Abstract
Background and Objectives
Information about the role of the stromal cell-derived factor-1α (SDF-1α)/chemokine receptor type 4 (CXCR4) axis in ischemic postconditioning (IPOC) is currently limited. We hypothesized that the SDF-1α/CXCR4 signaling pathway is directly involved in the cardioprotective effect of IPOC.
Methods
Isolated rat hearts were divided into four groups. The control group was subjected to 30-min of regional ischemia and 2-hour of reperfusion (n=12). The IPOC group was induced with 6 cycles of 10-second reperfusion and 10-second global ischemia (n=8) in each cycle. The CXCR4 antagonist, AMD3100, was applied before reperfusion in the IPOC group (AMD+IPOC group, n=11) and control group (AMD group, n=9). Hemodynamic changes with electrocardiography were monitored and infarct size was measured. The SDF-1α, lactate dehydrogenase (LDH) and creatine kinase (CK) concentrations in perfusate were measured. We also analyzed extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt phosphorylation state expression.
Results
IPOC significantly reduced infarct size, but AMD3100 attenuated the infarct reducing effect of IPOC. IPOC significantly decreased LDH and CK, but these effects were reversed by AMD3100. ERK1/2 and Akt phosphorylation increased with IPOC and these effects were blocked by AMD3100.
Conclusion
Based on the results of this study, SDF-1α/CXCR4 signaling may be involved in IPOC cardioprotection and this signaling pathway couples to the ERK1/2 and Akt pathways.
Keywords: Reperfusion injury, Ischemic postconditioning, Stromal cell-derived factor-1α, CXCR4 receptors
INTRODUCTION
Ischemic postconditioning (IPOC) may offer beneficial cardioprotection against myocardial ischemia/reperfusion (I/R) injury through repetitive episodes of I/R at the beginning of reperfusion after a prolonged period of ischemia, by intrinsic prosurvival signaling cascade activation.1),2),3)
Over the past two decades, there has been increasing interest in IPOC because of its comparable cardioprotective effect to ischemic preconditioning, which is achieved by brief periods of I/R injury before a prolonged period of ischemia. This approach may provide greater therapeutic potential since it could be performed at the time of reperfusion therapy, for instance percutaneous coronary intervention for acute myocardial infarction, or at the time of cardiac surgery, for example in coronary artery bypass surgery and heart transplantation. Underlying mechanisms for IPOC have been proposed, including, endothelial nitric oxide, free radicals, survival kinases, opioids, and ATP-sensitive potassium channels.4),5),6)
In comparison, stromal cell-derived factor-1α (SDF-1α or CXCL12) is a chemotactic cytokine that promotes angiogenesis as well as recruiting stem and progenitors cells to the site of injured organs through cognate chemokine receptor type 4 (CXCR4).7),8) Furthermore, recent studies have indicated that activation of the SDF-1α/CXCR4 axis has a direct role in cell protection and survival, not only against hypoxia/reoxygenation damage in vitro but also myocardial I/R injury in vivo.9) In addition, the SDF-1α/CXCR4 axis is linked to a series of downstream signaling pathways through activation of prosurvival kinase against I/R injury.4),10)
However, even though the SDF-1α/CXCR4 axis has been studied extensively, information about its role in IPOC is limited. In this study, we hypothesized that the SDF-1α/CXCR4 signaling pathway is directly involved in the cardioprotective effect of IPOC, and this study was designed to address this issue. We used a highly selective CXCR4 antagonist (AMD3100) in an IPOC-induced isolated rat heart model to remove any potential confounding effects derived from systemic effects on the infarcted heart.
METHODS
The experimental procedures and protocols used in this study were reviewed and approved by our Institutional Animal Care and Use Committee (PNU-2011-000292).
