Abstract
Background
Sarcopenia and proteinuria are significant health difficulties in the elderly; however, few studies have investigated their relationship. In this study, we investigated the association between sarcopenia and proteinuria in Korean subjects over 60 years old.
Methods
We included data from the Korean National Health and Nutrition Examination Survey, a cross-sectional, nationally representative survey conducted from 2009 to 2011 (n=4,008). Sarcopenia was defined using appendicular skeletal muscle mass as a percentage of body weight. Proteinuria was defined by a urine dipstick test result above trace levels.
Results
The overall proteinuria prevalence was 7.2%. The incidence of sarcopenia was significantly higher in subjects with proteinuria. The prevalence of proteinuria was significantly higher in the sarcopenic group (5.5% vs. 14.5% in the non-chronic kidney disease (CKD) group; 17.2% vs. 23.2% in the CKD group) than in the non-sarcopenic group. Furthermore, sarcopenic participants had worse metabolic parameters, such as higher body mass indexes, waist circumferences, and fasting glucose levels, and lower high-density lipoprotein cholesterol levels than those in the non-CKD group. After adjustment for confounders, the odds ratios (95% confidence interval) for proteinuria were 2.84 (1.92–4.18) in the sarcopenic non-CKD group, 3.70 (2.59–5.30) in the non-sarcopenic CKD group, and 5.19 (2.64–10.18) in the sarcopenic CKD group, compared to the non-sarcopenic, non-CKD group. Sarcopenia increased the proteinuria risk in elderly participants without CKD, even after adjustment for obesity, hypertension, diabetes, and metabolic syndrome.
Conclusion
These findings showed that sarcopenia was associated with dipstick proteinuria, especially in elderly participants without CKD, regardless of comorbidities.
Keywords: Sarcopenia, Proteinuria, Chronic Renal Insufficiency
INTRODUCTION
As the elderly population increases, the health of elderly citizens is becoming a priority for public health. One of the most important changes in old age is that in muscles. Sarcopenia is the age-related loss of muscle mass and strength. The prevalence of sarcopenia varies between 5%–13% in subjects over 60 years old, increasing sharply to 50% in those aged 80 or older.1,2,3) In sarcopenia, lost muscle tissue may be replaced by fat and connective tissue, causing chronic inflammation and catabolic cytokine production. These conditions are strongly associated with chronic diseases, such as hypertension, diabetes mellitus, and hyperlipidemia, as well as increased mortality.4,5,6)
Several previous studies have suggested that there might be an association between sarcopenia and decreased kidney function; these studies have primarily focused on chronic kidney disease (CKD). CKD can lead to sarcopenia, and vice versa; sarcopenia can also cause CKD, leading to a decreased glomerular filtration rate.7,8,9,10) The effect of sarcopenia on renal function can result in proteinuria in CKD. Considering that proteinuria is a risk factor prognostic indicator for CKD, it is vital to study the relationship between sarcopenia and proteinuria.11)
Proteinuria may also cause significant health difficulties in the elderly. It is a marker of kidney disease, particularly CKD.11,12) Persistent proteinuria is directly proportional to the extent of loss of renal function and is also a risk factor for cardiovascular diseases and mortality in older people.12,13) The best estimate of proteinuria is quantification using 24-hour urine collection and spot urine total protein or albumin levels that have been corrected for urine creatinine.11) However, these tests are difficult and time-consuming. In contrast, the urine dipstick test is a useful diagnostic tool to detect proteinuria because it is cheap, widely available, and fast.11)
In this study, we searched for an association between sarcopenia and dipstick proteinuria in elderly participants who were stratified by the presence of CKD, which is already known to be associated with proteinuria. We used data from the Korean National Health and Nutrition Examination Survey (KNHANES), a cross-sectional, nationally representative survey conducted from 2009–2011.
METHODS
1. Study Participants and Database
The KNHANES is a nationwide, population-based, cross-sectional health examination and survey regularly conducted by the Division of Chronic Disease Surveillance of the Korea Center for Disease Control and Prevention of the Ministry of Health and Welfare to monitor the general health and nutrition of South Koreans, as previously described in detail.14)
Of the 28,009 participants in the KNHANES 2009–2011, we initially selected 6,215 Koreans over the age of 60 years; 2,207 participants with missing data for their appendicular skeletal muscle mass (ASM), serum creatinine levels, or dipstick urine tests were excluded. In this study, participants diagnosed with stage 5 CKD based on the estimated glomerular filtration rate (eGFR) or who had end-stage renal disease (ESRD) were excluded; treatments for these conditions, like dialysis or kidney transplantation, may affect laboratory parameters and confound the outcome. A final total of 4,008 participants (1,794 men and 2,214 women) were included in the analysis. All participants in this study provided informed consent. The KNHANES was conducted following ethical approval by the Institutional Review Board of the Korea Center for Disease Control and Prevention (2009-01CON-03-2C, 2010-02CON-21-C, 2011-02CON-06-C).
