Abstract
After coronary stent implantation, neointima formation resembles the wound healing process as it involves the sequential processes of inflammation, granulation, and remodeling. Because antiproliferative drugs and polymers of drug-eluting stents (DESs) delay vascular healing compared with bare metal stents, fibrin deposition can remain long after stent implantation, or inflammation can be excessive. Delayed vascular healing can be associated with adverse clinical outcomes including DES thrombosis or restenosis, and poor endothelization of DES neointima can accelerate neoatherosclerotic change inside the neointima, further contributing to luminal restenosis or neointimal instability. Despite the lack of correlation between pathologic and optical coherence tomography (OCT) findings, OCT assessments of neointima under various circumstances can reveal vascular responses to stent therapy. Homogeneous, heterogeneous, and layered neointima patterns can be recognized by OCT and can change with time. Homogeneous neointima might be associated with better clinical outcomes after DES implantation, whereas non-homogeneous neointima or neoatherosclerotic change can be associated with poorer clinical outcomes. However, limited data are currently available, and further studies are required to comprehensively address these questions.
Keywords: Coronary artery disease, Drug-eluting stents, Optical coherence tomography
INTRODUCTION
Pathologic and intravascular ultrasound studies have revealed the mechanisms of coronary stent failure. Despite treatment with drug-eluting stents (DESs), neointimal hyperplasia is considered to be an important mechanism of stent therapy failure.1) Therefore, additional information about neointimal hyperplasia is required to develop novel devices and improve stent therapy. Recently, optical coherence tomography (OCT) has been used to assess the morphologies of implanted coronary stents in detail in various clinical situations. With higher resolution than intravascular ultrasound, OCT can evaluate strut-level coverage and assess neointima more accurately.2) Accordingly, insights from OCT studies regarding neointimal hyperplasia can be helpful to physicians, researchers, and developers.
The objective of this review is to describe the formation and transformation of neointima after DES implantation based on pathologic and OCT studies.
DELAYED VASCULAR HEALING OF DES
The earliest response after bare metal stent (BMS) implantation is deposition of platelets and fibrin around stent struts within 24 to 48 hours.3) Acute inflammatory cells such as neutrophils are concomitantly observed within platelet and fibrin aggregates. After the inflammatory phase, organization of the thrombus starts with migration and proliferation of smooth muscle cells in the granulation phase.4) Neointima containing smooth muscle cells can be recognized beginning about 14 days after stenting.3) At earlier times, synthetic smooth muscle cells appear and gradually re-differentiate into contractile α-actin-positive smooth muscle cells.4) In later phases, the smooth muscle cells rearrange along the lumen. The layer underneath the lumen is rich in smooth muscle cells and proteoglycans and tends to be compact with little matrix, while the area around stent struts tends to be less cellular and rich in proteoglycans.4) As tissue remodeling progresses, deposition of type I collagen increases with corresponding decreases in cellularity and extracellular matrix.4) Endothelization reaches 30% of the neointimal surface at 1 month and 80% to 100% at 3 to 4 months in BMS.5)
Compared to BMSs, DESs contain antiproliferative drugs that inhibit the migration and proliferation of smooth muscle cells. From a registry of 40 autopsies, Cypher® (Cordis Corp., Miami Lakes, FL, USA) and Taxus® (Boston Scientific Corp., Marlborough, MA, USA) DESs showed a greater delay in healing, characterized by persistent fibrin deposition and poorer endothelization, than BMSs.5) The persistence of fibrin and incomplete endothelization far beyond 30 days from the time of stenting result in the critical pathologic substrate underlying late stent thrombosis.5) Currently available second-generation cobalt-chromium everolimus-eluting stents demonstrated greater strut coverage with less inflammation, less fibrin deposition, and less late and very late stent thromboses than first-generation Cypher® and Taxus® DESs.6) Although second-generation DESs share certain properties with first-generation DESs, the second-generation DESs have thin stent struts and are made of biocompatible polymers that are thromboresistent, which results in less flow disturbance, greater endothelial cell coverage, and less thrombus formation.6),7),8) Nevertheless, endothelial maturation appears later in everolimus-eluting stents compared with BMSs.6),9)
