Abstract
Shigella spp. and Escherichia coli are closely related; both belong to the family Enterobacteriaceae. Phenotypically, Shigella spp. and E. coli share many common characteristics, yet they have separate entities in epidemiology and clinical disease, which poses a diagnostic challenge. We collated information for the best possible approach to differentiate clinically relevant E. coli from Shigella spp. We found that a molecular approach is required for confirmation. High discriminatory potential is seen with whole genome sequencing analysed for k-mers and single nucleotide polymorphism. Among these, identification using single nucleotide polymorphism is easy to perform and analyse, and it thus appears more promising. Among the nonmolecular methods, matrix-assisted desorption ionization–time of flight mass spectrometry may be applicable when data analysis is assisted with advanced analytic tools.
Keywords: 16S rRNA, k-mer, MALDI-TOF MS, single nucleotide polymorphism, whole genome sequencing
Introduction
Diarrhoeal disease is not uncommon in both developing and developed countries. Shigella spp. are among the most important enteric pathogens causing bacillary dysentery worldwide, mainly in humans. Differentiation of Shigella spp. from Escherichia coli is challenging because of their close genetic relatedness. Brenner et al. [1] determined that the nucleotide similarity between Shigella and E. coli was 80% to 90%, whereas other Escherichia species are genetically distant [2]. Shigellae are phylogenetically E. coli that were later classified as separate species on the bases of biochemical characteristics and clinical relevance [3], [4].
Biochemical characteristics and serotyping are usually used to identify the species. However, many isolates cannot be distinguished as either E. coli or Shigella spp. Molecular methods such as 16S rRNA gene sequencing and protein signature–based matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF MS) are unable to differentiate Shigella spp. from E. coli [4]. Further, Shigella-like strains of E. coli (enteroinvasive E. coli, EIEC) causing invasive dysenteric diarrhoeal illness make clinical and laboratory diagnoses difficult. In addition, the change in antimicrobial resistance patterns with the change in the serogroup/serotype further highlights the need for accurate identification of Shigella spp. so that appropriate antimicrobial therapy may be administered [5].
We attempted to accurately identify E. coli and Shigella spp., and trace the evolution of facts contributing to the masking of discrimination between E. coli and Shigella spp. We discuss the challenges and the possible methods to differentiate E. coli and Shigella spp. using protein signature and molecular tools.
Evolution of Shigella Species
At present, Shigella and Escherichia genera are considered to be unique genomospecies. Unlike E. coli, Shigella strains are nonmotile as a result of deletion in the fliF operon (flagellar coding region) or an ISI insertion mutation in the flhD operon. Also, Shigella does not ferment lactose, as S. flexneri [1], [3] and S. bodyii [2], [4] do not contain any of the lac genes (lacY, lacA and lacZ) required for fermentation. S. dysenteriae 1 was known to have only lacY and lacA. S. sonnei has all three genes but is unable to ferment as a result of lack of permease activity. These observations are one such example for the multiple origins of the Shigella phenotype by convergent evolution [6].
Earlier reports suggested that the arrival of a virulence plasmid into an E. coli strain gave rise to a monophyletic group from which all Shigella and E. coli groups descended. This led to the occurrence of highly diversified and pathogenic virotypes, which includes EIEC, Shiga toxin–producing E. coli (STEC; includes enterohemorrhagic E. coli, EHEC), enteropathogenic E. coli (EPEC), enteroaggregative E. coli (EAEC) and enterotoxigenic E. coli (ETEC) [7]. Interestingly, commensal E. coli strains may not become pathogenic Shigella on acquiring a virulence plasmid, as it does not seem to transmit horizontally among E. coli and Shigella strains [7].
STEC that is able to cause haemorrhagic colitis and haemolytic uremic syndrome is referred to as EHEC. This causes pancolitis due to toxigenic noninvasive (EHEC) infection, whereas EIEC causes proctocolitis via a nontoxigenic invasive mechanism similar to Shigella [8]. EIEC serotypes have been suggested as being ancestral to the different Shigella serogroups contributing to these differences [9]. However, supporting evidence for evolution of STEC is not clear. Similarly, limited information is available on the origins of other virotypes of E. coli.