2. Definition of Sarcopenia and Proteinuria
ASM was measured using whole-body, dual-energy X-ray absorptiometry (Discovery-W; Hologic Inc., Waltham, MA, USA). To define sarcopenia, we used ASM as a percentage of body weight (Wt), a procedure that was modified from the studies of Janssen et al.15) Sarcopenia was defined as a result that was 2 standard deviations below the sex-specific mean for healthy adults aged 20–39 years (1,054 men and 1,338 women), as previously described.16) The cut-off ASM/Wt value for sarcopenia was 27.2% in men and 21.3% in women. Urine dipstick analysis was performed using a Urisys 2400 automated urine analyzer (Roche Diagnostics GmbH, Mannheim, Germany), and proteinuria was defined as trace or greater.
3. Measurement of Clinical Parameters and Biochemical Analysis
We collected participant demographic data, including information on anthropometrics, smoking history, and physical activity, using standardized health questionnaires. Regular exercise was defined as engaging in moderate or vigorous exercise on a regular basis (≥20 minutes at a time and at least 3 times per week). Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). For biochemical analysis, blood samples were obtained in the morning following an overnight fast. Specimens were immediately processed and transported in cold storage to the Central Testing Institute (Neodin Medical Institute, Seoul, Korea). All blood samples were analyzed within 24 hours of transportation.
Plasma glucose, total cholesterol, triglyceride (TG), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein cholesterol levels were measured using a Hitachi 7600 automated chemistry analyzer (Hitachi, Tokyo, Japan) according to the manufacturer's protocol.
Insulin levels were determined using a gamma counter (1470 Wizard; Perkin Elmer, Turku, Finland) with an immunoradiometric assay (Biosource, Nivelles, Belgium). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as the fasting insulin (mIU/mL)×fasting glucose (mg/dL)/405.17) The serum creatinine concentration was measured using the kinetic Jaffe method, and the interassay coefficient of variation was less than 5%. Because the serum creatinine assay was not calibrated to be traceable using isotope dilution mass spectrometry, the eGFR was calculated using the original modification of diet in renal disease equation, as follows: eGFR=186.3×(serum creatinine)−1.154×(age)−0.203×0.742 (for women).18) CKD was defined in participants with an eGFR between 15 and 60 mL/min/1.73 m2.18)
The presence of other clinical conditions was assessed, including obesity, hypertension, diabetes mellitus, and metabolic syndrome, which are all associated with proteinuria. Obesity was defined as a BMI ≥25 kg/m2.19) Hypertension was defined in those who both reported a diagnosis of hypertension and currently received antihypertensive medications. Diabetes mellitus was determined in participants who were receiving either insulin or oral hypoglycemic agents. Metabolic syndrome was defined in people who met 3 or more of the following criteria for Asian populations: a waist circumference over 90 cm in men or 80 cm in women, blood pressure over 130/85 mm Hg, serum TG level over 150 mg/dL, serum HDL cholesterol level less than 40 mg/dL in men or 50 mg/dL in women, and fasting blood sugar over 110 mg/dL, according to the National Cholesterol Education Program ATP III definitions.20)
4. Statistical Analysis
Analyses for differences were performed using the IBM SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA). Participant characteristics were analyzed using a Student t-test or a one-way analysis of variance with Bonferroni post hoc analysis for continuous variables and the chi-square test for categorical variables. All tests for statistical significance were two-tailed and results were considered significant if P<0.05. Univariate and multivariate logistic regression analyses were carried out to identify factors associated with proteinuria. The confounding variables were age, sex, smoking status, and exercise status.
