Abstract
Background and Objectives
The aging population is rapidly increasing, and atrial fibrillation (AF) is becoming a significant public health burden in Asia, including Korea. This study evaluated current treatment patterns and guideline adherence of AF treatment.
Methods
In a prospective observational registry (COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation [CODE-AF] registry), 6,275 patients with nonvalvular AF were consecutively enrolled between June 2016 and April 2017 from 10 tertiary hospitals in Korea.
Results
The AF type was paroxysmal, persistent, and permanent in 65.3%, 30.0%, and 2.9% of patients, respectively. Underlying structural heart disease was present in 11.9%. Mean CHA2DS2-VASc was 2.7±1.7. Oral anticoagulation (OAC), rate control, and rhythm control were used in 70.1%, 53.9%, and 54.4% of patients, respectively. OAC was performed in 82.7% of patients with a high stroke risk. However, antithrombotic therapy was inadequately used in 53.4% of patients with a low stroke risk. For rate control in 192 patients with low ejection fraction (<40%), β-blocker (65.6%), digoxin (5.2%), or both (19.3%) were adequately used in 90.1% of patients; however, a calcium channel blocker was inadequately used in 9.9%. A rhythm control strategy was chosen in 54.4% of patients. The prescribing rate of class Ic antiarrythmics, dronedarone, and sotalol was 16.9% of patients with low ejection fraction.
Conclusion
This study shows how successfully guidelines can be applied in the real world. The nonadherence rate was 17.2%, 9.9%, and 22.4% for stroke prevention, rate control, and rhythm control, respectively.
Keywords: Atrial fibrillation, Anticoagulant agent, Guidelines adherence, Registry
INTRODUCTION
Atrial fibrillation (AF) is the most common, sustained cardiac arrhythmia, occurring in 1–2% of the general population,1) and is associated with increased morbidity and mortality.2),3) Because AF increases with advancing age, it is becoming a significant public health burden in Asia, including Korea, as the aging population rapidly increases. There are 3 therapeutic approaches in the treatment of AF: stroke prevention, rate control, and rhythm control.
AF is associated with a 5-fold increase in stroke risk, and 1 in 5 cases of stroke is attributed to this arrhythmia.4) Oral anticoagulation (OAC) treatment can prevent ischemic stroke in most patients with AF and prolong their lives.5) It is superior to no treatment or aspirin in patients with different stroke risk profiles.6) The net clinical benefit is almost universal, with the exception of patients with a very low stroke risk; therefore, OAC should be used in most patients with AF. Despite this evidence, underuse or premature termination of OAC therapy is still common. The effort required to monitor and adjust the dose of vitamin K antagonist (VKA) therapy is one of the most common reasons for withholding or ending OAC.7) However, the considerable stroke risk without OAC often exceeds the bleeding risk with OAC, even in the elderly, patients with cognitive dysfunction, and patients with frequent falls or frailty.8) Recently, non-VKA OAC (NOAC) was approved in Korea for stroke prevention in patients with AF. However, little contemporary data is currently available on temporal trends in anticoagulation therapy in Korea.
Rate control is an integral part of the AF management and is often sufficient to improve AF-related symptoms. Compared with stroke prevention and rhythm control, very little robust evidence exists to identify the best type and intensity of rate control treatment, with most data being derived from short-term crossover trials and observational studies.9),10) Pharmacological rate control can be achieved for acute or long-term rate control with β-blockers, digoxin, calcium channel blockers (CCBs), or combination therapy. Restoring and maintaining sinus rhythm is also an integral part of AF management. Antiarrhythmic drugs (AADs) approximately double the rate of sinus rhythm compared with placebo.11) Catheter ablation or combination therapy is often effective when AADs fail.12),13) Currently, rhythm control therapy is indicated to improve symptoms in patients with AF who remain symptomatic despite adequate rate control.
Recent advances in the management of AF include treatment strategies (rate vs. rhythm control), stroke prevention scales (validation of a new stroke risk index, the CHA2DS2-VASc score — congestive heart failure [HF]/left ventricular [LV] dysfunction, hypertension, age ≥75 years [doubled], diabetes, stroke [doubled], vascular disease, age 65–74 years, and sex category [female]), and publication of new guidelines for AF management.14),15) We hypothesized that adherence to anticoagulation guidelines will improve after the introduction of NOACs for the treatment of Korean AF patients. We also hypothesized that the usage of rate control medication will decrease after the introduction of loose rate control guidelines. The objectives of this study were to examine the patterns of treatment and the rates of adherence to guidelines for AF treatment, including antithrombotic, rate control, and rhythm control therapies in Korea, and to identify predictive factors for appropriate treatment.
