Abstract
Fontan circulation is generally characterized by high central venous pressure, low cardiac output, and slightly low arterial oxygen saturation, and it is quite different from normal biventricular physiology. Therefore, when a patient with congenital heart disease is selected as a candidate for this type of circulation, the ultimate goals of therapy consist of 2 components. One is a smooth adjustment to the new circulation, and the other is long-term circulatory stabilization after adjustment. When either of these goals is not achieved, the patient is categorized as having “failed” Fontan circulation, and the prognosis is dismal. For the first goal of smooth adjustment, a lot of effort has been made to establish criteria for patient selection and intensive management immediately after the Fontan operation. For the second goal of long-term circulatory stabilization, there is limited evidence of successful strategies for long-term hemodynamic stabilization. Furthermore, there have been no data on optimal hemodynamics in Fontan circulation that could be used as a reference for patient management. Although small clinical trials and case reports are available, the results cannot be generalized to the majority of Fontan survivors. We recently reported the clinical and hemodynamic characteristics of early and late failing Fontan survivors and their association with all-cause mortality. This knowledge could provide insight into the complex Fontan pathophysiology and might help establish a management strategy for long-term hemodynamic stabilization.
Keywords: Fontan procedure, Hemodynamics, Cardiac output, Vascular resistance, Mortality
INTRODUCTION
The Fontan operation is a definitive palliative procedure for patients with complex cyanotic congenital heart disease (CHD) who are not suitable for biventricular repair, including those with tricuspid atresia, univentricular heart, or hypoplastic left heart syndrome.1) The introduction of the Fontan operation dramatically improved both the prognosis and quality of life for patients with complex cyanotic CHD.2),3) However, because of their unique hemodynamics, long-term morbidity and mortality in these patients are still high compared to those who underwent biventricular repair.4),5) The causes of morbidity after the Fontan operation include heart failure (HF), arrhythmia,6) protein losing enteropathy (PLE),7),8) pulmonary arteriovenous fistulae (PAVF),9) thromboembolism,10),11) renal dysfunction,12) and Fontan-associated liver disease (FALD).13),14) These conditions are now considered major determinants of post-operative outcome for long-term Fontan survivors.15)
Our objective was to help post-Fontan patients adapt smoothly to their unique hemodynamics and to keep this adaptation optimal to avoid future complications. However, the definition of “optimal” with regard to Fontan hemodynamics is unclear, and we know little about late Fontan hemodynamics or its impact on Fontan pathophysiology, including the prognosis. In this review, we include recent data to focus on hemodynamic issues and their association with Fontan pathophysiology and prognosis, which has not been addressed in detail in the existing Fontan literature.
CHARACTERISTICS OF FONTAN HEMODYNAMICS
The primary characteristics of Fontan hemodynamics is a lack of subpulmonary ventricle,1),16) which automatically lead to high central venous pressure (CVP). This creates additional driving pressure for the pulmonary circulation and diminished cardiac preload for the systemic ventricle (SV), resulting in chronically low cardiac output (CO). These 2 consequences are considered inevitable in CHD patients with Fontan circulation. Mild but significant low arterial blood oxygen saturation (SaO2) is also a major hemodynamic feature, which probably results from intrapulmonary ventilation-perfusion mismatch17) as well as the development of veno-venous collaterals.18) All of these abnormal conditions are ultimately associated with reduced exercise capacity.17),19)
Thus, pathophysiologic complications after the Fontan operation consist mainly of the following 3 conditions: 1) multi-end-organ congestion due to high venous pressure, 2) chronic HF due to low CO, and 3) mild but significant hypoxia. With an elevated CVP, low CO can result in low systemic arterial blood pressure, leading to low systemic perfusion pressure (PP). Often, the pressure difference between the systemic blood pressure and CVP is an indicator of PP for multiple end organs.20)
One of the main goals of the circulatory system is to supply sufficient oxygen and other vital substances to the organs. Accordingly, the maintenance of adequate oxygen content and PP is crucial. To accomplish this goal, the circulatory system in Fontan patients appears to adjust to the inconvenient Fontan hemodynamics through the following adaptations commonly observed in patients with HF: 1) raising systemic artery resistance (Rs), and 2) redistributing CO to vital organs, such as the brain and heart, with increased oxygen content (hemoglobin concentration) to compensate for limited CO.20),21)
“BETTER” FONTAN HEMODYNAMICS
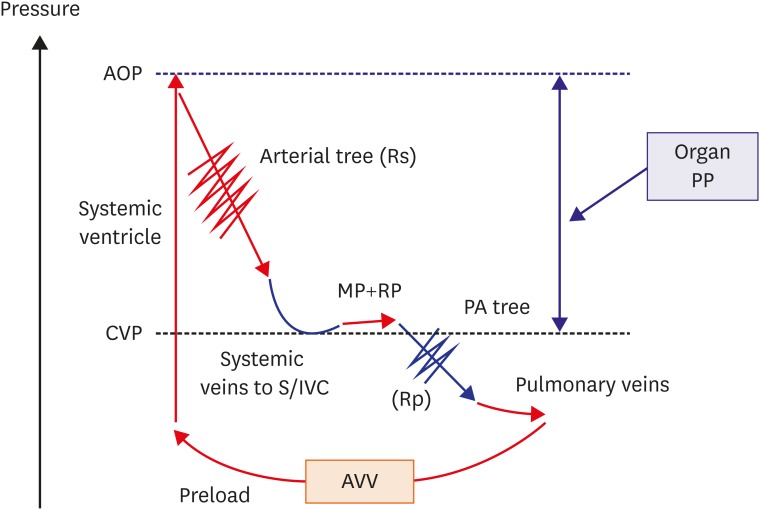
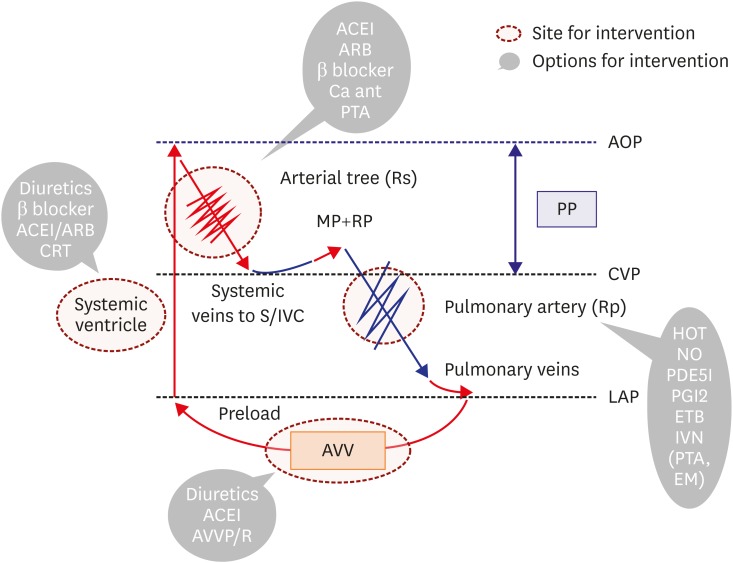
In general, Fontan circulation would be ideal if the hemodynamics was closer to that of a normal person, i.e., hemodynamics without an inappropriately high or low CVP and with sufficiently high Rs to maintain PP without significant hypoxia (Figure 1).