CORRELATIONS BETWEEN HISTOPATHOLOGIC FINDINGS AND OCT ASSESSMENTS OF NEOINTIMA
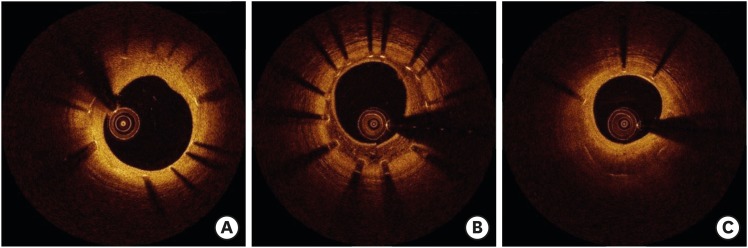
Gonzalo et al.10) described OCT patterns of stent restenosis. Homogeneous neointima has uniform optical properties and does not show focal variations in backscattering pattern. Heterogeneous neointima has focally changing optical properties and shows various backscattering patterns. Layered neointima consists of concentric layers with different optical properties: an adluminal high scattering layer and an abluminal low scattering layer (Figure 1).10) Normal neointima, defined as smooth muscle cell-rich tissue with extracellular matrix containing collagens and proteoglycans, is seen as homogeneous and has a smooth luminal surface. The peak intensity of optical frequency domain imaging is in the moderate range, and the attenuation rate is mild to moderate. However, areas of fibrin deposition or inflammation (hypersensitivity) that appear during the development of neointima normally have a dark appearance.11) According to a histopathological validation study, a homogeneous pattern correlates most often with smooth muscle cells and a collagenous/proteoglycan matrix, while a heterogeneous pattern is frequently associated with stent-induced hypersensitivity vasculitis. A layered pattern is most commonly correlated to healed neointimal rupture/erosion. However, OCT pattern does not indicate a specific histologic finding even though OCT can visualize neointima in detail. Table 1 summarizes OCT patterns and corresponding histologic findings.12)
Figure 1.
Homogeneous (A), heterogeneous (B), and layered patterns (C) observed by OCT.
OCT = optical coherence tomography.
Table 1. OCT patterns and corresponding histologic findings.
| OCT patterns | Histologic findings |
|---|---|
| Homogeneous neointima | Smooth muscle cells and extracellular matrix |
| Organized thrombus | |
| Heterogeneous neointima | Hypersensitivity vasculitis related to stent |
| Smooth muscle cells in rich extracellular matrix | |
| Layered neointima | Healed neointimal rupture/erosion |
| Peristrut neovascularization | |
| Smooth muscle cells in rich extracellular matrix | |
| Neointimal calcification |
OCT = optical coherence tomography.
OBSERVATION OF DEVELOPMENT OF DES NEOINTIMA USING OCT
Because pathologic evaluations are not always possible in various clinical situations, assessment of neointima using OCT could provide additional information despite a lack of histologic validation. In addition, possible OCT findings related to neointimal development can be made based on the optical properties of neointimal components. During the inflammatory phase, depositions or aggregates of platelets and fibrin appear as dark areas on OCT. As the migration and proliferation of smooth muscle cells progress, bright areas underneath the lumen can increase. In the case of DESs, deposition of platelets/fibrin or inflammation (hypersensitivity) can be persistent over time, resulting in a heterogeneous OCT pattern. As smooth muscle cells proliferate, the neointimal pattern can become more homogeneous. If there is a large number of platelet/fibrin aggregates or elevated inflammation, the proliferation of smooth muscle cells beneath the lumen can cause the neointima to have a layered appearance on OCT. Notably, given the formation process of neointima, it is important to note that the neointimal pattern assessed by OCT might not be constant and should be interpreted across the neointimal formation continuum. Also of significance is that neointimal patterns classified by OCT examination can have various pathologies and are thus not conclusive.