METHODS
Study design and centers
The COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) is a prospective, multicenter, observational study performed in patients >18 years with AF at any of 10 tertiary centers, encompassing all geographical regions of Korea. The study enrollment period started in June 2016 and will end in October 2018. The primary objective of the CODE-AF registry is to generate a prospective, multicenter AF registry to evaluate the outcomes of medical treatment such as anticoagulation, rate control, and rhythm control treatments. The secondary object of the study was to describe the clinical epidemiology of patients with AF and to determine the diagnostic and therapeutic processes (including the organization of programs for AF management) applied in these patients and the clinical outcomes. The registry was designed and coordinated by the Korea Heart Rhythm Society, which provides support to related committees, national coordinators, and participating centers. Data are entered in a common electronic database that limits inconsistencies and errors and provides online help for key variables. Each center has access to its own data and data from all other participating centers. The number of patients enrolled from each center is shown in Supplementary Figure 1. The study was approved by the ethics committee of each center, and all patients provided informed consent for inclusion. This study was registered at ClinicalTrials.gov (NCT02786095).
Patients
This study included 6,275 consecutive patients enrolled from June 2016 to April 2017. Each center enrolled patients who were >18 years with nonvalvular AF, attended the outpatient clinic, and were hospitalized on the same day for AF. Data collection was performed according to the same criteria and was usually carried out by personnel with no clinical activity assigned to the project. The CHA2DS2-VASc and hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio (INR), elderly, concomitant drugs/alcohol (HAS-BLED) scores were calculated for all patients with nonvalvular AF.
A follow-up visit was scheduled every 6 months, either through personal interview or telephone contact (data not presented).
Definition of guideline adherence
In this population, the most robust recommendations are the 2016 European Society of Cardiology and 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines for patients with AF.14),15) Both guidelines recommend estimating the stroke risk in patients with AF based on the CHA2DS2-VASc score.14),15) In general, patients without clinical stroke risk factors or low risk (CHA2DS2-VASc 0 or 1 [female]) do not need antithrombotic therapy, whereas patients with high stroke risk (i.e., CHA2DS2-VASc ≥2) should be treated with OAC.14),15) However, because the recommendations of the 2 guidelines were inconsistent in patients with intermediate stroke risk or a CHA2DS2-VASc score of 1 (male), guideline adherence was not evaluated in these patients.16) Patients with hypertrophic cardiomyopathy (HCMP) were excluded from this study.
Beta-blockers and/or digoxin are recommended for rate control in patients with AF with left ventricular ejection fraction (LVEF) <40%. Beta-blockers, digoxin, diltiazem, or verapamil are recommended for rate control in patients with AF with LVEF ≥40%.14),16)
For rhythm control of AF, dronedarone, flecainide, propafenone, or sotalol are recommended for preventing recurrent symptomatic AF in patients with normal LV function and without pathological left ventricular hypertrophy (LVH). Dronedarone, sotalol, or amiodarone are recommended for preventing recurrent symptomatic AF in patients with stable coronary artery disease (CAD) without HF. Amiodarone is recommended for preventing recurrent symptomatic AF in patients with HF. Catheter ablation can be used in all patients.14),16)
Finally, in patients with CHA2DS2-VASc score ≤1, anticoagulation can be used due to cardioversion, radiofrequency ablation (RFCA), and underlying HCMP. Second, for rhythm control in patients with no structural heart disease, amiodarone is recommended as the second line agent. However, in the above situations, we could not tell which patients were non-adherent to the guideline.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation, and categorical variables are reported as frequency (percentage). Statistical analyses were performed with SPSS 21.0 statistical software (SPSS Inc., Chicago, IL, USA). All p values were 2-tailed, and values <0.050 were considered statistically significant.
RESULTS
Baseline characteristics
The patient characteristics are summarized in Table 1. The mean patient age was 67.4±10.8 years (median, 66.0 years; range, 10–100 years). Male patients were more predominant (n=3,966, 63.2%). The type of AF was paroxysmal, persistent, and permanent in 65.3%, 30.0%, and 2.9% of patients, respectively. Underlying structural heart disease presented in 11.9%, including CAD (2.2%) and HF (10.1%). The mean CHA2DS2-VASc and HAS-BLED scores were 2.7±1.7 and 1.9±1.1, respectively.