Figure 1.
Fontan hemodynamics without failure. In Fontan patients, the SV supports systemic circulation. High CVP is the driving pressure of the pulmonary circulation, and the MP and RP play significant roles in pulmonary circulation. Organ PP, or the pressure difference between CVP and AOP, is low due to high CVP and low systemic pressure from diminished cardiac preload. Rs (red jagged line) appropriately increases to maintain PP, while the Rp (blue jagged line) should remain low.
AOP = aortic pressure; AVV = atrioventricular valve; CVP = central venous pressure; MP = muscle pump; PA = pulmonary artery; PP = perfusion pressure; RP = respiratory pump; Rp = pulmonary artery resistance; Rs = systemic artery resistance; S/IVC = superior vena cava/inferior vena cava; SV = systemic ventricle.
Possible causes of elevated CVP include pre-capillary factors, like stenosis of the Fontan route and high pulmonary artery resistance (Rp), and post-capillary factors, like systolic and/or diastolic SV dysfunction and atrioventricular valve (AVV) impairment. In addition, excess water retention, development of aorto-pulmonary collaterals,22) and inappropriately high CO could also be responsible for high CVP.23)
On the other hand, because low CO situation might be inevitable due to the lack of a subpulmonary ventricle, adaptive mechanisms for inappropriately high Rs and redistribution of the limited CO might be essential for better hemodynamics, as mentioned above. In this regard, the autonomic nervous system and neurohormonal activation could play an important role in regulation of systemic vascular tone.21) However, these compensatory adaptations result in a vicious cycle in HF patients, for instance, worsening HF due to sympathetic nervous activation, and this could potentially occur in Fontan patients, leading to poor prognosis.12),24) Thus, these essential compensatory adaptations and the maintenance of adequate Fontan circulation, even if the hemodynamics seems “optimal,” are contradictory issues that should be addressed in these patients.
“FAILING” FONTAN CIRCULATION
Although the term “failing” has been frequently quoted in the literature, the definition remains unclear in certain settings.25),26) In general, major hemodynamic abnormalities, such as high CVP, low CO, and low SaO2, characterize “failing” Fontan hemodynamics. This pathophysiology is often accompanied by systolic and/or diastolic SV dysfunction with or without AVV dysfunction. In addition, because serious post-operative complications, such as supraventricular arrhythmias, PLE, PAVF, and thromboembolic problems often coexist, adaptive mechanisms for maintaining both “appropriate” Rs and the redistribution of limited CO might lead to “failing” and unacceptable pathophysiology. Furthermore, the recent concept of failure can include additional non-cardiac end-organ impairments, such as FALD,13),14),15),27) renal dysfunction,12),27),28),29) glucose metabolic abnormalities,30) and even bone and nutritional problems.31),32) Hepato-renal pathophysiology, in particular, has a strong impact on morbidity and mortality in these patients.14),29) Thus, these emerging pathophysiologic situations make the definition of “failing” more complex.
A clinical phenotype-based classification system for patients with Fontan failure has been proposed, and it includes the following types:33) type I for those with reduced ejection fraction (EF), type II for those with preserved EF, type III for those with normal hemodynamics, and type IV for those with abnormal lymphatics. However, it can be difficult to determine the appropriate type of failure for a given clinical presentation, as a significant portion of failing patients have overlapping phenotypes8) or a lack of established “normal” Fontan hemodynamics. In addition, there is no clear evidence of a significant association between Fontan hemodynamics and long-term outcomes, including mortality.
PROGNOSIS OF FONTAN HEMODYNAMICS
Knowing the prognosis of Fontan hemodynamics can guide clinicians when managing these patients. Although there have been a few studies addressing “optimal” or “normal” hemodynamic characteristics in Fontan patients, the prognosis of Fontan hemodynamics remains unclear.34),35) We recently clarified the association between distinct differences in some hemodynamic variables and their effects on all-cause mortality between early (0.5 to 5 years) and late (≥15 years) Fontan survivors.23)
CVP
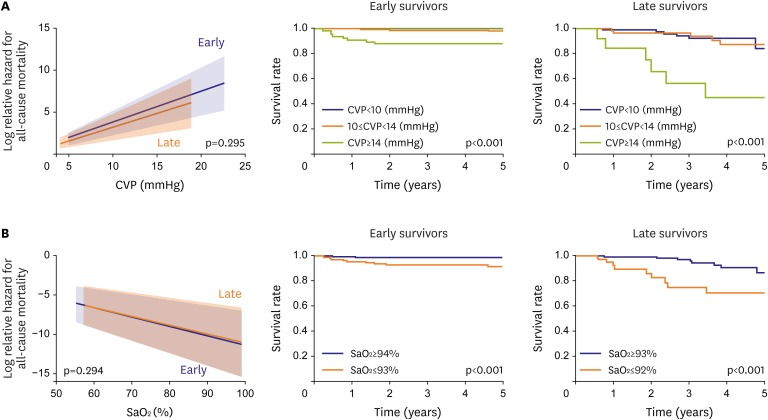
High CVP had an adverse impact on mortality in early and late Fontan survivors (Figure 2A). According to the receiver operating characteristics (ROC) curve, the cutoff value for mortality was 14 mmHg in both groups.
Figure 2.
(A) Effect of CVP on all-cause mortality in early and late Fontan survivors. High CVP has a significant adverse impact on mortality in both groups of survivors. The right upper figures show that Fontan survivors with high CVP (≥14 mmHg) have a high mortality. (B) Effect of arterial blood SaO2 on all-cause mortality in early and late Fontan survivors. Low SaO2 has a significant adverse impact on mortality in both groups of survivors. (A) and (B) are modified from those in reference 23. The right lower figures show that Fontan survivors with low SaO2 (≤93% in early survivors, ≤92% in late survivors) have high mortality.
CVP = central venous pressure; SaO2 = oxygen saturation.
SaO2
Low SaO2 had an adverse effect on mortality in early and late survivors (Figure 2B). According to the ROC curve, the cutoff value for mortality in early and late survivors was 93% and 92%, respectively.
