Abstract
Neuroketotherapeutics represent a class of bioenergetic medicine therapies that feature the induction of ketosis. These therapies include medium-chain triglyceride supplements, ketone esters, fasting, strenuous exercise, the modified Atkins diet, and the classic ketogenic diet. Extended experience reveals persons with epilepsy, especially pediatric epilepsy, benefit from ketogenic diets although the mechanisms that underlie its effects remain unclear. Data indicate ketotherapeutics enhance mitochondrial respiration, promote neuronal long-term potentiation, increase BDNF expression, increase GPR signaling, attenuate oxidative stress, reduce inflammation, and alter protein post-translational modifications via lysine acetylation and β-hydroxybutyrylation. These properties have further downstream implications involving Akt, PLCγ, CREB, Sirtuin, and mTORC pathways. Further studies of neuroketotherapeutics will enhance our understanding of ketone body molecular biology, and reveal novel central nervous system therapeutic applications.
Keywords: Ketone bodies, Ketogenic Diet, β-hydroxybutyrate, bioenergetics, Alzheimer’s disease, mitochondria
1. Introduction
Ketogenic therapies include any intervention that intentionally shifts the body into a state of ketone body production. Termed ketogenesis or ketosis, achieving a state of heightened ketone production has been used to treat epilepsy for nearly a century. The efficacy of ketogenic therapies to improve functional outcomes in epilepsy have increased interest in their translational potential to treat other neurologic disorders, including Alzheimer’s disease (AD) and stroke rehabilitation.
Ketogenesis can be achieved through multiple strategies including caloric restriction, administering medium chain triglycerides (MCT), strenuous exercise, and ketogenic diets (KD) which primarily feature fats and minimize carbohydrates (Gano et al., 2014). These generally well-tolerated interventions produce limited adverse effects. Ketotherapeutics still meet a considerable amount of scrutiny based on the association of ketones with the pathologic state of ketoacidosis, a complication of type I diabetes. It is important to recognize that these interventions produce a mild ketonemia, to about 5 mM, whereas ketoacidosis occurs when blood ketones enter a range of 10–25 mM. Ketoacidosis has not been found to manifest within the central nervous system microenvironment (Al-Mudallal et al., 1996).
While ketones can confer neurologic benefits, the mechanisms that underlie these benefits remain elusive. Here, we review the history of ketotherapeutics, potential mechanisms for their effects, and their therapeutic potential.
2. History of Ketotherapeutics
Ketogenic medicine perhaps dates back to ancient Greece. Most of the earliest writings on epilepsy were collected in On the Sacred Disease, part of the Hippocratic collection of works (Temkin, 1994; Wheless, 2008). This text illustrates that due to fears of demonic forces, society ostracized community members that suffered from fits, which today we understand to be epileptic seizures. Forced to fend for their meals in the wilderness, and being unequipped to accomplish this task, these persons would struggle to obtain adequate caloric intake. Interestingly, after undergoing forced prolonged fasts they experienced reduced seizure frequency and severity.
The Book of Matthew from the New Testament provides a similar account. In one passage, a father requested help from Jesus and his apostles in curing his son of fits. They advised him to pray and have the child fast, which resulted in the child’s recovery. The lower register of Raphael’s The Transfiguration depicts this story. This painting possibly represents the earliest graphical depiction of bioenergetic medicine in the historical record, even if that was not the intended purpose of the work.
Fasting therapies were more formally adopted for the treatment of epilepsy in 1911 by the French physicians Guelpa and Marie (Guelpa, 1911). Similar revivals were spearheaded in the United States by the physician Hugh Conklin and fitness celebrity Bernarr Macfadden (Wheless, 2008). H. Rawle Geyelin, an endocrinologist, was the first to report cognitive improvements occurred in patients adhering to fasting regimens (Geyelin, 1921). In the same year, fasting and low carbohydrate/high fat diets were found to increase levels of the ketone bodies β-hydroxybutyrate and acetoacetate in normal, healthy subjects and were suggested to mediate neurologic benefits in children (Wilder, 1921; Woodyatt, 1921). Later investigations into the efficacy of KDs to treat epilepsy in adults demonstrated seizures were completely controlled or improved in 56 of 100 patients (Barborka, 1928).
The KD remained a popular epilepsy treatment until the 1980’s, although its use gradually diminished with the advent of pharmaceutical therapies such as diphenylhydantoin, phenobarbital, and valproic acid. The KD enjoyed a resurgence in popularity following release of a made-for-TV movie, First Do No Harm, in 1997 and national media coverage on a television news program, Dateline, in 2000 (Wheless, 2008).
Increased popularity has expanded the number of ketogenic-related therapies. An increasing number of reports note the impact of ketotherapeutics on clinical phenotypes, cell physiology, and molecular physiology.
3. Molecular and Biochemical Ketone Biology
3.1. Ketogenesis
In mammals, ketosis results following prolonged periods of profound, reduced dietary intake. In this state, carbohydrate intake is low which results in decreased serum insulin and increased serum glucagon (Apfelbaum et al., 1972). This hormonal shift promotes hepatic glycogenolysis and gluconeogenesis to maintain euglycemia (Garber et al., 1974; Rui, 2014). A decline in insulin also promotes increased white adipose tissue lipolysis, which increases fatty acid circulation and β-oxidation (Vazquez-Vela et al., 2008).
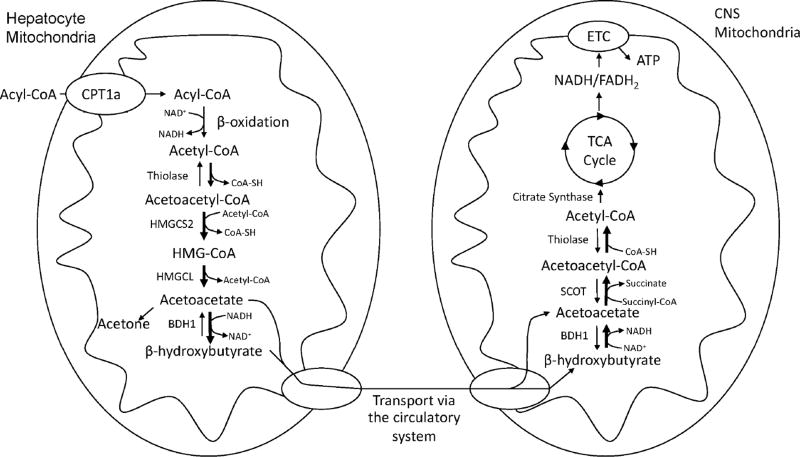
Avid fatty acid β-oxidation occurs in liver mitochondria, where it generates increased levels of acetyl-CoA as shown in Figure 1 (Drysdale and Lardy, 1953; Randle et al., 1963). Once the added acetyl-CoA surpasses the ability of the tricarboxylic acid (TCA) cycle to degrade it, it diverts to other needs such as cholesterol synthesis or ketogenesis (Baird et al., 1972; Garber et al., 1974). With ketogenesis, two molecules of acetyl-CoA are joined by thiolase to generate acetoacetyl-CoA (Middleton, 1972). A third molecule of acetyl-CoA is then added to produce β-hydroxy-β-methylglutaryl-CoA (HMG-CoA) in a reaction mediated by HMG-CoA synthase (Hegardt, 1999). This HMG-CoA synthase-catalyzed reaction represents the rate-limiting step of the ketogenesis pathway. HMG-CoA lyase then liberates a two-carbon group to produce one molecule each of acetyl-CoA and acetoacetate (Figure 2). Acetoacetate, therefore, represents, the first ketone body produced in the pathway (Puisac et al., 2012). Acetoacetate is further reduced by a molecule of NADH in a reaction mediated by β-hydroxybutyrate dehydrogenase 1 (BHD1) (Lehninger et al., 1960). This step produces the most abundant ketone found in circulation, β-hydroxybutyrate (Figure 2).
Figure 1. Ketogenesis and Ketolysis.
Hepatic mitochondria serve as the primary site for the production of serum ketone bodies. Acetyl-CoA generated from acyl-CoA β-oxidation is converted to HMG-CoA by the rate-limiting enzyme HMGCS2. HMG-CoA is further processed to the primary ketone bodies acetoacetate and β-hydroxybutyrate, which are released into the circulation via the monocarboxylate transporters (MCTs) 1 and 2. Ketone bodies enter the CNS via MCTs and are oxidized to acetyl-CoA through a series of reactions that require SCOT. Acetyl-CoA undergoes terminal oxidation through the TCA cycle to generate the high-energy electron carriers NADH and FADH2. Electrons enter the respiratory chain, leading to the generation of ATP through ATP synthase.
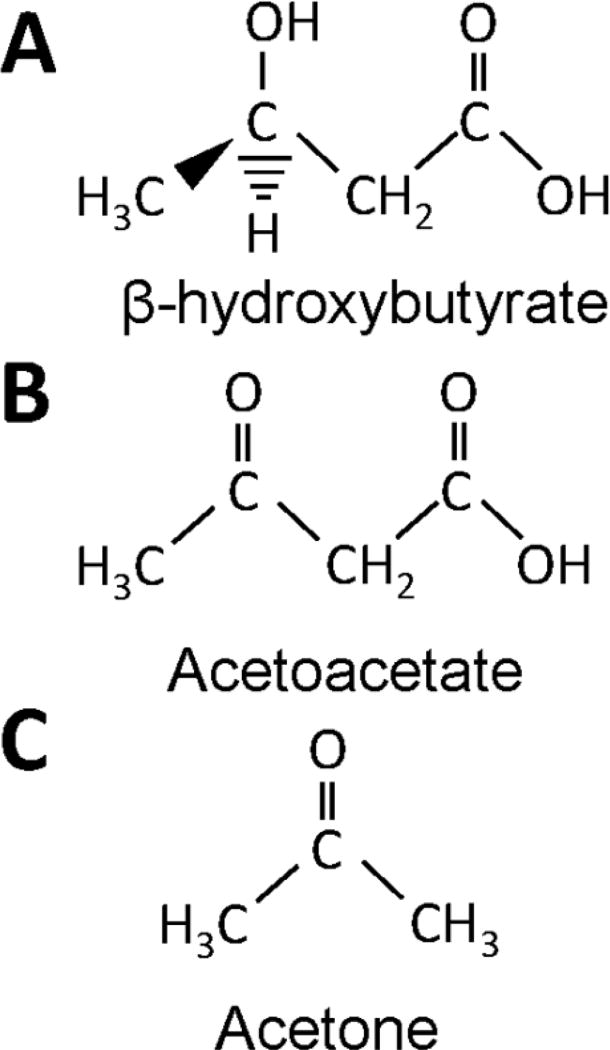
Figure 2. Chemical Structures of Biologically Relevant Ketone Bodies.