RESULTS
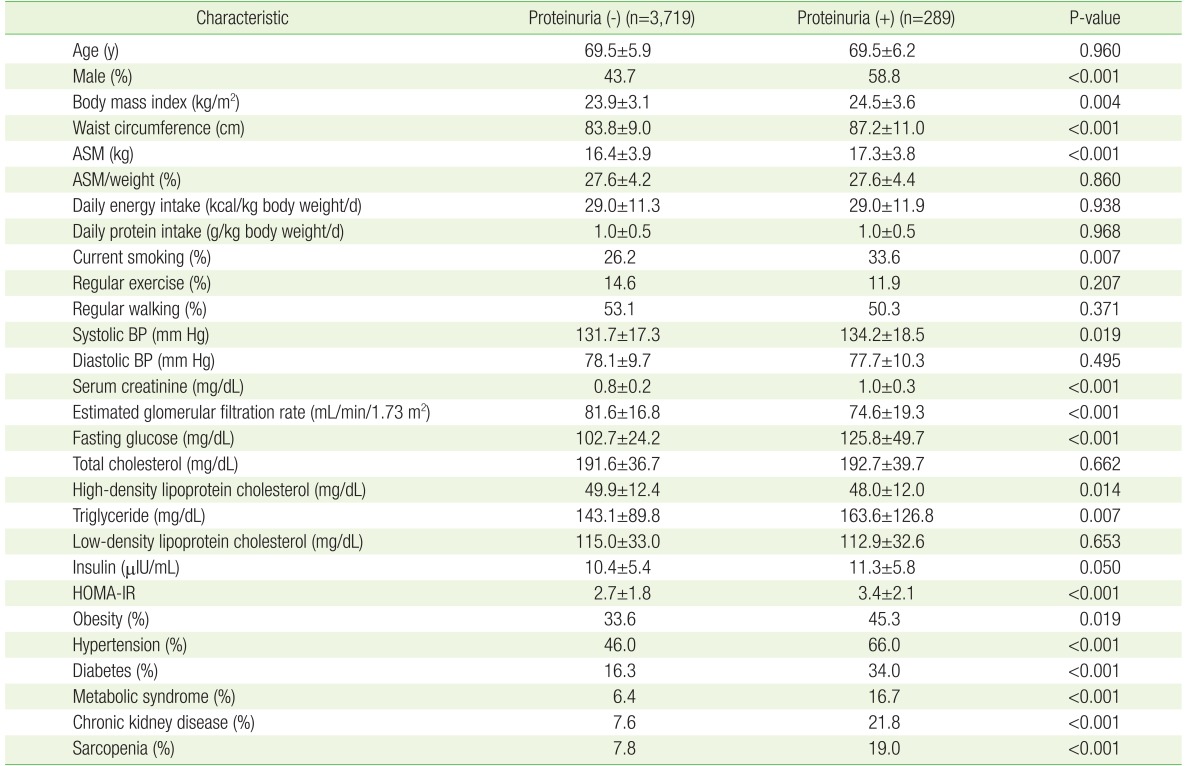
This study analyzed 4,008 participants. The clinical characteristics of participants according to the presence of proteinuria are summarized in Table 1. The overall prevalence of proteinuria was 7.2%. The group with proteinuria had a higher BMI, waist circumference, current smoking rate, and systolic blood pressure than the group without proteinuria. However, there was little difference regarding the extent of regular exercise and walking and the amount of daily energy and protein intake between the 2 groups. In addition, the group with proteinuria showed worse metabolic parameters, including higher fasting glucose, HOMA-IR, and TG levels, and lower HDL cholesterol, than the group without proteinuria. As expected, clinical conditions, such as the prevalence of obesity, hypertension, diabetes, metabolic syndrome, and CKD, were higher in the group with proteinuria. Most notably, the incidence of sarcopenia was significantly higher in the group with proteinuria, even though ASM/weight (%) was similar between the 2 groups.
Table 1. Baseline characteristics of the study participants.
Values are presented as mean±standard deviation or %. P-values were obtained using the Student t-test for continuous variables and the chi-square test for categorical variables. HOMA-IR was calculated using the insulin and fasting glucose levels as follows: fasting glucose (mg/dL)×insulin (µIU/mL)/405.
ASM, appendicular skeletal muscle mass; BP, blood pressure; HOMA-IR, homeostatic model assessment of insulin resistance.