End-diastolic systemic ventricular volume index (EDVI)
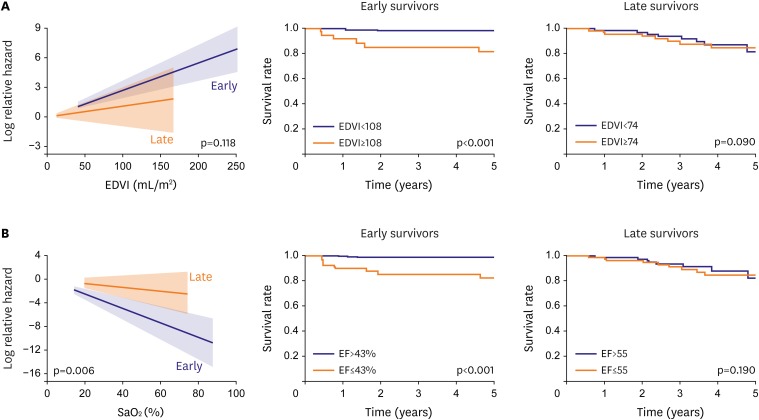
Large EDVI had an adverse effect on mortality only in early Fontan survivors, with a cutoff value of 108 mL/m2. However, this association was not observed in late Fontan survivors (Figure 3A).
Figure 3.
(A) Effect of end-diastolic volume of the SV (EDVI, mL/m2) on all-cause mortality in early and late Fontan survivors. Large EDVI has a significant adverse impact on mortality only in early survivors (the cutoff value was 108 mL/m2) (upper middle). However, large EDVI has no significant adverse impact on mortality in late survivors when they were divided into 2 groups based on a median EDVI value of 74 mL/m2 (upper right). (B) Effect of SV EF (%) on all-cause mortality in early and late Fontan survivors. Low EF has a significant adverse impact on mortality only in early survivors (cutoff value, 43%) (lower middle). However, low EF has no significant adverse impact on mortality in late survivors when late survivors were divided into 2 groups based on a median EF value of 53% (lower right). (A) and (B) are modified from those in reference 23).
EDVI = end-diastolic systemic ventricular volume index; EF = ejection fraction; SaO2 = oxygen saturation; SV = systemic ventricle.
EF of the systemic ventricle (SV)
As in the association between EDVI and mortality, low EF had an adverse effect on mortality only in early Fontan survivors, with a cutoff value of 43% (Figure 3B).
CO/cardiac index (CI)
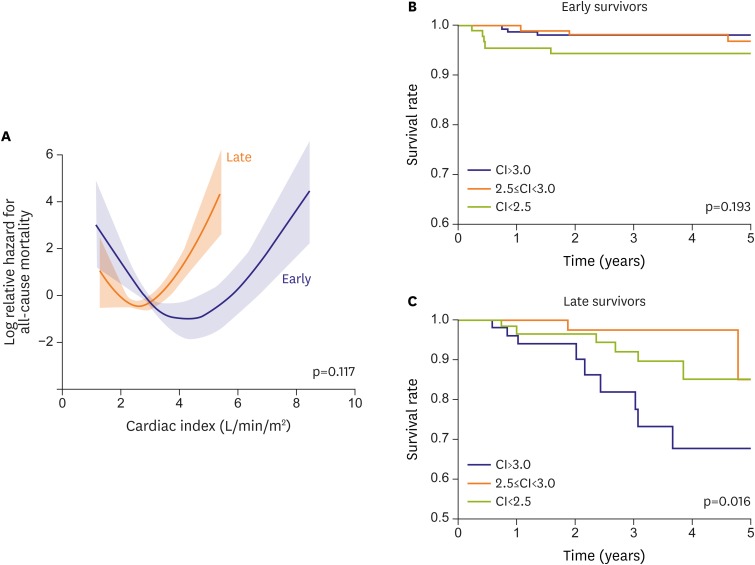
Based on Cox's hazard model, the CI had no impact on mortality in early Fontan survivors. However, a high CI predicted an adverse effect on mortality in late Fontan survivors (odds ratio [OR], 0.51; 95% confidence interval [95% CI], 0.26–0.99; p=0.048).23) Interestingly, the association with mortality was U-shaped in early and late Fontan survivors, with nadir CI values of 4.3 L/min/m2 and 2.6 L/min/m2, respectively (Figure 4A-C). Thus, high CO does not always indicate favorable physiology in Fontan circulation. Expectedly, low CO was associated with poor outcome in both groups.
Figure 4.
Effect of CI (L/min/m2) on all-cause mortality in early and late Fontan survivors (A). Associations between CI and all-cause mortality are U-shaped in early and late survivors with no group differences. Although a low CI tends to adversely impact all-cause mortality (B) in early survivors, a high CI has a significantly adverse impact on mortality in late survivors (C). (A) is modified from reference 23).
CI = cardiac index.
As described below, the associations between Rp and Rs and mortality were similar to the associations with CI.
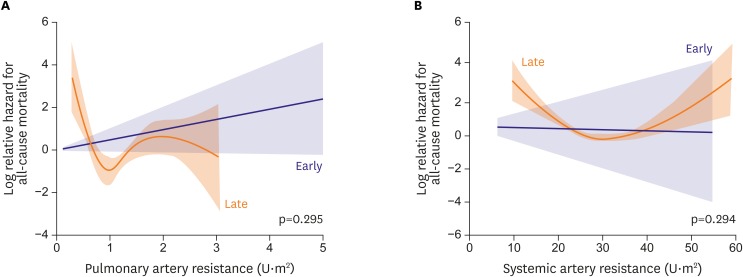
Pulmonary artery resistance (Rp)
Although it might be difficult to accurately estimate Rp in the Fontan pulmonary circulation,36) 37) there is no established methodology for measuring Fontan Rp other than conventional estimation with catheterization. Using this catheterization-based calculation, Rp had no significant impact on mortality in early or late Fontan survivors according to the Cox's hazard model. Although the associations were not significant, a low Rp seemed to be associated with high mortality (Figure 5A).
Figure 5.
Effects of pulmonary (A) and systemic (B) arterial resistance on all-cause mortality in early and late Fontan survivors. Although no significant associations between these variables and all-cause mortality were identified, the associations are unique and U-shaped in the late survivors. For systemic artery resistance, low resistance tended to have an adverse impact on mortality (HR, 0.94; 95% CI, 0.87–1.00; p=0.060).
95% CI = 95% confidence interval; HR = hazard ratio.
Systemic artery resistance (Rs)
Although Rs had no significant impact on mortality in early or late Fontan survivors, the association with mortality was also U-shaped, as observed with Rs (Figure 5B).