The physiologically relevant ketone bodies in order of decreasing serum concentrations are β-hydroxybutyrate (A), acetoacetate (B), and acetone (C). Acetone is highly volatile and is readily excreted via the pulmonary system or converted to lactate by the liver.
Deficiencies in HMG-CoA lyase prevent ketogenesis, and following periods of fasting HMG-CoA lyase deficiency produces the pathologic state of hypoketotic hypoglycemia. Interestingly, this condition also commonly associates with seizures (Fernandes et al., 2013; Fernandes et al., 2015).
Of minor note, a small portion of acetoacetate undergoes non-enzymatic decarboxylation to form acetone (Kalapos, 2003) (Figure 2). Acetone, while toxic in large concentrations, undergoes liver conversion via the methylglyoxal pathway. As acetone is highly volatile, when acetone production rates exceed conversion rates it is readily excreted by the pulmonary system. As a result, it does not reach appreciable levels under states of fasting or nutritional ketosis.
The net reaction is the synthesis of the two primary ketone bodies, β-hydroxybutyrate and acetoacetate, from two molecules of acetyl-CoA and the oxidation of one molecule of nicotinamide adenine dinucleotide (NADH). While the liver is certainly the primary organ for total body ketogenesis, though, other sites also produce ketone bodies. Increasing evidence reveals astrocytes perform ketogenesis when treated with medium chain triglycerides in vitro, which could play a role in regulating food intake (Le Foll et al., 2015a; Le Foll et al., 2014, 2015b; Le Foll and Levin, 2016). Ketones produced by astrocytes in response to increased dietary fat mediate this feedback by acting on ventromedial hypothalamic (VMH) neurons (Le Foll et al., 2014) that monitor nutrient status, including levels of glucose, lactate, and fatty acids.
Guzman and Blazquez proposed astrocyte-generated ketone bodies transfer to neurons (Guzman and Blazquez, 2001). Astrocytes are already known to perform a similar function, called the astrocyte-neuron lactate shuttle (ANLS), in which glucose is processed to lactate that then undergoes neuron consumption (Dienel, 2013). The possibility of an astrocyte-neuron ketone shuttle is certainly intriguing and underscores the need to investigate how metabolites influence distinct cell populations in the brain.
3.2. Ketolysis
Once produced by the liver, monocarboxylic acid transporters mediate the export of ketone bodies to the blood. This renders them available for extrahepatic catabolism and energy production (Hugo et al., 2012). The brain, heart, and muscle utilize ketone bodies (Fukao et al., 1997). Upon crossing the blood brain barrier, ketones are transported across cell plasma membranes via MCTs 1 and 2 in astrocytes and neurons, respectively (Vijay and Morris, 2014). Ketones are subsequently trafficked through the cytoplasm to the mitochondria, which serves as the primary site of ketone catabolism. Ketone catabolism largely features the reverse reactions that occur in ketogenesis, although specific parts of these opposing cycles are unique (Fukao et al., 1997).
The first step of ketone body catabolism is the oxidation of β-hydroxybutyrate to acetoacetate, with the concurrent reduction of NAD+ to produce NADH (Figure 1). BDH1 mediates this reaction. Acetoacetate is then converted to acetoacetyl-CoA by succinyl-CoA:3-ketoacid transferase (SCOT), also known as 3-oxoacid CoA-transferase 1 (OXCT1). As the name of this enzyme suggests, a CoA group is requisitioned from succinyl-CoA, thereby generating a molecule of succinate. The liver, which lacks SCOT, cannot consume ketones and this ensures its role as an exclusive ketone body producer. Lastly, acetoacetyl-CoA undergoes processing to two molecules of acetyl-CoA, which can then enter the tricarboxylic acid (TCA) cycle.
Ketones, upon contributing carbon to the TCA cycle, influence cell physiology in a number of ways. Forward progression through the TCA cycle generates the high-energy electron carriers NADH and flavin adenine dinucleotide (FADH2), which serve as substrates for the electron transport chain (ETC) and are necessary for the production of ATP from ADP in oxidative phosphorylation. This allows for a greater degree of nervous system bioenergetic plasticity, as it reduces glucose dependence and shifts the cell towards aerobic respiration. Indeed, ketone bodies have been shown to reduce glycolysis flux (LaManna et al., 2009). This likely occurs as part of a glycolysis negative feedback effect that arises due to increased NADH production.
Additionally, by supplying carbon to the TCA cycle ketone bodies increase TCA cycle anaplerosis (Owen et al., 2002). This in turn affects neurotransmitter levels as several TCA cycle intermediates serve as substrates for neurotransmitter synthesis. For example, α-ketoglutarate can exit the TCA cycle and undergo conversion to the major excitatory neurotransmitter, glutamate, or undergo decarboxylation to form an inhibitory neurotransmitter, γ-amino butyric acid (GABA). Acetyl-CoA itself combines with choline in the mitochondrial matrix to synthesize acetylcholine. Introducing ketone bodies to the nervous system not only effects bioenergetics, but also neurotransmitter levels.
3.3. Ketone bodies and mitochondrial function
How ketones influence mitochondrial efficiency is worth considering. In one study, supplementing the diet of C57BL/6J mice with a β-hydroxybutyrate-(R)-1,3-butanediol monoester ketone ester (KE) for 1 month was sufficient to induce ketonemia. The mice demonstrated increased expression of electron transport chain proteins, uncoupling protein (UCP) 1, and mitochondrial biogenesis signaling pathways in brown adipose tissue (Srivastava et al., 2012). Increased UCP expression in turn has multiple effects on mitochondrial physiology. First, in brown fat it increases heat production by shifting electron transfer energy away from ATP production. Second, increasing proton leak into the matrix can attenuate the generation of reactive oxygen nitrogen species (RONS) by helping to avoid matrix hyperpolarization. Data suggest that even a modest increase in proton leak can reduce hydrogen peroxide (H2O2) generation by as much as 70% (Echtay, 2007; Hansford et al., 1997; Votyakova and Reynolds, 2001).
A similar study examined the effects of a KE on the expression of brain UCP 4 and UCP 5 in Wistar rats. Brains of KE-supplemented rats exhibited a 1.5 fold increase in these UCPs (Kashiwaya et al., 2010). This is consistent with a prior study that reported caloric restriction increased rat brain UCP 4 expression, with neurons and astrocytes exhibiting the greatest and least expression of UCP4 respectively (Liu et al., 2006). A KD additionally upregulated UCP 2 and 4 expression in the brains and in particular the hippocampal dentate gyri of C3HeB/FeJ mice (Sullivan et al., 2004). Upregulation of UCP 4 and 5 in vitro protected against the complex I inhibitor 1-methyl-4-phenylpyridinium (MPP+), and the complex II inhibitor 3-nitroproprionic acid (3-NP) (Chu et al., 2009; Ho et al., 2006; Kwok et al., 2010; Ramsden et al., 2012; Wei et al., 2009). It appears that UCP-mediated neuroprotection requires signaling through the NF-κB pathway, and increased ATP production through increased complex II flux (Ho et al., 2012a; Ho et al., 2012b).
Ketotherapeutic-induced RONS attenuation extends beyond the upregulation of UCPs. Maintaining rats on a KD for 3 weeks increased levels of glutathione (GSH), a free radical scavenger. This increase was associated with an increase in glutamate cysteine ligase (GCL), the rate-limiting enzyme in GSH synthesis (Jarrett et al., 2008). It appears that KD-related increases in oxidative stress infrastructures are activated by initial increases in RONS, which induce oxidative stress response pathways through activation of its master regulator, nuclear factor erythroid 2-related factor (Nrf2) (Milder et al., 2010).
In rats, a KD increased brain expression of genes that accommodate oxidative phosphorylation and the TCA cycle including cytochrome c, isocitrate dehydrogenase, malate dehydrogenase, and subunits of succinate dehydrogenase, ATP synthase, cytochrome oxidase, and NADH dehydrogenase. This was associated with an electron microscopy-demonstrated rise in the number of hippocampal dentate gyrus mitochondria (Bough et al., 2006). It was unclear from this study, though, whether augmented mitochondrial mass was due to enhanced mitochondrial biogenesis, reduced mitophagy, or a combination of both.
3.4. Ketone bodies and post-translational protein modification
Increasing evidence suggests ketone bodies influence cell physiology independently, or at least indirectly, of their bioenergetic effects. Specifically, metabolites produced during ketone catabolism influence intracellular signaling by inducing changes in protein post-translational modifications (PTMs) (Newman and Verdin, 2014a, b).
This phenomenon was initially considered after it was recognized that butyrate, a short chain fatty acid whose structure differs from a ketone body only by the absence of a beta carbon hydroxyl group, affects histone acetylation (Stilling et al., 2016). In HEK293 cells and rodent kidney, increasing β-hydroxybutyrate levels inhibits histone deacetylases (HDACs) 1, 3, and 4 and consequently increases acetylation of key histone residues. This acetylation enhances FOXO3A-mediated gene transcription. A subset of the effected genes includes those responsible for mitigating oxidative stress such as manganese superoxide dismutase (MnSOD) and catalase (Shimazu et al., 2013). Similarly, in vitro exposure to β-hydroxybutyrate promotes activity of the EP300 family of lysine acetyltransferases (KATs) (Marosi et al., 2016). Since ketones appear to modulate a number of enzymes that control the cycling of protein acetylation in the cytoplasm and nucleus, it is likely that ketones alter other compartments in a similar manner. Ketones could influence activity of Sirtuin 3, the major mitochondrial deacetylase, especially considering their recognized trafficking into the mitochondrial matrix (Rardin et al., 2013).
Another exciting recent development in the area of ketone body-PTM relationships is the discovery that β-hydroxybutyrate itself can modify lysine residues (Xie et al., 2016). β-hydroxybutyrylation of histone lysines produces gene expression changes that recapitulate those of histone acetylation. TCA intermediates generated during ketone body metabolism-associated reactions may additionally function as epigenetic modifiers. In particular, changes in protein succinylation that arise through the actions of α-ketoglutarate dehydrogenase, or other succinylation enzymes under ketotic conditions, are suspected (Gibson et al., 2015).