1. Association between Sarcopenia and Proteinuria
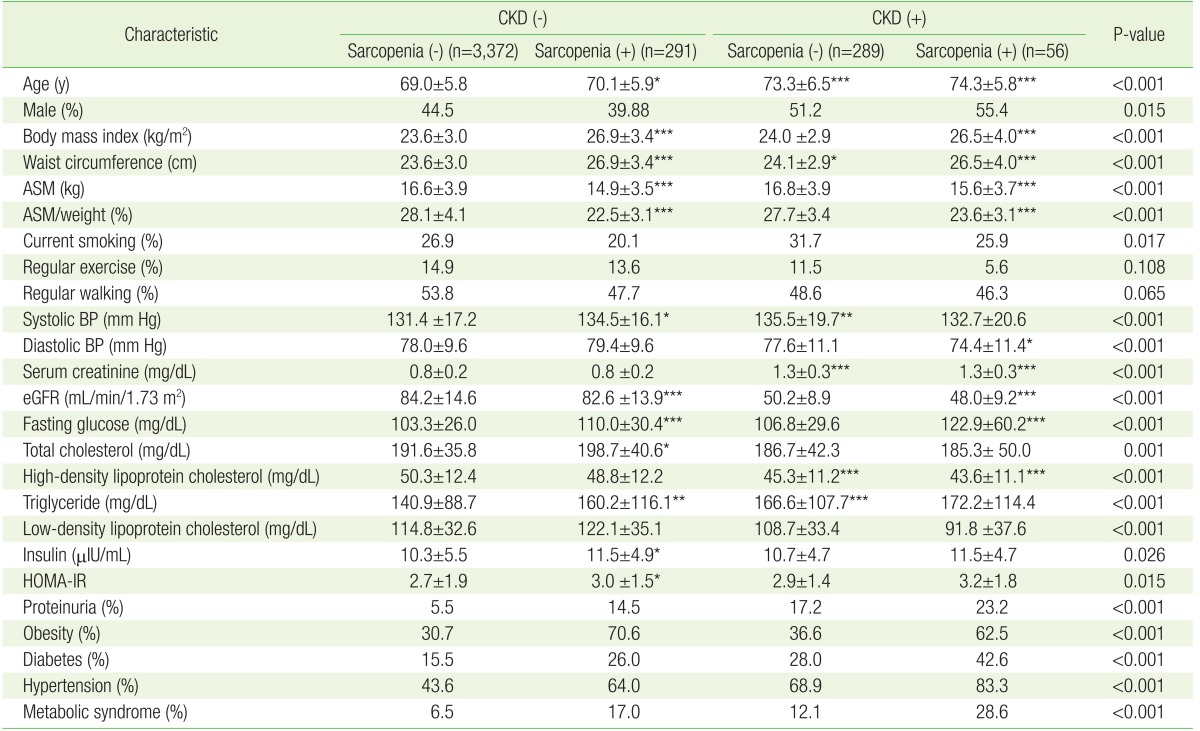
Because CKD is a well-established risk factor for proteinuria, we examined the independent association of sarcopenia with proteinuria after stratification according to CKD (Table 2). In the non-CKD group, sarcopenic participants had a higher BMI, waist circumference, and systolic blood pressure, along with worse metabolic parameters, such as fasting glucose, HOMA-IR, total cholesterol, and TG, than those without sarcopenia (reference group). In the CKD group, sarcopenic participants also exhibited worse metabolic parameters; however, there was a statistical significance only in BMI, waist circumference, and fasting glucose. Moreover, the prevalences of comorbid diseases like obesity, hypertension, diabetes, and metabolic syndrome were higher in the sarcopenic group. Notably, the incidence of proteinuria was also significantly higher in the sarcopenic group (5.5% versus 14.5% in the CKD group; 17.2% versus 23.2% in the non-CKD group) (P<0.001) than in the non-sarcopenic group (Table 2).
Table 2. Differences in clinical characteristics of participants according to their CKD and sarcopenia status.
Values are presented as mean±standard deviation or %. P-values were obtained using a one-way analysis of variance (with the Bonferroni post hoc test) for continuous variables and the chi-square test for categorical variables. eGFR is the estimated GFR calculated using the abbreviated modification of diet in renal disease equation: 175×(serum creatinine)−1.154×(age)−0.203×(0.742, for women). The HOMA-IR was calculated using the insulin and fasting glucose levels as follows: fasting glucose (mg/dL)×insulin (µIU/mL)/405.
CKD, chronic kidney disease; ASM, appendicular skeletal muscle mass; BP, blood pressure; eGFR, estimated glomerular filtration rate; HOMA-IR, homeostatic model assessment of insulin resistance.
*P<0.05, **P<0.01, and ***P<0.001 for comparison with the reference group with sarcopenia (-) and CKD (−).
