Abstract
We report a case of a patient with lymphocutaneous sporotrichosis in the right upper limb. The fungus was identified as Sporothrix schenckii senso stricto by calmodulin gene sequencing. The initial treatment was itraconazole (200 mg/day), but in vitro antifungal susceptibility demonstrated high resistant to this and another six antifungals, with exception to terbinafine. The lesions did not regress with itraconazole treatment. Thus, 500 mg/day of terbinafine was prescribed and clinical cure was obtained after four months
Keywords: Sporotrichosis, Sporothrix schenckii, Senso stricto, Itraconazole, Terbinafine, Antifungal susceptibility
1. Introduction
Sporotrichosis is a subacute or chronic infection caused by dimorphic fungi of the Sporothrix schenckii complex, which include: S. schenckii senso stricto, S. brasiliensis, S. globosa and S. luriei. Rarely species of the Sporothrix pallida complex may be the cause of the disease [1]. The main route of transmission of sporotrichosis is percutaneous, through traumatisms and bruises with contaminated surfaces (woods, thorns, splinters) [2], [3], [4]. Although it has a universal geographical distribution, sporotrichosis predominates in southern Africa, America (mainly in Brazil, Peru, Colombia, Guatemala, Mexico and the United States), Asia (Japan, India, China) and Oceania (Australia) [2], [4], [5]. In the state of Rio de Janeiro, Brazil, outbreaks of human sporotrichosis involving the transmission of the disease through contact with bites and scratches of infected cats are described. Other Brazilian states of greater prevalence are Rio Grande do Sul and São Paulo, mainly in men, adults, involved in activities that facilitate exposure to the etiological agent, such as rural workers [3], [4], [6].
Although some studies show differences in the susceptibility profile to antifungal in different isolates of the same species of Sporothrix [6], [7] little is known about the in vitro-in vivo correlation for determination of breakpoints for evaluation of antifungal sensitivity profile of the isolates of the genus [6]. This report presents a case of sporotrichosis in which the susceptibility test was important for the success of the therapy of a sporotrichosis caused by S. schenckii senso stricto.
2. Case
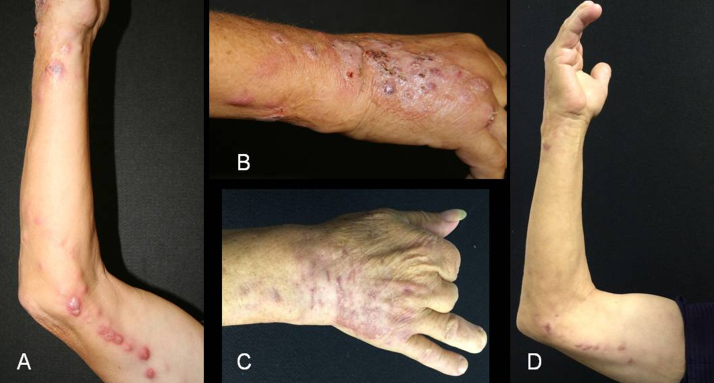
In March 2016, a male 63 years old patient, farmer, previously treated at the dermatology department of Santa Clara Hospital (posto G) in the Santa Casa de Misericórdia Hospital Complex of Porto Alegre, presenting, for more than 3 months, a verrucous plaque on the back of the right hand. In addition to papules and erythematous-purpura nodules, with suppurative tendency, in the forearm and ipsilateral arm, following ascending lymphatic path, suggesting sporotrichosis (Fig. 1A-B). Day zero, admission of the patient, was considered the first day he came to the hospital to seek help after 3 months of ineffective treatment. The patient reported that he had not previously used antifungal medication for the treatment of possible infection. On the same day of the consultation, a sample of purulent material from the patient's lesions for direct and cultural mycological examination was collected. Direct mycological examination was negative for the presence of yeast cells. However, due to clinical suspicion, treatment with itraconazole 200 mg/day was initiated. Five days after day zero, in the cultural examination with cultivation on Sabouraud dextrose agar at 25 °C, there was growth of whitish filamentous colony which, on microscopy, presented hyphae with conidiophore characteristic of Sporothrix sp., confirming the previous diagnosis. For identification at the species level, total genomic DNA was isolated using Power Soil DNA Isolation Kit (Mobio, USA). Partial sequencing of the nuclear calmodulin gene was performed with primers CL1 and CL2A, as described by Stopiglia et al. [6]. The PCR product was purified using the ExoSAP-IT (Affymetrix, USA) and sequenced in the ABIPRISM 3100 Genetic Analyzer (Applied Biosystems), according to the manufacturer's instructions. The sequence was compared with sequences of type strains reported in GenBank using the Basic Local Alignment Search Tool (BLAST) algorithm. The fungal was confirmed as Sporothrix schenckii, since it presented sequence identity at 97% and coverage at 97% with the type strain of this species CBS 359.36T. This strain was added to GenBank as number MF943129.
