Abstract
BACKGROUND
Vascular cognitive decline is critically important in the course of atherosclerosis and stroke.
OBJECTIVE
To explore the hypothesis that carotid endarterectomy (CEA) by removing an unstable plaque may slow the course of vascular cognitive decline in both symptomatic and asymptomatic patients.
METHODS
Patients with clinically significant (>60%) carotid stenosis were studied preop and 1 yr post-CEA for clinical symptoms, vascular cognitive decline, instability of carotid plaque—presence of microemboli, brain white matter changes, and medical risk factors.
RESULTS
Forty-six percent were classically symptomatic. All patients showed vascular cognitive decline at presentation which correlated with degree of plaque instability. Significant white matter hyperintensity changes (48.7%) and cerebral emboli (25%) were also seen at baseline in both classically symptomatic and asymptomatic. One year after CEA, both groups showed no decline in cognitive function and significant improvement in 2 tests (P = .028 and P = .013). Brain white matter hyperintensities were unchanged. Microemboli were reduced but remained present (17.86%). Improvement was predicted by the presence of hypertension (P = .001), or less advanced cognitive decline preoperatively (P = .009).
CONCLUSION
This study demonstrates the importance of vascular cognitive decline in atherosclerotic disease. This is a function of the degree of instability of the atherosclerotic plaque more than the presence of stroke symptoms. It further suggests that atherosclerotic vascular cognitive decline need not be inevitable, and may be modified by treating hypertension and removal of the unstable plaque. This highlights the need for continued research on the cognitive effects of cerebrovascular disease and the synergistic benefits of intensive medical and surgical therapy.
Keywords: Cerebrovascular disease, Stroke, Cognitive decline, Strain, Emboli
ABBREVIATIONS
- ANOVA
analysis of variance
- CEA
carotid endarterectomy
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- HITS
high-intensity transient signals
- MRI
magnetic resonance imaging
- SD
standard deviation
- TCD
transcranial Doppler
- TIA
transient ischemic attack
- WAIS
Wechsler Adult Intelligence Scale
- WMH
White matter hyperintensities
Vascular cognitive decline is perhaps the most important outcome for aging patients with cerebrovascular disease. Traditionally, we have highlighted speech, motor, and sensory symptomatology. We now understand that the cognitive symptomatology of “silent strokes” can be cumulative and devastating.1,2 For every overt transient ischemic attack (TIA) or stroke, it is felt that 5 to 8 other nondetected events may take place.2,3 Imaging studies suggest that microembolic and microvascular infarcts may number as many as 11 million/yr in the United States, well above the 800 000 easily recognizable events which are followed by standard clinical exams.3-5 Further, a number of studies have suggested that the cumulative impact of such silent cerebrovascular events over time is vascular cognitive decline.6-13
Studies have shown the relationship of silent emboli with cognitive decline after cardiac surgery and Doppler-detected carotid emboli.2,14,15 The risk factors for vascular cognitive decline and Alzheimer's disease are similar with the earlier presentation of vascular cognitive decline being decrease in executive cognitive function, creativity, decision making, and reasoning prior to the profound memory loss which defines Alzheimer's dementia. We have previously shown significant cognitive decline in patients with advanced (>60% stenosis) carotid atherosclerotic disease.6 We have suggested that both emboli from unstable carotid plaque and microvascular angiogenesis are active in this process.6,8-11,16-17 This cognitive decline is predicted by the presence of significant physical instability in the plaque, and not the presence or absence of overt motor symptoms.6 We have further reported that the more unstable the carotid plaque (plaque strain), the more cognitively declined was the patient.8,10 While we understand that other sources of vascular cognitive decline may still be present, we here test the hypothesis that removing an unstable plaque may alter patterns of microemboli, and over 1 year, slow the course of vascular cognitive decline in carotid cerebrovascular disease.