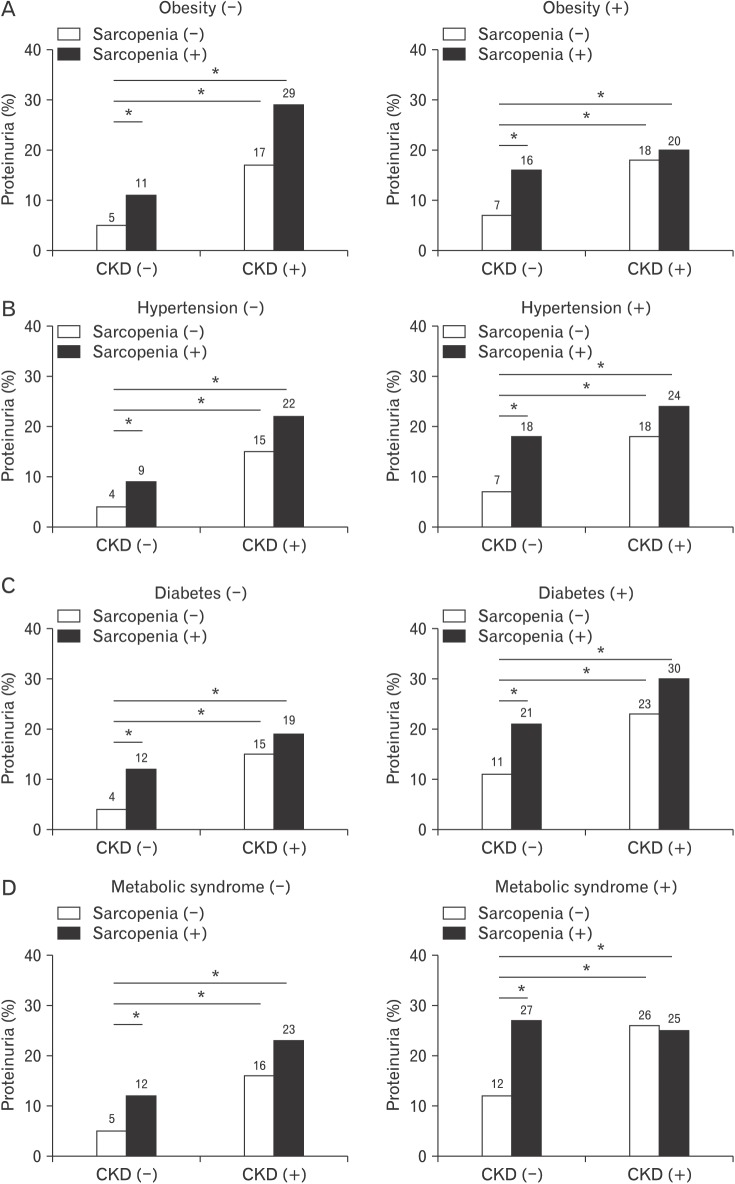
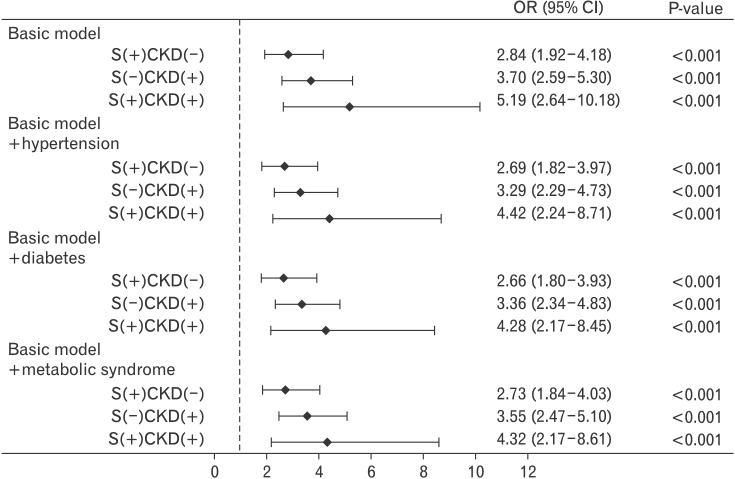
Because hypertension, diabetes, and metabolic syndrome are considered independent factors in kidney damage, we examined the independent association of proteinuria with sarcopenia after stratification according to these clinical conditions.21,22) The non-CKD group with sarcopenia showed an increased prevalence of proteinuria, regardless of whether they had obesity, hypertension, diabetes, or metabolic syndrome (Figure 1). However, in the CKD group, the prevalence of proteinuria was not different between the non-sarcopenic and sarcopenic groups. Notably, the odds ratio of sarcopenia without CKD for proteinuria increased to 2.84 (95% confidence interval [CI], 1.92 to 4.18; P<0.001) and was higher in CKD with sarcopenia as 5.19 (95% CI, 2.64 to 10.18) in the basic model, even after adjustments for clinical conditions such as hypertension, diabetes, and metabolic syndrome, as shown in Figure 2.