Table 1. Baseline characteristics of patients.
| Characteristics | Value | |
|---|---|---|
| Total | 6,275 | |
| Men (No.) | 3,966 (63.2) | |
| Age (years) | 67.4±10.8 | |
| BMI (kg/m2) | 24.6±3.3 | |
| Type of AF | ||
| Paroxysmal | 4,100 (65.3) | |
| Persistent | 1,883 (30) | |
| Permanent | 182 (2.9) | |
| Unknown | 110 (1.7) | |
| CHA2DS2-VASc score | 2.7±1.7 | |
| HAS-BLED score | 1.9±1.1 | |
| Valve disease | 643 (10.3) | |
| HF | 632 (10.1) | |
| Hypertension | 4,240 (67.6) | |
| Diabetes mellitus | 1,597 (25.5) | |
| History of stroke/TIA | 952 (15.2) | |
| History of MI/PAD | 533 (8.5) | |
| Cancer | 630 (10) | |
| CKD | 621 (9.9) | |
| ESRD | 101 (1.6) | |
| History of bleeding | 582 (9.3) | |
| Treatment | ||
| NOAC or warfarin | 4,399 (70.1) | |
| Rate control | 3,383 (53.9) | |
| Rhythm control | 3,414 (54.4) | |
Data are presented as mean±standard deviation or number (percentage).
AF = atrial fibrillation; BMI = body mass index; CKD = chronic kidney disease; ESRD = end-stage renal disease; HAS-BLED = hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, concomitant drugs/alcohol; HF = heart failure; MI = myocardial infarction; NOAC = non-vitamin K antagonist oral anticoagulation; PAD = peripheral artery disease; TIA = transient ischemic attack.
Transechocardiography data were obtained in 5,253 (82.4%) and 873 (13.9%) patients (Table 2). The mean LV ejection was 61.1±11.8, and the mean left atrial diameter was 43.8±10.4 mm.
Table 2. Baseline echocardiographic data.
| Characteristics | Value | ||
|---|---|---|---|
| Total | 6,275 | ||
| Transthoracic echocardiography | 5,253 (83.7) | ||
| LVEF (%) | 61.1±11.8 | ||
| <40% | 201 (3.8) | ||
| ≥40% | 5,031 (96.2) | ||
| LA diameter (mm) | 43.8±10.4 | ||
| LA volume index (mL/m2) | 47.5±25.9 | ||
| 29–33 | 510 (9.7) | ||
| ≥34 | 4,225 (80.4) | ||
| E/E' | 11.8±5.5 | ||
| Transesophageal echocardiography | 873 (13.9) | ||
| LV thrombi | 13 (1.5) | ||
Data are presented as mean±standard deviation or number (percentage).
LA = left atrium; LV = left ventricular; LVEF = left ventricular ejection fraction.
Pattern of and adherence to anticoagulation therapy
OAC was performed in 4,399 (70.1%) patients, with NOACs and warfarin in used 3,081 (49.1%) and 1,318 (21.0%) patients, respectively.
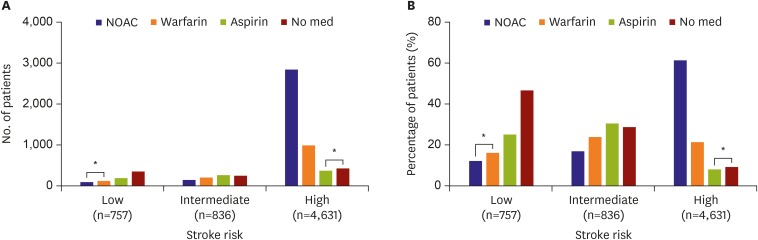
Figure 1 shows the pattern of stroke prevention in AF according to stroke risk. No antithrombotic medication was prescribed in 46.6% of 757 patients with a low stroke risk. However, OAC and aspirin were inadequately used in 28.3% and 25.1% of patients, respectively. In 863 patients with intermediate risk, OAC was prescribed in 40.8% of patients, and aspirin was prescribed in 30.5%. No antithrombotic medication was used in 28.7% of patients. In 4,631 patients with a high stroke risk (CHA2DS2-VASc score ≥2), anticoagulation was used in 3,832 (82.7%). NOACs and warfarin were used in 61.4% and 21.3% of patients, respectively, and were used simultaneously with aspirin in 3.1% of patients. However, only aspirin or no medication was used in 8.0% and 9.2% of patients, respectively. Overall, 1,203 patients (19.2%), including 799 (12.7%) undertreated patients with high stroke risk and 404 (6.4%) overtreated patients with low stroke risk, received treatment that did not adhere to the guidelines. Among 1,011 (76.7%) out of 1,318 patients taking warfarin, the INR was maintained in the optimal range between 2 and 3 in 33% of patients. However, the INR was less than 2 in 54.8% of patents.