3.5. Ketone bodies as extracellular signaling ligands
In addition to its ability to influence intracellular signaling, β-hydroxybutyrate may also function as an extracellular receptor ligand. It has been demonstrated to have agonistic effects on hydrocarboxylic acid receptor 2 (HCA2), otherwise known as the niacin receptor or G-Coupled Protein Receptor (GPR) 109A (Rahman et al., 2014). β-hydroxybutyrate is able to activate HCA2 with an EC50 near 700 µM, well below the approximately 5 mM concentrations observed in nutritional ketosis. HCA2 expression primarily occurs on the surface of cells derived from monocytes, including macrophages and microglia. Functioning as an inhibitory GPR, HCA2 activation suppresses cAMP levels, which ultimately reduces the production of proinflammatory cytokines. This suggests ketogenic therapies can produce a beneficial antiinflammatory effect, a prediction that has been experimentally verified (Selfridge et al., 2015). Other authors provide a more comprehensive review of the effects of HCA2 activation (Offermanns and Schwaninger, 2015).
Outside of the CNS, β-hydroxybutyrate has ligand effects on GPR41, also known as free fatty acid receptor 3 (FFA3). GPR41 is a Gi/o receptor previously shown to respond to the short chain fatty acid butyrate (Stilling et al., 2016; Won et al., 2013). Its expression is primarily restricted to sympathetic chain ganglia. Upon exposure to β-hydroxybutyrate, GPR41 suppresses the activity of the sympathetic nervous system through the Gβγ-PLCβ-MAPK signaling pathway (Kimura et al., 2011). Through this mechanism, ketones are able to modulate body energy expenditure and metabolic homeostasis.
3.6. Ketone bodies and activation of signaling pathways
Exogenous administration of ketones and ketones produced through vigorous exercise increase the expression of brain derived neurotrophic factor (BDNF) (Marosi et al., 2016; Sleiman et al., 2016; Swerdlow, 2014). BDNF acts as a ligand for TrkB family nerve growth factor (NGF) receptors, and exhibits weak agonistic effects on the p75 receptor. Upon binding TrkB, BDNF activates protein kinase B (Akt), phospholipase C gamma (PLCγ), and cAMP response element binding protein (CREB) signaling pathways (Baydyuk and Xu, 2014). Interestingly, BDNF expression increases following histone deacetylase inhibition in cortical neurons (Koppel and Timmusk, 2013). The deacetylase inhibition properties of ketones may in fact induce BDNF expression. The extent to which ketosis activates such pathways remains relatively understudied, and investigations into these interactions could potentially explain some ketotherapeutic effects.
Akt regulates cell growth through its downstream targets tuberous sclerosis (TSC) 1, TSC2, and mammalian target of rapamycin complex (mTORC) 1. It also enhances cell survival by inhibiting Bad, a pro-apoptotic factor. Akt interacts with the GABA receptor, ataxin-1, and the huntingtin protein (Manning and Cantley, 2007). Akt levels are typically low in the adult brain, but expression increases following injury in some models (Owada et al., 1997). Akt is protective in the setting of reduced trophic support, exposure to reactive oxygen species, and DNA damage (Chong et al., 2002, 2003; Chong et al., 2005; Henry et al., 2001; Kang et al., 2003; Matsuzaki et al., 1999).
PLCγ activation by BDNF signaling generates inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). IP3 and DAG, respectively, mobilize internal calcium stores and activate protein kinase C (PKC). Activation of these pathways has multiple effects including modulation of cell proliferation, migration, neurite outgrowth, synaptic plasticity, and survival. PLCγ expression primarily occurs in the cortex and hippocampus (Jang et al., 2013; Suh et al., 2008). PLCγ regulates N-methyl-D-aspartate (NMDA) receptor biology through neuregulin 1 (NRG1) (Gu et al., 2005). PLCγ facilitates hippocampal long-term potentiation (LTP), a process that facilitates the acquisition and storage of information at the synapse (Kandel, 2001; Minichiello et al., 2002). Given the role of PLCγ in cell survival and molecular mechanisms of learning, activation of PLCγ by ketone-induced BDNF signaling represents an intriguing potential mediator of ketotherapeutic effects.
CREB, typically activated by increases in cytosolic calcium or cAMP, has a recognized role in the regulation of circadian rhythms and memory formation (Silva et al., 1998). Disruption of CREB signaling pathways induces neurodegeneration of the hippocampus and dorsolateral striatum in mice lacking the related protein cAMP response element modulatory protein (CREM) (Mantamadiotis et al., 2002). Certain CREB isoforms are critical in learning and memory processes (Silva et al., 1998). For instance, on food preference tasks mice lacking CREBα show competent immediate memory but reduced memory at 24 hours (Kogan et al., 1997). Ketone-mediated modulation of CREB activity through BDNF could have important translational potential in age-related dementias.
It was recently demonstrated that BDNF also induces the expression of inhibin β-A by modifying nuclear responses to synaptic NMDA-associated calcium currents. Inhibin β-A subsequently inhibits extrasynaptic NMDA receptor-mediated calcium currents that can inhibit mitochondrial function and induce glutamate excitotoxicity-related cell death (Lau et al., 2015). Exploiting the protective effects of inhibin β-A could conceivably benefit conditions or diseases that feature reduced BDNF signaling.
4. Relevance to Development and Neurologic Disease
4.1. Ketones in Development
Ketone bodies play a critical role during normal brain development (Cotter et al., 2011; Cunnane et al., 2016). Oxidation of ketones by the brain begins during fetal development (Adam et al., 1975). Mammalian neonates are largely reliant on maternal breast milk as their primary nutrition source. Maternal milk is rich in dietary fats and has a high ketogenic ratio due to its abundance of medium chain fatty acids (Breckenridge and Kuksis, 1967; Hilditch, 1944; Insull and Ahrens, 1959; Nehlig, 1999). Unlike adult ketosis, neonatal ketosis occurs independently of whether or not the infant is in a fed state (Lindblad et al., 1977). Despite the fact that long chain fatty acids do not freely diffuse into the brain, a robust amount of neonate brain energy production relies on ketone metabolism (Nehlig and Pereira de Vasconcelos, 1993). Blocking ketogenesis enhances seizure severity in rat pups (Minlebaev and Khazipov, 2011).
The importance of ketones to the developing brain extend well beyond their role in bioenergetics. Ketones serve as the primary substrate for lipid synthesis during periods of rapid brain growth (Cunnane and Crawford, 2003; Freemantle et al., 2006). Indeed, neonatal ketogenesis appears so essential to development that even the neonatal intestine is capable of ketogenesis (Bekesi and Williamson, 1990).
4.2. Epilepsy
Epilepsy involves the aberrant, synchronous depolarization of neurons in the central nervous system. It generally manifests as paroxysmal disruptions of awareness, consciousness, and motor activity. Epilepsy affects 1% of the United States population and up to as many as 50 million people globally (Hirtz et al., 2007; Kobau et al., 2008; McNally and Hartman, 2012). As described previously, epilepsy represents the first condition that intentionally utilized ketotherapeutics. A modern randomized clinical trial in humans demonstrated that the KD was able to reduce seizures in 75% of pediatric epileptics over a three-month period (Neal et al., 2008). Despite their modern use extending back by as much as a century, its therapeutic mechanism remains a matter of much debate.
Anticonvulsant properties of acetoacetate were first demonstrated in rabbits administered thujone to produce seizures (Keith, 1935). Given that glutamate excitotoxicity represents one possible cause of neuronal damage from seizures, efforts were made to investigate whether ketotherapeutics worked by attenuating neuronal excitation by modulating neurotransmitter balance or release. There is some evidence to suggest that ketotherapies increase GABA. In rats, acetoacetate and β-hydroxybutyrate increased the accumulation of GABA in rat presynaptic vesicles (Erecinska et al., 1996). Magnetic resonance spectroscopy of humans maintained on a KD also showed increased brain GABA levels (Wang et al., 2003). Further, ketotic rats exhibit lower levels of glutamate in neurons but stable amounts of GABA. Since GABA is synthesized from glutamate, this indicates a greater proportion of glutamate may be converted to GABA, and thereby shift the total balance of these neurotransmitters towards inhibition (Melo et al., 2006).
Reducing glutamate release could also potentially reduce seizure-associated glutamate excitotoxicity. Acetoacetate reversibly inhibits glutamate release in mouse hippocampal slices and cultured rat neurons at high concentrations of 10 mM (Juge et al., 2010). Others have noted this effect may not have physiologic relevance as extracellular levels of β-hydroxybutyrate in the hippocampal microenvironment were determined to be between 40–50 µM following three weeks of KD (McNally and Hartman, 2012; Samala et al., 2011). In light of this, the role of ketotherapeutics in modulating neurotransmitter balance remains speculative.
Ketotherapeutics may modulate neuronal excitability independent of influencing neurotransmitter levels. As previously discussed, following their entry into the TCA cycle ketones enhance the production of ATP. ATP levels could influence the activity of membrane ion pumps and thus alter neuron membrane potential. In this regard, ATP-sensitive potassium (KATP) channels are of particular interest given their recognized activation upon changes in the ATP/ADP ratio, and their effects on membrane polarization. Tanner et al. demonstrated that β-hydroxybutyrate enhances KATP channel opening in cultured mouse hippocampal neurons (Tanner et al., 2011). Cultured GABAergic substantia nigra pars reticulata (SNpr) neurons exhibited reduced firing rates in the presence of either acetoacetate or βhydroxybutyrate. However, this effect disappeared following knockout of the potassium inward rectifier channel Kir6.2 or in the presence of the KATP channel inhibitor tolbutamide (Deransart et al., 2003). Consequently, it is possible that neuroprotection from seizure events could be mediated through reduced cell firing rates that result from changes in neuron membrane potential, and not the suppression or alteration of total neurotransmitter concentrations.
Voltage sensitive potassium channel Kv1.1 knockout mice maintained on a KD exhibited increased nuclear localization of the peroxisome proliferator activated receptor-γ 2 (PPARγ2) splice variant. This was associated with a 70% reduction in seizure frequency. The PPARγ antagonist GW9662 eliminated this benefit. Further, the anti-seizure effects of the KD were mimicked in Kv1.1 KO mice when administered the PPARγ agonist pioglitazone (Simeone et al., 2017). Modulation of PPARγ activity presents an attractive and readily available target for novel epilepsy therapeutics.