Figure 1. Prevalence of proteinuria according to the status of CKD and sarcopenia and the presence of obesity (A), hypertension (B), diabetes (C), and metabolic syndrome (D). Statistically significant differences across the status of sarcopenia and CKD were demonstrated by the chi-square test with linear-by-linear associations. CKD, chronic kidney disease. *P< 0.001.
Figure 2. Adjusted OR and 95% CI of proteinuria according to the status of CKD and sarcopenia and the presence of obesity, hypertension, diabetes, and metabolic syndrome. Values have been adjusted for age, gender, smoking status, and exercise status. OR, odds ratio; CI, confidence interval; CKD, chronic kidney disease.
DISCUSSION
Aging is an increasingly important issue for public health worldwide, with the sharp rise in the elderly population. Aging gradually causes a decline in physical function and activity, eventually leading to frailty. As one of the key components of frailty, sarcopenia is a progressive and generalized loss of skeletal muscle mass and strength.21) Sarcopenia is related to functional impairment, cardiopulmonary failure, and physical disability in the elderly.9) In addition to being an indicator of renal function, proteinuria is also a risk factor for cardiovascular disease and mortality in older people.23,24) Various studies have reported a strong relationship between sarcopenia and CKD or ESRD.9,10) However, there have been few studies on the relationship between sarcopenia and proteinuria using urinalysis with dipstick screening. We found that sarcopenia was significantly associated with dipstick proteinuria in elderly participants.
Recently, Foley et al.8) and Moon et al.10) reported that sarcopenia is associated with the stage of kidney disease in community-based populations in the United States and Korea, respectively. These studies emphasized that the higher the CKD stage, the larger the increase in sarcopenia.8,10) One mechanism for sarcopenia development in CKD is the inflammatory process. Chronic inflammation leads to a decrease in muscle mass, particularly in those with ESRD, resulting in sarcopenia in CKD.9) However, the present study showed that the prevalence of proteinuria was not different according to the presence or absence of sarcopenia in CKD, while it was significantly different in non-CKD group. This might be due to the small number of participants with CKD, and no difference in ASM (Wt) between the non-sarcopenic and sarcopenic subjects with CKD.
Verna et al. reported that aging is associated with a decline in renal function due to the decrease of functional glomeruli at a rate of approximately 0.75 mL/min/y.12) This tendency can be accelerated by comorbid diseases like hypertension, diabetes, atherosclerosis, and heart disease. As these diseases are common amongst the elderly, it is difficult to differentiate between pathological and physiological proteinuria. Normal aging of the kidney is related to an increased proportion of globally sclerotic glomeruli (glomerulosclerosis), increased arteriosclerosis (fibrointimal hyperplasia), medial hypertrophy, arteriolar hyalinosis, and tubular atrophy with surrounding areas of interstitial fibrosis.12,25) Proteinuria is caused by glomerular, tubular, and overflow glomerular dysfunction. Of these, glomerular dysfunction is the most common cause, and results in altered permeability of the glomerular basement membrane.26) For example, one of the most common etiologies of proteinuria and diabetic nephropathy includes glomerular dysfunction and glomerulonephropathy.26) The most important risk factors for sarcopenia include aging and female sex.9) Alterations in catabolic and anabolic stimuli, changes in growth hormone and insulin-like growth factor 1, and increased insulin resistance are also related to sarcopenia in the elderly. Moreover, chronic inflammation in aging muscles, which is similar to that observed in chronic diseases such as ESRD, diabetes, cognitive impairment, and mood disorders, is also related to sarcopenia in the elderly.9) In this study, sarcopenia was strongly associated with proteinuria, especially in non-CKD participants. As the study included subjects over 60 years old, sarcopenia and proteinuria were likely to have developed in this population; aging itself could be the cause of both sarcopenia and proteinuria. Otherwise, like other age-prevalent diseases, including hypertension, diabetes, and CKD, sarcopenia might also contribute to proteinuria in elderly patients without CKD. Sarcopenia is therefore an important disease in the elderly population. As a result, screening for and monitoring of sarcopenia in older people is needed. Further evaluations should be carried out to determine the etiology of sarcopenia and proteinuria.