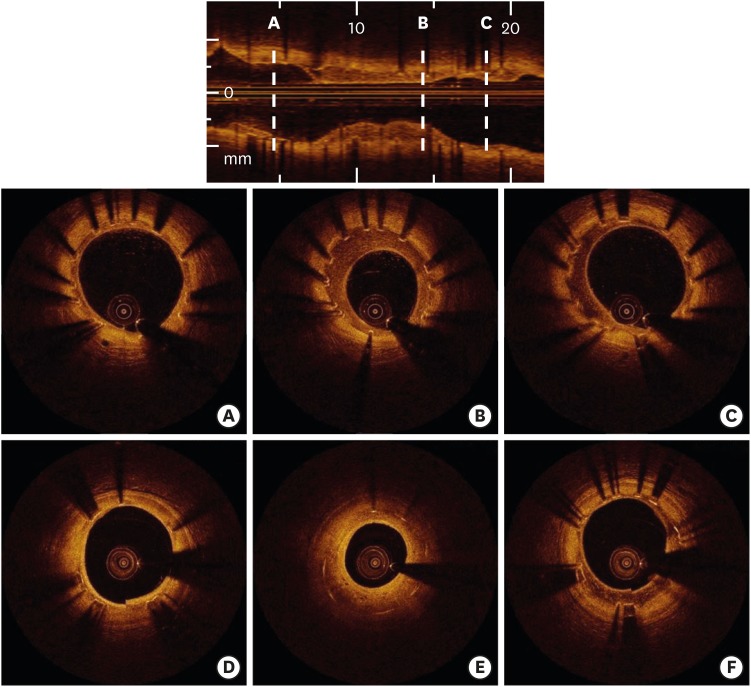
From an observational OCT study investigating 507 DES-treated lesions with a mean neointimal thickness >100 μm, homogeneous neointima was the most common finding 3 to 6 months after the index procedure.13) The frequency of heterogeneous neointima decreased gradually with time, and layered neointima was most frequently observed in restenotic DES lesions within 30 months after stenting.13) Figure 2 presents morphological changes in neointimal pattern during the follow-up period.14)
Figure 2.
Serial changes in neointimal patterns after DES implantation as assessed by OCT. A 58-year-old female patient treated with Nobori® DES underwent serial OCT evaluations between 9 months and 2 years after stenting. At the 9-month follow-up, heterogeneous neointima was diffusely identified through the longitudinal axis (A, B, C). At the 2-year follow-up, heterogeneous neointima was partly changed into homogeneous (D) or layered pattern (E). In contrast, heterogeneous neointima was persistent in another segment (F).
DES = drug-eluting stent; OCT = optical coherence tomography.
CLINICAL IMPLICATIONS OF OCT-DEFINED NEOINTIMAL PATTERNS
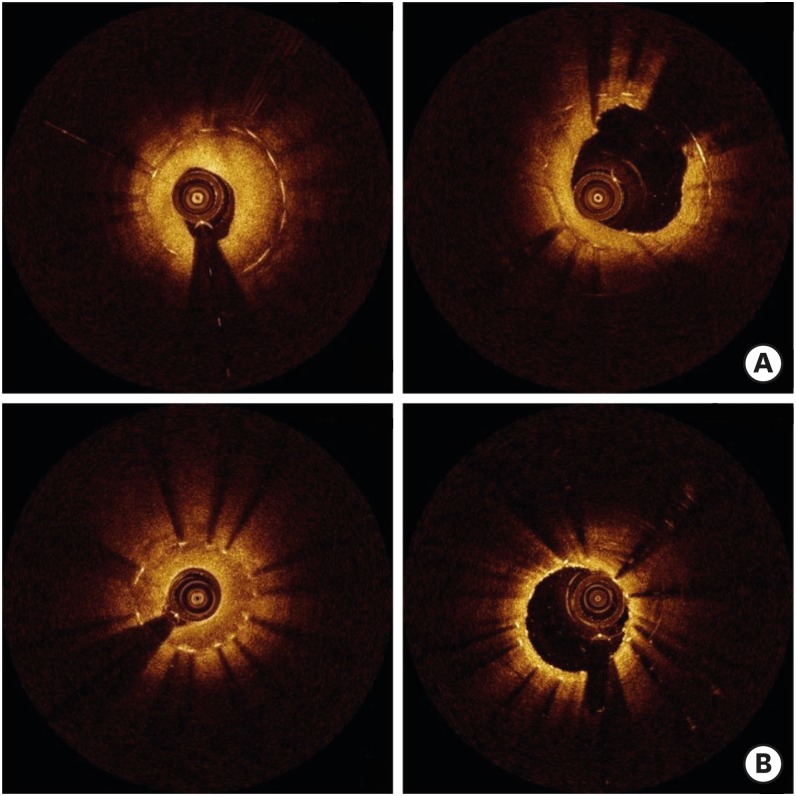
Non-homogeneous neointima patterns, including heterogeneous and layered patterns, were more commonly detected among restenotic DES lesions than a homogeneous neointima pattern. Non-homogeneous neointima accounted for about two-thirds of restenotic lesions, suggesting that early DES in-stent restenosis might be associated with delayed arterial healing.15) A speckled pattern similar to that of heterogeneous neointima decreased with time, consistent with previous observations.13),15) According to a serial OCT study, there was a decrease in the area of homogeneous neointima at the second follow-up 9 