Abstract
Objective
This study aims to test the effectiveness of a patient navigation (PN) intervention to reduce time to diagnostic resolution among older adults age ≥65 years versus those <65 years with abnormal breast, cervical, or colorectal cancer screening exams participating in the Ohio Patient Navigation Research Program (OPNRP).
Method
The OPNRP utilized a nested cohort group-randomized trial design to randomize 862 participants (n = 67 for ≥65 years; n = 795 for <65 years) to PN or usual care conditions. A shared frailty Cox model tested the effect of PN on time to resolution.
Results
Older adult participants randomized to PN achieved a 6-month resolution rate that was 127% higher than those randomized to usual care (p = .001). This effect was not significantly different from participants <65 years.
Discussion
PN significantly reduced time to diagnostic resolution among older adults beginning 6 months after an abnormal cancer screening exam. Health care systems should include this population in PN programs to reduce cancer disparities.
Keywords: patient navigation, older adults, diagnostic resolution, barriers to care, cancer health disparities
Introduction
Advancing age is the greatest risk factor for cancer, with older adults, defined as 65 years of age and older, accounting for 60% of newly diagnosed malignancies and 70% of all cancer deaths (American Cancer Society, 2016b; Berger, Savvides, & Koroukian, 2006). Between 2010 and 2030, a 67% increase in cancer incidence is expected for adults ≥65 years, compared with only an 11% increase in cancer incidence anticipated for adults <65 years (Smith, Smith, Hurria, Hortobagyi, & Buchholz, 2009). Thus, older adults are disproportionately affected by cancer, and because the number of older adults is projected to markedly increase over the next 30 years, cancer is expected to become the leading cause of death among older adults in America (Berger et al., 2006; Byers, 2010; Day, 1996; Kinsella & He, 2009). As the older adult population continues to increase in number and life expectancy with a simultaneous increase in cancer incidence rates, there is an emerging need for the health care system to address the increased burden of cancer incidence and mortality in the older adult population.
Despite improvements in prevention, early detection, diagnosis, and treatment of cancer, older adults continue to be disproportionately vulnerable to lack of access to health care, caused by individual and system-level barriers across the cancer care continuum (Fitzpatrick, Powe, Cooper, Ives, & Robbins, 2004). Such barriers include access to cancer care services, patient communication and satisfaction with health care providers, insurance coverage, and cost on both individual and system levels (Fitzpatrick et al., 2004; Thorpe, Thorpe, Kennelty, & Pandhi, 2011). Furthermore, interventions to address these barriers to care have been vastly understudied in the older adult population, compared with those of racial and ethnic minorities and low socioeconomic status.
Patient navigation (PN) has emerged as a model of health care coordination to help patients overcome barriers to care and prevent delays across the cancer care continuum to reduce cancer incidence and mortality (Dohan & Schrag, 2005; Paskett et al., 2012). PN was first introduced by Harold P. Freeman as a strategy to eliminate barriers to timely cancer screening, diagnosis, treatment, and supportive care among African American patients in a New York public hospital (Oluwole et al., 2003). Patient navigators, defined as care coordinators (Dohan & Schrag, 2005), have been found to reduce loss to follow-up after an abnormal cancer screening exam and improve timeliness to cancer-related diagnosis and treatment (Wells et al., 2008). Patient navigators have also been found to address both psychosocial and system navigation problems, as well as access problems (Ell, Vourlekis, Lee, & Xie, 2007), extending their role to function as patient advisors and educators to help reduce barriers to care.
Although PN studies have been shown to reduce delays in cancer care in low-income and minority patients as an intervention to eliminate cancer health disparities (Freund et al., 2008; Paskett, Harrop, & Wells, 2011; Paskett et al., 2012; Wells et al., 2008), with several studies specifically reporting reduced time to diagnostic resolution following abnormal cancer screening exams in low-income (Ell, Padgett, et al., 2002; Ell, Vourlekis, et al., 2002; Hiatt et al., 2001b) and medically underserved populations (Battaglia, Roloff, Posner, & Freund, 2007; Burhansstipanov et al., 1998; Clark et al., 2009; Ferrante, Chen, & Kim, 2008; Freeman, Muth, & Kerner, 1995; Frelix, Rosenblatt, Solomon, & Vikram, 1999; Maxwell, Jo, Crespi, Sundan, & Bastani, 2010; Paskett et al., 2012), no known prior studies have examined the effectiveness of PN on time from abnormal cancer screening to diagnostic resolution in the older adult population. Because timeliness and completion of recommended follow-up care have been associated with improvements in survival, especially in older adults (Geiger et al., 2007), a reduction in the time from abnormal cancer screening to diagnostic resolution may result in improved clinical outcomes for this population.
This study used a secondary analysis of the Ohio Patient Navigation Research Program (OPNRP), which was a large-scale study examining the role of PN in underserved populations. The goal of the larger study was to facilitate access to prompt, quality standard cancer care among persons with abnormal breast, cervical, or colorectal cancer screening exams across 18 primary care clinics located in central Ohio that primarily served underserved populations. Although the OPNRP showed that PN improved time to diagnostic resolution, defined in this study as confirmation of a benign condition or a cancer diagnosis, in participants with abnormal screening exams, the potential barriers encountered and the effect of PN in the older adult population have yet to be specifically studied (Paskett et al., 2012). Therefore, this article aims to (a) investigate whether the time to diagnostic resolution in older adult participants with abnormal breast, cervical, or colorectal cancer screening exams is reduced in those receiving PN and (b) identify potential barriers preventing recommended follow-up among the older adult participants receiving PN.