In the midst of changing evolution, there is a need for accurate identification of E. coli and Shigella spp. for appropriate clinical management and accurate epidemiologic data. The accuracy of identification using molecular methods (duplex real-time PCR, 16S rRNA, multilocus sequence typing (MLST) and whole genome sequencing (WGS)) and nonmolecular methods (matrix-assisted desorption ionization–time of flight mass spectrometry, MALDI-TOF MS) will be discussed.
Currently Used Molecular Methods for Differentiation of E. coli and Shigella spp.
Duplex real-time PCR
A duplex real-time PCR for differentiation of EIEC and Shigella spp. was reported by Pavlovic et al. [10]; this PCR amplified the genes encoding β-glucuronidase (uidA) and lactose permease (lacY). The gene uidA is common for E. coli and Shigella, while the latter (lacY) is present only in E. coli. Ninety-six isolates including 11 EIEC isolates of different serotypes and at least three representatives of each Shigella species were identified correctly. Likewise, Lobersli et al. [11] established a duplex real-time PCR (ipaH and lacY) to differentiate EIEC and Shigella spp., where lacY is specific to E. coli. This PCR target differentiated Shigella spp. and EIEC O121 and O124 groups, but not EIEC O164 group.
16S rRNA gene sequencing to differentiate E. coli from Shigella spp.
Molecular identification using 16S rRNA sequencing could not distinguish atypical E. coli and Shigella spp. [12], [13]. The 16S rRNA sequence similarities between various pathogenic strains of E. coli, EPEC (KR476716), EHEC (CP018252), STEC (CP015229), EIEC (AB604198), E. coli ATCC 25922 (KC429776), S. boydii (JQ073777), S. sonnei (HQ591457), S. flexneri (NR026331), S. flexneri 2a (CP012137), S. flexneri 5a (NZCM001474) and S. dysenteriae (NR026332) were calculated using the available reference 16S rRNA sequences from the National Center for Biotechnology Information (NCBI) database (Table 1).
Table 1.
16S rRNA sequence similarity between closely related Shigella serogroups, serotypes and virotypes of Escherichia coli
| E. coli ATCC 25922 | EPEC | EHEC | STEC | EIEC | S. dysenteriae | S. flexneri 2a | S. flexneri 5a | S. flexneri | S. boydii | S. sonnei | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli ATCC 25922 | 100 | ||||||||||
| EPEC | 98.89 | 100 | |||||||||
| EHEC | 99.04 | 98.89 | 100 | ||||||||
| STEC | 98.97 | 98.55 | 99.42 | 100 | |||||||
| EIEC | 99.63 | 98 | 98.41 | 98.47 | 100 | ||||||
| S. dysenteriae | 98.97 | 98.2 | 98.92 | 98.99 | 98.72 | 100 | |||||
| S. flexneri 2a | 99.63 | 98.06 | 98.91 | 98.97 | 99.53 | 98.86 | 100 | ||||
| S. flexneri 5a | 99.63 | 98 | 98.84 | 99.03 | 99.07 | 98.92 | 99.55 | 100 | |||
| S. flexneri | 99.78 | 98.2 | 98.99 | 99.13 | 99.6 | 99.13 | 99.73 | 99.8 | 100 | ||
| S. boydii | 99.56 | 98 | 98.8 | 98.87 | 99.66 | 98.79 | 99.93 | 99.47 | 99.66 | 100 | |
| S. sonnei | 99.56 | 97.93 | 98.78 | 98.97 | 99 | 98.86 | 99.49 | 99.68 | 99.73 | 99.4 | 100 |
EHEC, enterohaemorrhagic E. coli; EIEC, enteroinvasive E. coli; EPEC, enteropathogenic E. coli; STEC, Shiga toxin–producing E. coli.