to 18 months after DES implantation, whereas the amount of non-homogeneous neointima (heterogeneous plus layered patterns) did not change significantly.16) Given that neointimal regression was observed 6 months after BMS implantation,17) homogeneous neointima identified in DES might represent the natural healing process. In a clinical follow-up study of 336 patients with 368 DES-treated lesions, major adverse cardiac events, defined as the composite of cardiac death, nonfatal myocardial infarction, or target lesion revascularization, occurred less frequently in patients with homogeneous neointima during a median follow-up of 31 months. Patients with heterogeneous neointima underwent major adverse cardiac events most frequently, suggesting the possibility that heterogeneous neointima might predict a poor clinical outcome.18) In drug-coated balloon angioplasty for DES restenosis, post-interventional luminal enlargement was similar between homogeneous and non-homogeneous lesions. However, in homogeneous lesions, the luminal gain was driven primarily by an increase in stent area, whereas in non-homogeneous lesions, it more often resulted from a decrease in neointimal area.19) These findings suggest that mechanical resistance to balloon dilation can differ among neointimal patterns as assessed by OCT.20) Figure 3 shows neointimal changes before and after drug-coated balloon angioplasty in patients with DES restenosis.
Figure 3.
Serial changes in neointima before (left panel) and after (right panel) drug-coated balloon angioplasty in in-stent restenotic lesions. (A) Homogeneous neointima: acute luminal gain was mostly derived from stent overexpansion (68.0%, 1.7 mm2/2.5 mm2). (B) Heterogeneous neointima: acute luminal gain was mostly derived from neointimal compression (85.0%, 1.7 mm2/2.0 mm2).
