm.200505-810OC. [DOI] [PubMed] [Google Scholar]
- 5.Kuo W, Ciet P, Tiddens HA, Zhang W, Guillerman RP, van Straten M. Monitoring cystic fibrosis lung disease by computed tomography. Radiation risk in perspective. Am J Respir Crit Care Med. 2014;189(11):1328–1336. doi: 10.1164/rccm.201311-2099CI. [DOI] [PubMed] [Google Scholar]
- 6.Manson DE. MR imaging of the chest in children. Acta Radiol. 2013;54(9):1075–1085. doi: 10.1177/0284185113497475. [DOI] [PubMed] [Google Scholar]
- 7.Kangarloo H. Chest MRI in children. Radiol Clin North Am. 1988;26(2):263–275. [PubMed] [Google Scholar]
- 8.Siegel MJ. Chest applications of magnetic resonance imaging in children. Top Magn Reson Imaging. 1990;3(1):1–23. [PubMed] [Google Scholar]
- 9.Bisset GS., 3rd Pediatric thoracic applications of magnetic resonance imaging. J Thorac Imaging. 1989;4(2):51–57. doi: 10.1097/00005382-198904000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Laurin S, Williams JL, Fitzsimmons JR. Magnetic resonance imaging of the pediatric thorax: initial experience. Eur J Radiol. 1986;6(1):36–41. [PubMed] [Google Scholar]
- 11.Liszewski MC, Hersman FW, Altes TA, et al. Magnetic resonance imaging of pediatric lung parenchyma, airways, vasculature, ventilation, and perfusion: state of the art. Radiol Clin North Am. 2013;51(4):555–582. doi: 10.1016/j.rcl.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Goo HW. Advanced functional thoracic imaging in children: from basic concepts to clinical applications. Pediatr Radiol. 2013;43(3):262–268. doi: 10.1007/s00247-012-2466-3. [DOI] [PubMed] [Google Scholar]
- 13.Goo HW. State-of-the-art pediatric chest imaging. Pediatr Radiol. 2013;43(3):261. doi: 10.1007/s00247-012-2514-z. [DOI] [PubMed] [Google Scholar]
- 14.Manson DE. Magnetic resonance imaging of the mediastinum, chest wall and pleura in children. Pediatr Radiol. 2016;46(6):902–915. doi: 10.1007/s00247-016-3598-7. [DOI] [PubMed] [Google Scholar]
- 15.Hahn AD, Higano NS, Walkup LL, et al. Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system. J Magn Reson Imaging. 2016;45(2):463–471. doi: 10.1002/jmri.25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med. 2013;70(5):1241–1250. doi: 10.1002/mrm.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bannas P, Bell LC, Johnson KM. Pulmonary embolism detection with three-dimensional ultrashort echo time MR imaging: experimental study in canines. Radiology. 2016;278(2):413–421. doi: 10.1148/radiol.2015150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roach DJ, Crémillieux Y, Fleck RJ, et al. Ultrashort echo-time magnetic resonance imaging is a sensitive method for the evaluation of early cystic fibrosis lung disease. Ann Am Thorac Soc. 2016;13(11):1923–1931. doi: 10.1513/AnnalsATS.201603-203OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheikh K, Guo F, Capaldi DP, et al. Ultrashort echo time MRI biomarkers of asthma. J Magn Reson Imaging. 2016;45(4):1204–1215. doi: 10.1002/jmri.25503. [DOI] [PubMed] [Google Scholar]
- 20.Ma W, Sheikh K, Svenningsen S, et al. Ultra-short echo-time pulmonary MRI: evaluation and reproducibility in COPD subjects with and without bronchiectasis. J Magn Reson Imaging. 2015;41(5):1465–1474. doi: 10.1002/jmri.24680. [DOI] [PubMed] [Google Scholar]
- 21.Ohno Y, Nishio M, Koyama H, et al. Pulmonary 3 T MRI with ultrashort TEs: influence of ultrashort echo time interval on pulmonary functional and clinical stage assessments of smokers. J Magn Reson Imaging. 2014;39(4):988–997. doi: 10.1002/jmri.24232. [DOI] [PubMed] [Google Scholar]
- 22.Dournes G, Menut F, Macey J, et al. Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur Radiol. 2016;26(11):3811–3820. doi: 10.1007/s00330-016-4218-5. [DOI] [PubMed] [Google Scholar]