The differentiation of E. coli and Shigella spp. could not be achieved using 16S rRNA gene sequences as a result of the narrow (<1%) divergence between EHEC, EIEC and Shigella spp. Jenkins et al. [14] concur with this finding; their 16S rRNA gene comparison could not distinguish between E. coli and Shigella spp. as a result of >99% sequence identity. We therefore deem this approach to be unacceptable to differentiate certain inter- and intraspecies identity.
Exploration of MLST for differentiation of E. coli and Shigella spp.
The Pasteur and Warwick MLST databases use highly conserved housekeeping genes that are the same for both E. coli and Shigella spp. Hence, sequence types are assigned irrespective of E. coli and Shigella spp. A study by Li et al. [15] involving MLST for clinical S. flexneri isolates found that different serotypes (1–5, X and Y) were clustered together in a group, while a single serotype formed a distinct group. Li et al. reported the inability of MLST method to differentiate the evolutionary relationship between virotypes of E. coli and Shigella spp. However, there have been reports focusing directly on sequence data from the housekeeping genes rather than the allelic profile for clonal diversification. The discrimination based on difference in one MLST housekeeping gene sequence from the founder genotype is termed single-locus variants, and diversification of two housekeeping genes is defined as double-locus variants (DLVs) [16], [17], [18], [19]. Until now, these variants were used to categorize clonal complexes to relate the phylogeny. Taking a cue from this knowledge, we made an attempt to use the direct sequence data of housekeeping genes to differentiate E. coli from Shigella spp.
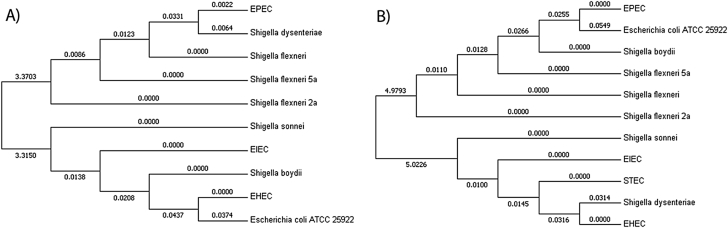
Interestingly, we could identify the variations among Shigella spp. and E. coli virotypes beyond their sequence types utilizing the DLV approach (Fig. 1). Accurate identification was achieved using rpoB and mdh genes. rpoB, a protein-encoding housekeeping gene, has several potential advantages over other molecular methods. The rpoB gene occurs as a single copy in all prokaryotes, it functions as a housekeeping gene, it is less susceptible to some lateral gene transfer and its genetic divergence provides enhanced resolution for species identification. 16S rRNA gene copy number, however, varies among species and shows heterogeneity among intragenomic gene copies. rpoB is therefore the better marker to distinguish interspecies relationships between and within E. coli and Shigella spp. than 16S rRNA sequences [20]. Similarly, housekeeping gene malate–lactate dehydrogenase (mdh) was reported to provide good subtype discrimination between various subspecies [21], which reveals the evolutionary histories of Salmonella and E. coli chromosomes.
Fig. 1.
Genotypic diversification of various Escherichia coli and Shigella spp. based on highly conserved housekeeping genes mdh (A) and rpoB (B). EHEC, EIEC, EPEC, STEC and ATCC 25922 E. coli form E. coli group; S. dysenteriae, S. flexneri 2a, S. flexneri 5a, S. flexneri, S. boydii and S. sonnei from Shigella group were used to construct double-locus variant–based phylogeny. EHEC, enterohaemorrhagic E. coli; EIEC, enteroinvasive E. coli; EPEC, enteropathogenic E. coli; STEC, Shiga toxin–producing E. coli.
WGS for differentiation of E. coli and Shigella spp.