METHODS
Participants
Forty-six patients underwent carotid endarterectomy (CEA) for established clinical indications (>60%) carotid stenosis. All patients were initially screened by a faculty member of the Comprehensive Stroke Program. Inclusion criteria were as follows: symptomatic carotid stenosis by North American Symptomatic Carotid Endarterectomy Trial criteria or asymptomatic Asymptomatic Carotid Atherosclerotic Study criteria; native English speaker; age 18 yr or older; preop for CEA under general anesthesia. Exclusion criteria were previous history of open carotid or endovascular surgery; considered unsuitable for CEA, and/or cervical radiation or lacked consent capacity. Twenty-five were symptomatic by previous stroke or TIA and positive computed tomography or magnetic resonance imaging (MRI) scans (M age = 69.52; standard deviation [SD] = 11.03; range = 43-85); 21 were identified as asymptomatic (mean age = 69.95, SD = 7.75; range = 59-84). All patients underwent a clinical screening for family history (cardiac/coronary, stroke/TIA, Alzheimer's), clinical demographics (gender, age, height, and weight), and medical history (hypertension, hyperlipidemia, diabetes, cancer, peripheral vascular disease, hypothyroid, surgical history, intake of statins, aspirin, etc). Traditional risk factors including blood pressure and diabetes are similar in both groups and did not predict cognition. No other differences in demographic variables were found across groups (see Table 1) ClinicalTrials.gov identifier: NCT02476396.
TABLE 1.
Participant Characteristics Displayed By Group
| Symptomatic | Asymptomatic | |
|---|---|---|
| Variable | (n = 25) | (n = 21) |
| Age (years) | 69.52 (11.03) | 69.95 (7.75) |
| *Gender (no./% female) | 8 (32.0%) | 13 (61.9%) |
| Estimated FSIQ | 107.59.35 (8.45) | 103.35 (7.12) |
| % Stenosis | 70.69 | 77.85 |
FSIQ: full-scale intelligence quotient.
*Significant (P ≤ .05) difference(s) across participant groups.
Neuropsychological Assessment
All patients were assessed before CEA. Follow-up testing occurred 1 yr following surgery and was identical to baseline testing. After the Institutional Review Board approval and informed consent, each participant underwent a 60-min neuropsychological test protocol following guidelines of the National Institute of Neurological Disorders and Canadian Stroke Network with tests of verbal and nonverbal memory, language, speeded psychomotor, executive function/attention, and visuospatial skills. For the current study, widely used verbal and performance IQ measures [Wechsler Adult Intelligence Scale (WAIS) version IV Information, Digit Span, and Block Design] were added (Table 2).
TABLE 2.
Neuropsychological Tests by Domain
| Domain | Ability | Test | References |
|---|---|---|---|
| Executive/attention | Semantic fluency | Animal naming | Isaacs and Kennie, 197318 |
| Letter fluency | COWAT: F-A-S | Benton, Hamsher, and de S Sivan, 199419; Ober et al, 198620 | |
| WAIS-IV: Digit Span | Wechsler, 200821 | ||
| Speeded psychomotor | Motor sequencing | Trail making test | Alexander, Stuss, and Fansabedian, 200322 |
| Coding | WAIS-IV: Digit Symbol-Coding | Wechsler, 200821 | |
| Language | Confrontation naming | BNT: second Edition, short form | Franzen, Haut, Rankin, and Keefover, 199523 |
| General knowledge | WAIS-IV: Information | Wechsler, 200821 | |
| Visuospatial | Figure reproduction | ROCFT: Copy | Rey, 194124; Osterrieth, 194425 |
| Visuospatial construction | WAIS-IV: Block design | Wechsler, 200821 | |
| Memory | Verbal memory | HVLT-R | Brandt and Benedict, 200126 |
| Nonverbal memory | ROCFT: 3' and 30' delayed recall | Rey, 194124; Osterrieth, 194425 |
COWAT: Controlled Oral Word Associates Test; WAIS-IV: Wechsler Adult Intelligence Scale—version IV; BNT: Boston Naming Test; HVLT-R: Hopkins Verbal Learning Test—revised; ROCFT: Rey-Osterrieth Complex Figure Test.