Fig. 1.
Lesions of sporotrichosis, containing nodule following the ascending lymphatic path (A) with verrucous plaque in right hand, initial site of infection (B); After four months of treatment with terbinafine (500 mg/day), showing scars of the lesions (C, D).
An antifungal susceptibility test was requested, since the infection presented extensive cutaneous involvement. In this test, the minimum inhibitory concentration (MIC) of seven antifungal agents was evaluated by the 96-well plate microdilution method according to protocol M38-A2 do Clinical & Laboratory Standards Institute (CLSI) [8]. The MICs (μg / ml) obtained were: terbinafine (0.25); posaconazole (2.0); ketoconazole (4.0); amphotericin B (8.0); itraconazole (16.0); voriconazole (> 16.0); fluconazole (> 64.0). In view of the possible in vitro resistance of the isolate to itraconazole and the high MICs of all other antifungal agents, except for terbinafine, the patient was reassessed after 48 days of treatment with itraconazole. As the patient showed no signs of clinical improvement, itraconazole was replaced by terbinafine 500 mg/day. After 4 months of treatment with terbinafine, the patient was discharged with clinical cure of the disease, presenting slight residual atrophy in the areas of previous skin lesions (Fig. 1C-D) one year after the end of treatment, there was no recurrence of the disease.
3. Discussion
Sporothrix schenkii was identified more than a century ago [4] and is considered as the only species that causes the disease until the appearance of identification through the sequencing of regions of the fungus DNA, such as partial calmodulin gene, that allows the identification of the different species of the Sporothrix genus [1], [6].
In the direct mycological examination, the yeasts of Sporothrix spp. are rarely observed. Thus, cultural mycological examination is the reference method for the diagnostic confirmation of sporotrichosis [4], [5], [9], with the identification of the fungus generally limited to genus level in clinical practice. However, species-level identification is becoming important for the determination of possibly resistant isolates, since epidemiological cutoff values (ECVs) of MICs of some antifungals have been stipulated for the most prevalent species, S. schenckii and S. brasiliensis [7].
S. schenckii senso stricto has universal geographical distribution [3], [4] and the other species are related to different geographical origins [6]. In Brazil, S. brasiliensis has been reported as a more frequent species [3], [4], [10], [11]. However, these studies are linked to isolates from epidemic outbreaks with zoonotic transmission from the metropolitan region of the state of Rio de Janeiro. In Rio Grande do Sul, S. schenckii senso stricto, which was the species that caused the disease in the present patient, has been considered the most frequent species in 92.5% of sporotrichosis cases [6]. In addition, other clinical-epidemiological data from this report are compatible with the regional literature, since the patient is an adult male, a farmer, and had upper limb lesions in the clinical form of lymphobuccal disease, which is the site and most common form of the disease [4], [9], [12], [13].
The treatment of choice for strict cutaneous sporotrichosis and lymphobutanation in many countries is itraconazole 200 mg/day for 3–6 months. This antifungal can also be used in the disseminated form of the disease [14]. For cutaneous forms, alternatively, oral potassium iodide solution could be used, since it is considered a first-choice drug in developing countries, due to its high efficiency, low cost and safety profile [2], [15], [16]. However, the occurrence of adverse effects with iodine (such as gastrointestinal intolerance and metallic taste) and the more convenient dosage of more modern drugs represent some of the factors that limit its use [17]. In this case, a recent history of treatment for “bowel lesions” reported by the patient beyond the convenience of use, itraconazole was initially prescribed. Subsequently, we knew that the lesions were benign intestinal tumors.