Currently, the most accurate test for proteinuria is quantification using timed (usually 24 hours) urine collection. However, this method is not only extremely time-consuming but may also be imprecise. Therefore, current clinical practice guidelines recommend “spot urine total protein or albumin” corrected for urine creatinine as the optimal method for evaluation of proteinuria, which is also time-consuming and expensive. In contrast, the urine dipstick test is widely used as an initial screening tool for the detection of proteinuria because of its low cost, wide availability, and rapid results. Despite the advantages of the urine dipstick test, few studies have used this method to detect proteinuria. 11,27) Most studies of CKD have defined the disease according to the eGFR (eGFR <60 mL/min/1.73 m2), although CKD is technically defined as renal dysfunction or proteinuria (dipstick urinary protein scores ≥1+).22) Lim et al.11) explored the accuracy of urine dipstick testing for the detection of proteinuria in elderly subjects. They concluded that if it is intended to set an albumin/creatinine ratio (ACR) ≥300 mg/g or a protein/creatinine ratio (PCR) as the reference standard for proteinuria, urine dipstick testing can be recommended for screening in the elderly.11) Although there is no reference standard for ACR and PCR proteinuria that can be compared with urine dipstick testing in this study, proteinuria can be sufficiently measured using the urine dipstick test.11)
In a previous report, regardless of hypertension, diabetes, or obesity, a higher prevalence of proteinuria was found in sarcopenic patients than in those who were non-sarcopenic.21) Very recently, Kim et al.28) and Han et al.29) also reported that sarcopenia is associated with albuminuria independently of comorbidities such as hypertension and diabetes in Korean adults aged over 19 years. In this study, we focused on the association between sarcopenia and proteinuria according to the presence or absence of CKD using a simple dipstick urine test. Even after adjusting for obesity, hypertension, diabetes, and metabolic syndrome, sarcopenia increased the risk of proteinuria, especially in elderly participants without CKD.
The current study had some limitations. First, the study was crosssectional, so it is impossible to assess any cause and effect relationship. Further prospective research will be needed to identify any causal relationship between kidney proteinuria and sarcopenia. Second, the European Working Group on Sarcopenia in Older People made it clear that age-related sarcopenia must include both low muscle mass and low muscle function; however, we only used the measurements of a participant's skeletal muscle mass to define sarcopenia.10,21) Third, the accuracy of this study's determination of proteinuria may be doubtful because it defined using a one-time urine dipstick test. The sensitivity for trace or greater proteinuria depends on factors including the standard ACR ratio and the automated reading of dipsticks.11) To overcome this, dipstick screening must be repeated; ACR standards for urinestick proteinuria and the accurate reading of dipsticks should also be developed. Finally, due to the nature of the survey questionnaires, there may have been an information bias in the survey responses.
Nonetheless, the current study also had some strengths. First, our study provided strong evidence of a close relationship between sarcopenia and proteinuria in an elderly population. Previous studies have reported an association between kidney function and sarcopenia in patients with CKD.8,10) This study enabled us to begin defining the relevance of kidney function and sarcopenia in participants according to the presence or absence of CKD based on population demographics. Second, we used urine dipstick testing to assess proteinuria in the study. Although measurement of protein in a timed urine collection might traditionally be the gold standard for the assessment of proteinuria, an easier and more convenient detection method is necessary. Urine dipstick assessment can be an effective way to improve awareness of proteinuria in the elderly.27) Finally, this was a large, population-based analysis using well-examined national data, which strengthens the statistical reliability of the results and the generalizability of the data.