Cognitive Data Analysis and Statistics
We have previously reported baseline cognitive deficits in a subset of these patients relative to controls.6 In the current analysis, we formally examined effects of patient group (symptomatic status: yes/no) and time (baseline/follow-up) using a mixed analysis of variance (ANOVA) model. Published testing materials were used to age- and sex-norm all scores. We were primarily interested in the presence of group × time interactions, which would indicate that 1 group of patients showed an accelerated rate of decline or improvement relative to the other group in the 1-yr period after CEA. Associations between cognitive test scores and maximal plaque strain instability (axial, lateral, shear) values were assessed using partial correlations, controlling for age and sex. We next examined several demographic and clinical variables and their relation to change in cognitive performance from baseline to 1-yr follow-up. To form a metric of overall cognition, scores from each test were standardized and then subtracted from a common measure of premorbid IQ. The North Amercian Adult Reading Test (revised: NAART-R). Data for both baseline and follow-up testing sessions were used to create difference scores indicating change over 1 yr; all individual test data were then ranked and summed across tests using O’Brian's nonparametric rank sum method. Patients whose overall cognitive rank improved from baseline (“improvers”) were then compared to patients who showed movement in the opposite direction (“decliners”) to determine if clinical risk factors would predict outcome.
Ultrasound Strain and Transcranial Doppler Methods
Both ultrasound and transcranial Doppler (TCD) were done on all patients to determine the degree of plaque instability or strain, and presence of TCD recorded embolic high-intensity transient signals (HITS) by methods we have previously published.7,8,10 These data were reviewed by a blinded reviewer to quantify plaque instability and presence of ipsilateral and contralateral HITS on middle artery cerebral recording.
Estimation of the Ultrasound Strain Indices
Ultrasound B-mode from reconstructed radiofrequency data were acquired with an 18L6 linear array transducer and Siemens S2000 system (Siemens, Munich, Germany) at 40 Mhz. Plaque and adventitia were segmented utilizing the Medical Imaging Interaction Toolkit (Figure 1). Segmented plaque regions manually outlined on the end diastolic frame was utilized for semiautomated deformation tracking over 2 complete cardiac cycles to obtain displacement and strain maps using a hierarchical block-matching algorithm developed by our laboratory.
FIGURE 1.
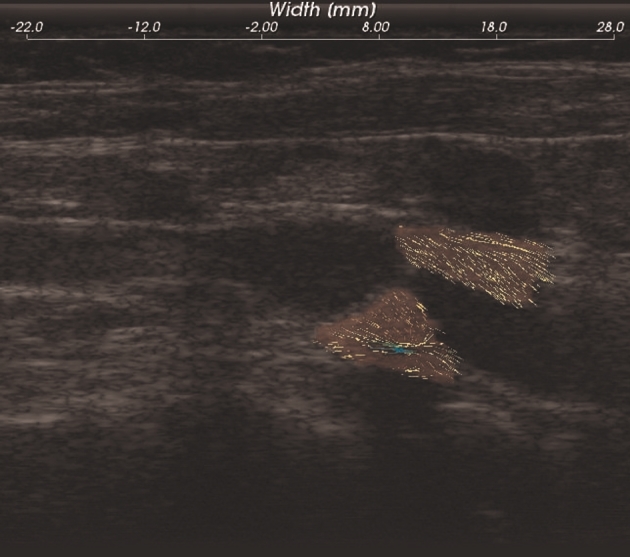
B-mode and segmented plaque with adventitia.
Strain is the gradient of displacement. Axial strain is defined as 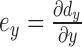 , lateral strain is defined as
, lateral strain is defined as 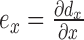 , and shear strain is defined as
, and shear strain is defined as 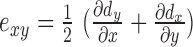 , where dy and dx represent the axial and lateral displacements, respectively. Positive strains indicate tissue expansion, while negative strain indicates tissue compression. The absolute value of the maximum strain over a cardiac cycle is used in our analysis.