Despite frequent non-solicitation of antifungal susceptibility tests in clinical practice, studies demonstrate variable results of antifungal susceptibilities among isolates of the same species of Sporothrix [6], [7], [11], [18]. Therefore, it is important to perform a susceptibility test to aid in the choice of treatment, especially in cases refractory to initial treatment or in more severe forms of the disease [18]. For S. schenckii senso stricto, 9.8% of clinical isolates from various regions of the world [7] and 6.5% of Brazilian isolates [6] had MICs equal to or greater than 4 μg/mL for itraconazole, considered possibly resistant, based on ECV of 2 μg/mL for itraconazole [7].
In a study by Espinel-Ingroff et al. [7], terbinafine had its ECV stipulated at 0.12 μg/mL for S. brasiliensis, but for S. schenckii senso stricto, no ECV was stipulated, since the data were considered insufficient. The present study shows that MIC of 0.25 μg/mL in vitro led to cure of the disease in 4 months of terbinafine monotherapy in the total dosage of 500 mg/day. A case report of sporotrichosis with failed therapy using itraconazole and success with terbinafine relating to in vitro results for both antifungal was previously published [19]. However, the species was not identified at the species level and also presented MIC lower than the present study for terbinafine. Therefore, the results of the present study may help to determine the ECV of terbinafine in S. schenckii senso stricto, as well as to help in decision making in cases similar to these studies, in which high MICs of the antifungal were obtained, except to the MIC of terbinafine [19].
In addition to case reports evidencing the success of terbinafine in lymphocutaneous sporotrichosis [19], [20], the clinical trial of Francesconi et al. [12] compared the use of itraconazole 100 mg/day with terbinafine 250 mg/day in the treatment of sporotrichosis in fixed cutaneous and lymphocutaneous forms in a total of 304 patients, with no statistically significant difference in efficacy between terbinafine and itraconazole (92.7% and 92%, respectively). Furthermore, the frequency of relapses was similar in both treatments (less than 2%). In addition to the clinical results equivalent to itraconazole observed, terbinafine has less drugs interactions than itraconazole [12], making it preferable to use in elderly patients, since they generally use many medications of continuous use [19].
In this case, the antifungal susceptibility test prevented an ineffective treatment of sporotrichosis with itraconazole to continue, which reduced time to resolution of the disease, in addition to possible future drug interactions and treatment costs. The use of terbinafine, although still restricted, was based on the antifungal susceptibility test of the patient isolate and was supported by the success in the therapy with terbinafine in clinical trials and case reports found in the literature, which ensured the safety of the clinical staff in altering the therapy of the patient, being successful in this clinical decision making.
Acknowledgements
The authors thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) (Daiane Heidrich - CAPES 1562252/Carina Thimoteo - CAPES 1646655/Danielle Machado Pagani - CAPES 1693871) for the scholarships.
Acknowledgments
Conflict of interest
There are none.