ATHEROSCLEROTIC CHANGE INSIDE NEOINTIMA
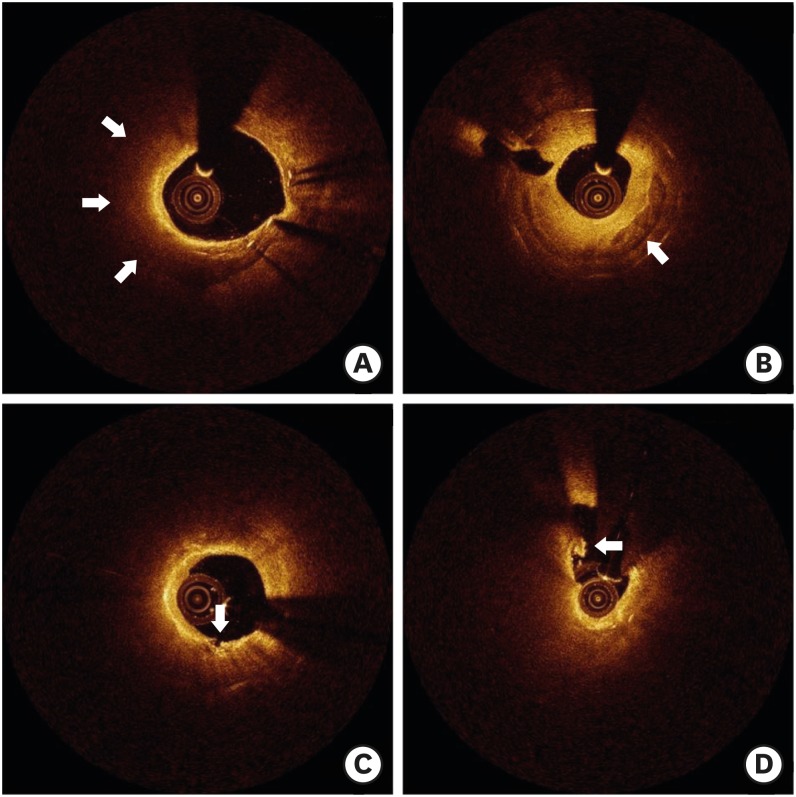
Although the pathogenesis of neoatherosclerosis remains unknown, it is speculated that incomplete or immature endothelization allows a greater amount of lipoproteins to enter the neointima, leading to neoatherosclerosis.21) Accordingly, this process can be accelerated in the context of the greater delay in vascular healing associated with DESs than BMSs.22) It is also plausible that neoatherosclerosis following stent implantation seems to occur more rapidly than atherosclerosis in native coronary arteries.21) Assessment of neoatherosclerosis using OCT is challenging because accumulations of lipids have the same appearance as fibrin deposition or excessive inflammation.11) In addition, optical attenuation by red thrombi or macrophage accumulations, artifacts such as shadowing caused by blood inside of catheters, or tangential signal dropout can falsely identify lipidic neointima.23) However, as shown in Figure 4, a non-homogeneous pattern with an invisible stent strut on OCT imaging can identify the presence of lipids within neointima.24)
Figure 4.
Various neoatherosclerotic neointima observed by OCT. (A) Neointima with lipid (arrow). (B) Neointima with calcification (arrow). (C) Lipidic neointima with disruption (arrow). (D) Ruptured neointima with thrombi (arrow).
OCT = optical coherence tomography.
Clinical implications of neoatherosclerosis are consistent across pathologic and OCT studies; neoatherosclerosis contributes to late failure of stent therapy. The prevalence of very late stent thrombosis from neoatherosclerosis increases with time in both BMSs and first-generation DESs.21),25),26),27) Neoatherosclerosis causes one-third of very late stent thrombosis cases.25),26),27) Notably, the timing of very late stent thrombosis from in-stent plaque rupture is substantially earlier in first-generation DESs than BMSs.22) Neoatherosclerosis has also been identified in both BMS and DES restenoses.21) Neoatherosclerosis generally increases the neointimal burden and contributes to development of in-stent restenosis.21) Among 152 lesions with >50% neointimal stenosis, neoatherosclerosis was found in one-third.28) Patients with neoatherosclerosis (vs. those without neoatherosclerosis) had higher rates of target lesion revascularization and stent thrombosis.28) In a serial OCT study, the frequency of neointima with neoatherosclerosis increased between 9 months and 2 years, with a simultaneous increase in mean neointimal thickness.29) Accordingly, the late contribution of neoatherosclerosis to neointimal growth can possibly explain a late renarrowing phase of a triphasic luminal response that was found in a previous angiographic study.30) Figure 5 shows neoatherosclerotic changes of the neointima during the follow-up period. Clinical factors such as hypertension, current smoking, chronic kidney disease, and low-density lipoprotein cholesterol >70 mg/dL increased the risk of neoatherosclerosis, while the use of angiotensin-converting enzyme inhibitors/angiotensin II receptor blockade reduced the risk of neoatherosclerosis.31),32) Although limited data are currently available, the frequency of neoatherosclerosis was comparable between first- and second-generation DESs according to both pathologic and OCT studies.6),32),33) This finding suggests that an innate antiproliferative property of DESs might play an important role in the development of neoatherosclerosis. Neoatherosclerosis was also observed in patients who had in-scaffold restenosis after successful treatment with bioresorbable vascular scaffold.34),35) To date, observed predictors for neoatherosclerosis, with the exception of DESs and older stent age, are not consistent across studies, and optimal prevention and treatment strategies for patients with neoatherosclerosis remain unknown.