, where dy and dx represent the axial and lateral displacements, respectively. Positive strains indicate tissue expansion, while negative strain indicates tissue compression. The absolute value of the maximum strain over a cardiac cycle is used in our analysis.
TCD and Cardiac Echo Imaging—Methods
The TCD examination was performed with a 2.0-Mhz transducer.
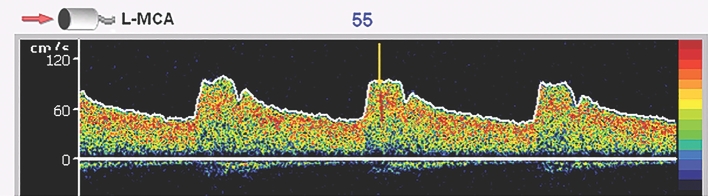
The following criteria were utilized to differentiate middle cerebral artery HITS suggesting microemboli from artifacts a transient (less than 300 ms in duration), unidirectional, high-intensity signal (detected by the ultrasound system; Figure 2), audible chirp (per the Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium), and a complex mode change. A physician and 2 observers reviewed all HITS for accuracy.
FIGURE 2.
Figure demonstrates Doppler signal from left middle cerebral artery with the HIT identified by the yellow arrow. This HIT was also accompanied by an audible chirp, a change in complex, and was less than 300 ms in duration.
MRI Methods
A subset of 24 subjects underwent 3-Tesla MRI on a GE x750 scanner. T1-weighted and T2 fluid-attenuated inversion recovery (FLAIR) scans were acquired in the axial and sagittal planes, respectively. The T1 was acquired with a 3-D fast spoiled gradient echo sequence, echo time (TE) = 3.2 ms, repetition time (TR) = 8.1 ms, inversion time (TI) = 450 ms; flip angle + 12° acquisition matrix = 256 × 256 mm; slice thickness = 1.0 mm; field of view (FOV) = 256 mm.
TI = 1868 ms; TR = 6000 ms; FOV = 256 mm; slice thickness = 2.0 mm; TE = 123 ms; acquisition matrix = 256 × 256 mm; flip angle = 90°; and no gap which yielded a voxel resolution of 1 mm × 1 mm × 2 mm.7
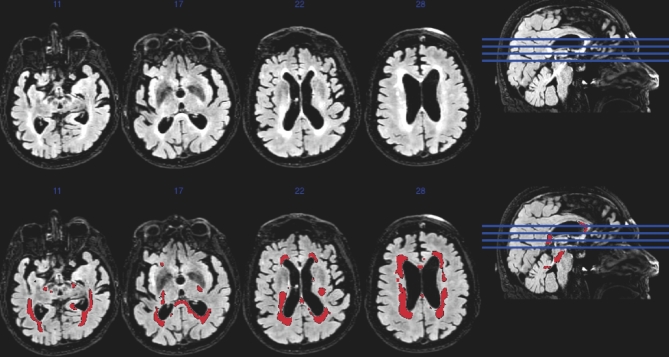
The Lesion Segmentation Toolbox segments the White matter hyperintensities (WMH) lesions.27 The T1 scan is used to determine the tissue class of all the voxels (cerebrospinal fluid, white matter, gray matter) and the T2-FLAIR is used to identify hyperintense voxels. These hyperintense voxels represent WMH, and subsequently, cumulative vascular injury (Figure 3).28 As a means to determine the relative degree of WMH ischemic injury in our pre-CEA population, we compared these subjects from the Alzheimer's Disease Research Center, a cognitively normal cohort (mean age 69, 57.84% female, 45.3% hypertensive, 15.6% diabetic, total cholesterol 190). Importantly, this cohort was acquired on the same scanner using the same sequences as were the subjects in the present study.7
FIGURE 3.
White matter hyperintensities in a symptomatic patient.
RESULTS
Clinical Course and Emboli
Of 46 study patients at 1 yr after surgery, 1 patient showed restenosis and 1 patient had a TIA neurological event since surgery. There were no strokes. TCD emboli HITS were recorded preoperatively and 1 yr after CEA in a subset of 28 subjects. HITS were well distributed through both symptomatic and asymptomatic patients and were present preoperatively in 25.0% (on the surgical side) of patients studied for 1 h. One year after CEA, emboli were present in 17.86% of patients treated.