Method
Study Design
The OPNRP has been described elsewhere, but a brief description of the methods is detailed below (Paskett et al., 2012). The OPNRP utilized a group-randomized nested cohort design and randomized 18 medical clinics in Columbus, OH, to specific study conditions, PN or usual care without PN, as the control. Clinics were paired based on type (e.g., primary care, Federally Qualified Health Center, gynecology, gastrointestinal specialty, general internal medicine, and family medicine clinics) and then randomized from within pairs to study conditions, PN or usual care, which resulted in nine intervention clinics and nine control clinics. Individual participants (n = 862) were contacted, screened for eligibility, consented if eligible, and completed baseline and end-of-study questionnaires. The surveys gathered information regarding participant demographics (e.g., age, gender, race; see Table 1), in addition to information from the Quality of Life Index, the Trust in Physician Scale, the Beck Anxiety Inventory, the Center for Epidemiological Studies Depression Scale (CES-D), and the Perceived Social Support-Friends and Perceived Social Support-Family (Paskett et al., 2012). These scales measured factors that were related to barriers and/or adherence to care and were part of the common data elements for the Patient Navigation Research Program (PNRP) decided by the steering committee of PNRP (Freund et al., 2008). Resolution of each participant’s abnormality and time to diagnostic resolution was obtained through patient medical record reviews. The Ohio State University Institutional Review Board approved this study.
Table 1.
OPNRP Categories of Barriers to Care and Corresponding Examples.
| Major OPNRP category |
Individual barrier | Examples |
|---|---|---|
| Intrapersonal | Financial issues | Insurance, employment issues, financial issues, housing |
| Comorbidities | Patient disability, medical/mental health comorbidities | |
| Beliefs and attitudes | Attitudes toward providers, perceptions/beliefs at test or treatment, fear | |
| Interpersonal | Transportation | Out of town/country, location of health care facility, transportation |
| Interpersonal relationships (demands from others for care) | Social/practical support, child care, adult care | |
| System | Logistical within health care | Problem with scheduling, system proactive needed |
| Communication | Coaching, communication concerns with health care providers, literacy, language/interpretation |
Note. OPNRP = Ohio Patient Navigation Research Program.
To be eligible for participation in this study, participants must have been (a) 18 years and older; (b) a regular patient of the primary care practice receiving medical care from their health care provider within the past 3 years; (c) not cognitively impaired as noted in their medical record and interviewer’s judgment at screening and enrollment; (d) able to give informed consent; (e) identified as having an abnormal screening exam, an abnormal diagnostic exam, or an abnormal clinical finding leading to diagnostic testing for cervical, breast, or colorectal cancer; (f) without a history of cancer, except for nonmelanoma skin cancer; (g) living outside a nursing home or institutional setting; (h) without a history of medical navigation; and (i) able to speak and understand English or Spanish.
PN intervention
Participants in PN clinics interacted with one of the three trained lay patient navigators employed through the university. First, the assigned patient navigator contacted each participant through phone or in person within 5 days of the assignment. Second, the patient navigator identified each participant’s barriers to care by directly asking for barriers and going through a barrier checklist with each participant and addressed them according to their specific needs, which included connecting patients to community and social support services, facilitating interaction and communication with health care providers and their team, and providing health education materials. This checklist of barriers was developed by the steering committee of PNRP and assessed across all PNRP sites. If a participant reported no barriers to care, the patient navigator thanked the participant and reminded them that they would be contacted again to complete the end-of-study questionnaire and study closeout. Process measures were recorded for each encounter by patient navigators through a form enumerating the number and types of barriers a participant experienced, as well as action steps to be taken by the participant and/or patient navigator and the amount of time spent per encounter.
Comparison condition
Participants in usual care clinics received mailed educational materials focusing on their specific cancer test and/or abnormality within 1 month of completing their baseline questionnaire.
The end-of-study questionnaires were conducted when each participant’s abnormality was resolved or at the end of the study period. To ensure the accuracy of the time to resolution information, trained research staff reviewed paper records and/or electronic records at clinics. If the resolution was confirmed, copies of the procedure records and pathology reports were obtained as source documentation of the resolution diagnosis. Baseline and end-of-study questionnaires collected similar demographic and psychosocial information as previously specified.
Data Analyses
Baseline demographic characteristics of participants in the PN and usual care arms by age group (≥65 versus <65 years) were compared using means for continuous variables and percentages for categorical variables. Kaplan–Meier methods were used to estimate survival curves in four strata—older adult participants receiving PN, older adult participants receiving usual care, younger participants receiving PN, and younger participants receiving usual care. A Cox proportional hazards regression model with a shared frailty parameter (random clinic effect) was used to test for differences in time to diagnostic resolution between the PN and usual care arms. A mixed effects logistic regression model was used to compare arms for the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) benchmark of having a diagnostic resolution within 60 days of an abnormal screening exam. Fixed effects were included for age group, arm, and the interaction of age and arm. As nonproportionality was present in the PN effect over time, interactions between these three factors and the logarithm of time were included.
The hypothesis of whether PN affected older adult participants differently than younger participants was tested using a 2-degree-of-freedom (df) Wald test on the interactions of arm by age and time by arm by age. A covariate-adjusted Cox model was fit that included the participant characteristics of race, marital status, education, housing status, and income in addition to age, arm, and the relevant interactions included in the unadjusted model.