4.3. Alzheimer’s Disease
AD is the most common form of dementia and involves progressive neurodegeneration that causes impaired memory and a reduced ability to perform activities of daily living. Theories vary regarding the underlying etiology of the overall disease process, but glucose hypometabolism, mitochondrial dysfunction, extracellular amyloid-β (Aβ) plaque accumulation, and intracellular tau protein neurofibrillary tangles are recognized biochemical and histological hallmarks (Swerdlow, 2011, 2012a, b).
Emerging evidence suggests defects in mitochondrial function and a consequential decline in respiratory chain function alter amyloid precursor protein (APP) processing to favor the production of the pathogenic Aβ fragment (Wilkins and Swerdlow, 2016). Several groups have shown that both APP and Aβ co-localize with mitochondria, suggesting the possibility that mitochondrial function and APP biology interact (Devi et al., 2006; Hansson Petersen et al., 2008). Ketone bodies block mitochondrial amyloid entry and improve cognition (Yin et al., 2016). This ability would predictably ameliorate Aβ-mediated suppression of respiratory chain function and could perhaps to some rescue the bioenergetic hypometabolism observed in AD brains (Swerdlow, 2012c). Alternatively, improving mitochondrial performance outright could reduce the production of Aβ and increase the production of soluble APPα, a molecule with trophic properties capable of binding the BDNF receptor p75 to promote neurite growth (Hasebe et al., 2013).
Fluorodeoxyglucose positron emission tomography (FDG-PET) studies find asymptomatic individuals with an increased AD risk show less prefrontal cortex, posterior cingulate, entorhinal cortex, and hippocampal glucose uptake than normal-risk individuals (de Leon et al., 2001; Ishii et al., 1997; Mosconi et al., 2011; Mosconi et al., 2008a; Mosconi et al., 2008b; Mosconi et al., 2009; Mosconi et al., 2008c; O’Dwyer et al., 2011; Reiman et al., 2004; Rosenbloom et al., 2011; Spulber et al., 2010; Villain et al., 2010; Vlassenko et al., 2010). This reduction associates with the downregulation of the glucose transporter GLUT1 in the AD brain (Winkler et al., 2015). Further, increasing evidence suggests that increased insulin resistance contributes to the development of sporadic AD (de la Monte, 2009).
Clinical studies suggest ketogenic therapies may benefit AD patients. Medium chain triglyceride and ketone ester supplements raised plasma ketone levels and improved cognition in AD subjects (Henderson, 2008; Newport et al., 2015; Reger et al., 2004). This is in agreement with epidemiological studies that report positive associations between ketotherapeutic use and reduced AD risk (Henderson, 2008; Morris, 2005).
Preclinical studies performed using the triple transgenic mouse model of AD demonstrated reduced pathology following the administration of the ketogenic modulator 2-deoxy-D-glucose (2-DG), which inhibits glycolysis and increases cell reliance on respiratory chain oxidative phosphorylation (Yao et al., 2011a; Yao et al., 2011b). Further, PET studies using radiolabeled acetoacetate and β-hydroxybutyrate demonstrated single compartment kinetics of brain ketone body utilization, matching levels and uptake over a large range of concentrations (Blomqvist et al., 2002; Blomqvist et al., 1995). Importantly, brain uptake of acetoacetate did not significantly differ either globally or regionally in early stage AD patient brains, as compared to age-matched controls (Castellano et al., 2015). Of note, increased astrocyte metabolism appeared to mitigate Aβ-related impairment of memory function in day old chicks, which implies bioenergetic benefits require astrocyte function (Gibbs et al., 2009). It remains unclear if the brain’s capacity to utilize ketones in general declines with age.
Catabolism of white matter lipids, with the purpose of providing substrate for astrocytic ketogenesis, may occur in the aging female brain (Klosinski et al., 2015). This could mitigate neurocognitive decline by compensating metabolism defects with ketolysis (Bartzokis et al., 2004a; Bartzokis et al., 2004b; Brinton, 2008; Carmichael et al., 2010; Kuczynski et al., 2010). While ketone compensation may delay the onset of clinical symptoms, this process could lead to white matter demyelination and its associated clinical consequences (Morris, 2005). It would be interesting to know if providing increased dietary fats sustains ketogenesis and reduces white matter scavenging in the aged brain, thereby extending the window of ketone compensation and delaying the onset of AD symptoms. In support of this possibility, rats fed a high-fat diet rich in lipoic acid reportedly improved their performance on a novel object recognition task (Rodriguez-Perdigon et al., 2016).
4.4. Stroke
Stroke typically refers to an acute interruption of blood flow to a region of the brain that results in cell injury and cell death. Stroke carries significant morbidity and societal impact, and it is currently the fourth leading cause of death in the United States (Ovbiagele and Nguyen-Huynh, 2011). The majority of approved therapies to prevent and treat stroke focus on the prevention and dissolution of blood clots to maintain patent blood vessels. Therapeutics that can improve biochemical plasticity and confer resistance to the sequelae of ischemia could provide a strategy for reducing injury during and improving rehabilitation after stroke.
Supplying an oxidative phosphorylation-promoting intermediate would seem unlikely to enhance energy production within the ischemic core of an infarct. However, as discussed the physiologic role of ketones extend far beyond their direct role in bioenergetic pathways. Providing ketone bodies to the penumbra, the region of brain tissue lying directly adjacent to the ischemic core, may spare glucose for the core, thereby helping to sustain energy production in the absence of molecular oxygen (Gibson et al., 2012).
Preclinical studies in mice demonstrated that the KD and BHB reduce total infarct volume induced by permanent middle cerebral artery occlusion (MCAO) (Puchowicz et al., 2008; Rahman et al., 2014). Interestingly, this effect does not occur in HCA2 knockout mice, which suggests this benefit may rely on anti-inflammation signaling pathways. A similar phenomenon was reported in rodent models of ischemic stroke treated with BHB, which led to reductions in infarct volume, reduced brain edema, and improvements in motor function (Suzuki et al., 2002; Suzuki et al., 2001). This study did not determine, though, whether HCA2 signaling mediated these effects.
4.5. Traumatic Brain Injury
Epidemiologic studies link traumatic brain injury (TBI) to AD, PD, ALS and the recently described condition of chronic traumatic encephalopathy (CTE) (Bruce et al., 2015). Current therapies for TBI primarily consist of behavioral therapy and the pharmaceutical treatment of associated symptoms such as depression. We need therapies that can enhance TBI recovery, or can precondition high-risk individuals towards lesser injuries.
In rats, post-TBI administration of β-hydroxybutyrate reduced contusion volume in an age-dependent manner (Prins et al., 2005; Prins and Matsumoto, 2014). This associated with a reversal of injury-related ATP reduction (Prins et al., 2004). Recently, activation of the Akt/GSK-3β/β-catenin signaling pathway was found to enhance neuron survival in a TBI rat model (Zhao et al., 2012a). Ketone activation of Akt signaling, therefore, could offer a potential strategy for preserving neuron integrity.
It is important to note a study reported β-hydroxybutyrate increased blood-brain barrier permeability in a TBI rat model (Orhan et al., 2016). This may contribute to increased inflammation due to the exposure of brain parenchyma to pro-inflammatory blood components. The cost-benefit utility of ketotherapeutics in TBI, therefore, requires careful consideration.
4.6. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a neurodegenerative disorder that features the demise of upper and lower motor neurons (Ji et al., 2017). Disease progression manifests as progressive muscle weakness and paralysis. Once respiratory muscle involvement occurs, ventilation compromise ensues and ultimately the disease concludes in death. As is the case with AD, the majority of ALS cases are sporadic but a subset of familial cases arise from autosomal dominant mutations. One well-studied causal mutation occurs in the gene that encodes superoxide dismutase 1 (SOD1). Two ketogenic therapy studies, which evaluated a KD and an MCT, reported improved motor performance in SOD1-G93A transgenic mice. In both studies, ketotherapeutics reduced the loss of lower motor neurons in the spinal cord ventral horn. However, neither study appeared to benefit survival outcomes (Zhao et al., 2012b; Zhao et al., 2006).
Another common feature of the disease, one that it shares with frontotemporal dementia (FTD), is a cytoplasmic accumulation of the ubiquitinated nuclear protein transactive response DNA-binding protein 43 (TDP-43). Recent studies demonstrate the toxic mechanism of TDP-43 requires its localization to mitochondria, where it reduces the expression of mitochondrial DNA-encoded proteins (Wang et al., 2016). As ketone bodies seem able to modify mitochondria-Aβ interactions, it would be interesting to see whether they similarly affect mitochondria-TDP43 interactions.
4.7. Huntington’s Disease
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder that presents with involuntary choreiform movements, impaired cognition, and psychiatric symptoms. It effects 5 to 8 people per 100,000 in Europe and North America. Disease pathogenesis involves trinucleotide repeat expansion of CAG residues in the huntingtin gene. The resulting polyglutamine-expanded protein undergoes errors in protein folding and toxic accumulation that ultimately leads to impaired neuron function and cell death. It primarily affects structures in the basal ganglia, which manifests grossly as caudate atrophy (Ross and Tabrizi, 2011).
Lim et al. provided initial support for the therapeutic potential of ketone bodies in HD. The investigators administered subcutaneous BHB to 3-nitroproprionic acid (3-NP) toxin and R6/2 genetic mouse models of HD. BHB attenuated motor dysfunction in both models. BHB-treated R6/2 mice also saw an extension of lifespan, as well as increased striatal histone acetylation (Lim et al., 2011). Further investigation in PC12 cells demonstrated the increase in histone acetylation occurred independent of mitochondrial function or HDAC inhibition. Additional testing of ketogenic therapies in HD appears warranted.
4.8. Parkinson’s Disease
Parkinson’s disease (PD) affects between 100 to 200 people per 100,000 over the age of 40, and its incidence increases with age. Disease pathogenesis involves a progressive neurodegeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The loss of these neurons manifests as progressive bradykinesia, tremors, and rigidity. Late stages of disease often feature increasing cognitive dysfunction and dementia (Kalia and Lang, 2015).
Preclinical studies in the MPTP mouse model show that infusing BHB or a KD reduces dopaminergic neuron degeneration, improves motor deficits, reduces microglial activation, and reduces expression of pro-inflammatory cytokines (Tieu et al., 2003; Yang and Cheng, 2010). Similarly, a KD reduces dopamine neuron loss in the 6-hydroxydopamine PD rat model (Cheng et al., 2009).