MRI Brain White Matter Change
Brain white matter hyperintensity changes correlate to vascular cognitive decline and microvascular disease.29,30 Preoperatively, we have previously shown that carotid plaque instability and maximum shear strain predicted white matter changes with a R2 value of 0.287.7 In present sample of 24 patients, we saw significant white matter hyperintensities of 48.7 mL in preoperative patients. This compares to 25.3 mL in controls P < .001. Fourteen of the 24 patients with imaging underwent follow-up MRI at time 2. We did not see a statistically significant change in white matter burden 1 yr after CEA.
Cognition and Plaque Instability
Baseline cognition in these patients was assessed by administration of 14 standard neuropsychological test measures. It was decreased in all measures. The relationship between baseline cognition and strain was assessed by partial correlations (Table 3). Significance was assessed at P < .05. At least 1 maximum strain variable (lateral, axial, shear) predicted cognitive scores on 9 of 14 tests; the best predictor from the 3 strain variables is given below after each variable, along with the correlation coefficient. All analysis control for age and sex. Importantly, poor performance on tasks of category fluency (r = –.384, P = .014), speeded psychomotor processing (Trails A; r = –.478, P = .002), complex motor/executive function (Digit Symbol-Coding; r = 701, P < .001) and immediate verbal memory (r = –.367, P = .025) was associated with increased maximum shear strain. All other significant correlations were between cognition and maximum lateral strain: complex motor/executive function (Trails B; r = −.584, P < .001), working memory (Digit Span; r = –.363, P = .027), figure copy (r = −.353, P = .030), immediate nonverbal memory (r = −.352, P = .033), and delayed nonverbal memory (r = −.389, P = .017). All relationships were negative: high strain was always associated with poor cognition.8
TABLE 3.
Cognitive Improvement Over 1 Yr and Association With Clinical Factors
| Baseline variable | Correlation | Significance |
|---|---|---|
| Hypertension | 0.38 | .008 |
| Hyperlipidemia | –0.11 | .486 |
| Diabetes | 0.06 | .718 |
| Previous smoker | 0.17 | .300 |
| Fam Hx stroke/TIA | 0.11 | .486 |
| Preop cognition | 0.87 | <.001 |
Cognition at Baseline and 1 Yr Postop
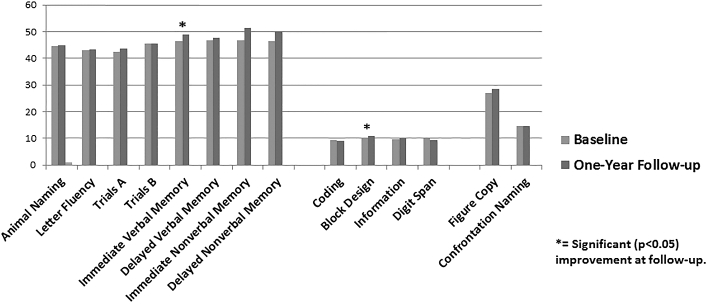
Preoperative test scores were rescaled to allow direct comparison with estimated premorbid IQ. Paired sample t-tests revealed that participant scores at presentation were lower for all tests that would be expected (with the exception of confrontation naming, which showed no difference) based on their estimated IQ (range of ts: 2.80-6.72; range of Ps: .0001-.008). In the current analysis, symptomatic and asymptomatic patients did not significantly differ on any measures of baseline cognitive decline. The effects of CEA over time were examined using a 2 (Group: symptomatic vs asymptomatic) x 2 (time: baseline and 1-yr follow-up) mixed-design ANOVA. Two tests, measures of visuoconstruction {WAIS-IV Block Design} (F[1, 38] = 6.855, P = .013) and immediate verbal memory (F[1,43] = 5.171, P = .028), showed a main effect for time. In both cases, patient performance improved at 1-yr postsurgery (Figure 4) compared to baseline. No measure of cognition significantly declined at year 1 after surgery. No test showed a symptomatic status × time interaction, indicating that both symptomatic and asymptomatic patient groups showed equivalent changes (or lack of change) over time in cognition.