Barriers to receiving recommended care were identified among those in the PN arm and divided into three groups according to Table 1 as previously determined by PNRP—intrapersonal (e.g., financial issues, beliefs and attitudes, and comorbidities), interpersonal (e.g., transportation and interpersonal relationships), and system-focused barriers (e.g., health care system and communication). The barriers among those in the PN arm were then compared between older adult participants and younger participants, according to the number and types of barriers the participant encountered, as well as action steps taken by the participant and/or patient navigator, and the time spent completed for each encounter. Barriers to care were analyzed by measuring frequency of individual barriers, frequency of major PNRP barrier categories, and the sum of total barriers.
Results
Characteristics of Participants
A total of 862 participants from 18 clinics enrolled in the OPNRP, and these participants were further divided into subgroups based on age in the study, consisting of 67 older adult participants age 65 to 89 years and 795 younger participants age 18 to 64 years. The baseline characteristics for the enrolled participants by study group and age are shown in Table 2. Similar sociodemographic patterns were found across both groups with no significant differences in sociodemographic characteristics between the older adult and younger groups. The average age among older adult participants was 72 years, whereas the average age among younger participants was 42 years. The majority of participants among both age groups were female (97%) and had abnormal screening exams for breast cancer (55.8%). Overall, both groups were White (70.7%), college-educated (80.9%), homeowners (58.3%), and had an annual household income exceeding $50,000 (47.3%). Although nearly half (49.3% older adult; 46.9% younger) of the participants among both groups were married, there was a nearly equivalent proportion of participants in the older adult group who were also divorced/widowed (44.7%) that was not observed in the younger group (21%). In addition, a majority of participants in the older adult group were retired (67.2%) and had public insurance (91%), whereas participants in the younger group were mainly employed full-time (56%) and had private insurance (72.6%).
Table 2.
Participant Characteristics by Age and Study Site Group, OPNRP (n = 862).
| Older adult participants (≥65 years) |
Younger participants (<65 years) |
|||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variable | Level | Navigation (n = 36) |
Control (n = 31), n (%) |
Navigation (n = 449), n (%) |
Control (n = 346), n (%) |
Total (n = 862), n (%) |
| Age at consent (years), M (SD) | 71.9 (5.3) | 71.8 (6.9) | 43.8 (12.6) | 40.8 (12.8) | 44.8 (14.7) | |
| Anatomical site | Breast | 33 (56.9) | 25 (43.1) | 249 (58.9) | 174 (41.1) | 481 (55.8) |
| Cervix | 3 (75.0) | 1 (25.0) | 173 (54.7) | 143 (45.3) | 320 (37.1) | |
| Colorectal | 0 (0.0) | 5 (100.0) | 27 (48.2) | 29 (51.8) | 61 (7.1) | |
| Sex | Female | 36 (55.4) | 29 (44.6) | 437 (56.7) | 334 (43.3) | 836 (97.0) |
| Male | 0 (0.0) | 2 (100.0) | 12 (50.0) | 12 (50.0) | 26 (3.0) | |
| Race | White | 27 (56.2) | 21 (43.8) | 320 (57.5) | 237 (42.5) | 605 (70.7) |
| Black | 7 (50.0) | 7 (50.0) | 91 (52.9) | 81 (47.1) | 186 (21.7) | |
| Other | 2 (50.0) | 2 (50.0) | 38 (62.3) | 23 (37.7) | 65 (7.6) | |
| Primary language, English | No | 0 (0.0) | 3 (100.0) | 18 (47.4) | 20 (52.6) | 41 (4.8) |
| Yes | 36 (57.1) | 27 (42.9) | 431 (57.1) | 324 (42.9) | 818 (95.2) | |
| Marital status | Single | 0 (0.0) | 3 (100.0) | 137 (54.2) | 116 (45.8) | 256 (29.8) |
| Married | 19 (57.6) | 14 (42.4) | 222 (59.5) | 151 (40.5) | 406 (47.3) | |
| Divorced/widowed | 17 (56.7) | 13 (43.3) | 90 (53.9) | 77 (46.1) | 197 (22.9) | |
| Education level | Less than high school | 2 (66.7) | 1 (33.3) | 26 (59.1) | 18 (40.9) | 47 (5.5) |
| High school | 4 (66.7) | 2 (33.3) | 75 (67.6) | 36 (32.4) | 117 (13.6) | |