In a five participant pilot study of PD patients, a 28-day KD improved unified Parkinson’s disease rating scale (UPDRS) scores. The small size of this study and the lack of a control group, though, precludes conclusive inferences (Vanitallie et al., 2005).
4.9. Multiple Sclerosis
Multiple sclerosis (MS) features an autoimmune-associated demyelination of CNS axons. It most often presents in a relapsing-remitting fashion that can include varying involvement of optic, sensory, motor, or coordination systems. After trauma, MS is the next most common CNS-related cause of young adult permanent disability (Noseworthy et al., 2000; Ramagopalan and Sadovnick, 2011).
Preclinical data from the mouse experimental autoimmune encephalomyelitis (EAE) multiple sclerosis model indicate a KD improves outcomes. In their 2012 study, Kim et al. demonstrated a KD reduced brain inflammation, mitigated motor disability, preserved CA1 long-term potentiation, and improved spatial learning and memory as assessed by the Morris water maze. The KD also induced a reduction in reactive oxygen species in conjunction with reduced pro-inflammatory cytokines (Kim et al., 2012). The anti-inflammatory properties of the KD are in line with suggested benefits as discussed previously. It would be interesting to know if the HCA2 pathway modulates these effects.
5. Conclusions
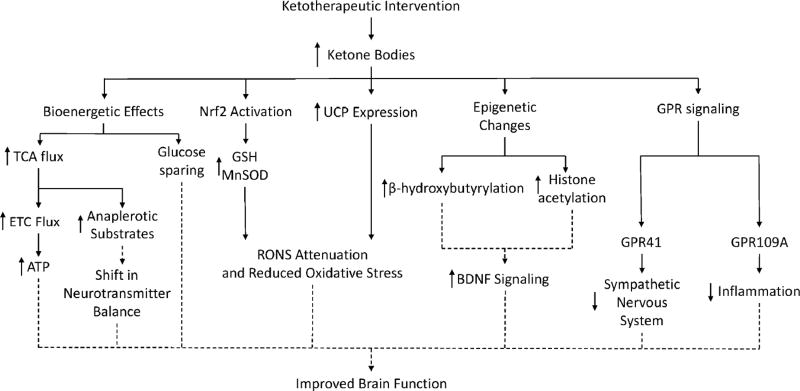
Fatty acid β-oxidation by hepatocytes and astrocytes generates the primary ketone bodies, β-hydroxybutyrate and acetoacetate. These ketones are taken up and catabolized by neurons, and help produce energy via oxidative phosphorylation. Recent work suggests that ketone bodies or interventions that serve to produce or introduce ketone bodies provide neurologic benefits that extend beyond their direct role in bioenergetic fluxes. Specifically, ketones affect protein post-translational modifications, attenuate RONS production, increase anti-oxidant stress response pathway expression, modulate GPR and BDNF signaling pathways, contribute to anaplerosis, and exhibit anti-inflammatory effects (Figure 3).
Figure 3. Pleiotropic Effects and Hypothesized Benefits of Ketotherapeutics.
Multiple ketotherapeutics produce an increase in serum ketone bodies. Ketone bodies increase oxidative metabolism, reduce oxidative stress, alter epigenetic protein post-translational modifications, increase BDNF signaling, and signal through GPRs in various model systems. Many of these effects require further study to determine the underlying mechanism of action and ultimate therapeutic value. Observed and predicted effects are indicated with solid and dashed lines respectively.
Ketotherapeutic approaches, therefore, may influence the nervous system through a variety of mechanisms. Their potential for treating a variety of neurologic conditions warrants consideration. It is further worth considering whether ketotherapeutics can promote healthy aging, an effect that could consequently delay the development or onset of age-dependent neurologic diseases.
Highlights.
Several approaches exist for achieving ketosis
Ketosis approaches produce pervasive molecular effects
These approaches may potentially benefit multiple neurologic conditions
Acknowledgments
The authors are supported by the University of Kansas Alzheimer’s Disease Center (NIA P30AG035982), a Ruth L. Kirschstein National Research Service Award (T32 HD057850), and a Mabel A. Woodyard Fellowship from the University of Kansas Institute for Neurological Discoveries.
Abbreviations
- 2-DG
2-deoxy-D-glucose
- Aβ
amyloid-β
- Acetyl-CoA
acetyl-coenzyme A
- ADP
adenosine diphosphate
- Akt
protein kinase B
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- AMP
adenosine monophosphate
- APP
amyloid precursor protein
- ANLS
astrocyte-neuron lactate shuttle
- ATP
adenosine triphosphate
- BDNF
brain derived neurotrophic factor
- BHB
β-hydroxybutyrate
- BHD1
β-hydroxybutyrate dehydrogenase 1
- cAMP
cyclic AMP
- CNS
central nervous system
- CREB
cAMP response element-binding protein
- CREM
cAMP response element modulatory protein
- CTE
chronic traumatic encephalopathy
- DAG
diacylglycerol
- EAE
experimental autoimmune encephalomyelitis
- ETC
electron transport chain
- FADH2
flavin adenine dinucleotide
- FDG-PET
fluorodeoxyglucose positron emission tomography
- FFA3
free fatty acid receptor 3
- FTD
frontotemporal dementia
- GABA
v-aminobutyric acid
- GCL
glutamate cysteine ligase
- GPR
G-coupled protein receptor
- GSH
glutathione
- GSK-3β
glycogen synthase kinase-3β
- HCA2
hydrocarboxylic acid receptor 2
- HD
Huntington’s disease
- HDAC
histone deacetylase
- HMG-CoA
β-hydroxy-β-methylglutaryl-coenzyme A
- H2O2
hydrogen peroxide
- IP3
inositol 1,4,5-triphosphate
- KAT
lysine acetyl-transferase
- KATP
ATP-sensitive potassium channels
- KD
ketogenic diet
- KE
ketone ester
- Kir
potassium inward rectifier channel
- KV
voltage sensitive potassium channel
- LTP
long term potentiation
- MAPK
mitogen activated protein kinase
- MCAO
middle cerebral artery occlusion
- MCT
medium chain triglyceride
- MnSOD
manganese superoxide dismutase
- MPP+
1-methyl-4-phenylpyridinium
- MS
multiple sclerosis
- mTORC
mammalian target of rapamycin complex
- NADH
nicotinamide adenine dinucleotide
- NF-kB
nuclear factor kappa-light chain-enhancer of activated B cells
- NG1
neuregulin 1
- NGF
nerve growth factor
- NMDA
N-methyl-D-aspartate
- NP-3
3-nitroproprionic acid
- Nrf2
nuclear factor erythroid 2-related factor
- OXCT1
3-oxoacid coenzyme A transferase 1
- PD
Parkinson’s disease
- PKC
protein kinase C
- PLCβ
phospholipase-C β
- PLCγ
phospholipase-C γ
- PPARγ
peroxisome proliferator activated receptor-γ
- PTM
post-translational modification
- RONS
reactive oxygen nitrogen species
- SCOT
succinyl-coenzyme A:3-ketoacid transferase
- Sirt
Sirtuin
- SNpc
substantia nigra pars compacta
- SNpr
substantia nigra pars reticulata
- TBI
traumatic brain injury
- TCA
tricarboxylic acid
- TDP-43
transactive response DNA binding protein 43 kDa
- TSC
tuberous sclerosis
- UCP
uncoupling protein
- UPDRS
unified Parkinson’s disease rating scale
- VMH
ventromedial hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors have no disclosures.