In conclusion, we identified a strong association between sarcopenia and dipstick proteinuria in elderly subjects without CKD. In addition, sarcopenia was also an important factor in proteinuria, even after adjustment for obesity, hypertension, diabetes, and metabolic syndrome. Considering the worldwide aging population, prospective studies are needed to determine the relationship between low skeletal muscle mass and proteinuria in the elderly subjects.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle. 2014;5:253–259. doi: 10.1007/s13539-014-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morley JE. Sarcopenia in the elderly. Fam Pract. 2012;29(Suppl 1):i44–i48. doi: 10.1093/fampra/cmr063. [DOI] [PubMed] [Google Scholar]
- 4.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–159. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 5.Chin SO, Rhee SY, Chon S, Hwang YC, Jeong IK, Oh S, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS One. 2013;8:e60119. doi: 10.1371/journal.pone.0060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–559. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 8.Foley RN, Wang C, Ishani A, Collins AJ, Murray AM. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27:279–286. doi: 10.1159/000101827. [DOI] [PubMed] [Google Scholar]
- 9.Ozkayar N, Altun B, Halil M, Kuyumcu ME, Arik G, Yesil Y, et al. Evaluation of sarcopenia in renal transplant recipients. Nephrourol Mon. 2014;6:e20055. doi: 10.5812/numonthly.20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon SJ, Kim TH, Yoon SY, Chung JH, Hwang HJ. Relationship between stage of chronic kidney disease and sarcopenia in Korean aged 40 years and older using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008-2011. PLoS One. 2015;10:e0130740. doi: 10.1371/journal.pone.0130740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim D, Lee DY, Cho SH, Kim OZ, Cho SW, An SK, et al. Diagnostic accuracy of urine dipstick for proteinuria in older outpatients. Kidney Res Clin Pract. 2014;33:199–203. doi: 10.1016/j.krcp.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma V, Kant R, Sunnoqrot N, Gambert SR. Proteinuria in the elderly: evaluation and management. Int Urol Nephrol. 2012;44:1745–1751. doi: 10.1007/s11255-012-0252-7. [DOI] [PubMed] [Google Scholar]
- 13.Yamagata K, Iseki K, Nitta K, Imai H, Iino Y, Matsuo S, et al. Chronic kidney disease perspectives in Japan and the importance of urinalysis screening. Clin Exp Nephrol. 2008;12:1–8. doi: 10.1007/s10157-007-0010-9. [DOI] [PubMed] [Google Scholar]
- 14.Hwang S, Choi HS, Kim KM, Rhee Y, Lim SK. Associations between serum 25-hydroxyvitamin D and bone mineral density and proximal femur geometry in Koreans: the Korean National Health and Nutrition Examination Survey (KNHANES) 2008-2009. Osteoporos Int. 2015;26:163–171. doi: 10.1007/s00198-014-2877-0. [DOI] [PubMed] [Google Scholar]
- 15.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim MK, Baek KH, Song KH, Il Kang M, Park CY, Lee WY, et al. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J Clin Endocrinol Metab. 2011;96:3250–3256. doi: 10.1210/jc.2011-1602. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. Geneva: World Health Organization; 2000. [Google Scholar]
- 20.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 21.Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008-2011) Hepatology. 2016;63:776–786. doi: 10.1002/hep.28376. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa K, Takahashi K, Okutani T, Yamada R, Kinaga T, Matsumoto M, et al. Risk of chronic kidney disease in non-obese individuals with clustering of metabolic factors: a longitudinal study. Intern Med. 2015;54:375–382. doi: 10.2169/internalmedicine.54.3092. [DOI] [PubMed] [Google Scholar]
- 23.Culleton BF, Larson MG, Parfrey PS, Kannel WB, Levy D. Proteinuria as a risk factor for cardiovascular disease and mortality in older people: a prospective study. Am J Med. 2000;109:1–8. doi: 10.1016/s0002-9343(00)00444-7. [DOI] [PubMed] [Google Scholar]
- 24.Iseki K. Role of urinalysis in the diagnosis of chronic kidney disease (CKD) Japan Med Assoc J. 2011;54:27–30. [Google Scholar]
- 25.Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 2012;82:270–277. doi: 10.1038/ki.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YH, Kim JE, Roh YH, Choi HR, Rhee Y, Kang DR, et al. The combination of vitamin D deficiency and mild to moderate chronic kidney disease is associated with low bone mineral density and deteriorated femoral microarchitecture: results from the KNHANES 2008-2011. J Clin Endocrinol Metab. 2014;99:3879–3888. doi: 10.1210/jc.2013-3764. [DOI] [PubMed] [Google Scholar]
- 27.Wen CP, Yang YC, Tsai MK, Wen SF. Urine dipstick to detect trace proteinuria: an underused tool for an underappreciated risk marker. Am J Kidney Dis. 2011;58:1–3. doi: 10.1053/j.ajkd.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Kim TN, Lee EJ, Hong JW, Kim JM, Won JC, Kim MK, et al. Relationship between sarcopenia and albuminuria: the 2011 Korea National Health and Nutrition Examination Survey. Medicine (Baltimore) 2016;95:e2500. doi: 10.1097/MD.0000000000002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han E, Lee YH, Kim G, Kim SR, Lee BW, Kang ES, et al. Sarcopenia is associated with albuminuria independently of hypertension and diabetes: KNHANES 2008-2011. Metabolism. 2016;65:1531–1540. doi: 10.1016/j.metabol.2016.07.003. [DOI] [PubMed] [Google Scholar]