According to our recent data, we summarize our findings as follows: 1) high CVP and low SaO2 are closely associated with poor outcome regardless of the follow-up period, 2) SV dysfunction adversely affects mortality only in early survivors, and 3) the CI gradually decreases as Fontan survivors age,38) showing a fixed U-shaped association with mortality.23) This implies that optimal CO gradually decreases, at least during a follow-up period of 15 years after the Fontan operation, and that high CO is not always favorable in late Fontan survivors. Thus, in addition to the traditional failing Fontan phenotype characterized by high CVP and low CO, we have recognized a new failing Fontan hemodynamic phenotype characterized by high CVP, inappropriately high CO, and low SaO2.23),33)
Interestingly, this new failing hemodynamic phenotype consists of preserved SV function; more importantly, patients with this phenotype have even higher mortality than those with the traditional failing phenotype.23) Low Rp and Rs, especially inappropriately low Rs, might be major causes of high CO. Advanced FALD could be responsible for low Rs,23),33),39),40),41) and FALD-associated development of PAVF exacerbates the failing conditions due to hypoxia-induced dilatation of the systemic arteries42) that further increases CO with preserved cardiac preload and SV function. Thus, this FALD-associated pathophysiology, rather than low Rp itself, could be responsible for the high mortality in patients with high CVP and high CO.23)
HEMODYNAMICS OF CLINICALLY “EXCELLENT” FONTAN SURVIVORS
From the mortality point of view, preserved SV function, low CVP, and high SaO2 with a CI of approximately 4.3 L/min/m2 best characterize “optimal” hemodynamics in early Fontan survivors, whereas low CVP and high SaO2 with a relatively low CI of approximately 2.6 L/min/m2 might be characteristic of the same in late Fontan survivors. However, it is still unclear whether these hemodynamic characteristics are equivalent to those of clinically “excellent” Fontan survivors. In this regard, knowing the Fontan hemodynamics in clinically “excellent” long-term survivors could help guide clinicians to better manage other Fontan patients.
We previously and arbitrarily defined “excellent” post-operative Fontan patients (n=18) as those without any unexpected hospitalization or all-cause mortality during a ≥15-year post-operative follow-up period. Additionally, we compared their hemodynamic variables to those of non-excellent survivors (n=43).38)
According to our data on “excellent” Fontan survivors, the CVP increased slightly at post-operative year 5, then gradually decreased to a value of 10 at the end of the 15-year follow-up (mmHg). The CI continued to decrease during the entire follow-up period with a final value of 2.6 L/min/m2 15 years after the operation. Additionally, Rs increased steadily to compensate for decreasing CO and to maintain sufficient systemic PP, while Rp steadily decreased to preserve ventricular preload. The final mean values for Rp and Rs were approximately 1.2 U·m2 and 30 U·m2, respectively. Thus, Fontan hemodynamics changes significantly over time.38) In addition, we should be aware that, because these hemodynamic values depend on the anesthetic condition during catheterization, i.e., general or local anesthesia, these values should be compared under the same condition.
When assessing SV function in “excellent” survivors, the indexed end-diastolic volume decreased immediately after surgery, then stabilized for the rest of the follow-up period, with a mean value of approximately 70 mL/m2. The EF for the SV also seemed to be stable for the entire follow-up period, with a mean value of approximately 55%. Additional hemodynamic characteristics for “excellent” survivors included a high SaO2 of approximately 94% without significant AVV regurgitation.38)
Importantly, low CVP and improved atrioventricular function independently predicted “excellent” long-term Fontan survival. Late post-operative CO in “excellent” survivors could be equivalent to the “optimal” CO for late survivors.
Based on our analyses of Fontan hemodynamics, including all-cause mortality and “excellent” long-term outcomes, “optimal” Fontan hemodynamics are characterized in Table 1. In early survivors, low CVP without hypoxia, preserved SV function, and high CO are important factors for “optimal” hemodynamics. In excellent late survivors, a relatively low CO of approximately 2.6 L/min/m2 is a common feature in addition to low CVP without hypoxia.
Table 1. Comparison of “optimal” hemodynamics and systemic ventricular function in early, late and excellent Fontan survivors.
| Goals | Free from all-cause mortality | Excellent survivors | |
|---|---|---|---|
| Follow-up (years) | Early survivors (0.5–5) | Late survivors (≥15) | Late survivors (≥15) |
| CVP (mmHg) | The lower, the better | 10 | |
| Arterial SaO2 (%) | The higher, the better | 94 | |
| CI (L/min/m2) | ≈4.2 | ≈2.6 | 2.6 |
| EDVI (mL/m2) | The smaller, the better | - | ≈70 |
| EF of the SV (%) | The higher, the better | - | ≈55 |
CI = cardiac index; CVP = central venous pressure; EDVI = end-diastolic systemic ventricular volume index; EF = ejection fraction; SaO2 = oxygen saturation; SV = systemic ventricle.
HEMODYNAMICS-BASED MANAGEMENT FOR PATIENTS WITH FONTAN CIRCULATION
According to the associations of Fontan hemodynamics with all-cause mortality, the following subgrouping might be clinically beneficial for managing failing hemodynamics. One failing group consists of patients who cannot smoothly adjust to Fontan circulation during the early phase after the operation, and the other failing group consists of those who cannot maintain Fontan circulation because of pathological interactions between the cardiovascular system and non-cardiac end-organs and/or cardiovascular dysfunction due to long-lasting Fontan-associated insults to end-organs.23),25),43) Although clinically relevant arrhythmias increase in incidence over time in Fontan patients and constitute another major failing phenotype,44),45) we focus on hemodynamic management. Additionally, this arrhythmia-induced failure is extensively described elsewhere.46)
We should also be aware that the management strategy for patients with failing hemodynamics might not be applicable to those without hemodynamic failure.
Patients without hemodynamic failure
There is no established management strategy for non-failing Fontan patients. In our “excellent” long-term Fontan survivors, the use of diuretics gradually decreased. Only one patient (8%) continued receiving diuretics, while 5 patients (39%) received angiotensin converting enzyme inhibitors or angiotensin receptor blockers (ACEI/ARBs).38) Of note, 50% of the survivors were not taking any cardiac medication. We have to be careful when introducing new interventions to stable survivors because Fontan patients without any major complications rarely experience clinical events.47) It might be better to avoid any new intervention that is not yet justified in Fontan patients.
Patients with hemodynamic failure
We proposed a new simple hemodynamics-based classification for failing Fontan patients that might be helpful for hemodynamic management regardless of the incidence of major complications.23)
High CVP with low CO
This is the traditional phenotype for Fontan failure. In general, circulation in these patients is unable to adjust to the Fontan circulation during the early post-operative phase. This maladaptation is mainly due to cardiopulmonary problems that include SV dysfunction, AVV impairment, high Rp, and/or markedly high Rs.