Differentiation of species based on WGS can be attained by two methods, k-mers and whole genome single nucleotide polymorphism (SNP). Chattaway et al. utilized k-mers (substrings of k nucleotides in DNA sequence data) to predict the species based on the number of co-occurring k-mers in two bacterial genomes as a measure of evolutionary relatedness. This accurately identified the strains to the species level [22], [23], [24]. Among 1297 isolates, 18 were misidentified by conventional biochemicals and serotyping. Of these, 15 were intragenomic misidentifications and three were intergenomic misidentifications. These 18 isolates were then correctly identified by the k-mer approach. The phylogenetic relation of the clonal complexes derived from MLST and a minimum spanning tree confirmed that the k-mer method was accurate in discriminating Shigella spp. from E. coli.
Recently the use of whole genome SNPs for drawing phylogenetic relationships has been gaining attention. Pettengill et al. [25] reported the ability of SNPs to accurately identify EIEC and Shigella spp. from WGS data. This method used 404 SNP markers for differentiating Shigella and EIEC lineages. Further, Ashton et al. [26] proved classification of Shigella serotypes using SNPs with their evolutionary phylogenetic relationships. This seems to be an easier and more promising approach.
Identification based on ribosomal protein signature
MALDI-TOF MS is used for early species-level identification. However, the power of discrimination is still considered to be low for Shigella spp. [27]. In 2013, Khot and Fisher [4] reported that conventional MALDI-TOF MS failed to distinguish Shigella spp. from E. coli. However, they reported that MALDI-TOF MS with an automated data analysis approach could distinguish inactive and other non-lactose-fermenting E. coli from Shigella species [4]. This special approach included the use of ClinPro software's database and analysis tool functions like data preparation, model generation and spectra classification. Classification of unknown spectra for identification was achieved by using the ‘Classify’ function in ClinProTools, in which, if two or more of three spectra per isolate were assigned to the same class, the identification was accepted [16].
Table 2 compares the ability of each molecular method to differentiate E. coli and Shigella serogroups.
Table 2.
List of nonmolecular and molecular methods for accurate differentiation of Escherichia coli and Shigella spp
| Method for differentiation | Target | Level of differentiation between E. coli and Shigella spp. | References |
|---|---|---|---|
| MALDI-TOF MS | Biomarker-based classifiers using their protein signature | Conventional MALDI-TOF MS fails to distinguish E. coli from Shigella spp. However, advanced software analytic tools like ClinPro could distinguish inactive and other non-lactose-fermenting E. coli from Shigella spp. | Francisco et al.[16]; Khot and Fischer [4] |
| Duplex real-time PCR | uidA, lacY | Method is based on target-specific real-time PCR. EIEC and Shigella spp. can be differentiated because lacY is specific for E. coli | Pavlovic et al.[10] |
| 16S rRNA sequencing | 16S rRNA | Unacceptable for discrimination of E. coli and Shigella spp. because sequence similarities were >99% for EIEC, EHEC and Shigella spp. | Edwards et al.[12]; Chen et al.[13] |
| MLST (conventional) | Housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, recA) | Allele-based sequence type identification within E. coli and Shigella spp. without differentiating between them | Li et al.[15] |
| Specific locus variants | Housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, recA) | Uses sequence data of housekeeping genes rather than MLST allelic profiles. Can differentiate within sequence types using single-locus variant and double-locus variant approach | Gibreel et al.[17]; Otero et al.[18]; Shahsavan et al.[19] |
| k-mer | k-mer regions | Serotype-level identification and differentiation of E. coli and Shigella spp. is performed using co-occurring k-mers | Hasman et al.[23]; Larsen et al.[24]; Chattaway et al.[22] |
| SNP | SNP markers | Specific SNP markers were used for classification using SNPs with their evolutionary phylogenetic relationships | Ashton et al.[26]; Pettengill et al.[25] |
EHEC, enterohaemorrhagic Escherichia coli; EIEC, enteroinvasive Escherichia coli; MALDI-TOF MS, matrix-assisted desorption ionization–time of flight mass spectrometry; MLST, multilocus sequence typing; SNP, single nucleotide polymorphism.