Figure 1.
Anticoagulation and adherence to anticoagulation guidelines according to stroke risk: (A) number of patients and (B) percentage of patients.
med = medicine; NOAC = non-vitamin K antagonist oral anticoagulation.
*Patients who were non-adherent to the guidelines are marked.
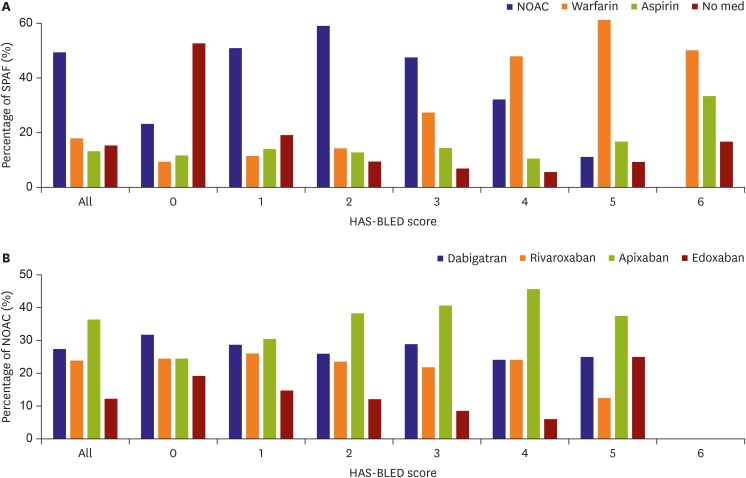
Figure 2 shows the pattern of stroke prevention in AF and the percentage of NOAC use according to HAS-BLED score. Whereas warfarin was more frequently used than NOACs in patients with HAS-BLED score ≥4, the opposite was true in patients with HAS-BLED score ≤3. Apixaban was used in 41.5% of patients with HAS-BLED score ≥3. Dose reduction was performed in accordance to the manufacturer's recommendation in 2,064 (70.0%) patients.
Figure 2.
SPAF and NOAC according to HAS-BLED score: (A) percentage of SPAF according to HAS-BLED score and (B) percentage of NOAC.
HAS-BLED = hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, concomitant drugs/alcohol; NOAC = non-vitamin K antagonist oral anticoagulation; SPAF = stroke prevention for atrial fibrillation.
Pattern of and adherence to rate control therapy
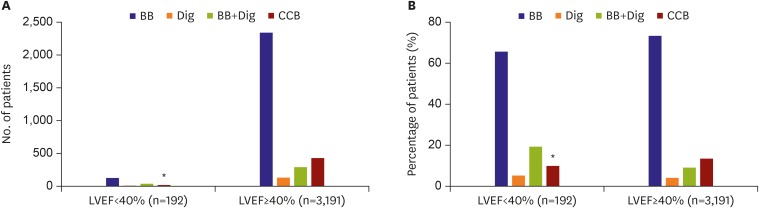
Rate control was performed in 3,383 (53.9%) patients. Rate control medication was prescribed in 192 of 244 (85.7%) patients with LVEF <40% and in 3,191 of 3,383 (94.3%) patients with LVEF ≥40%.
Figure 3 shows the pattern of and adherence to rate control therapy. In patients with LVEF <40%, β-blockers, digoxin, and both were adequately used in 65.6%, 5.2%, and 19.3% of patients, respectively. However, a CCB was inadequately used in 9.9% of patients. The mean heart rate was 79.9±18.1 beats per minutes (bpm).
Figure 3.
Rate control therapy according to LVEF: (A) number of patients and (B) percentage of patients.
BB = β-blocker; CCB = calcium channel blocker; Dig = digoxin; LVEF = left ventricular ejection fraction.