| Some college/associate’s degree | 13 (48.1) | 14 (51.9) | 145 (53.9) | 124 (46.1) | 296 (34.5) | |
| College graduate/graduate school | 17 (56.7) | 13 (43.3) | 203 (55.2) | 165 (44.8) | 398 (46.4) | |
| Housing status | Rent | 4 (40.0) | 6 (60.0) | 157 (53.8) | 135 (46.2) | 302 (35.2) |
| Own | 30 (57.7) | 22 (42.3) | 264 (58.9) | 184 (41.1) | 500 (58.3) | |
| Live with family, friends, other | 2 (50.0) | 2 (50.0) | 27 (51.9) | 25 (48.1) | 56 (6.5) | |
| Country of birth United States | No | 5 (62.5) | 3 (37.5) | 40 (56.3) | 31 (43.7) | 79 (9.2) |
| Yes | 31 (53.4) | 27 (46.6) | 409 (56.6) | 313 (43.4) | 780 (90.8) | |
| Number of dependents | None | 31 (52.5) | 28 (47.5) | 231 (55.9) | 182 (44.1) | 472 (55.1) |
| One | 5 (71.4) | 2 (28.6) | 97 (55.7) | 77 (44.3) | 181 (21.1) | |
| Two | 0 (0.0) | 0 (0.0) | 76 (58.5) | 54 (41.5) | 130 (15.2) | |
| Three or more | 0 (0.0) | 0 (0.0) | 44 (59.5) | 30 (40.5) | 74 (8.6) | |
| Household size, including self | One | 14 (51.9) | 13 (48.1) | 88 (55.7) | 70 (44.3) | 185 (21.5) |
| Two | 20 (58.8) | 14 (41.2) | 153 (54.3) | 129 (45.7) | 316 (36.8) | |
| Three | 2 (100.0) | 0 (0.0) | 102 (62.2) | 62 (37.8) | 166 (19.3) | |
| Four | 0 (0.0) | 2 (100.0) | 63 (57.3) | 47 (42.7) | 112 (13.0) | |
| Five or more | 0 (0.0) | 1 (100.0) | 43 (54.4) | 36 (45.6) | 80 (9.3) | |
| Employment status | Full-time | 3 (42.9) | 4 (57.1) | 232 (52.1) | 213 (47.9) | 452 (52.9) |
| Part-time | 4 (57.1) | 3 (42.9) | 62 (55.4) | 50 (44.6) | 119 (13.9) | |
| Retired | 24 (53.3) | 21 (46.7) | 34 (79.1) | 9 (20.9) | 88 (10.3) | |
| Disabled | 2 (66.7) | 1 (33.3) | 43 (62.3) | 26 (37.7) | 72 (8.4) | |
| Unemployed | 3 (75.0) | 1 (25.0) | 76 (63.3) | 44 (36.7) | 124 (14.5) | |
| Annual household income in past year | Less than $10K | 3 (50.0) | 3 (50.0) | 47 (50.5) | 46 (49.5) | 99 (11.5) |
| $10K–$29,999 | 6 (54.5) | 5 (45.5) | 77 (53.5) | 67 (46.5) | 155 (18.0) | |
| $30K–$49,999 | 3 (42.9) | 4 (57.1) | 78 (58.2) | 56 (41.8) | 141 (16.4) | |
| $50K+ | 17 (58.6) | 12 (41.4) | 220 (58.2) | 158 (41.8) | 407 (47.3) | |
| Don’t know | 7 (53.8) | 6 (46.2) | 27 (60.0) | 18 (40.0) | 58 (6.7) | |
| Insurance | Uninsured | 0 (0.0) | 0 (0.0) | 28 (71.8) | 11 (28.2) | 39 (4.7) |
| Private | 3 (60.0) | 2 (40.0) | 322 (55.8) | 255 (44.2) | 582 (69.5) | |
| Public | 33 (54.1) | 28 (45.9) | 89 (57.1) | 67 (42.9) | 217 (25.9) | |
Note. Not all variables total n = 862 due to missing data. OPNRP = Ohio Patient Navigation Research Program.
Primary Outcome: Time to Diagnostic Resolution
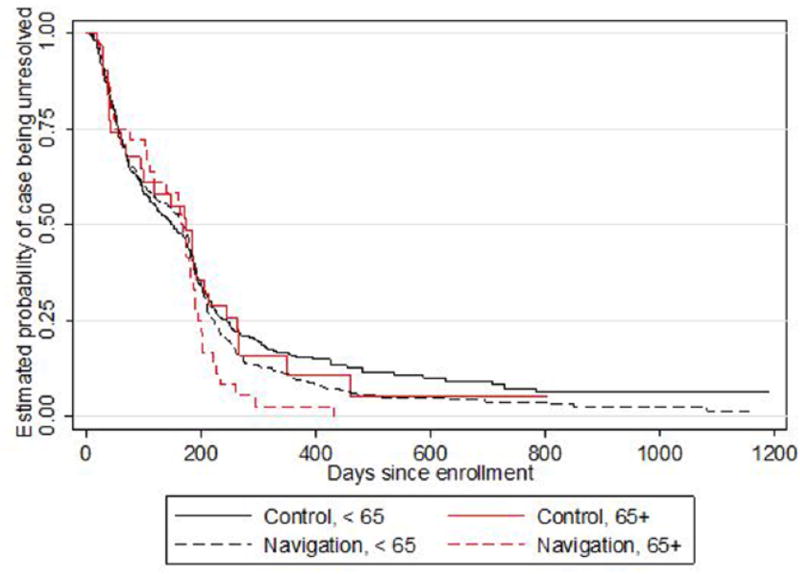
The Kaplan–Meier survival curves for time to diagnostic resolution by PN versus usual care arms in the older adult and younger groups are shown in Figure 1. The results showed that the proportion of resolved cases throughout the first 6 months was approximately the same across both study arms in the older adult group, with a greater proportion of resolved cases observed among navigated older adult participants beginning roughly at 6 months compared with older adult participants in the usual care arm. The same was true for the younger age groups.
Figure 1.
Estimated Kaplan–Meier curves for time to diagnostic resolution by PN versus usual care arms.
Further investigation indicated that time to diagnostic resolution decreased at a significantly faster rate among older adult participants in the PN arm compared with those in the usual care arm. Conditional on the clinic random effect, the rate of resolution among older adult participants in the PN arm was 43% faster (hazard ratio [HR] = 1.43, p = .13) than usual care at 3 months and 127% faster (HR = 2.27, p = .001) at 6 months. This pattern is displayed in Table 3, which provides estimated HR and p values of the PN effect from the model at several time points of follow-up and for each age group. Although it appears that older adult participants may have benefited more from PN than those younger than 65 years, this difference was not significant (2-df Wald p = .56).
Table 3.