FIGURE 4.
Cognitive performance at baselina and 1-yr follow-up. Patients showed significant improvement on tests of immediate verbal memory and block design; no tests indicated significant worsening over one year.
Effect of Traditional Risk Factors on Outcome
Finally, we examined the clinical risk factors to see if they predicted response to treatment. We found that baseline hyperlipidemia, diabetes, history of smoking, and family history of stroke/TIA did not predict the response of cognition 1 yr after treatment. However, we found that baseline hypertension (P = .009) and better baseline cognitive performance (P < .001) correlated with better cognitive outcome (Table 3).
While the populations showed a significant presence of these risk factors, it was equally distributed in symptomatic and asymptomatic patients (80.4% are hypertensive, 19.6% are diabetic, 76.1% history of smoking, 78.2% have hyperlipidemia). Both groups were maximally treated for these conditions.
DISCUSSION
We have previously shown the importance of instability or strain within a pulsating carotid plaque as being more important than the presence of stroke, TIA, or degree of stenosis in predicting the degree of vascular cognitive decline.8,10 This suggests that vascular wall strain is a measure of instability as well as an overall measure of systemic degree of atherosclerotic progression. In this report, a carefully studied group of patients were followed for their cognitive performance both before and 1 yr after endarterectomy. We have earlier shown that a surprising loss in cognitive function compared to estimated premorbid IQ and age-matched norms regardless of whether classically symptomatic or asymptomatic suggesting silent events, not visible to the typical neurological examination in asymptomatics. We have suggested that this cognitive decline may be related to emboli as well as atherosclerotic load and cerebral small vessel disease.6-11 In this report, the striking result is the similarity of the classically symptomatic and classically asymptomatic patients regarding cognition. Across the 14 cognitive test measures examined preoperatively, on no test did the symptomatic patients perform more poorly than asymptomatic patients. However, both groups were equally and significantly worse their estimated premorbid IQ across all cognitive areas. We have shown that strain predicts cognition, suggesting instability of the plaque either caused or heralded the vascular cognitive decline.6,8,10 Purandare and colleagues14,15 have shown that the presence of any middle cerebral artery emboli in 1 h testing premorbid is associated with vascular cognitive decline.
We do not believe the removal of the plaque alone is sufficient to explain all of the cognitive improvement. In our study, emboli are reduced, but not significantly. The microvascular changes which are described are present not only in the vessel wall of the unstable plaque, but in the brain white matter, suggesting a systemic disease that must be treated both surgically and medically. Nevertheless, reduction of emboli is a probable contributor to improvement.
Age-related cognitive decline is traditionally felt to be relentless. These results suggest that this may not be the case for vascular cognitive decline. We use 14 parameters of cognitive decline. Patients are declined in all of them at presentation. At 1 yr after CEA, no patient group, symptomatic or asymptomatic is further declined. All are stable, and when you look at the entire group, 2 of the major cognitive parameters are actually improved. This is a profound difference from the expected natural course and not a simple multitest variable change.
In looking at individuals rather than groups, the only baseline characteristics that correlated with improved cognition 1 yr after surgery were the presence of hypertension and lesser cognitive decline at presentation. All of these patients received intensive medical treatment for their risk factors from the time of coming to attention at time 1. The benefit of treating a modifiable risk factor like hypertension suggests that these are patients in whom additional medical interventions may have a synergistic benefit. This suggests that any cognitive benefits seen 1 yr after CEA are a result of not only the surgical results, but also the intensive medical management. The second predictor is similar to the findings seen in epilepsy surgery where benefits of surgery are best seen early in the process before irreversible damage takes place.31,32 Our results suggest that a similar process in the pathophysiology of vascular cognitive decline is present in patients with significant carotid atherosclerosis.