Thus, hemodynamic management could be focused on these cardiopulmonary problems. Combined with appropriate water balance, conventional HF medications, such as ACEI/ARBs, β blockers, and aldosterone antagonists,48),49) could be effective for managing systolic SV dysfunction. Additionally, re-synchronization therapy for ventricular dyssynchrony can be used.50),51),52) Furthermore, valvuloplasty or mechanical valve replacement should be considered in patients with AVV dysfunction.53),54) When patients have relatively high Rp, pulmonary vasodilators can be effective in select patients.55) These options are summarized in Figure 6.
Figure 6.
Traditional failing Fontan hemodynamics (high CVP with low output) and possible therapeutic options. For systemic ventricular systolic dysfunction, conventional anti-HF strategies might be successful. In addition, pulmonary artery dilators could also be effective. For AVV impairment, surgical options should be considered.
ACEI = angiotensin converting enzyme inhibitor; AOP = aortic pressure; ARB = angiotensin II receptor blocker; AVV = atrioventricular valve; AVVP/R = atrioventricular valvuloplasty or replacement; CRT = cardiac resynchronization therapy; CVP = central venous pressure; EM = coil embolization to collateral vessels; ETB = endothelin receptor blocker; HF = heart failure; HOT = home oxygen therapy; IVN = catheter intervention; LAP: functional left atrial pressure; MP = muscle pump; NO = nitric oxide; PDE5I = phosphodiesterase 5 inhibitor; PGI2 = prostaglandin I2; PTA = percutaneous transluminal angioplasty; RP = respiratory pump; Rp = pulmonary artery resistance; Rs = systemic artery resistance; S/IVC = superior vena cava/inferior vena cava.
When these management strategies fail, patients might be eligible for cardiac transplantation.56),57)
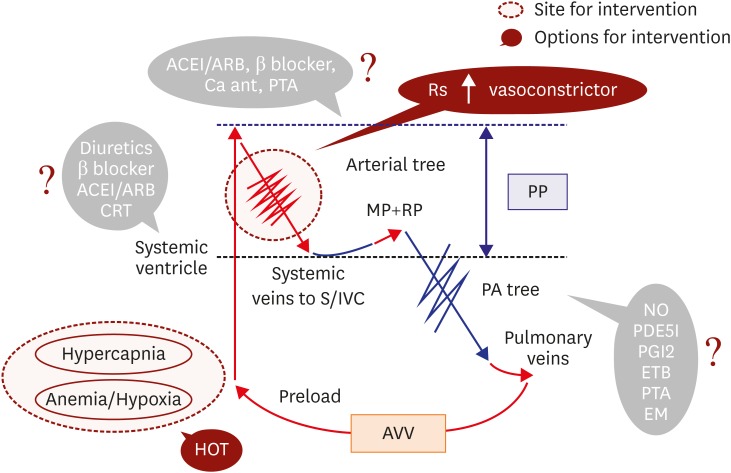
High CVP with high CO
This failing hemodynamic phenotype is not well recognized nor is the detailed pathophysiology well understood.23),27),33),34),35) This failure group often consists of long-term adult Fontan survivors because the phenotype can develop after a period of stabilized Fontan circulation due to multiple end-organ dysfunction, such as FALD. This hemodynamic phenotype is mainly characterized by inappropriately low Rs,23),34) resulting in an even higher CO to maintain PP, which remains low.23) In addition, PAVF often develops,23) further exacerbating the secondary hypoxia,42) which is another characterizing feature of this failure group. Furthermore, the prognosis of this group is worse than that of traditional failing Fontan patients,23) and the condition can ultimately lead to hepatorenal syndrome.58)
Thus, conventional HF management for systolic SV dysfunction can exacerbate these failing hemodynamics, and a different management strategy is required. In this phenotype, pulmonary artery dilators might not be successful, because Rp is often already low enough for adequate pulmonary circulation,23) and the dilators can further dilate the systemic artery. In this phenotype, it might be important to increase Rs to maintain effective PP. In addition to oxygen therapy for the coexisting hypoxia, arterial vasoconstrictors, such as α agonists, norepinephrine, and vasopressin, might be effective. If necessary, the use of ACEI/ARBs and/or β blockers should be reduced to regain systemic arterial constriction. Although short-term hemodynamic stability can be achieved,58) the long-term efficacy of these strategies is unknown, and we should be aware that this management strategy is only feasible in patients with preserved SV systolic function and adequate AVV function. These options are summarized in Figure 7.
Figure 7.
Newly recognized failing Fontan hemodynamics (high CVP with high output) and possible therapeutic options. For this failing Fontan hemodynamic phenotype, traditional therapeutic approaches might be questionable and can even be harmful. Inappropriately low Rs requires vasoconstrictors, such as alpha 1 receptor agonists (midodrine). For coexistent hypoxia, oxygen inhalation might be effective.
ACEI/ARBs = angiotensin converting enzyme inhibitors or angiotensin receptor blockers; AVV = atrioventricular valve; CRT = cardiac resynchronization therapy; CVP = central venous pressure; EM = coil embolization to collateral vessels; ETB = endothelin receptor blocker; HOT = home oxygen therapy; MP = muscle pump; NO = nitric oxide; PA = pulmonary artery; PDE5I = phosphodiesterase 5 inhibitor; PGI2 = prostaglandin I2; PP = perfusion pressure; PTA = percutaneous transluminal angioplasty; RP = respiratory pump; Rs = systemic artery resistance; S/IVC = superior vena cava/inferior vena cava.
Pathophysiologic interactions between the cardiovascular system and advanced non-cardiovascular end-organ dysfunction play major roles in this failing phenotype. For instance, the influence of liver cirrhosis on the cardiovascular system39),40),41),59) can cause cardiac transplantation to not be a suitable treatment option. Therefore, it is important to determine the optimal timing for cardiac transplantation,57) and more importantly, much effort should be made to avoid development of this failing phenotype.
Finally, although information on management of failing Fontan patients has accumulated on a case-by-case basis60),61),62),63),64),65),66),67),68),69),70),71),72),73) and based on small randomized controlled trials,74),75),76),77),78),79),80) this data cannot be generalized to the majority of Fontan patients, especially those with failing hemodynamics. In addition, we now realize that Fontan hemodynamics changes significantly over time, as patients develop12) multiple end-organ interactions. A significant shift occurs in the major determinants of morbidity and mortality from cardiac factors to non-cardiac factors as patients age,38) even though the majority of Fontan survivors are still in their 20s or 30s. Thus, much more research is needed to establish long-term management strategies with a broader perspective of Fontan pathophysiology as a multi-organ disease.
Footnotes
Conflict of Interest: The author has no financial conflicts of interest.