Model-Estimated HRs and 95% CIs for Diagnostic Resolution by PN Versus Usual Care by Age Group.
| Older adult participants ≥65 years | Younger participants <65 years | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
|
|
|
|
|
|||||
| Interval (months) |
Estimated HR (95% CI) |
p | Estimated HR (95% CI) |
p | Estimated HR (95% CI) |
p | Estimated HR (95% CI) |
p |
| 3 | 1.43 [0.90, 2.26] | .1257 | 1.19 [0.75, 1.88] | .4591 | 1.20 [0.88, 1.62] | .2444 | 1.17 [0.94, 1.46] | .1702 |
| 6 | 2.27 [1.39, 3.71] | .0010 | 1.88 [1.17, 3.01] | .0089 | 1.38 [1.01, 1.88] | .0461 | 1.36 [1.07, 1.73] | .0122 |
| 9 | 2.96 [1.63, 5.37] | .0004 | 2.43 [1.35, 4.40] | .0032 | 1.49 [1.06, 2.09] | .0209 | 1.48 [1.13, 1.95] | .0049 |
| 12 | 3.61 [1.80, 7.25] | .0003 | 2.96 [1.46, 6.01] | .0027 | 1.58 [1.10, 2.28] | .0135 | 1.58 [1.16, 2.15] | .0034 |
Note. The data shown are categorized as unadjusted (n = 862) and adjusted (n = 797) for race (White, Black, Other), marital status (single, married, divorced/widowed), education (less than high school, high school, some college/associate’s degree, college graduate/graduate degree), housing status (rent, own, live with family/friends/other), and income (less than $10K, $10K–$29,999, $30K–$49,999, $50K+). HR = hazard ratio; CI = confidence interval; PN = patient navigation.
Table 3 also shows that similar results were found after adjusting for participant demographics, indicated by a rate of resolution at 6 months that was 88% higher among older adult participants randomized to PN than those randomized to usual care (HR = 1.88, p = .009). This result suggests that there was no meaningful confounding of the age effect by race, marital status, education, housing status, and income. Similar to the pattern observed in the older adult group, younger participants with an abnormal cancer screening exam in the PN arm also experienced a significantly reduced time to diagnostic resolution in both the unadjusted (HR = 1.38, p = .046) and adjusted (HR = 1.36, p = .012) analyses compared with the usual care arm by 6 months of follow-up.
In addition, in comparison to the standards set by the NBCCEDP (Richardson et al., 2010), PN did not significantly improve the likelihood of diagnostic resolution within 60 days after an abnormal cancer screening exam in either the older (odds ratio [OR] = 1.07, p = .92) or younger (OR = 1.12, p = .68) age groups (Table 4).
Table 4.
Impact of PN on Diagnostic Resolution Within 60 Days After an Abnormal Cancer Screening Exam.
| Older adult participants ≥65 years | Younger participants <65 years | ||
|---|---|---|---|
|
|
|
||
| Estimated odds ratio (95% CI) |
p | Estimated odds ratio (95% CI) |
p |
| 1.07 [0.32, 3.57] | .92 | 1.12 [0.65, 1.93] | .68 |
Note. Odds ratios are in terms of PN over usual care. In accordance with the standards established by the National Breast and Cervical Cancer Early Detection Program. PN = patient navigation; CI = confidence interval.
Secondary Outcome: Navigation Process
More than half (58.3%) of the 475 participants in the PN arm reported no barriers, with approximately 60% of participants in both the younger (58.1%) and older adult (61.1%) groups reporting a lack of barriers to care. Among those reporting barriers in the older adult group (n = 14, 38.9%), eight (57.1%) older adult participants reported only one barrier and six (42.9%) older adult participants reported two or more barriers. Among those reporting barriers in the younger group (n = 184, 41.9%), 91 (49.5%) reported only one barrier and 93 (50.5%) reported two or more barriers. Interestingly, the three most frequently reported barriers among both groups were misperceptions about a test or treatment (15%), communication concerns with medical personnel (13.1%), and system problems with scheduling care (10.5%), which were categorized as intrapersonal and systemic barriers.
Patient navigators reported 1,152 total encounters with the participants in the PN arm, which consisted of 84 encounters with the older adult group and 1,068 encounters with the younger group. The mean number of encounters per navigated participant was similar among participants in the younger group (2.4 ± 3.1, range 1–38) and the older adult group (2.33 ± 2.0, range 1–10). The majority of these encounters among participants in both the older adult and younger groups with a patient navigator lasted less than 15 min (90.5% and 89.7%, respectively) and commonly included referrals or direct contact and support from the patient navigator.
Discussion
The primary objective of this study was to test the efficacy of PN on improving time to diagnostic resolution and identifying barriers to care among older adults in the OPNRP with abnormal cancer screening exams to determine the utility of PN as an effective strategy to facilitate access to prompt and quality standard cancer care in this population. Analyses revealed that PN significantly reduced time to diagnostic resolution among older adult participants beginning at roughly 6 months after an abnormal cancer screening exam, regardless of participants’ race, marital status, education, housing status, and income. This difference increased over time, resulting in a larger proportion of abnormal cancer screening exams resolved among navigated older adult participants compared with older adult participants receiving usual care. In addition, although similar trends were observed when comparing the rates of resolution between PN and usual care arms in both the older adult and younger groups, a higher rate of resolution was found at 6 months in the PN arm for older adult participants (88%) compared with younger navigated participants (36%). However, the difference in the rates of resolution was nonsignificant, limited by the small sample size of older adult participants. This result suggests that despite the similar trends observed between older adult and younger navigated participants, PN may be more effective in reducing time to diagnostic resolution among older adults, which provides evidence demonstrating the necessity of PN programs targeted to the older adult population.