References
- Adam PA, Raiha N, Rahiala EL, Kekomaki M. Oxidation of glucose and D-B-OH-butyrate by the early human fetal brain. Acta Paediatr Scand. 1975;64:17–24. doi: 10.1111/j.1651-2227.1975.tb04375.x. [DOI] [PubMed] [Google Scholar]
- Al-Mudallal AS, LaManna JC, Lust WD, Harik SI. Diet-induced ketosis does not cause cerebral acidosis. Epilepsia. 1996;37:258–261. doi: 10.1111/j.1528-1157.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- Apfelbaum M, Lacatis D, Reinberg A, Assan R. Persisting circadian rhythm in insulin, glucagon, cortisol etc. of healthy young women during caloric restriction (protein diet) Rev Med Chir Soc Med Nat Iasi. 1972;76:123–130. [PubMed] [Google Scholar]
- Baird GD, Heitzman RJ, Hibbitt KG. Effects of starvation on intermediary metabolism in the lactating cow. A comparison with metabolic changes occurring during bovine ketosis. Biochem J. 1972;128:1311–1318. doi: 10.1042/bj1281311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barborka CJ. Ketogenic diet treatment of epilepsy in adults. Journal of the American Medical Association. 1928;91:73–78. [Google Scholar]
- Bartzokis G, Lu PH, Mintz J. Quantifying age-related myelin breakdown with MRI: novel therapeutic targets for preventing cognitive decline and Alzheimer’s disease. J Alzheimers Dis. 2004a;6:S53–59. doi: 10.3233/jad-2004-6s604. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol Aging. 2004b;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Baydyuk M, Xu B. BDNF signaling and survival of striatal neurons. Frontiers in Cellular Neuroscience. 2014;8:254. doi: 10.3389/fncel.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekesi A, Williamson DH. An explanation for ketogenesis by the intestine of the suckling rat: the presence of an active hydroxymethylglutaryl-coenzyme A pathway. Biol Neonate. 1990;58:160–165. doi: 10.1159/000243256. [DOI] [PubMed] [Google Scholar]
- Blomqvist G, Alvarsson M, Grill V, Von Heijne G, Ingvar M, Thorell JO, Stone-Elander S, Widen L, Ekberg K. Effect of acute hyperketonemia on the cerebral uptake of ketone bodies in nondiabetic subjects and IDDM patients. Am J Physiol Endocrinol Metab. 2002;283:E20–28. doi: 10.1152/ajpendo.00294.2001. [DOI] [PubMed] [Google Scholar]
- Blomqvist G, Thorell JO, Ingvar M, Grill V, Widen L, Stone-Elander S. Use of R-beta-[1-11C]hydroxybutyrate in PET studies of regional cerebral uptake of ketone bodies in humans. Am J Physiol. 1995;269:E948–959. doi: 10.1152/ajpendo.1995.269.5.E948. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Breckenridge WC, Kuksis A. Molecular weight distributions of milk fat triglycerides from seven species. J Lipid Res. 1967;8:473–478. [PubMed] [Google Scholar]
- Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer’s disease. Adv Drug Deliv Rev. 2008;60:1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce ED, Konda S, Dean DD, Wang EW, Huang JH, Little DM. Neuroimaging and traumatic brain injury: State of the field and voids in translational knowledge. Mol Cell Neurosci. 2015;66:103–113. doi: 10.1016/j.mcn.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR, Jr, Weiner M, DeCarli C. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano CA, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G, Imbeault H, Turcotte E, Fulop T, Cunnane SC. Lower brain 18F–fluorodeoxyglucose uptake but normal 11C–acetoacetate metabolism in mild Alzheimer’s disease dementia. J Alzheimers Dis. 2015;43:1343–1353. doi: 10.3233/JAD-141074. [DOI] [PubMed] [Google Scholar]
- Cheng B, Yang X, An L, Gao B, Liu X, Liu S. Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res. 2009;1286:25–31. doi: 10.1016/j.brainres.2009.06.060. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histology and histopathology. 2005;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu AC, Ho PW, Kwok KH, Ho JW, Chan KH, Liu HF, Kung MH, Ramsden DB, Ho SL. Mitochondrial UCP4 attenuates MPP+ - and dopamine-induced oxidative stress, mitochondrial depolarization, and ATP deficiency in neurons and is interlinked with UCP2 expression. Free Radic Biol Med. 2009;46:810–820. doi: 10.1016/j.freeradbiomed.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Cotter DG, d’Avignon DA, Wentz AE, Weber ML, Crawford PA. Obligate Role for Ketone Body Oxidation in Neonatal Metabolic Homeostasis. The Journal of Biological Chemistry. 2011;286:6902–6910. doi: 10.1074/jbc.M110.192369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane SC, Courchesne-Loyer A, St-Pierre V, Vandenberghe C, Pierotti T, Fortier M, Croteau E, Castellano CA. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann N Y Acad Sci. 2016;1367:12–20. doi: 10.1111/nyas.12999. [DOI] [PubMed] [Google Scholar]
- Cunnane SC, Crawford MA. Survival of the fattest: fat babies were the key to evolution of the large human brain. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:17–26. doi: 10.1016/s1095-6433(03)00048-5. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB Rep. 2009;42:475–481. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deransart C, Hellwig B, Heupel-Reuter M, Leger JF, Heck D, Lucking CH. Single-unit analysis of substantia nigra pars reticulata neurons in freely behaving rats with genetic absence epilepsy. Epilepsia. 2003;44:1513–1520. doi: 10.1111/j.0013-9580.2003.26603.x. [DOI] [PubMed] [Google Scholar]
- Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Astrocytic energetics during excitatory neurotransmission: What are contributions of glutamate oxidation and glycolysis? Neurochem Int. 2013;63:244–258. doi: 10.1016/j.neuint.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale GR, Lardy HA. Fatty acid oxidation by a soluble enzyme system from mitochondria. J Biol Chem. 1953;202:119–136. [PubMed] [Google Scholar]
- Echtay KS. Mitochondrial uncoupling proteins--what is their physiological role? Free Radic Biol Med. 2007;43:1351–1371. doi: 10.1016/j.freeradbiomed.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Daikhin Y, Yudkoff M. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J Neurochem. 1996;67:2325–2334. doi: 10.1046/j.1471-4159.1996.67062325.x. [DOI] [PubMed] [Google Scholar]
- Fernandes CG, da Rosa MS, Seminotti B, Pierozan P, Martell RW, Lagranha VL, Busanello EN, Leipnitz G, Wajner M. In vivo experimental evidence that the major metabolites accumulating in 3-hydroxy-3-methylglutaryl-CoA lyase deficiency induce oxidative stress in striatum of developing rats: a potential pathophysiological mechanism of striatal damage in this disorder. Mol Genet Metab. 2013;109:144–153. doi: 10.1016/j.ymgme.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Fernandes CG, Pierozan P, Soares GM, Ferreira F, Zanatta A, Amaral AU, Borges CG, Wajner M, Pessoa-Pureur R. NMDA Receptors and Oxidative Stress Induced by the Major Metabolites Accumulating in HMG Lyase Deficiency Mediate Hypophosphorylation of Cytoskeletal Proteins in Brain From Adolescent Rats: Potential Mechanisms Contributing to the Neuropathology of This Disease. Neurotox Res. 2015;28:239–252. doi: 10.1007/s12640-015-9542-z. [DOI] [PubMed] [Google Scholar]
- Freemantle E, Vandal M, Tremblay-Mercier J, Tremblay S, Blachere JC, Begin ME, Brenna JT, Windust A, Cunnane SC. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukot Essent Fatty Acids. 2006;75:213–220. doi: 10.1016/j.plefa.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Fukao T, Song XQ, Mitchell GA, Yamaguchi S, Sukegawa K, Orii T, Kondo N. Enzymes of ketone body utilization in human tissues: protein and messenger RNA levels of succinyl-coenzyme A (CoA):3-ketoacid CoA transferase and mitochondrial and cytosolic acetoacetyl-CoA thiolases. Pediatr Res. 1997;42:498–502. doi: 10.1203/00006450-199710000-00013. [DOI] [PubMed] [Google Scholar]
- Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res. 2014;55:2211–2228. doi: 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber AJ, Menzel PH, Boden G, Owen OE. Hepatic ketogenesis and gluconeogenesis in humans. J Clin Invest. 1974;54:981–989. doi: 10.1172/JCI107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyelin HR. Fasting as a method for treating epilepsy. Med Rec. 1921;99:1037–1039. [Google Scholar]
- Gibbs ME, Gibbs Z, Hertz L. Rescue of Abeta(1–42)-induced memory impairment in day-old chick by facilitation of astrocytic oxidative metabolism: implications for Alzheimer’s disease. J Neurochem. 2009;109(Suppl 1):230–236. doi: 10.1111/j.1471-4159.2009.05800.x. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Murphy AN, Murphy SP. Stroke outcome in the ketogenic state--a systematic review of the animal data. J Neurochem. 2012;123(Suppl 2):52–57. doi: 10.1111/j.1471-4159.2012.07943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Xu H, Chen HL, Chen W, Denton TT, Zhang S. Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines. J Neurochem. 2015;134:86–96. doi: 10.1111/jnc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelpa GMA. La lutte contre l’e’pilepsie par la de’ sintoxication et par la re’e’ducation alimentaire. Rev Ther Medico-Chirurgicale. 1911;78:8–13. [Google Scholar]
- Guzman M, Blazquez C. Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol Metab. 2001;12:169–173. doi: 10.1016/s1043-2760(00)00370-2. [DOI] [PubMed] [Google Scholar]
- Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29:89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe N, Fujita Y, Ueno M, Yoshimura K, Fujino Y, Yamashita T. Soluble beta-amyloid Precursor Protein Alpha binds to p75 neurotrophin receptor to promote neurite outgrowth. PLoS One. 2013;8:e82321. doi: 10.1371/journal.pone.0082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegardt FG. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J. 1999;338(Pt 3):569–582. [PMC free article] [PubMed] [Google Scholar]
- Henderson ST. Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics. 2008;5:470–480. doi: 10.1016/j.nurt.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MK, Lynch JT, Eapen AK, Quelle FW. DNA damage-induced cell-cycle arrest of hematopoietic cells is overridden by activation of the PI-3 kinase/Akt signaling pathway. Blood. 2001;98:834–841. doi: 10.1182/blood.v98.3.834. [DOI] [PubMed] [Google Scholar]
- Hilditch TP. The component acids of milk fats of the goat, ewe and mare. Biochem J. 1944;38:443–447. doi: 10.1042/bj0380443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- Ho JW, Ho PW, Liu HF, So DH, Chan KH, Tse ZH, Kung MH, Ramsden DB, Ho SL. UCP4 is a target effector of the NF-kappaB c-Rel prosurvival pathway against oxidative stress. Free Radic Biol Med. 2012a;53:383–394. doi: 10.1016/j.freeradbiomed.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Ho PW, Chu AC, Kwok KH, Kung MH, Ramsden DB, Ho SL. Knockdown of uncoupling protein-5 in neuronal SH-SY5Y cells: Effects on MPP+-induced mitochondrial membrane depolarization, ATP deficiency, and oxidative cytotoxicity. J Neurosci Res. 2006;84:1358–1366. doi: 10.1002/jnr.21034. [DOI] [PubMed] [Google Scholar]
- Ho PW, Ho JW, Tse HM, So DH, Yiu DC, Liu HF, Chan KH, Kung MH, Ramsden DB, Ho SL. Uncoupling protein-4 (UCP4) increases ATP supply by interacting with mitochondrial Complex II in neuroblastoma cells. PLoS One. 2012b;7:e32810. doi: 10.1371/journal.pone.0032810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo SE, Cruz-Garcia L, Karanth S, Anderson RM, Stainier DYR, Schlegel A. A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting. Genes & Development. 2012;26:282–293. doi: 10.1101/gad.180968.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insull W, Jr, Ahrens EH., Jr The fatty acids of human milk from mothers on diets taken ad libitum. Biochem J. 1959;72:27–33. doi: 10.1042/bj0720027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Sasaki M, Kitagaki H, Yamaji S, Sakamoto S, Matsuda K, Mori E. Reduction of cerebellar glucose metabolism in advanced Alzheimer’s disease. J Nucl Med. 1997;38:925–928. [PubMed] [Google Scholar]