- 23.Teufel M, Ketelsen D, Fleischer S, et al. Comparison between high-resolution CT and MRI using a very short echo time in patients with cystic fibrosis with extra focus on mosaic attenuation. Respiration. 2013;86(4):302–311. doi: 10.1159/000343085. [DOI] [PubMed] [Google Scholar]
- 24.Robison RK, Anderson AG, 3rd, Pipe JG. Three-dimensional ultrashort echo-time imaging using a FLORET trajectory. Magn Reson Med. 2016 doi: 10.1002/mrm.26500. in press. [DOI] [PubMed] [Google Scholar]
- 25.Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multispoke inversion recovery pulse sequence. Magn Reson Med. 2016;76(2):577–582. doi: 10.1002/mrm.25823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med. 2006;55(3):575–582. doi: 10.1002/mrm.20796. [DOI] [PubMed] [Google Scholar]
- 27.Vasanawala SS, Nguyen KL, Hope MD. Safety and technique of ferumoxytol administration for MRI. Magn Reson Med. 2016;75(5):2107–2111. doi: 10.1002/mrm.26151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai LM, Cheng JY, Alley MT, Zhang T, Lustig M, Vasanawala SS. Feasibility of ferumoxytol-enhanced neonatal and young infant cardiac MRI without general anesthesia. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25482. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanneman K, Kino A, Cheng JY, Alley MT, Vasanawala SS. Assessment of the precision and reproducibility of ventricular volume, function, and mass measurements with ferumoxytol-enhanced 4D flow MRI. J Magn Reson Imaging. 2016;44(2):383–392. doi: 10.1002/jmri.25180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng JY, Hanneman K, Zhang T, et al. Comprehensive motion-compensated highly accelerated 4D flow MRI with ferumoxytol enhancement for pediatric congenital heart disease. J Magn Reson Imaging. 2016;43(6):1355–1368. doi: 10.1002/jmri.25106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ning P, Zucker EJ, Wong P, Vasanawala SS. Hemodynamic safety and efficacy of ferumoxytol as an intravenous contrast agents in pediatric patients and young adults. Magn Reson Imaging. 2016;34(2):152–158. doi: 10.1016/j.mri.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Vasanawala SS, Hanneman K, Alley MT, Hsiao A. Congenital heart disease assessment with 4D flow MRI. J Magn Reson Imaging. 2015;42(4):870–886. doi: 10.1002/jmri.24856. [DOI] [PubMed] [Google Scholar]
- 33.Ruangwattanapaisarn N, Hsiao A, Vasanawala SS. Ferumoxytol as an off-label contrast agent in body 3T MR angiography: a pilot study in children. Pediatr Radiol. 2015;45(6):831–839. doi: 10.1007/s00247-014-3226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T, Cheng JY, Chen Y, Nishimura DG, Pauly JM, Vasanawala SS. Robust self-navigated body MRI using dense coil arrays. Magn Reson Med. 2016;76(1):197–205. doi: 10.1002/mrm.25858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the golden ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007;26(1):68–76. doi: 10.1109/TMI.2006.885337. [DOI] [PubMed] [Google Scholar]
- 36.Kazantsev IG, Matej S, Lewitt RM. Optimal ordering of projections using permutation matrices and angles between projection subspaces. Electron Notes Discret Math. 2005;20:205–216. [Google Scholar]
- 37.Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magn Reson Med. 2004;51(1):93–102. doi: 10.1002/mrm.10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng JY, Zhang T, Ruangwattanapaisarn N, et al. Free-breathing pediatric MRI with nonrigid motion correction and acceleration. J Magn Reson Imaging. 2015;42(2):407–420. doi: 10.1002/jmri.24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson KM, Block WF, Reeder SB, Samsonov A. Improved least squares MR image reconstruction using estimates of k-space data consistency. Magn Reson Med. 2012;67(6):1600–1608. doi: 10.1002/mrm.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uecker M, Ong F, Tamir JI, et al. Berkeley Advanced Reconstruction Toolbox. Proc. Intl. Soc. Mag. Reson. Med; Toronto, Ontario, Canada. 2015. p. 2486. [Google Scholar]