References
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.d’Udekem Y, Iyengar AJ, Cochrane AD, et al. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation. 2007;116:I157–I164. doi: 10.1161/CIRCULATIONAHA.106.676445. [DOI] [PubMed] [Google Scholar]
- 3.Ohuchi H, Kagisaki K, Miyazaki A, et al. Impact of the evolution of the Fontan operation on early and late mortality: a single-center experience of 405 patients over 3 decades. Ann Thorac Surg. 2011;92:1457–1466. doi: 10.1016/j.athoracsur.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 4.Khairy P, Fernandes SM, Mayer JE, Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 5.Diller GP, Kempny A, Alonso-Gonzalez R, et al. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow-up at a large tertiary centre. Circulation. 2015;132:2118–2125. doi: 10.1161/CIRCULATIONAHA.115.017202. [DOI] [PubMed] [Google Scholar]
- 6.Quinton E, Nightingale P, Hudsmith L, et al. Prevalence of atrial tachyarrhythmia in adults after Fontan operation. Heart. 2015;101:1672–1677. doi: 10.1136/heartjnl-2015-307514. [DOI] [PubMed] [Google Scholar]
- 7.John AS, Johnson JA, Khan M, Driscoll DJ, Warnes CA, Cetta F. Clinical outcomes and improved survival in patients with protein-losing enteropathy after the Fontan operation. J Am Coll Cardiol. 2014;64:54–62. doi: 10.1016/j.jacc.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Ohuchi H, Yasuda K, Miyazaki A, et al. Haemodynamic characteristics before and after the onset of protein losing enteropathy in patients after the Fontan operation. Eur J Cardiothorac Surg. 2013;43:e49–e57. doi: 10.1093/ejcts/ezs714. [DOI] [PubMed] [Google Scholar]
- 9.Moore JW, Kirby WC, Madden WA, Gaither NS. Development of pulmonary arteriovenous malformations after modified Fontan operations. J Thorac Cardiovasc Surg. 1989;98:1045–1050. [PubMed] [Google Scholar]
- 10.Ohuchi H, Yasuda K, Miyazaki A, et al. Prevalence and predictors of haemostatic complications in 412 Fontan patients: their relation to anticoagulation and haemodynamics. Eur J Cardiothorac Surg. 2015;47:511–519. doi: 10.1093/ejcts/ezu145. [DOI] [PubMed] [Google Scholar]
- 11.Egbe AC, Connolly HM, Niaz T, et al. Prevalence and outcome of thrombotic and embolic complications in adults after Fontan operation. Am Heart J. 2017;183:10–17. doi: 10.1016/j.ahj.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Ohuchi H, Yasuda K, Miyazaki A, et al. Comparison of prognostic variables in children and adults with Fontan circulation. Int J Cardiol. 2014;173:277–283. doi: 10.1016/j.ijcard.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Assenza GE, Graham DA, Landzberg MJ, et al. MELD-XI score and cardiac mortality or transplantation in patients after Fontan surgery. Heart. 2013;99:491–496. doi: 10.1136/heartjnl-2012-303347. [DOI] [PubMed] [Google Scholar]
- 14.Pundi K, Pundi KN, Kamath PS, et al. Liver disease in patients after the Fontan operation. Am J Cardiol. 2016;117:456–460. doi: 10.1016/j.amjcard.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Asrani SK, Warnes CA, Kamath PS. Hepatocellular carcinoma after the Fontan procedure. N Engl J Med. 2013;368:1756–1757. doi: 10.1056/NEJMc1214222. [DOI] [PubMed] [Google Scholar]
- 16.Ohuchi H. Adult patients with Fontan circulation: what we know and how to manage adults with Fontan circulation? J Cardiol. 2016;68:181–189. doi: 10.1016/j.jjcc.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Ohuchi H, Ohashi H, Takasugi H, Yamada O, Yagihara T, Echigo S. Restrictive ventilatory impairment and arterial oxygenation characterize rest and exercise ventilation in patients after Fontan operation. Pediatr Cardiol. 2004;25:513–521. doi: 10.1007/s00246-003-0652-7. [DOI] [PubMed] [Google Scholar]
- 18.Ohuchi H, Yasuda K, Hasegawa S, et al. Influence of ventricular morphology on aerobic exercise capacity in patients after the Fontan operation. J Am Coll Cardiol. 2001;37:1967–1974. doi: 10.1016/s0735-1097(01)01266-9. [DOI] [PubMed] [Google Scholar]
- 19.Heinemann M, Breuer J, Steger V, Steil E, Sieverding L, Ziemer G. Incidence and impact of systemic venous collateral development after Glenn and Fontan procedures. Thorac Cardiovasc Surg. 2001;49:172–178. doi: 10.1055/s-2001-14339. [DOI] [PubMed] [Google Scholar]
- 20.Levine TB, Levine AB. Regional blood flow supply and demand in heart failure. Am Heart J. 1990;120:1547–1551. doi: 10.1016/0002-8703(90)90057-5. [DOI] [PubMed] [Google Scholar]
- 21.Drexler H. Reduced exercise tolerance in chronic heart failure and its relationship to neurohumoral factors. Eur Heart J. 1991;12(Suppl C):21–28. doi: 10.1093/eurheartj/12.suppl_c.21. [DOI] [PubMed] [Google Scholar]
- 22.Latus H, Gummel K, Diederichs T, et al. Aortopulmonary collateral flow is related to pulmonary artery size and affects ventricular dimensions in patients after the Fontan procedure. PLoS One. 2013;8:e81684. doi: 10.1371/journal.pone.0081684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohuchi H, Miyazaki A, Negishi J, et al. Hemodynamic determinants of mortality after Fontan operation. Am Heart J. 2017;189:9–18. doi: 10.1016/j.ahj.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Benedict CR, Shelton B, Johnstone DE, et al. Prognostic significance of plasma norepinephrine in patients with asymptomatic left ventricular dysfunction. SOLVD Investigators. Circulation. 1996;94:690–697. doi: 10.1161/01.cir.94.4.690. [DOI] [PubMed] [Google Scholar]
- 25.Deal BJ, Jacobs ML. Management of the failing Fontan circulation. Heart. 2012;98:1098–1104. doi: 10.1136/heartjnl-2011-301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Rita F, Crossland D, Griselli M, Hasan A. Management of the failing Fontan. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2015;18:2–6. doi: 10.1053/j.pcsu.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Mori M, Aguirre AJ, Elder RW, et al. Beyond a broken heart: circulatory dysfunction in the failing Fontan. Pediatr Cardiol. 2014;35:569–579. doi: 10.1007/s00246-014-0881-y. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno M, Ohuchi H, Matsuyama TA, Miyazaki A, Ishibashi-Ueda H, Yamada O. Diverse multi-organ histopathologic changes in a failed Fontan patient. Pediatr Int. 2016;58:1061–1065. doi: 10.1111/ped.13054. [DOI] [PubMed] [Google Scholar]