Utilizing the NBCCEDP standard of having a diagnosis within 60 days of an abnormal cancer screening exam (Richardson et al., 2010), PN did not significantly improve time to diagnostic resolution within 60 days of an abnormal cancer screening exam in this study. This nonsignificant finding may be attributed to a number of factors including the homogeneity of the participants (e.g., mostly White, female, breast cancer survivors) and comorbidities. Comorbidities were not identified as a major barrier to care for either age group, and this study did not adjust for the number of comorbidities. Thus, the true impact of the existing comorbidities and corresponding disease management may have complicated the effectiveness of PN which focused on a timely diagnosis of cancer (Williams et al., 2016).
Other potential reasons for the nonsignificant difference in resolution within 60 days may have been differences in provider skills and characteristics, miscommunication between patients and their providers regarding compliance with screening exams and proper follow-up procedures following abnormal screening exams, and waiting times within the health care system, as posited in previous PN studies (Ganry, Peng, & Dubreuil, 2004; Richardson et al., 2010). Consequently, it may be difficult to distinguish between patient-, physician-, and system-related delays, as shown in prior studies that have attempted to meet this benchmark (Ganry et al., 2004; Richardson et al., 2010). For example, a study by Freund et al. (2014) reported similar diagnostic delays, indicating that some participants were not contacted by their patient navigator within 60 days, whereas other participants were able to overcome barriers without a patient navigator. Because no prior studies have examined the effect on PN on improving time to diagnostic resolution in older adults, it remains difficult to propose potential reasons as they may not equate to possible reasons for delays in diagnostic resolution among older adults. Further investigation is needed to discern the factors that contributed to not meeting the NBCCEDP’s benchmark to determine additional strategies to reduce delays to achieving diagnostic resolution in this population.
This study is novel in that it is the first study of PN as an intervention to reduce the time to diagnostic resolution following an abnormal cancer screening exam for the older adult population. Previous studies have shown continued benefits of PN in other areas along the cancer continuum such as cancer screenings and treatments in the older adult population. For example, several studies of PN in the older adult population have shown that PN improved cancer screening rates, although these studies focused on specific demographic factors such as race and immigration status (Marshall et al., 2016; Percac-Lima, Milosavljevic, Oo, Marable, & Bond, 2012). Another study found that PN significantly decreased time to consultation for treatment following a breast cancer diagnosis for women older than 60 years (Basu et al., 2013). Although these aforementioned studies demonstrated the effectiveness of PN in the older adult population, they focused on a specific cancer, demographic characteristic, or aspect of the cancer care continuum and lacked generalizability to the heterogeneous older adult cancer patient population. Furthermore, the absence of additional studies involving PN in the older adult population limits the ability to evaluate its effect as a potential intervention to reduce delays in care. More research is needed among older adult populations to better understand how to integrate PN throughout all aspects of care on the cancer care continuum to reduce health disparities in this population.
Future PN interventions among older adult populations also need to take into account that the current cancer screening guidelines for older adults are varied and unclear. For example, the American Cancer Society recommends that women 55 years and older should transition to biennial screening or have the opportunity to continue screening annually. In addition, they suggest that women should continue screening mammography as long as their overall health is good and they have a life expectancy of 10 years or longer (American Cancer Society, 2016a). On the contrary, the United States Preventive Task Force (USPTF) recommends that women age 50 to 74 years should receive biennial screening mammography. However, the USPTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening mammography in women age 75 years or older (USPTF, 2016). Thus, older adults and their clinicians should consider the benefit-to-harm ratio for cancer screenings and should recognize cancer screenings as an individualized decision, taking into account their life expectancy, functional status, comorbidities, and personal preferences (Eckstrom, Feeny, Walter, Perdue, & Whitlock, 2013).
This study found that both older and younger participants who received PN resolved significantly more quickly compared with usual care, demonstrating that both age groups can benefit from PN. Therefore, it was important to identify potential patient and systemic barriers to care for individual participants within each age group to appropriately tailor future PN programs to address age-specific needs. Although both the older adult and younger groups reported similar proportions and types of barriers, the older adult participants reported a higher percentage of barriers involving system-level barriers, such as communication concerns with medical personnel, whereas younger participants more often reported patient-based barriers, such as misperceptions about a test or treatment. One potential explanation is that older adult patients are more likely than younger patients to have lower health and document literacy, to communicate poorly with health care professionals, and to avoid asking questions about their health care (Basu et al., 2013; Dunlop, Manheim, & Chang, 2002; Tariman, Berry, Cochrane, Doorenbos, & Schepp, 2012), perhaps, in part, due to their preference of a physician-centered model of care (Cutilli, 2010). Because older adult patients are more likely to seek health information from health care professionals than the Internet (Cutilli, 2010)and often have less social support in cancer decisions compared with younger patients (Krok-Schoen, Palmer-Wackerly, Dailey, Wojno, &Krieger, 2016), older adult patients may be more likely to find necessary support from a patient navigator (Basu et al., 2013). This study also found that almost half of older adult participants encountered two or more barriers that frequently required more than two encounters with a patient navigator. This could also explain why a larger proportion of abnormal cancer screening exams resolved among older adult navigated participants over time compared with older adult participants receiving usual care. The combination of these findings provides insight as to how the health care system may address barriers to care and health care behaviors and beliefs specific to the older adult population to help older adult patients effectively utilize necessary health care services.
Strengths/Limitations
This study possesses several strengths, including a group-randomized trial design, a mix of clinic types, utilization of lay navigators from the participants’ communities, and an examination of barriers to care in this sample of older adult participants. An additional study strength is the inclusion of several cancer types including breast, cervical, and colorectal. Despite these criteria, the small sample size of older adult participants limited our findings to mostly White female participants with abnormal breast cancer screening exams and public insurance. Future studies should consider the effectiveness of PN interventions among other racial and ethnic groups, the uninsured, and those without a primary care provider to better identify potential disparities within older cancer patient populations. In addition, this study only examined the time to diagnostic resolution and barriers to care of participants in the OPNRP. Future studies with older adult participants may benefit from investigating delays to care across the cancer continuum, including prevention efforts, screening exams, treatments, and long-term survivorship.