References
- 1.Suzuki R., Yikelamu A., Tanaka R., Igawa K., Yolozeki H., Yaguchi T. Studies in phylogeny, development of rapid identification methods, antifungal susceptibility, and growth rates of clinical strains of Sporothrix schenckii complex in Japan. Med. Mycol. J. 2016:E47–E57. doi: 10.3314/mmj.16-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bonifaz A. 4th ed. Mcgraw-Hill Interamericana; México: 2012. Micología Médica Básica; pp. 214–230. [Google Scholar]
- 3.Chakrabarti A., Bonifaz A., Gutierrez-Galhardo M.C., Mochizuki T., Li S. Global epidemiology of sporotrichosis. Med. Mycol. 2015;53:3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- 4.JMR Yuil, Candiani J.O. Página; Buenos Aires: 2016. Manual de Dermatologia Infecciosa; pp. 360–367. [Google Scholar]
- 5.Lacaz . 9th ed. Sarvier; São Paulo: 2002. Tratado de Micologia Médica Lacaz. [Google Scholar]
- 6.Stopiglia C.D.O., Magagnin C.M., Castrillón M.R., Mendes S.D., Heidrich D., Valente P., Scroferneker P., Antifungal M.L. Susceptibilities and identification of species of the Sporothrix schenckii complex isolated in Brazil. Med. Mycol. 2014;52:56–64. doi: 10.3109/13693786.2013.818726. [DOI] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff A., Abreu A.P.B., Almeida-Paes R., Brilhante R.S.N., Chakrabarti A., Chowdhary A. Multicenter and international study of MIC/MEC distributions for definition of epidemiological cutoff values (ECVs) for species of Sporothrix identified by molecular methods. Antimicrob. Agents Chemother. 2017 doi: 10.1128/AAC.01057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute (CLSI) 2nd ed. Clinical and Laboratory Standards Institute (CLSI); Wayne, PA: 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. (Approved Standard M38-A2) [Google Scholar]
- 9.Zaitz C., Marques S.A., Ruiz L.R.B., Framil V.M.S. 2th ed. Guanabara Koogan; Rio de Janeiro: 2012. Compêndio de Micologia Médica. [Google Scholar]
- 10.Mahajan V.K. Sporotrichosis: an overview and therapeutic options. Dermatol. Res. Pract. 2014 doi: 10.1155/2014/272376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marimon R., Cano J., Gené J., Sutton D.A., Kawasaki M., Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol. 2007;45:3198–3206. doi: 10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francesconi G., do Valle A.C.F., Passos S.L., Barros M.B.L., Paes R.P.A., Curi A.L. Comparative study of 250 mg/day terbinafine and 100 mg/day itraconazole for the treatment of cutaneous sporotrichosis. Mycopathologia. 2011;171:349–354. doi: 10.1007/s11046-010-9380-8. [DOI] [PubMed] [Google Scholar]
- 13.da Rosa A.C., Scroferneker M.L., Vettorato R., Gervini R.L., Vettorato G., Weber A. Epidemiology of sporotrichosis: a study of 304 cases in Brazil. J. Am. Acad. Dermatol. 2005;52:451–459. doi: 10.1016/j.jaad.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 14.De Silva R.F., Bonfitto M., Silva Junior F.I.M., Ameida M.T.G., Silva R.C. Sporotrichosis in a liver transplant patient: a case report and literature review. Med. Mycol. Case Rep. 2017;17:25–27. doi: 10.1016/j.mmcr.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauffman C.A., Bustamante B., Chapman S.W., Pappas P.G. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007;45:1255–1265. doi: 10.1086/522765. [DOI] [PubMed] [Google Scholar]
- 16.Landell M.F., Stopiglia C.D.O., Billodre R.G., Heidrich D., Sorrentino J.M., Vainstein M.H. Evaluation of the origin of a sample of Sporothrix schenckii that caused contamination of a researcher in Southern Brazil. Mycopathologia. 2011;171:203–207. doi: 10.1007/s11046-010-9361-y. [DOI] [PubMed] [Google Scholar]
- 17.Costa R.O., Macedo P.M., Carvalhal A., Bernardes-Engemann A.R. Use of potassium iodide in dermatology: updates on an old drug. Ann. Bras. Dermatol. 2013;88:396–402. doi: 10.1590/abd1806-4841.20132377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marimon R., Serena C., Gené J., Cano J. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob. Agents Chemother. 2008;52:732–734. doi: 10.1128/AAC.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidrich D., Stopiglia C.D.O., Senter L., Vettorato G., Valente P., Scroferneker M.L. Successful treatment of terbinafine in a case of sporotrichosis. Ann. Bras. Dermatol. 2011;86:S182–S185. doi: 10.1590/s0365-05962011000700047. [DOI] [PubMed] [Google Scholar]
- 20.Hull P.R., Vismer H.F. Treatment of cutaneous sporotrichosis with terbinafine. Br. J. Dermatol. 1992;126:51–55. doi: 10.1111/j.1365-2133.1992.tb00011.x. [DOI] [PubMed] [Google Scholar]