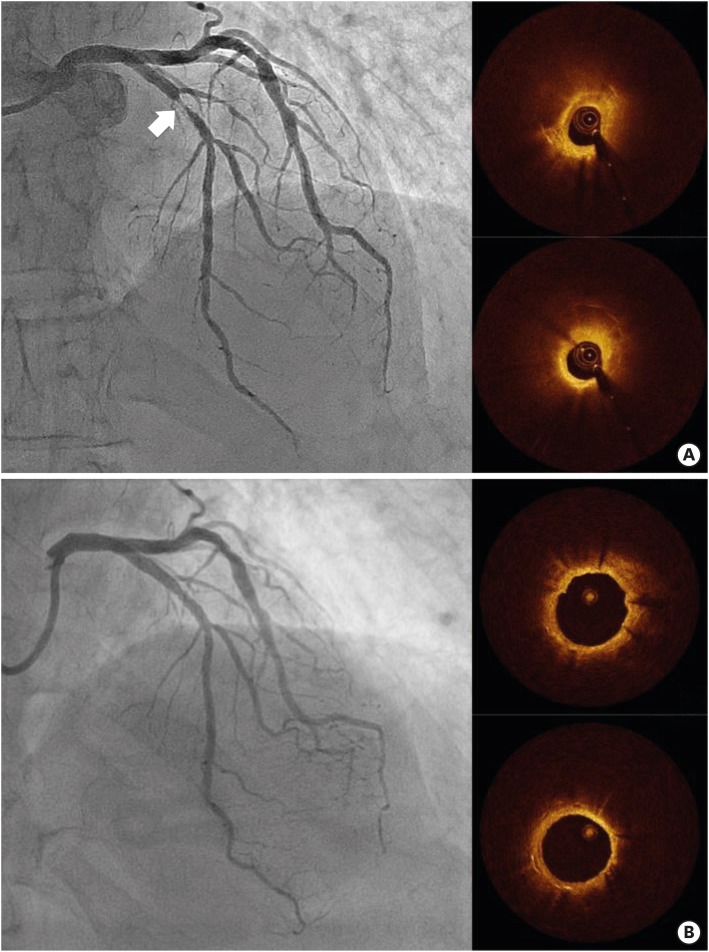
Figure 5.
Development of in-stent neoatherosclerosis that caused DES restenosis. A 77-year-old male patient with exertional chest pain showed restenosis (arrow) of Cypher® DES in the left anterior descending artery (A). OCT evaluation showed lipidic neointima at the site of minimal luminal area (A). Three years prior, this patient underwent OCT evaluation that demonstrated relatively homogeneous neointima without significant luminal narrowing (B). The patient was successfully treated with Xience® DES implantation.
DES = drug-eluting stent; OCT = optical coherence tomography.
CONCLUSION
Despite lack of histopathologic validation, OCT can be used to assess the presence of delayed vascular healing after DES implantation, keeping in mind the formation process of neointima. Homogeneous neointima can be related to better clinical outcomes, while non-homogeneous neointima or neoatherosclerotic change within the neointima can be associated with unfavorable clinical outcomes, such as restenosis and stent thrombosis.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Lee SY, Hong MK, Jang Y.
- Writing - original draft: Lee SY, Hong MK, Jang Y.
- Writing - review & editing: Lee SY, Hong MK, Jang Y.