- 29.Ohuchi H, Negishi J, Hayama Y, Miyazaki A, Shiraishi I, Ichikawa H. Renal resistive index reflects Fontan pathophysiology and predicts mortality. Heart. 2017 doi: 10.1136/heartjnl-2016-310812. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Ohuchi H, Miyamoto Y, Yamamoto M, et al. High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J. 2009;158:30–39. doi: 10.1016/j.ahj.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Avitabile CM, Leonard MB, Zemel BS, et al. Lean mass deficits, vitamin D status and exercise capacity in children and young adults after Fontan palliation. Heart. 2014;100:1702–1707. doi: 10.1136/heartjnl-2014-305723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avitabile CM, Goldberg DJ, Zemel BS, et al. Deficits in bone density and structure in children and young adults following Fontan palliation. Bone. 2015;77:12–16. doi: 10.1016/j.bone.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Book WM, Gerardin J, Saraf A, Marie Valente A, Rodriguez F., 3rd Clinical phenotypes of Fontan failure: implications for management. Congenit Heart Dis. 2016;11:296–308. doi: 10.1111/chd.12368. [DOI] [PubMed] [Google Scholar]
- 34.Hebson CL, McCabe NM, Elder RW, et al. Hemodynamic phenotype of the failing Fontan in an adult population. Am J Cardiol. 2013;112:1943–1947. doi: 10.1016/j.amjcard.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori M, Hebson C, Shioda K, et al. Catheter-measured hemodynamics of adult Fontan circulation: associations with adverse event and end-organ dysfunctions. Congenit Heart Dis. 2016;11:589–597. doi: 10.1111/chd.12345. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell MB, Campbell DN, Ivy D, et al. Evidence of pulmonary vascular disease after heart transplantation for Fontan circulation failure. J Thorac Cardiovasc Surg. 2004;128:693–702. doi: 10.1016/j.jtcvs.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Henaine R, Vergnat M, Bacha EA, et al. Effects of lack of pulsatility on pulmonary endothelial function in the Fontan circulation. J Thorac Cardiovasc Surg. 2013;146:522–529. doi: 10.1016/j.jtcvs.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 38.Ohuchi H, Ono S, Tanabe Y, et al. Long-term serial aerobic exercise capacity and hemodynamic properties in clinically and hemodynamically good, “excellent”, Fontan survivors. Circ J. 2012;76:195–203. doi: 10.1253/circj.cj-11-0540. [DOI] [PubMed] [Google Scholar]
- 39.Iwakiri Y, Tsai MH, McCabe TJ, et al. Phosphorylation of eNOS initiates excessive NO production in early phases of portal hypertension. Am J Physiol Heart Circ Physiol. 2002;282:H2084–H2090. doi: 10.1152/ajpheart.00675.2001. [DOI] [PubMed] [Google Scholar]
- 40.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–S131. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 41.Song D, Liu H, Sharkey KA, Lee SS. Hyperdynamic circulation in portal-hypertensive rats is dependent on central c-fos gene expression. Hepatology. 2002;35:159–166. doi: 10.1053/jhep.2002.30417. [DOI] [PubMed] [Google Scholar]
- 42.Waypa GB, Schumacker PT. Hypoxia-induced changes in pulmonary and systemic vascular resistance: where is the O2 sensor? Respir Physiol Neurobiol. 2010;174:201–211. doi: 10.1016/j.resp.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rychik J, Veldtman G, Rand E, et al. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium. Pediatr Cardiol. 2012;33:1001–1012. doi: 10.1007/s00246-012-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carins TA, Shi WY, Iyengar AJ, et al. Long-term outcomes after first-onset arrhythmia in Fontan physiology. J Thorac Cardiovasc Surg. 2016;152:1355–1363.e1. doi: 10.1016/j.jtcvs.2016.07.073. [DOI] [PubMed] [Google Scholar]
- 45.Pundi KN, Pundi KN, Johnson JN, et al. Sudden cardiac death and late arrhythmias after the Fontan operation. Congenit Heart Dis. 2017;12:17–23. doi: 10.1111/chd.12401. [DOI] [PubMed] [Google Scholar]
- 46.Li D, Fan Q, Hirata Y, Ono M, An Q. Arrhythmias after Fontan operation with intra-atrial lateral tunnel versus extra-cardiac conduit: a systematic review and meta-analysis. Pediatr Cardiol. 2017;38:873–880. doi: 10.1007/s00246-017-1595-8. [DOI] [PubMed] [Google Scholar]
- 47.Allen KY, Downing TE, Glatz AC, et al. Effect of Fontan-associated morbidities on survival with intact Fontan circulation. Am J Cardiol. 2017;119:1866–1871. doi: 10.1016/j.amjcard.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Nair AP, Timoh T, Fuster V. Contemporary medical management of systolic heart failure. Circ J. 2012;76:268–277. doi: 10.1253/circj.cj-11-1424. [DOI] [PubMed] [Google Scholar]
- 49.Wojnowich K, Korabathina R. Heart failure update: outpatient management. FP Essent. 2016;442:18–25. [PubMed] [Google Scholar]
- 50.Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370:1694–1701. doi: 10.1056/NEJMoa1401426. [DOI] [PubMed] [Google Scholar]
- 51.Enomoto Y, Aoki M, Nakamura Y, Hagino I, Fujiwara T, Nakajima H. Successful Fontan completion after cardiac resynchronization therapy. Circulation. 2012;125:e655–e658. doi: 10.1161/CIRCULATIONAHA.111.070979. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi D, Asagai S, Ishihara K, Nakanishi T. Successful Fontan conversion combined with cardiac resynchronization therapy for a case of failing Fontan circulation with ventricular dysfunction. Eur J Cardiothorac Surg. 2014;46:913–915. doi: 10.1093/ejcts/ezu084. [DOI] [PubMed] [Google Scholar]
- 53.Honjo O, Atlin CR, Mertens L, et al. Atrioventricular valve repair in patients with functional single-ventricle physiology: impact of ventricular and valve function and morphology on survival and reintervention. J Thorac Cardiovasc Surg. 2011;142:326–335.e2. doi: 10.1016/j.jtcvs.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 54.King G, Gentles TL, Winlaw DS, et al. Common atrioventricular valve failure during single ventricle palliation. Eur J Cardiothorac Surg. 2017;51:1037–1043. doi: 10.1093/ejcts/ezx025. [DOI] [PubMed] [Google Scholar]