Chemicals
AMD3100, the highly selective CXCR4 antagonist, 1,1′-[1,4-phenylenebis-(methylene)]-bis-(1,4,8,11-tetraazacyclotetradecane) octahydrochloride was purchased from Tocris Bioscience (Ellisville, MO, USA). 2,3,5-triphenyltetrazolium chloride (TTC) was obtained from Sigma-Aldrich Chemical (St. Louis, MO, USA). Fluorescent polymer microspheres were purchased from Duke Scientific (Palo Alto, CA, USA). Antibodies against extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). Other chemicals were obtained from Sigma-Aldrich Chemical.
Langendorff-perfused heart isolation
Male Sprague-Dawley rats that weighed 280–330 g were obtained from KOATECH Co., Cheongju, Korea. They received 50 mg/kg of pentobarbital sodium (Entobar®, Yongin, Korea) and 300 IU of heparin intraperitoneally. A median thoracotomy was performed after anesthesia and hearts were isolated and immediately connected to the Langendorff system via the aorta. Hearts were then perfused with modified Krebs-Henseleit buffer solution that contained (in mM) 118.5 NaCl, 4.7 KCl, 1.2 MgSO4, 1.8 CaCl2, 24.8 NaHCO3, 1.2 KH2PO4, and 10 glucose, as described previously.5) The proximal portion of the left coronary artery (LCA) was first localized between the left atrial appendage and the right ventricular outflow tract to induce regional ischemia. A 6–0 polypropylene suture was then passed around the major trunk or prominent branches of the LCA. The ends of the thread were passed through a small piece of PE50 tube to form a snare. All hearts were allowed to stabilize for at least 30 minutes. Ischemia was induced by pulling the snare then fixing it by clamping the tube with a small hemostat and confirmed by regional cyanosis, a substantial decrease in left ventricular developed pressure (LVDP), or a fall in coronary flow (CF). Reperfusion was initiated by releasing the snare. Hearts that experienced ventricular fibrillation (VF) after reperfusion typically spontaneously reverted to sinus rhythm. VF that lasted more than 45 seconds was treated with finger flick cardioversion until a perfusing rhythm was obtained. No pharmacological agents were used for defibrillation.
Experimental protocol
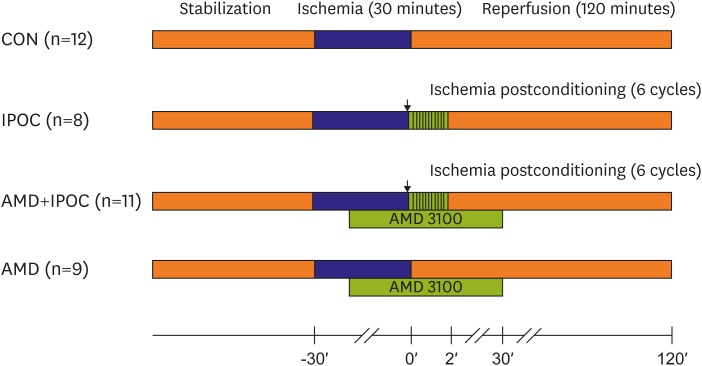
Hearts were randomly divided into 4 groups to investigate the involvement of the SDF-1α/CXCR4 axis in the IPOC-induced infarct limitation effect: 1) CON group (n=12), untreated control hearts subjected to 30 minutes of regional ischemia and 120 minutes of reperfusion; 2) IPOC group (n=8), 6 cycles of 10 seconds of reperfusion followed by 10 seconds of global ischemia immediately after the index ischemia; 3) AMD+IPOC group (n=11), AMD3100 pretreatment in the IPOC group; and 4) AMD group (n=9), AMD3100 in control hearts (Figure 1).5) In the AMD+IPOC and AMD groups, 1 µM of AMD3100 was perfused, starting 20 minutes before reperfusion and allowed up to 30 minutes of reperfusion. The AMD3100 concentration was based on our previous report on isolated working rat hearts.11)
Figure 1.