References
- 1.Kang SJ, Mintz GS, Park DW, et al. Mechanisms of in-stent restenosis after drug-eluting stent implantation: intravascular ultrasound analysis. Circ Cardiovasc Interv. 2011;4:9–14. doi: 10.1161/CIRCINTERVENTIONS.110.940320. [DOI] [PubMed] [Google Scholar]
- 2.Cho YK, Hur SH. Practical application of coronary imaging devices in cardiovascular intervention. Korean Circ J. 2015;45:87–95. doi: 10.4070/kcj.2015.45.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farb A, Sangiorgi G, Carter AJ, et al. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999;99:44–52. doi: 10.1161/01.cir.99.1.44. [DOI] [PubMed] [Google Scholar]
- 4.Nakano M, Virmani R. Histopathology of vascular response to drug-eluting stents: an insight from human autopsy into daily practice. Cardiovasc Interv Ther. 2015;30:1–11. doi: 10.1007/s12928-014-0281-5. [DOI] [PubMed] [Google Scholar]
- 5.Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 6.Otsuka F, Vorpahl M, Nakano M, et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014;129:211–223. doi: 10.1161/CIRCULATIONAHA.113.001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon C, Palmaz JC, Sprague EA. Influence of topography on endothelialization of stents: clues for new designs. J Long Term Eff Med Implants. 2000;10:143–151. doi: 10.1615/jlongtermeffmedimplants.v10.i12.120. [DOI] [PubMed] [Google Scholar]
- 8.Kolandaivelu K, Swaminathan R, Gibson WJ, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–1409. doi: 10.1161/CIRCULATIONAHA.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalo N, Serruys PW, Okamura T, et al. Optical coherence tomography patterns of stent restenosis. Am Heart J. 2009;158:284–293. doi: 10.1016/j.ahj.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Nakano M, Vorpahl M, Otsuka F, et al. Ex vivo assessment of vascular response to coronary stents by optical frequency domain imaging. JACC Cardiovasc Imaging. 2012;5:71–82. doi: 10.1016/j.jcmg.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Lutter C, Mori H, Yahagi K, et al. Histopathological differential diagnosis of optical coherence tomographic image interpretation after stenting. JACC Cardiovasc Interv. 2016;9:2511–2523. doi: 10.1016/j.jcin.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Shin DH, Kim JS, et al. Optical coherence tomographic observation of morphological features of neointimal tissue after drug-eluting stent implantation. Yonsei Med J. 2014;55:944–952. doi: 10.3349/ymj.2014.55.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SY, Hong MK, Mintz GS, et al. Temporal course of neointimal hyperplasia following drug-eluting stent implantation: a serial follow-up optical coherence tomography analysis. Int J Cardiovasc Imaging. 2014;30:1003–1011. doi: 10.1007/s10554-014-0437-5. [DOI] [PubMed] [Google Scholar]
- 15.Habara M, Terashima M, Nasu K, et al. Morphological differences of tissue characteristics between early, late, and very late restenosis lesions after first generation drug-eluting stent implantation: an optical coherence tomography study. Eur Heart J Cardiovasc Imaging. 2013;14:276–284. doi: 10.1093/ehjci/jes183. [DOI] [PubMed] [Google Scholar]
- 16.Fukuhara K, Okura H, Kume T, Yamada R, Neishi Y, Uemura S. In-stent neointimal characteristics and late neointimal response after drug-eluting stent implantation: a preliminary observation. J Cardiol. 2016;67:437–441. doi: 10.1016/j.jjcc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Kimura T, Yokoi H, Nakagawa Y, et al. Three-year follow-up after implantation of metallic coronary-artery stents. N Engl J Med. 1996;334:561–566. doi: 10.1056/NEJM199602293340903. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Lee JH, Shin DH, et al. Long-term outcomes of neointimal hyperplasia without neoatherosclerosis after drug-eluting stent implantation. JACC Cardiovasc Imaging. 2014;7:788–795. doi: 10.1016/j.jcmg.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Lee SY, Hong MK, Shin DH, et al. Mechanisms of postintervention and 9-month luminal enlargement after treatment of drug-eluting in-stent restenosis with a drug-eluting balloon. Am J Cardiol. 2014;113:1468–1473. doi: 10.1016/j.amjcard.2014.01.424. [DOI] [PubMed] [Google Scholar]