- Jang HJ, Yang YR, Kim JK, Choi JH, Seo YK, Lee YH, Lee JE, Ryu SH, Suh PG. Phospholipase C-gamma1 involved in brain disorders. Adv Biol Regul. 2013;53:51–62. doi: 10.1016/j.jbior.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Jarrett SG, Milder JB, Liang LP, Patel M. The ketogenic diet increases mitochondrial glutathione levels. J Neurochem. 2008;106:1044–1051. doi: 10.1111/j.1471-4159.2008.05460.x. [DOI] [PubMed] [Google Scholar]
- Ji AL, Zhang X, Chen WW, Huang WJ. Genetics insight into the amyotrophic lateral sclerosis/frontotemporal dementia spectrum. J Med Genet. 2017 doi: 10.1136/jmedgenet-2016-104271. [DOI] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalapos MP. On the mammalian acetone metabolism: from chemistry to clinical implications. Biochimica et Biophysica Acta (BBA) - General Subjects. 2003;1621:122–139. doi: 10.1016/s0304-4165(03)00051-5. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE. Parkinson’s disease. The Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003;74:37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- Kashiwaya Y, Pawlosky R, Markis W, King MT, Bergman C, Srivastava S, Murray A, Clarke K, Veech RL. A ketone ester diet increases brain malonyl-CoA and Uncoupling proteins 4 and 5 while decreasing food intake in the normal Wistar Rat. J Biol Chem. 2010;285:25950–25956. doi: 10.1074/jbc.M110.138198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith H. Experimental convulsions induced by administration of thujone. Archives Neurological Psychiatry. 1935;34:1022–1040. [Google Scholar]
- Kim DY, Hao J, Liu R, Turner G, Shi FD, Rho JM. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One. 2012;7:e35476. doi: 10.1371/journal.pone.0035476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci U S A. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosinski LP, Yao J, Yin F, Fonteh AN, Harrington MG, Christensen TA, Trushina E, Brinton RD. White Matter Lipids as a Ketogenic Fuel Supply in Aging Female Brain: Implications for Alzheimer’s Disease. EBioMedicine. 2015;2:1888–1904. doi: 10.1016/j.ebiom.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobau R, Zahran H, Thurman DJ, Zack MM, Henry TR, Schachter SC, Price PH. Epilepsy surveillance among adults--19 States, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ. 2008;57:1–20. [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- Koppel I, Timmusk T. Differential regulation of Bdnf expression in cortical neurons by class-selective histone deacetylase inhibitors. Neuropharmacology. 2013;75:106–115. doi: 10.1016/j.neuropharm.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Kuczynski B, Targan E, Madison C, Weiner M, Zhang Y, Reed B, Chui HC, Jagust W. White matter integrity and cortical metabolic associations in aging and dementia. Alzheimers Dement. 2010;6:54–62. doi: 10.1016/j.jalz.2009.04.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok KH, Ho PW, Chu AC, Ho JW, Liu HF, Yiu DC, Chan KH, Kung MH, Ramsden DB, Ho SL. Mitochondrial UCP5 is neuroprotective by preserving mitochondrial membrane potential, ATP levels, and reducing oxidative stress in MPP+ and dopamine toxicity. Free Radic Biol Med. 2010;49:1023–1035. doi: 10.1016/j.freeradbiomed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Salem N, Puchowicz M, Erokwu B, Koppaka S, Flask C, Lee Z. KETONES SUPPRESS BRAIN GLUCOSE CONSUMPTION. Adv Exp Med Biol. 2009;645:301–306. doi: 10.1007/978-0-387-85998-9_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D, Bengtson CP, Buchthal B, Bading H. BDNF Reduces Toxic Extrasynaptic NMDA Receptor Signaling via Synaptic NMDA Receptors and Nuclear-Calcium-Induced Transcription of inhba/Activin A. Cell Reports. 2015;12:1353–1366. doi: 10.1016/j.celrep.2015.07.038. [DOI] [PubMed] [Google Scholar]
- Le Foll C, Dunn-Meynell AA, Levin BE. Role of FAT/CD36 in fatty acid sensing, energy, and glucose homeostasis regulation in DIO and DR rats. Am J Physiol Regul Integr Comp Physiol. 2015a;308:R188–198. doi: 10.1152/ajpregu.00367.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll C, Dunn-Meynell AA, Miziorko HM, Levin BE. Regulation of hypothalamic neuronal sensing and food intake by ketone bodies and fatty acids. Diabetes. 2014;63:1259–1269. doi: 10.2337/db13-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll C, Dunn-Meynell AA, Miziorko HM, Levin BE. Role of VMH ketone bodies in adjusting caloric intake to increased dietary fat content in DIO and DR rats. Am J Physiol Regul Integr Comp Physiol. 2015b;308:R872–878. doi: 10.1152/ajpregu.00015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll C, Levin BE. Fatty acid-induced astrocyte ketone production and the control of food intake. Am J Physiol Regul Integr Comp Physiol. 2016;310:R1186–1192. doi: 10.1152/ajpregu.00113.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger AL, Sudduth HC, Wise JB. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J Biol Chem. 1960;235:2450–2455. [PubMed] [Google Scholar]
- Lim S, Chesser AS, Grima JC, Rappold PM, Blum D, Przedborski S, Tieu K. D-beta-hydroxybutyrate is protective in mouse models of Huntington’s disease. PLoS One. 2011;6:e24620. doi: 10.1371/journal.pone.0024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad BS, Settergren G, Feychting H, Persson B. Total parenteral nutrition in infants. Blood levels of glucose, lactate, pyruvate, free fatty acids, glycerol, d-beta-hydroxybutyrate, triglycerides, free amino acids and insulin. Acta Paediatr Scand. 1977;66:409–419. doi: 10.1111/j.1651-2227.1977.tb07920.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Chan SL, de Souza-Pinto NC, Slevin JR, Wersto RP, Zhan M, Mustafa K, de Cabo R, Mattson MP. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med. 2006;8:389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schutz G. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet. 2002;31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- Marosi K, Kim SW, Moehl K, Scheibye-Knudsen M, Cheng A, Cutler R, Camandola S, Mattson MP. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. 2016;139:769–781. doi: 10.1111/jnc.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Mitsuda N, Namikawa K, Kiyama H, Miyake S, Tohyama M. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J Neurochem. 1999;73:2037–2046. [PubMed] [Google Scholar]
- McNally MA, Hartman AL. Ketone bodies in epilepsy. J Neurochem. 2012;121:28–35. doi: 10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem Int. 2006;48:498–507. doi: 10.1016/j.neuint.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Middleton B. The existence of ketoacyl-CoA thiolases of differing properties and intracellular localization in ox liver. Biochem Biophys Res Commun. 1972;46:508–515. doi: 10.1016/s0006-291x(72)80168-2. [DOI] [PubMed] [Google Scholar]
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238–244. doi: 10.1016/j.nbd.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Khazipov R. Antiepileptic effects of endogenous beta-hydroxybutyrate in suckling infant rats. Epilepsy Res. 2011;95:100–109. doi: 10.1016/j.eplepsyres.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Morris AA. Cerebral ketone body metabolism. J Inherit Metab Dis. 2005;28:109–121. doi: 10.1007/s10545-005-5518-0. [DOI] [PubMed] [Google Scholar]
- Mosconi L, de Leon M, Murray J, E L, Lu J, Javier E, McHugh P, Swerdlow RH. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer’s disease. J Alzheimers Dis. 2011;27:483–490. doi: 10.3233/JAD-2011-110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, Rich KE, Switalski R, Mehta PD, Pratico D, Zinkowski R, Blennow K, de Leon MJ. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008a;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008b;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72:513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, Reiman EM, Holthoff V, Kalbe E, Sorbi S, Diehl-Schmid J, Perneczky R, Clerici F, Caselli R, Beuthien-Baumann B, Kurz A, Minoshima S, de Leon MJ. Multicenter standardized 18F–FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med. 2008c;49:390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- Nehlig A. Age-dependent pathways of brain energy metabolism: the suckling rat, a natural model of the ketogenic diet. Epilepsy Res. 1999;37:211–221. doi: 10.1016/s0920-1211(99)00073-x. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Pereira de Vasconcelos A. Glucose and ketone body utilization by the brain of neonatal rats. Prog Neurobiol. 1993;40:163–221. doi: 10.1016/0301-0082(93)90022-k. [DOI] [PubMed] [Google Scholar]
- Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends in endocrinology and metabolism: TEM. 2014a;25:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Verdin E. β-hydroxybutyrate: Much more than a metabolite. Diabetes research and clinical practice. 2014b;106:173–181. doi: 10.1016/j.diabres.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport MT, VanItallie TB, Kashiwaya Y, King MT, Veech RL. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer’s disease. Alzheimers Dement. 2015;11:99–103. doi: 10.1016/j.jalz.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- O’Dwyer L, Lamberton F, Bokde AL, Ewers M, Faluyi YO, Tanner C, Mazoyer B, O’Neill D, Bartley M, Collins DR, Coughlan T, Prvulovic D, Hampel H. Multiple indices of diffusion identifies white matter damage in mild cognitive impairment and Alzheimer’s disease. PLoS One. 2011;6:e21745. doi: 10.1371/journal.pone.0021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S, Schwaninger M. Nutritional or pharmacological activation of HCA(2) ameliorates neuroinflammation. Trends Mol Med. 2015;21:245–255. doi: 10.1016/j.molmed.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Orhan N, Ugur Yilmaz C, Ekizoglu O, Ahishali B, Kucuk M, Arican N, Elmas I, Gurses C, Kaya M. Effects of beta-hydroxybutyrate on brain vascular permeability in rats with traumatic brain injury. Brain Res. 2016;1631:113–126. doi: 10.1016/j.brainres.2015.11.038. [DOI] [PubMed] [Google Scholar]
- Ovbiagele B, Nguyen-Huynh MN. Stroke Epidemiology: Advancing Our Understanding of Disease Mechanism and Therapy. Neurotherapeutics. 2011;8:319–329. doi: 10.1007/s13311-011-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owada Y, Utsunomiya A, Yoshimoto T, Kondo H. Expression of mRNA for Akt, serine-threonine protein kinase, in the brain during development and its transient enhancement following axotomy of hypoglossal nerve. J Mol Neurosci. 1997;9:27–33. doi: 10.1007/BF02789392. [DOI] [PubMed] [Google Scholar]
- Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- Prins ML, Fujima LS, Hovda DA. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J Neurosci Res. 2005;82:413–420. doi: 10.1002/jnr.20633. [DOI] [PubMed] [Google Scholar]
- Prins ML, Lee SM, Fujima LS, Hovda DA. Increased cerebral uptake and oxidation of exogenous betaHB improves ATP following traumatic brain injury in adult rats. J Neurochem. 2004;90:666–672. doi: 10.1111/j.1471-4159.2004.02542.x. [DOI] [PubMed] [Google Scholar]