Conclusion
Among the molecular methods, we deem 16S rRNA to be unacceptable, while duplex real-time PCR and DLV using sequence data of the conserved housekeeping genes rpoB and mdh may be used. A high discriminatory potential is evident with WGS that analyses k-mers and SNPs. Among these two WGS modalities, identification using SNPs is easy to perform and analyse, and we think it is more promising. Among the nonmolecular methods, MALDI-TOF MS may be applicable when data analysis is assisted with advanced analytic tools.
Acknowledgement
Supported in part by the Indian Council of Medical Research, New Delhi, India (AMR/TF/55/13ECDII).
Conflict of Interest
None declared.
References
- 1.Brenner D.J., Fanning G.R., Steigerwalt A.G., Orskov I., Orskov F. Polynucleotide sequence relatedness among three groups of pathogenic Escherichia coli strains. Infect Immun. 1972;6:308–315. doi: 10.1128/iai.6.3.308-315.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van den Beld M.J., Reubsaet F.A. Differentiation between Shigella, enteroinvasive Escherichia coli (EIEC) and noninvasive Escherichia coli. Eur J Clin Microbiol Infect Dis. 2012;31:899–904. doi: 10.1007/s10096-011-1395-7. [DOI] [PubMed] [Google Scholar]
- 3.Connor T.R., Barker C.R., Baker K.S., Weill F.X., Talukder K.A., Smith A.M. Species-wide whole genome sequencing reveals historical global spread and recent local persistence in Shigella flexneri. Elife. 2015;4:e07335. doi: 10.7554/eLife.07335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khot P.D., Fisher M.A. Novel approach for differentiating Shigella species and Escherichia coli by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol. 2013;51:3711–3716. doi: 10.1128/JCM.01526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shakya G., Acharya J., Adhikari S., Rijal N. Shigellosis in Nepal: 13 years review of nationwide surveillance. J Health Popul Nutr. 2016;35:36. doi: 10.1186/s41043-016-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pupo G.M., Lan R., Reeves P.R. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc Natl Acad Sci U S A. 2000;197:10567–10572. doi: 10.1073/pnas.180094797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escobar-Paramo P., Giudicelli C., Parsot C., Denamur E. The evolutionary history of Shigella and enteroinvasive Escherichia coli revised. J Mol Evol. 2003;57:140–148. doi: 10.1007/s00239-003-2460-3. [DOI] [PubMed] [Google Scholar]
- 8.Johnson J.R. Shigella and E. coli at the crossroads: Machiavellian masqueraders or taxonomic treachery? J Med Microbiol. 2000;49:583–585. doi: 10.1099/0022-1317-49-7-583. [DOI] [PubMed] [Google Scholar]
- 9.Lukjancenko O., Wassenaar T.M., Ussery D.W. Comparison of 61 sequenced Escherichia coli genomes. Microb Ecol. 2010;60:708–720. doi: 10.1007/s00248-010-9717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlovic M., Luze A., Konrad R., Berger A., Sing A., Busch U. Development of a duplex real-time PCR for differentiation between E. coli and Shigella spp. J Appl Microbiol. 2011;110:1245–1251. doi: 10.1111/j.1365-2672.2011.04973.x. [DOI] [PubMed] [Google Scholar]
- 11.Lobersli I., Wester A.L., Kristiansen A., Brandal L.T. Molecular differentiation of Shigella spp. from enteroinvasive E. coli. Eur J Microbiol Immunol. 2016;6:197–205. doi: 10.1556/1886.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards K.J., Logan J.M.J., Langham S., Swift C., Gharbia S.E. Utility of real-time amplification of selected 16S rRNA gene sequences as a tool for detection and identification of microbial signatures directly from clinical samples. J Med Microbiol. 2012;61:645–652. doi: 10.1099/jmm.0.041764-0. [DOI] [PubMed] [Google Scholar]