Experimental protocol. CON group was subjected to 30 minutes of regional ischemia followed by 120 minutes of reperfusion. IPOC group was induced by 6 cycles of 10 seconds reperfusion and 10 seconds global ischemia immediately after reperfusion. CXCR4 antagonist AMD3100 was administered from 20 minutes before reperfusion to 30 minutes after reperfusion.
CON = untreated control hearts (n=12); CXCR4 = chemokine receptor type 4; IPOC = ischemic postconditioning (n=8).
Cardiac function assessment
An air-bubble free, fluid-filled latex balloon was inserted into the left ventricle (LV) through the left atrial appendage in isolated hearts to assess cardiac function. Balloon volume was adjusted with the BIOPAC system (BIOPAC Systems Inc., Goleta, CA, USA) to provide and sustain a left ventricular end-diastolic pressure (LVEDP) of 5–10 mmHg. LVDP was calculated as the difference between the left ventricular systolic pressure (LVSP) and LVEDP. Rate-pressure product (RPP) was calculated as the LVDP × heart rate (HR). CF was measured by timing the perfusate that dripped from the right heart into a graduated cylinder. Cardiodynamic data including HR, LVSP, LVEDP, and the maximum first derivative of left ventricular pressure (+dP/dtmax) was obtained and analyzed using analysis software, BSL v3.7.3 (BIOPAC Systems Inc.).
Area at risk (AR) and infarct size
After 2 hours of reperfusion, the AR and area of necrosis (AN) were demarcated by diluted fluorescent polymer microspheres and TTC stain, as described previously.11) The LV was removed from the remaining tissue. The AR and AN zones in the LV were quantified with Image Tool (UTHSCSA Image Tool version 3.0; University of Texas Health Science Center, San Antonio, TX, USA) and were converted into volumes by multiplying the areas by the slice thickness (2 mm). The volume of AN was expressed as a percentage of the AR volume. All measurements were blinded.
Myocardial necrosis
Myocardial necrosis in the CON, IPOC, and AMD+IPOC groups was evaluated by measuring the release of lactate dehydrogenase (LDH) and creatine kinase (CK) in the coronary effluent collected at 60 minutes after reperfusion. Assays for LDH and CK involved ultraviolet (UV)-rate methods and were based on manufacturer's instructions (TBA-200 FR NEO; Denka Seiken Co., Tokyo, Japan). Units are expressed as IU/L and U/L, respectively.
Enzyme-linked immunosorbent assay (ELISA) test for SDF-1α
Concentrations of SDF-1α in perfusate were measured at 60 minutes after reperfusion using the rat SDF-1α ELISA kit, according to manufacturer's recommendations (Cusabio Biotech Co., Ltd., Hubei, China). Optical density (OD) was measured using a spectrophotometer at a wavelength of 450±2 nm and the concentration of SDF-1α in the samples was determined by comparing the OD of the samples to the standard curve. The SDF-1α was expressed in pg/mL. The SDF-1α concentration in each sample was the average of 2 independent experiments, each from a different heart. Additionally, the concentration of SDF-1α in non-infarcted sham hearts (n=8) was measured for comparison with infarcted groups.
Confocal immunofluorescence microscopy
The heart tissues were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS), then permeabilized with 0.1% Triton X-100 in PBS for 10 minutes, and then blocked with 2% bovine serum albumin (BSA). The SDF-1 primary antibody was incubated at a 1:200 dilution overnight at 4°C. Next, the hearts were repeatedly washed with PBS and incubated with Alexa Fluor 488 goat anti-rabbit immunoglobulin G (IgG) (H+L) for 2 hours at room temperature. The tissues were repeatedly washed in PBS and then stained with 100 ng/mL 4',6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich Chemical) in PBS for 10 minutes. Stained heart tissues were analyzed using an upright spectral laser scanning confocal microscope (Model FV 1000; Olympus, Tokyo, Japan) equipped with a blue argon (for DAPI) and green argon (for Alexa Fluor 488) laser. Image processing, analysis, and the extent of colocalization were evaluated with FV10-ASW software.