- Prins ML, Matsumoto JH. The collective therapeutic potential of cerebral ketone metabolism in traumatic brain injury. J Lipid Res. 2014;55:2450–2457. doi: 10.1194/jlr.R046706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchowicz MA, Zechel J, Valerio J, Emancipator D, Xu K, Pundik S, LaManna JC, Lust WD. Neuroprotection in Diet Induced Ketotic Rat Brain Following Focal Ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:1907–1916. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puisac B, Ramos M, Arnedo M, Menao S, Gil-Rodriguez MC, Teresa-Rodrigo ME, Pie A, de Karam JC, Wesselink JJ, Gimenez I, Ramos FJ, Casals N, Gomez-Puertas P, Hegardt FG, Pie J. Characterization of splice variants of the genes encoding human mitochondrial HMG-CoA lyase and HMG-CoA synthase, the main enzymes of the ketogenesis pathway. Mol Biol Rep. 2012;39:4777–4785. doi: 10.1007/s11033-011-1270-8. [DOI] [PubMed] [Google Scholar]
- Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Muller-Fielitz H, Pokorna B, Vollbrandt T, Stolting I, Nadrowitz R, Okun JG, Offermanns S, Schwaninger M. The beta-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- Ramagopalan SV, Sadovnick AD. Epidemiology of multiple sclerosis. Neurol Clin. 2011;29:207–217. doi: 10.1016/j.ncl.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Ramsden DB, Ho PW, Ho JW, Liu HF, So DH, Tse HM, Chan KH, Ho SL. Human neuronal uncoupling proteins 4 and 5 (UCP4 and UCP5): structural properties, regulation, and physiological role in protection against oxidative stress and mitochondrial dysfunction. Brain Behav. 2012;2:468–478. doi: 10.1002/brb3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, Schilling B, Mooney SD, Kahn CR, Verdin E, Gibson BW. Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci U S A. 2013;110:6601–6606. doi: 10.1073/pnas.1302961110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25:311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Perdigon M, Solas M, Moreno-Aliaga MJ, Ramirez MJ. Lipoic acid improves neuronal insulin signalling and rescues cognitive function regulating VGlut1 expression in high-fat-fed rats: Implications for Alzheimer’s disease. Biochim Biophys Acta. 2016;1862:511–517. doi: 10.1016/j.bbadis.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MH, Alkalay A, Agarwal N, Baker SL, O’Neil JP, Janabi M, Yen IV, Growdon M, Jang J, Madison C, Mormino EC, Rosen HJ, Gorno-Tempini ML, Weiner MW, Miller BL, Jagust WJ, Rabinovici GD. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011;76:1789–1796. doi: 10.1212/WNL.0b013e31821cccad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samala R, Klein J, Borges K. The ketogenic diet changes metabolite levels in hippocampal extracellular fluid. Neurochem Int. 2011;58:5–8. doi: 10.1016/j.neuint.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Selfridge JE, Wilkins HM, E L, Carl SM, Koppel S, Funk E, Fields T, Lu J, Tang EP, Slawson C, Wang W, Zhu H, Swerdlow RH. Effect of one month duration ketogenic and non-ketogenic high fat diets on mouse brain bioenergetic infrastructure. J Bioenerg Biomembr. 2015;47:1–11. doi: 10.1007/s10863-014-9570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV, Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Simeone TA, Matthews SA, Samson KK, Simeone KA. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp Neurol. 2017;287:54–64. doi: 10.1016/j.expneurol.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, Stringer T, Ulja D, Karuppagounder SS, Holson EB, Ratan RR, Ninan I, Chao MV. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife. 2016:5. doi: 10.7554/eLife.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spulber G, Niskanen E, MacDonald S, Smilovici O, Chen K, Reiman EM, Jauhiainen AM, Hallikainen M, Tervo S, Wahlund LO, Vanninen R, Kivipelto M, Soininen H. Whole brain atrophy rate predicts progression from MCI to Alzheimer’s disease. Neurobiol Aging. 2010;31:1601–1605. doi: 10.1016/j.neurobiolaging.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Kashiwaya Y, King MT, Baxa U, Tam J, Niu G, Chen X, Clarke K, Veech RL. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. Faseb j. 2012;26:2351–2362. doi: 10.1096/fj.11-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Suh PG, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yun S, Ryu SH. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 2008;41:415–434. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Suzuki M, Kitamura Y, Mori S, Sato K, Dohi S, Sato T, Matsuura A, Hiraide A. Beta-hydroxybutyrate, a cerebral function improving agent, protects rat brain against ischemic damage caused by permanent and transient focal cerebral ischemia. Jpn J Pharmacol. 2002;89:36–43. doi: 10.1254/jjp.89.36. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Suzuki M, Sato K, Dohi S, Sato T, Matsuura A, Hiraide A. Effect of beta-hydroxybutyrate, a cerebral function improving agent, on cerebral hypoxia, anoxia and ischemia in mice and rats. Jpn J Pharmacol. 2001;87:143–150. doi: 10.1254/jjp.87.143. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Brain aging, Alzheimer’s disease, and mitochondria. Biochim Biophys Acta. 2011;1812:1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Alzheimer’s disease pathologic cascades: who comes first, what drives what. Neurotox Res. 2012a;22:182–194. doi: 10.1007/s12640-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. beta-Apptists and Tauists, it is time for a sermon from the book of biogenesis. J Neurochem. 2012b;120:347–349. doi: 10.1111/j.1471-4159.2011.07561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria and cell bioenergetics: increasingly recognized components and a possible etiologic cause of Alzheimer’s disease. Antioxid Redox Signal. 2012c;16:1434–1455. doi: 10.1089/ars.2011.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Comment: BDNF, fitness, and the brain. Neurology. 2014;83:1351. doi: 10.1212/WNL.0000000000000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner GR, Lutas A, Martinez-Francois JR, Yellen G. Single K ATP channel opening in response to action potential firing in mouse dentate granule neurons. J Neurosci. 2011;31:8689–8696. doi: 10.1523/JNEUROSCI.5951-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin O. The Falling Sickness: A History of Epilepsy from the Greeks to the Beginnings of Modern Neurology. Johns Hopkins University Press; 1994. [Google Scholar]
- Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003;112:892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB. Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology. 2005;64:728–730. doi: 10.1212/01.WNL.0000152046.11390.45. [DOI] [PubMed] [Google Scholar]
- Vazquez-Vela ME, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res. 2008;39:715–728. doi: 10.1016/j.arcmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Vijay N, Morris ME. Role of Monocarboxylate Transporters in Drug Delivery to the Brain. Curr Pharm Des. 2014;20:1487–1498. doi: 10.2174/13816128113199990462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain N, Fouquet M, Baron JC, Mezenge F, Landeau B, de La Sayette V, Viader F, Eustache F, Desgranges B, Chetelat G. Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain. 2010;133:3301–3314. doi: 10.1093/brain/awq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci U S A. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang L, Lu J, Siedlak SL, Fujioka H, Liang J, Jiang S, Ma X, Jiang Z, da Rocha EL, Sheng M, Choi H, Lerou PH, Li H, Wang X. The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat Med. 2016;22:869–878. doi: 10.1038/nm.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Bergqvist C, Hunter JV, Jin D, Wang DJ, Wehrli S, Zimmerman RA. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy--exploration of GABA levels in a ketogenic diet. Magn Reson Med. 2003;49:615–619. doi: 10.1002/mrm.10429. [DOI] [PubMed] [Google Scholar]
- Wei Z, Chigurupati S, Bagsiyao P, Henriquez A, Chan SL. The brain uncoupling protein UCP4 attenuates mitochondrial toxin-induced cell death: role of extracellular signal-regulated kinases in bioenergetics adaptation and cell survival. Neurotox Res. 2009;16:14–29. doi: 10.1007/s12640-009-9039-8. [DOI] [PubMed] [Google Scholar]
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):3–5. doi: 10.1111/j.1528-1167.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- Wilder RM. The effect of ketonemia on the course of epilepsy. Mayo Clinic Bulletin. 1921;2:307. [Google Scholar]
- Wilkins HM, Swerdlow RH. Amyloid precursor protein processing and bioenergetics. Brain Res Bull. 2016 doi: 10.1016/j.brainresbull.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, Sullivan JS, Zhao Z, Meiselman HJ, Wenby RB, Soto J, Abel ED, Makshanoff J, Zuniga E, De Vivo DC, Zlokovic BV. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won YJ, Lu VB, Puhl HL, 3rd, Ikeda SR. beta-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J Neurosci. 2013;33:19314–19325. doi: 10.1523/JNEUROSCI.3102-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodyatt RT. Objects and method of diet adjustment in diabetes. Archives of Internal Medicine. 1921;28:125–141. [Google Scholar]
- Xie Z, Zhang D, Chung D, Tang Z, Huang H, Dai L, Qi S, Li J, Colak G, Chen Y, Xia C, Peng C, Ruan H, Kirkey M, Wang D, Jensen LM, Kwon OK, Lee S, Pletcher SD, Tan M, Lombard DB, White KP, Zhao H, Li J, Roeder RG, Yang X, Zhao Y. Metabolic Regulation of Gene Expression by Histone Lysine beta-Hydroxybutyrylation. Mol Cell. 2016;62:194–206. doi: 10.1016/j.molcel.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Cheng B. Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J Mol Neurosci. 2010;42:145–153. doi: 10.1007/s12031-010-9336-y. [DOI] [PubMed] [Google Scholar]
- Yao J, Chen S, Mao Z, Cadenas E, Brinton RD. 2-Deoxy-D-glucose treatment induces ketogenesis, sustains mitochondrial function, and reduces pathology in female mouse model of Alzheimer’s disease. PLoS One. 2011a;6:e21788. doi: 10.1371/journal.pone.0021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Rettberg JR, Klosinski LP, Cadenas E, Brinton RD. Shift in brain metabolism in late onset Alzheimer’s disease: implications for biomarkers and therapeutic interventions. Mol Aspects Med. 2011b;32:247–257. doi: 10.1016/j.mam.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JX, Maalouf M, Han P, Zhao M, Gao M, Dharshaun T, Ryan C, Whitelegge J, Wu J, Eisenberg D, Reiman EM, Schweizer FE, Shi J. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol Aging. 2016;39:25–37. doi: 10.1016/j.neurobiolaging.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Zhao S, Fu J, Liu X, Wang T, Zhang J, Zhao Y. Activation of Akt/GSK-3beta/beta-catenin signaling pathway is involved in survival of neurons after traumatic brain injury in rats. Neurol Res. 2012a;34:400–407. doi: 10.1179/1743132812Y.0000000025. [DOI] [PubMed] [Google Scholar]
- Zhao W, Varghese M, Vempati P, Dzhun A, Cheng A, Wang J, Lange D, Bilski A, Faravelli I, Pasinetti GM. Caprylic triglyceride as a novel therapeutic approach to effectively improve the performance and attenuate the symptoms due to the motor neuron loss in ALS disease. PLoS One. 2012b;7:e49191. doi: 10.1371/journal.pone.0049191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, Humala N, Thiyagarajan M, Wang J, Pasinetti GM. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]