*Patients who were non-adherent to the guidelines are marked.
In patients with LVEF ≥40%, β-blocker, CCB, digoxin, and combination therapy were used in 73.4%, 13.4%, 4.1%, and 9.1% patients, respectively. The mean heart rate was 75.8±16.5 bpm in patients with LVEF ≥40%.
Rhythm control
A rhythm control strategy was chosen in 3,414 (54.4%) of patients, including AADs and catheter ablation in 51.8% and 16.2%, respectively.
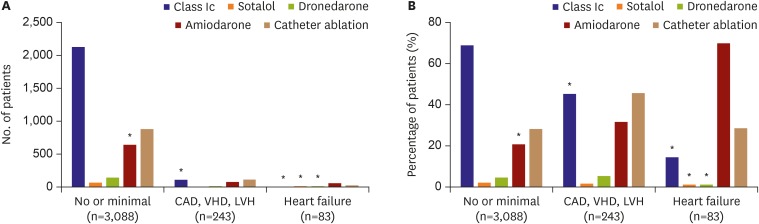
Figure 4 shows the pattern of and adherence to rhythm control therapy. Amiodarone was used in 20.4% of 3,088 patients with no or minimal signs of structural heart disease. Class Ic drugs were inadequately prescribed in 41.5% of 243 patients with CAD, valvular heart disease (VHD), or LVH. In 83 patients with HF on rhythm control medication, amiodarone and catheter ablation were adequately used in 69.9% and 28.9%, respectively. However, 16.9% were prescribed inadequate AADs, including 14.5% who took a class Ic drug, 1.2% who took dronedarone, and 1.2% who took sotalol.
Figure 4.
Rhythm control therapy: (A) number of patients and (B) percentage of patients.
CAD = coronary artery disease; LVH = left ventricular hypertrophy; VHD = valvular heart disease.
*Patients who were non-adherent to the guidelines are marked.
Finally, among 3,414 patients with rhythm control medication, nonadherence to guidelines was observed in 765 (12.2%), including 641 (18.8%) patients with no or minimal signs of structural heart disease, 110 with CAD (VHD or LVH), and 14 with HF.
DISCUSSION
This study shows how successfully guidelines can be applied in the real world. Stroke prevention, rate control, and rhythm control were performed in 70.1%, 53.9%, and 54.4% of patients, respectively. Anticoagulation was used adequately in 82.7% and 71.5% of patients with high and low stroke risk. However, stroke prevention for AF was inadequately performed in 19.2%. For rate control in patients with LVEF <40%, although β-blocker or digoxin was adequately used in 90.1%, a CCB was inadequately used in 9.9%. Finally, rhythm control was inadequately performed in 22.4% of patients.
Asian AF patients are included in several global AF registries or randomized NOAC trials. Among 1,063 patients enrolled in the Phase I Global Registry on Long-term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) registry, 713 (67.1%) Chinese AF patients were included (not Korean).17) Among 15,641 patients enrolled in the Phase II GLORIA-AF registry, 3,071 (20.3%) Asian AF patients from China, Hong Kong, Russia, Singapore, South Korea, and Taiwan were included.18) In all 4 major NOAC trials, the proportion of Asian patients is around 10–15%.19),20),21),22) Therefore, Asian AF data is still insufficient. Even in Asia, the characteristics of stroke differ between countries.4),23) Moreover, because several types of NOACs are not allowed in Taiwan or Australia, the usage of NOACs cannot be universally applied for Korean AF patients. The most unique and novel feature of this study is the inclusion of the largest, most recent Korean AF registry, which can identify the clinical and treatment characteristics of Korean AF patients.
VKAs have been the mainstay of OAC treatment for several decades and reduce the relative risk of stroke in patients with AF by approximately 64%.24) However, compared with warfarin, NOAC agents have all demonstrated at least similar efficacy and safety in stroke prevention.19),20),21),22) With respect to stroke prevention, warfarin underuse is well recognized in patients with AF.25) The underuse of antithrombotic therapy and inadequate anticoagulation can lead not only to ischemic stroke, but also to death or severe disability.26) Using a sample cohort from the Korean National Health Insurance Data Sample Cohort (K-NHID-Sample Cohort) from 2004 through 2013, we reported that, although the mean CHA2DS2-VASc score increased from 3.5 to 4.4 in the AF population, the proportion of patients who received anticoagulation therapy with warfarin was 18.2–16.7%, showing no significant change for the decade.1) In this study, OAC was adequately used in 82.7% of patients with a high stroke risk (CHA2DS2-VASc score ≥2). This result is consistent with the result of the GLORIA-AF registry phase 2,18) which reported an OAC rate of 83.2% in the same risk group. However, the usage rate of NOACs was higher in this study (61.4%) than in the GLORIA-AF registry phase 2 (49.9%).