- 55.Agnoletti G, Gala S, Ferroni F, et al. Endothelin inhibitors lower pulmonary vascular resistance and improve functional capacity in patients with Fontan circulation. J Thorac Cardiovasc Surg. 2017;153:1468–1475. doi: 10.1016/j.jtcvs.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 56.Tabarsi N, Guan M, Simmonds J, et al. Meta-analysis of the effectiveness of heart transplantation in patients with a failing Fontan. Am J Cardiol. 2017;119:1269–1274. doi: 10.1016/j.amjcard.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Matsuda H, Ichikawa H, Ueno T, Sawa Y. Heart transplantation for adults with congenital heart disease: current status and future prospects. Gen Thorac Cardiovasc Surg. 2017;65:309–320. doi: 10.1007/s11748-017-0777-x. [DOI] [PubMed] [Google Scholar]
- 58.de Mattos ÁZ, de Mattos AA, Méndez-Sánchez N. Hepatorenal syndrome: current concepts related to diagnosis and management. Ann Hepatol. 2016;15:474–481. [PubMed] [Google Scholar]
- 59.Ruiz-del-햞bol L Serradilla R. Cirrhotic cardiomyopathy. World J Gastroenterol. 2015;21:11502–11521. doi: 10.3748/wjg.v21.i41.11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uzun O, Wong JK, Bhole V, Stumper O. Resolution of protein-losing enteropathy and normalization of mesenteric Doppler flow with sildenafil after Fontan. Ann Thorac Surg. 2006;82:e39–e40. doi: 10.1016/j.athoracsur.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 61.Bhagirath KM, Tam JW. Resolution of protein-losing enteropathy with low-molecular weight heparin in an adult patient with Fontan palliation. Ann Thorac Surg. 2007;84:2110–2112. doi: 10.1016/j.athoracsur.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 62.Hoashi T, Ichikawa H, Ueno T, Kogaki S, Sawa Y. Steroid pulse therapy for protein-losing enteropathy after the Fontan operation. Congenit Heart Dis. 2009;4:284–287. doi: 10.1111/j.1747-0803.2009.00274.x. [DOI] [PubMed] [Google Scholar]
- 63.Straver B, Wagenaar LJ, Blom NA, et al. Percutaneous tricuspid valve implantation in a Fontan patient with congestive heart failure and protein-losing enteropathy. Circ Cardiovasc Interv. 2011;4:112–113. doi: 10.1161/CIRCINTERVENTIONS.110.958736. [DOI] [PubMed] [Google Scholar]
- 64.John AS, Driscoll DJ, Warnes CA, Phillips SD, Cetta F. The use of oral budesonide in adolescents and adults with protein-losing enteropathy after the Fontan operation. Ann Thorac Surg. 2011;92:1451–1456. doi: 10.1016/j.athoracsur.2011.03.103. [DOI] [PubMed] [Google Scholar]
- 65.Okano S, Sugimoto M, Takase M, Iseki K, Kajihama A, Azuma H. Effectiveness of high-dose spironolactone therapy in a patient with recurrent protein-losing enteropathy after the Fontan procedure. Intern Med. 2016;55:1611–1614. doi: 10.2169/internalmedicine.55.6303. [DOI] [PubMed] [Google Scholar]
- 66.António M, Gordo A, Pereira C, Pinto F, Fragata I, Fragata J. Thoracic duct decompression for protein-losing enteropathy in failing Fontan circulation. Ann Thorac Surg. 2016;101:2370–2373. doi: 10.1016/j.athoracsur.2015.08.079. [DOI] [PubMed] [Google Scholar]
- 67.Friedland-Little JM, Gajarski RJ, Schumacher KR. Dopamine as a potential rescue therapy for refractory protein-losing enteropathy in Fontan-palliated patients. Pediatr Transplant. 2017;21:e12925. doi: 10.1111/petr.12925. [DOI] [PubMed] [Google Scholar]
- 68.Wakeham MK, Van Bergen AH, Torero LE, Akhter J. Long-term treatment of plastic bronchitis with aerosolized tissue plasminogen activator in a Fontan patient. Pediatr Crit Care Med. 2005;6:76–78. doi: 10.1097/01.PCC.0000149320.06424.1D. [DOI] [PubMed] [Google Scholar]
- 69.Apostolopoulou SC, Papagiannis J, Rammos S. Bosentan induces clinical, exercise and hemodynamic improvement in a pre-transplant patient with plastic bronchitis after Fontan operation. J Heart Lung Transplant. 2005;24:1174–1176. doi: 10.1016/j.healun.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 70.Heath L, Ling S, Racz J, et al. Prospective, longitudinal study of plastic bronchitis cast pathology and responsiveness to tissue plasminogen activator. Pediatr Cardiol. 2011;32:1182–1189. doi: 10.1007/s00246-011-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dori Y, Keller MS, Rome JJ, et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation. 2016;133:1160–1170. doi: 10.1161/CIRCULATIONAHA.115.019710. [DOI] [PubMed] [Google Scholar]
- 72.Opocher F, Varnier M, Sanders SP, et al. Effects of aerobic exercise training in children after the Fontan operation. Am J Cardiol. 2005;95:150–152. doi: 10.1016/j.amjcard.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 73.Brassard P, Poirier P, Martin J, et al. Impact of exercise training on muscle function and ergoreflex in Fontan patients: a pilot study. Int J Cardiol. 2006;107:85–94. doi: 10.1016/j.ijcard.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 74.Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29:1681–1687. doi: 10.1093/eurheartj/ehn215. [DOI] [PubMed] [Google Scholar]
- 75.Goldberg DJ, French B, McBride MG, et al. Impact of oral sildenafil on exercise performance in children and young adults after the Fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation. 2011;123:1185–1193. doi: 10.1161/CIRCULATIONAHA.110.981746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schuuring MJ, Vis JC, van Dijk AP, et al. Impact of bosentan on exercise capacity in adults after the Fontan procedure: a randomized controlled trial. Eur J Heart Fail. 2013;15:690–698. doi: 10.1093/eurjhf/hft017. [DOI] [PubMed] [Google Scholar]
- 77.Cordina RL, O'Meagher S, Karmali A, et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol. 2013;168:780–788. doi: 10.1016/j.ijcard.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 78.Rhodes J, Ubeda-Tikkanen A, Clair M, et al. Effect of inhaled iloprost on the exercise function of Fontan patients: a demonstration of concept. Int J Cardiol. 2013;168:2435–2440. doi: 10.1016/j.ijcard.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van De Bruaene A, La Gerche A, Claessen G, et al. Sildenafil improves exercise hemodynamics in Fontan patients. Circ Cardiovasc Imaging. 2014;7:265–273. doi: 10.1161/CIRCIMAGING.113.001243. [DOI] [PubMed] [Google Scholar]
- 80.Hebert A, Mikkelsen UR, Thilen U, et al. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (treatment with endothelin receptor antagonist in Fontan patients, a randomized, placebo-controlled, double-blind study measuring peak oxygen consumption) study. Circulation. 2014;130:2021–2030. doi: 10.1161/CIRCULATIONAHA.113.008441. [DOI] [PubMed] [Google Scholar]