Conclusion
This study was the first to explore PN as an intervention to reduce the time to diagnostic resolution following an abnormal cancer screening exam for the older adult population. The results indicated that PN was an effective intervention to reduce time to diagnostic resolution among older adults with abnormal breast, cervical, or colorectal cancer screening exams. This study also found that older adults often experience system-level barriers to care such as communication concerns with medical personnel. These findings imply the need for clinicians to consider the risks and benefits associated with cancer screenings among older adults as the screening guidelines are unclear. The findings in this study were from a hypothesis-generating study, and recruiting a broader, more heterogeneous older adult population while accounting for their unique health statuses (e.g., comorbidities) is critical to developing a larger, more generalizable evidence base to guide future implementation of PN among older adult patient populations. Further research is also needed to assess the cost and effectiveness of different approaches of PN tailored to the older adult population to eliminate the increasing burden of cancer incidence and mortality.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this project was provided by The Ohio State University College of Medicine Roessler Research Scholarship, the American Cancer Society (112190-SIRSG-05-253-01), and NCI (P30CA016058).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Cancer Society. American Cancer Society guidelines for the early detection of cancer. 2016a Retrieved from http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer.
- American Cancer Society. Cancer facts and figures, 2016. Atlanta, GA: Author; 2016b. [Google Scholar]
- Basu M, Linebarger J, Gabram SG, Patterson SG, Amin M, Ward KC. The effect of nurse navigation on timeliness of breast cancer care at an academic comprehensive cancer center. Cancer. 2013;119:2524–2531. doi: 10.1002/cncr.28024. [DOI] [PubMed] [Google Scholar]
- Battaglia TA, Roloff K, Posner MA, Freund KM. Improving follow-up to abnormal breast cancer screening in an urban population: a patient navigation intervention. Cancer. 2007;109:359–367. doi: 10.1002/cncr.22354. [DOI] [PubMed] [Google Scholar]
- Berger NA, Savvides P, Koroukian SM. Cancer in the elderly. Transactions of the American Clinical and Climatological Association. 2006;117:147–156. [PMC free article] [PubMed] [Google Scholar]
- Burhansstipanov L, Wound DB, Capelouto N, Goldfarb F, Harjo L, Hatathlie L, White M. Culturally relevant “Navigator” patient support. The Native sisters. Cancer Practice. 1998;6:191–194. doi: 10.1046/j.1523-5394.1998.006003191.x. [DOI] [PubMed] [Google Scholar]
- Byers T. Two decades of declining cancer mortality: Progress with disparity. Annual Review of Public Health. 2010;31:121–132. doi: 10.1146/annurev.publhealth.121208.131047. [DOI] [PubMed] [Google Scholar]
- Clark CR, Baril N, Kunicki M, Johnson N, Soukup J, Ferguson K REACH 2010 Breast and Cervical Cancer Coalition. Addressing social determinants of health to improve access to early breast cancer detection: Results of the Boston REACH 2010 Breast and Cervical Cancer Coalition Women’s Health Demonstration Project. Journal of Women’s Health. 2009;18:677–690. doi: 10.1089/jwh.2008.0972. [DOI] [PubMed] [Google Scholar]
- Cutilli CC. Seeking health information: What sources do your patients use? Orthopedic Nursing. 2010;29:214–219. doi: 10.1097/NOR.0b013e3181db5471. [DOI] [PubMed] [Google Scholar]
- Day JC. Population projections of the United States by age, sex, race, and Hispanic origin: 1995–2050. Washington, DC: U.S. Bureau of the Census; 1996. [Google Scholar]
- Dohan D, Schrag D. Using navigators to improve care of underserved patients: current practices and approaches. Cancer. 2005;104:848–855. doi: 10.1002/cncr.21214. [DOI] [PubMed] [Google Scholar]
- Dunlop DD, Manheim LM, Chang RW. Gender and ethnic/racial disparities in health care utilization among older adults. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences. 2002;57:S221–S233. doi: 10.1093/geronb/57.4.s221. [DOI] [PubMed] [Google Scholar]
- Eckstrom E, Feeny DH, Walter LC, Perdue LA, Whitlock EP. Individualizing cancer screening in older adults: A narrative review and framework for future research. Journal of General Internal Medicine. 2013;28:292–298. doi: 10.1007/s11606-012-2227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ell K, Padgett D, Vourlekis B, Nissly J, Pineda D, Sarabia O, Lee PJ. Abnormal mammogram follow-up: a pilot study in women with low income. Cancer Practice. 2002;10:130–138. doi: 10.1046/j.1523-5394.2002.103009. [DOI] [PubMed] [Google Scholar]
- Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Preventive Medicine. 2007;44:26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Ell K, Vourlekis B, Muderspach L, Nissly J, Padgett D, Pineda D, Lee PJ. Abnormal cervical screen follow-up among low-income Latinas: Project SAFe. Journal of Women's Health & Gender-Based Medicine. 2002;11:639–651. doi: 10.1089/152460902760360586. [DOI] [PubMed] [Google Scholar]
- Ferrante JM, Chen PH, Kim S. The effect of patient navigation on time to diagnosis, anxiety, and satisfaction in urban minority women with abnormal mammograms: a randomized controlled trial. Journal of Urban Health. 2008;85:114–124. doi: 10.1007/s11524-007-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Powe NR, Cooper LS, Ives DG, Robbins JA. Barriers to health care access among the elderly and who perceives them. American Journal of Public Health. 2004;94:1788–1784. doi: 10.2105/ajph.94.10.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Practice. 1995;3:19–30. [PubMed] [Google Scholar]