- 13.Chen L., Cai Y., Zhou G., Shi X., Su J., Chen G. Rapid Sanger sequencing of the 16S rRNA gene for identification of some common pathogens. PLoS One. 2014;9:e88886. doi: 10.1371/journal.pone.0088886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins C., Ling C.L., Ciesielczuk H.L., Lockwood J., Hopkins S., McHugh T.D. Detection and identification of bacteria in clinical samples by 16S rRNA gene sequencing: comparison of two different approaches in clinical practice. J Med Microbiol. 2012;61:483–488. doi: 10.1099/jmm.0.030387-0. [DOI] [PubMed] [Google Scholar]
- 15.Li S., Sun Q., Wei X., Klena J.D., Wang J., Liu Y. Genetic characterization of Shigella flexneri isolates in Guizhou province, China. PLoS One. 2015;10:e0116708. doi: 10.1371/journal.pone.0116708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francisco A.P., Bugalho M., Ramirez M., Carriço J.A. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinformatics. 2009;10:152. doi: 10.1186/1471-2105-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibreel T.M., Dodgson A.R., Cheesbrough J., Fox A.J., Bolton F.J., Upton M. Population structure, virulence potential and antibiotic susceptibility of uropathogenic Escherichia coli from Northwest England. J Antimicrob Chemother. 2012;67:346–356. doi: 10.1093/jac/dkr451. [DOI] [PubMed] [Google Scholar]
- 18.Otero V., Rodriguez-Calleja J.M., Otero A., Garcia-Lopez M.L., Santos J.A. Genetic characterization of atypical enteropathogenic Escherichia coli isolates from ewes' milk, sheep farm environments, and humans by multilocus sequence typing and pulsed-field gel electrophoresis. Appl Environ Microbiol. 2013;79:5864–5869. doi: 10.1128/AEM.01809-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahsavan S., Nobakht M., Rastegar-Lari A., Owlia P., Bakhshi B. Multi-locus sequence type analysis of Shigella spp. isolates from Tehran, Iran. Iranian J Microbiol. 2016;8:298–306. [PMC free article] [PubMed] [Google Scholar]
- 20.Vos M., Quince C., Pijl A.S., de Hollander M., Kowalchuk G.A. A Comparison of rpoB and 16S rRNA as markers in pyrosequencing studies of bacterial diversity. PLoS One. 2012;7:e30600. doi: 10.1371/journal.pone.0030600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown E.W., Mammel M.K., LeClerc J.E., Cebula T.A. Limited boundaries for extensive horizontal gene transfer among Salmonella pathogens. Proc Natl Acad Sci U S A. 2003;100:15676–15681. doi: 10.1073/pnas.2634406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chattaway M.A., Schaefer U., Tewolde R., Dallman T.J., Jenkins C. Identification of Escherichia coli and Shigella species from whole-genome sequences. J Clin Microbiol. 2017;55:616–623. doi: 10.1128/JCM.01790-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasman H., Saputra D., Sicheritz-Ponten T., Lund O., Svendsen C.A., Frimodt-Moller N. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples. J Clin Microbiol. 2014;52:139–146. doi: 10.1128/JCM.02452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen M.V., Cosentino S., Lukjancenko O., Saputra D., Rasmussen S., Hasman H. Benchmarking of methods for genomic taxonomy. J Clin Microbiol. 2014;52:1529–1539. doi: 10.1128/JCM.02981-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettengill E.A., Pettengill J.B., Binet R. Phylogenetic analyses of Shigella and enteroinvasive Escherichia coli for the identification of molecular epidemiological markers: whole-genome comparative analysis does not support distinct genera designation. Front Microbiol. 2015;6:1573. doi: 10.3389/fmicb.2015.01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashton P.M., Baker K.S., Gentle A., Wooldridge D.J., Thomson N.R., Dallman T.J. Draft genome sequences of the type strains of Shigella flexneri held at Public Health England: comparison of classical phenotypic and novel molecular assays with whole genome sequence. Gut Pathog. 2014;6:7. doi: 10.1186/1757-4749-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dekker J., Frank K. Salmonella, Shigella, and Yersinia. Clin Lab Med. 2015;35:225–246. doi: 10.1016/j.cll.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]