It is likely that the other classic risk factors are active in the initiation of atherosclerosis, but not as important as vascular instability in predicting the progression to cognitive decline. In our studies, all patients were maximally medically treated regarding their traditional risk factors, both at time of initiation and at time 2. It is, therefore, not surprising that they would not be able to independently show a cognitive difference between these groups from time 1 to time 2 by simply treating these risk factors. It is, however, interesting that adding the factors of plaque stability and surgical intervention did affect cognition, suggesting that these are very important factors in the cognitive disease process, even though they are not the only factors.
CONCLUSION
Further studies and larger groups will need to be done to fully understand the nature of this benefit of CEA on cognition, but the most profound result of this study is the recognition of the importance of vascular cognitive decline in cerebrovascular disease, its vital importance to the well-being our patients, and the possibility that combined medical and surgical interventions may halt or reverse some aspect of this disease. The present impact of cognitive decline in this population of atherosclerotic patients is devastating. Continued research is vital to ameliorate this condition.
Disclosures
This work was supported by the National Institutes of Health [grant number RO1 NS064034 PI: R. Dempsey], the Rath Distinguished Graduate Foundation Research Award (funded author Sara Berman), The Medical Scientist Training Program AH (funded author Sara Berman), Neuroscience Training Program MH, T32GM007507 (funded author Sara Berman), the Wisconsin's Alzheimer Disease Research Center P50-AG03351 (funded authors Sterling Johnson and Sara Berman). Dr Mitchell has Davies Publishing authorship for 2 Echocardiography textbooks, currently under review, may have future royalties, and Elsevier, Wolters Kluwer author textbook chapters may have future royalties. Dr Varghese has Research Agreement for Use of Ultrasound Research Interface with Siemens Ultrasound with no financial benefits. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgement
The authors would like to extend their deepest gratitude to all individuals who participated in this study.
REFERENCES
- 1. Dempsey RJ, Varghese T, Jackson DC et al. Carotid Atherosclerotic plaque instability and cognition determined by ultrasound-measured plaque strain in asymptomatic patients with significant stenosis. [Epub ahead of print Mar 10 2017] J Neurosurg. 2017. doi: 10.3171/2016.10.JNS161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dempsey RJ, Vemuganti R, Varghese T, Hermann BP. A review of carotid atherosclerosis and vascular cognitive decline: a new understanding of the keys to symptomology. Neurosurgery. 2010;67(2):484-493; discussion 493-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215-1222. [DOI] [PubMed] [Google Scholar]
- 4. Tyas SL, Salazar JC, Snowdon DA et al. Transitions to mild cognitive impair-ments, dementia, and death: findings from the Nun Study. Am J Epidemiol. 2007;165(11):1231-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leary MC, Saver JL. Annual incidence of first silent stroke in the United States: a preliminary estimate. Cerebrovasc Dis. 2003;16(3):280-285. [DOI] [PubMed] [Google Scholar]
- 6. Jackson DC, Sandoval-Garcia C, Rocque BG et al. Cognitive deficits in symptomatic and asymptomatic carotid endarterectomy surgical candidates. Arch Clin Neuropsychol. 2016;31(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berman SE, Wang X, Mitchell CC et al. The relationship between carotid artery plaque stability and white matter ischemic injury. Neuroimage Clin. 2015;(9):216-222. doi: 10.1016/j.nicl.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang X, Jackson DC, Mitchell CC et al. Classification of symptomatic and asymptomatic patients with and without cognitive decline using non-invasive carotid plaque strain indices as biomarkers. Ultrasound Med Biol. 2016;42(4):909-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X, Mitchell CC, Varghese T et al. Improved correlation of strain indices with cognitive dysfunction with inclusion of adventitial layer with carotid plaque. Ultrason Imaging. 