- 20.Itoh T, Fusazaki T, Kimura T, et al. Clinical and pathological characteristics of homogeneous and nonhomogeneous tissue of in-stent restenosis visualized by optical coherence tomography. Coron Artery Dis. 2015;26:201–211. doi: 10.1097/MCA.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 21.Otsuka F, Byrne RA, Yahagi K, et al. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J. 2015;36:2147–2159. doi: 10.1093/eurheartj/ehv205. [DOI] [PubMed] [Google Scholar]
- 22.Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57:1314–1322. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 24.Imanaka T, Fujii K, Hao H, et al. Ex vivo assessment of neointimal characteristics after drug-eluting stent implantation: optical coherence tomography and histopathology validation study. Int J Cardiol. 2016;221:1043–1047. doi: 10.1016/j.ijcard.2016.07.110. [DOI] [PubMed] [Google Scholar]
- 25.Souteyrand G, Amabile N, Mangin L, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J. 2016;37:1208–1216. doi: 10.1093/eurheartj/ehv711. [DOI] [PubMed] [Google Scholar]
- 26.Taniwaki M, Radu MD, Zaugg S, et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. 2016;133:650–660. doi: 10.1161/CIRCULATIONAHA.115.019071. [DOI] [PubMed] [Google Scholar]
- 27.Lee SY, Ahn JM, Mintz GS, et al. Characteristics of earlier versus delayed presentation of very late drug-eluting stent thrombosis: an optical coherence tomographic study. J Am Heart Assoc. 2017;6:e005386. doi: 10.1161/JAHA.116.005386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SY, Shin DH, Mintz GS, et al. Optical coherence tomography-based evaluation of in-stent neoatherosclerosis in lesions with more than 50% neointimal cross-sectional area stenosis. EuroIntervention. 2013;9:945–951. doi: 10.4244/EIJV9I8A158. [DOI] [PubMed] [Google Scholar]
- 29.Kim JS, Hong MK, Shin DH, et al. Quantitative and qualitative changes in DES-related neointimal tissue based on serial OCT. JACC Cardiovasc Imaging. 2012;5:1147–1155. doi: 10.1016/j.jcmg.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 30.Kimura T, Abe K, Shizuta S, et al. Long-term clinical and angiographic follow-up after coronary stent placement in native coronary arteries. Circulation. 2002;105:2986–2991. doi: 10.1161/01.cir.0000019743.11941.3b. [DOI] [PubMed] [Google Scholar]
- 31.Yonetsu T, Kato K, Kim SJ, et al. Predictors for neoatherosclerosis: a retrospective observational study from the optical coherence tomography registry. Circ Cardiovasc Imaging. 2012;5:660–666. doi: 10.1161/CIRCIMAGING.112.976167. [DOI] [PubMed] [Google Scholar]
- 32.Lee SY, Hur SH, Lee SG, et al. Optical coherence tomographic observation of in-stent neoatherosclerosis in lesions with more than 50% neointimal area stenosis after second-generation drug-eluting stent implantation. Circ Cardiovasc Interv. 2015;8:e001878. doi: 10.1161/CIRCINTERVENTIONS.114.001878. [DOI] [PubMed] [Google Scholar]
- 33.Otsuka F, Sakakura K, Yahagi K, et al. TCT-655 Contribution of in-stent neoatherosclerosis to late stent failure following bare metal and 1st- and 2nd-generation drug-eluting stent placement: an autopsy study. J Am Coll Cardiol. 2014;64:B190–B191. [Google Scholar]
- 34.Mangiameli A, Ohno Y, Attizzani GF, Capodanno D, Tamburino C. Neoatherosclerosis as the cause of late failure of a bioresorbable vascular scaffold. JACC Cardiovasc Interv. 2015;8:633–634. doi: 10.1016/j.jcin.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Hiltrop N, Jorge C, Bennett J, Adriaenssens T. Late neoatherosclerotic scaffold failure: an unexpected achilles heel for current bioresorbable scaffold technology? Int J Cardiol. 2016;223:133–135. doi: 10.1016/j.ijcard.2016.08.076. [DOI] [PubMed] [Google Scholar]