The high frequency of inadequate antithrombotic therapy in 54.6% patients with a low stroke risk might be explained by cardioversion and catheter ablation. However, the relatively high inadequate use of aspirin of 25.1% in patients with a low stroke risk should be corrected.
Other studies reported a risk-treatment paradox in which OAC decreased with increasing stroke risk.27) However, this study shows that the OAC rate was consistently maintained even in patients with a high bleeding risk. Interestingly, warfarin was more frequently used than NOACs in patients with a high HAS-BLED score ≥4. Dabigatran was the most common NOAC used in the GLORIA-AF registry phase 218) in all regions including Asia. However, in this study, apixaban was most commonly used.
The optimal heart rate target in patients with AF is unclear. A lenient heart rate target of 110 bpm is an acceptable initial approach regardless of HF status, unless symptoms call for stricter rate control. There was no difference in the composite of clinical events (14.9% in the strict rate control group, 12.9% in the lenient group),28) New York Heart Association class, or hospitalizations.28) Consistently, according to the K-NHID-Sample Cohort, the proportion of patients who received rate control therapy rate decreased from 53.7% to 46.1% in 2004 to 2013.1) In this study, rate control was performed in 53.9% of patients. The proportion of rate control therapy is less than that in a 2007 study on pharmacotherapy in Medicare beneficiaries with AF, which showed that most enrollees were on rate-control agents (74.0%).29)
Rate control with β-blockers or nondihydropyridine CCBs is a class I guideline recommendation for the management of patients with AF.14) Among patients receiving a rate control agent, 65.6% with LVEF <40% and 73.4% with LVEF ≥40% were taking a β-blocker. The proportion of CCB use was relatively low (13.4% patients with LVEF ≥40%).
In this study, 54.4% of enrollees were receiving AAD therapy, consistent with large AF registries, which often exceed 50%.30) Among the AADs, class Ic drugs were the most commonly prescribed. Because amiodarone is often considered a last-resort drug for AF, the preponderance of amiodarone use might be related to age and lack of response to other agents. Physicians might also prescribe amiodarone earlier in older patients. In this study, amiodarone use was greater among patients with AF and HF, consistent with guidelines.14)
Our study has several limitations. First, our analysis of medication use focused on prescriptions filled in the first few months of the calendar year (the first 4 months), and we were unable to ascertain longitudinal adherence. Second, since all patients were enrolled from tertiary centers, the current registry is not free from referral bias.
Medication use for AF varies according to underlying risks and comorbid diseases. In the recently developed Korean AF registry, the nonadherence rate was 17.2%, 9.9%, and 22.4% for stroke prevention, rate control, and rhythm control, respectively. This study shows how successfully guidelines can be applied in the real world in Korean AF patients.
Footnotes
Funding: This study was supported by a grant from the Korean Healthcare Technology R & D project funded by the Ministry of Health and Welfare (HI15C1200).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Joung B.
- Data curation: Kim H, Park H.
- Formal analysis: Kim T.
- Funding acquisition: Joung B.
- Investigation: Park JK.
- Methodology: Kang K.
- Project administration: Shim J, Choi E, Kim J, Kim C.
- Resources: Uhm J.
- Software: Park JB.
- Supervision: Lee Y.
- Validation: Cha M.
- Visualization: Lee J.
- Writing - original draft: Kim T.
- Writing - review & editing: Kim J.
Supplementary Material
The number of patients enrolled from each center.