2016;38(3):194-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Jackson DC, Varghese T et al. Correlation of cognitive function with ultrasound strain indices in carotid plaque. Ultrasound Med Biol. 2014;40(1):78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang X, Jackson DC, Mitchell CC et al. Estimation of ultrasound strain indices in carotid plaque and correlation to cognitive dysfunction. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:5627-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gorelick PB, Farooq MU. Cerebral microbleeds, cognition, and therapeutic impli-cations. JAMA Neurol. 2016;73(8):908-910. [DOI] [PubMed] [Google Scholar]
- 13. Akoudad S, Wolters FJ, Viswanathan A et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73(8):934-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Purandare N, Voshaar RC, Morris J et al. Asymptomatic spontaneous cerebral emboli predict cognitive and functional decline in dementia. Biol Psychiatry. 2007;62(4):339-344. [DOI] [PubMed] [Google Scholar]
- 15. Purandare N, Burns A. Cerebral microemboli in the genesis of dementia. J Neurol Sci. 2009;283(1-2):17-20. [DOI] [PubMed] [Google Scholar]
- 16. Türeyen K, Vemuganti R, Salamat MS, Dempsey RJ. Increased angiogenesis and angiogenic gene expression in carotid artery plaques from symptomatic stroke patients. Neurosurgery. 2006;58(5):971-977; discussion 971–977. [DOI] [PubMed] [Google Scholar]
- 17. Vemuganti R. All's well that transcribes well: non-coding RNAs and post-stroke brain damage. Neurochem Int. 2013;63(5):438-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Isaacs B, Kennie AT. The set test as an aid to the detection of dementia in old people. Br J Psychiatry. 1973;123(575):467-470. [DOI] [PubMed] [Google Scholar]
- 19. Benton A, Hamsher K, de S, Sivan A. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- 20. Ober BA, Dronkers NF, Koss E et al. Retrieval from semantic memory in Alzheimer-type dementia. J Clin Exp Neuropsychol. 1986;8(1):75-92. [DOI] [PubMed] [Google Scholar]
- 21. Wechsler D. Wechsler Adult Intelligence Scale – IV Edition. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 22. Alexander MP, Stuss DT, Fansabedian N. California Verbal Learning Test: performance by patients with focal frontal and non-frontal lesions. Brain. 2003;126(Pt 6):1493-1503. [DOI] [PubMed] [Google Scholar]
- 23. Franzen M, Haut M, Rankin E et al. Empirical comparison of alternative forms of the Boston naming test. Clin Neuropsychol. 1995;9:225-229. [Google Scholar]
- 24. Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Arch de Psychologie. 1941;28:286-340. [Google Scholar]
- 25. Osterrieth P. Le test de copie d’une figure complexe. Arch de Psychologie. 1944;30:206-356. [Google Scholar]
- 26. Brandt J, Benedict R. Verbal Learning Test-Revised Professional Manual. Lutz, FL: Psychological Assessment Resources, INC; 2001. [Google Scholar]
- 27. Schmidt P, Gaser C, Arsic M et al. An automated tool for detection of FLAIR-hyperintense whitematter lesions in multiple sclerosis. Neuroimage. 2012;59(4):3774-3783. [DOI] [PubMed] [Google Scholar]
- 28. Basic identification criteria of Doppler microembolic signals Consensus committee of the ninth international cerebral hemodynamic symposium. Stroke. 1995;26(6):1123. [PubMed] [Google Scholar]
- 29. Brickman AM, Siedlecki KL, Muraskin J et al. White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging. 2011;32(9):1588-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Birdsill AC, Koscik RL, Jonaitis EM et al. Regional white matter hyperin-tensities: aging, Alzheimer's disease risk, and cognitive function. Neurobiol Aging. 2014;35(4):769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skirrow C, Cross JH, Harrison S et al. Temporal lobe surgery in childhood and neuroanatomical predictors of long-term declarative memory outcome. Brain. 2015;138(pt 1):80-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puka K, Smith ML. Where are they now? Psychosocial, educational, and vocational outcomes after epilepsy surgery in childhood. Epilepsia. 2016;57(4):574-581. [DOI] [PubMed] [Google Scholar]