- Frelix GD, Rosenblatt R, Solomon M, Vikram B. Breast cancer screening in underserved women in the Bronx. Journal of the National Medical Association. 1999;91:195–200. [PMC free article] [PubMed] [Google Scholar]
- Freund KM, Battaglia TA, Calhoun E, Darnell JS, Dudley DJ, Fiscella K Writing Group of the Patient Navigation Research Program. Impact of patient navigation on timely cancer care: The Patient Navigation Research Program. Journal of the National Cancer Institute. 2014;106(6):dju115. doi: 10.1093/jnci/dju115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund KM, Battaglia TA, Calhoun E, Dudley DJ, Fiscella K, Paskett ED The Patient Navigation Research Program Group. National Cancer Institute Patient Navigation Research Program. Cancer. 2008;113:3391–3399. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganry O, Peng J, Dubreuil A. Influence of abnormal screens on delays and prognostic indicators of screen-detected breast carcinoma. Journal of Medical Screening. 2004;11(1):28–31. doi: 10.1177/096914130301100107. [DOI] [PubMed] [Google Scholar]
- Geiger AM, Thwin SS, Lash TL, Buist DS, Prout MN, Wei F, Silliman RA. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007;109:966–974. doi: 10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- Hiatt RA, Pasick RJ, Stewart S, Bloom J, Davis P, Gardiner P, Stroud F. Community-based cancer screening for underserved women: Design and baseline findings from the Breast and Cervical Cancer Intervention Study. Preventive Medicine. 2001;33:190–203. doi: 10.1006/pmed.2001.0871. [DOI] [PubMed] [Google Scholar]
- Kinsella K, He W. An aging world: 2008. Washington, DC: U.S. Bureau of the Census; 2009. [Google Scholar]
- Krok-Schoen JL, Palmer-Wackerly A, Dailey PM, Wojno JC, Krieger JL. Age differences in cancer treatment decision making and social support. Journal of Aging and Health. 2016;29:187–205. doi: 10.1177/0898264316628488. [DOI] [PubMed] [Google Scholar]
- Marshall JK, Mbah OM, Ford JG, Phelan-Emrick D, Ahmed S, Bone L, Pollack CE. Effect of patient navigation on breast cancer screening among African American Medicare beneficiaries: a randomized controlled trial. Journal of General Internal Medicine. 2016;31:68–76. doi: 10.1007/s11606-015-3484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AE, Jo AM, Crespi CM, Sundan M, Bastani R. Peer navigation improves diagnostic follow-up after breast cancer screening among Korean American women: results of a randomized trial. Cancer Causes Control. 2010;21:1931–1940. doi: 10.1007/s10552-010-9621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluwole SF, Ali AO, Adu A, Blane BP, Barlow B, Oropeza R. Impact of a cancer screening program on breast cancer stage at diagnosis in a medically underserved urban community. Journal of the American College of Surgeons. 2003;196:180–188. doi: 10.1016/S1072-7515(02)01765-9. [DOI] [PubMed] [Google Scholar]
- Paskett ED, Harrop JP, Wells KJ. Patient navigation: An update on the state of the science. CA: A Cancer Journal for Clinicians. 2011;61:237–249. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskett ED, Katz ML, Post DM, Pennell ML, Young GS, Seiber EE, Murray DM. The Ohio Patient Navigation Research Program: does the American Cancer Society patient navigation model improve time to resolution in patients with abnormal screening tests? Cancer Epidemiology, Biomarkers and Prevention. 2012;21:1620–1628. doi: 10.1158/1055-9965.EPI-12-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percac-Lima S, Milosavljevic B, Oo SA, Marable D, Bond B. Patient navigation to improve breast cancer screening in Bosnian refugees and immigrants. Journal of Immigrant and Minority Health. 2012;14:727–730. doi: 10.1007/s10903-011-9539-5. [DOI] [PubMed] [Google Scholar]
- Richardson LC, Royalty J, Howe W, Helsel W, Kammerer WK, Benard VB. Timeliness of breast cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program, 1996–2005. American Journal of Public Health. 2010;100:1769–1776. doi: 10.2105/AJPH.2009.160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. Journal of Clinical Oncology. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- Tariman JD, Berry DL, Cochrane B, Doorenbos A, Schepp K. Physician, patient, and contextual factors affecting treatment decisions in older adults with cancer and models of decision making: A literature review. Oncology Nursing Forum. 2012;39(1):E70–E83. doi: 10.1188/12.ONF.E70-E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe JM, Thorpe CT, Kennelty KA, Pandhi N. Patterns of perceived barriers to medical care in older adults: a latent class analysis. Biomed Central Health Services Research. 2011;11:181. doi: 10.1186/1472-6963-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Final recommendation statement: Breast cancer: Screening. Author; 2016. Nov, Retrieved from https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. [Google Scholar]
- Wells KJ, Battaglia TA, Dudley DJ, Garcia R, Greene A, Calhoun E. Patient navigation: state of the art or is it science? Cancer. 2008;113:1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR, Mackenzie A, Magnuson A, Olin R, Chapman A, Mohile S, Holmes H. Comorbidity in older adults with cancer. Journal of Geriatric Oncology. 2016;7:249–257. doi: 10.1016/j.jgo.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]