References
- 1.Lee H, Kim TH, Baek YS, et al. The trends of atrial fibrillation-related hospital visit and cost, treatment pattern and mortality in Korea: 10-year nationwide sample cohort data. Korean Circ J. 2017;47:56–64. doi: 10.4070/kcj.2016.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J, Park CH, Uhm JS, Pak HN, Lee MH, Joung B. A thin left atrial antral wall around the pulmonary vein reflects structural remodeling by atrial fibrillation and is associated with stroke. Yonsei Med J. 2017;58:282–289. doi: 10.3349/ymj.2017.58.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TH, Yang PS, Uhm JS, et al. Cha2ds2-vasc score (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female) for stroke in Asian patients with atrial fibrillation: a Korean nationwide sample cohort study. Stroke. 2017;48:1524–1530. doi: 10.1161/STROKEAHA.117.016926. [DOI] [PubMed] [Google Scholar]
- 5.Lip GY, Al-Khatib SM, Cosio FG, et al. Contemporary management of atrial fibrillation: what can clinical registries tell us about stroke prevention and current therapeutic approaches? J Am Heart Assoc. 2014;3:e001179. doi: 10.1161/JAHA.114.001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hart RG, Pearce LA, Aguilar MI. Adjusted-dose warfarin versus aspirin for preventing stroke in patients with atrial fibrillation. Ann Intern Med. 2007;147:590–592. doi: 10.7326/0003-4819-147-8-200710160-00018. [DOI] [PubMed] [Google Scholar]
- 7.Gorst-Rasmussen A, Skjøth F, Larsen TB, Rasmussen LH, Lip GY, Lane DA. Dabigatran adherence in atrial fibrillation patients during the first year after diagnosis: a nationwide cohort study. J Thromb Haemost. 2015;13:495–504. doi: 10.1111/jth.12845. [DOI] [PubMed] [Google Scholar]
- 8.Donzé J, Clair C, Hug B, et al. Risk of falls and major bleeds in patients on oral anticoagulation therapy. Am J Med. 2012;125:773–778. doi: 10.1016/j.amjmed.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Al-Khatib SM, Allen LaPointe NM, Chatterjee R, et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160:760–773. doi: 10.7326/M13-1467. [DOI] [PubMed] [Google Scholar]
- 10.Nikolaidou T, Channer KS. Chronic atrial fibrillation: a systematic review of medical heart rate control management. Postgrad Med J. 2009;85:303–312. doi: 10.1136/pgmj.2008.068908. [DOI] [PubMed] [Google Scholar]
- 11.Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Bergmann JF. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2007:CD005049. doi: 10.1002/14651858.CD005049.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Anselmino M, Matta M, D'Ascenzo F, et al. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014;7:1011–1018. doi: 10.1161/CIRCEP.114.001938. [DOI] [PubMed] [Google Scholar]
- 13.Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 15.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 16.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College Of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Huisman MV, Ma CS, Diener HC, et al. Antithrombotic therapy use in patients with atrial fibrillation before the era of non-vitamin K antagonist oral anticoagulants: the Global Registry on Long-term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) Phase I cohort. Europace. 2016;18:1308–1318. doi: 10.1093/europace/euw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huisman MV, Rothman KJ, Paquette M, et al. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF Registry Phase 2. J Am Coll Cardiol. 2017;69:777–785. doi: 10.1016/j.jacc.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 19.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 20.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 21.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 22.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 23.Chao TF, Wang KL, Liu CJ, et al. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol. 2015;66:1339–1347. doi: 10.1016/j.jacc.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 25.Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in medicare beneficiaries with atrial fibrillation. Stroke. 2006;37:1070–1074. doi: 10.1161/01.STR.0000208294.46968.a4. [DOI] [PubMed] [Google Scholar]
- 26.Indredavik B, Rohweder G, Lydersen S. Frequency and effect of optimal anticoagulation before onset of ischaemic stroke in patients with known atrial fibrillation. J Intern Med. 2005;258:133–144. doi: 10.1111/j.1365-2796.2005.01512.x. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: the risk--treatment paradox in patients with newly diagnosed non-valvular atrial fibrillation. Heart. 2011;97:2046–2050. doi: 10.1136/heartjnl-2011-300901. [DOI] [PubMed] [Google Scholar]
- 28.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 29.Piccini JP, Mi X, DeWald TA, Go AS, Hernandez AF, Curtis LH. Pharmacotherapy in Medicare beneficiaries with atrial fibrillation. Heart Rhythm. 2012;9:1403–1408. doi: 10.1016/j.hrthm.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Heuzey, JY, Breithardt G, Camm J, et al. The RecordAF study: design, baseline data, and profile of patients according to chosen treatment strategy for atrial fibrillation. Am J Cardiol. 2010;105:687–693. doi: 10.1016/j.amjcard.2009.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number of patients enrolled from each center.