Tissue lysate preparation and western blot analysis
Additional hearts were randomly assigned into the CON, IPOC, and AMD+IPOC groups (each n=6). Myocardial samples were taken 10 minutes after reperfusion from isolated rat hearts and were homogenized in ice-cold lysis buffer. Equal amounts of protein (50 µg/lane) were loaded and electrophoresed on sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. Membranes were blocked with nonfat milk and then incubated at 4°C overnight with primary antibodies to facilitate Akt and ERK1/2 phosphorylation. Primary antibody binding was detected with a secondary anti-rabbit antibody and visualized with enhanced chemiluminescence. Data for total- and phospho-ERK1/2 represent the sum of the 42- and 44-kDa bands.
Statistical analysis
Data are expressed as mean±standard deviation (SD). Data analysis was performed with a statistical software package (SPSS for Windows, Release 12.0; SPSS Inc., Chicago, IL, USA). Data were analyzed using 1-way and 2-way analysis of variance (ANOVA) followed by post-hoc multiple comparison tests. A value of p<0.050 was considered significant.
RESULTS
CF and cardiodynamics
Table 1 summarizes the CF and hemodynamic parameters, including HR, LVDP, RPP, and +dP/dtmax in all groups determined at baseline, and at 30 minutes and 120 minutes after reperfusion. There were no significant differences in baseline CF and cardiodynamic variables. There were no significant differences in CF and cardiodynamic variables after reperfusion.
Table 1. CF and cardiodynamics in isolated hearts.
| Parameters | Baseline | Reperfusion (30 minutes) | Reperfusion (120 minutes) | |
|---|---|---|---|---|
| CF (mL/min/g) | ||||
| CON | 12.7±3.5 | 7.8±4.8 | 7.5±5.0 | |
| IPOC | 11.2±2.8 | 8.5±2.7 | 5.9±2.7 | |
| AMD+IPOC | 11.3±1.8 | 6.9±2.9 | 4.3±3.2 | |
| AMD | 13.0±2.6 | 8.2±6.8 | 5.6±5.1 | |
| HR (beats/min) | ||||
| CON | 272.4±32.5 | 235.2±43.1 | 202.4±74.5 | |
| IPOC | 257.6±16.7 | 228.7±29.7 | 176.0±54.4 | |
| AMD+IPOC | 258.7±43.1 | 186.4±67.7 | 158.8±62.5 | |
| AMD | 284.5±49.3 | 189.3±58.0 | 177.0±57.4 | |
| LVDP (mmHg) | ||||
| CON | 124.8±20.6 | 77.8±24.4 | 47.1±19.2 | |
| IPOC | 122.6±14.3 | 88.2±22.9 | 53.1±25.7 | |
| AMD+IPOC | 124.7±25.0 | 71.3±33.8 | 46.0±24.4 | |
| AMD | 127.4±27.5 | 53.3±19.8 | 46.9±16.9 | |
| RPP (mmHg·beats/min·103) | ||||
| CON | 34.0±7.0 | 19.0±8.8 | 10.4±7.4 | |
| IPOC | 31.7±5.1 | 20.4±6.5 | 10.4±6.1 | |
| AMD+IPOC | 32.1±7.5 | 14.5±9.9 | 7.4±5.6 | |
| AMD | 36.3±10.8 | 9.3±5.3 | 8.9±5.2 | |
| +dP/dtmax (mmHg/sec·103) | ||||
| CON | 2.6±1.0 | 1.5±0.5 | 0.9±0.3 | |
| IPOC | 2.6±0.3 | 1.7±0.4 | 1.1±0.5 | |
| AMD+IPOC | 2.4±0.7 | 1.3±0.6 | 0.8±0.3 | |
| AMD | 2.5±0.4 | 1.0±0.3 | 0.7±0.4 | |
Data expressed as mean±standard deviation.
AMD = AMD3100 treatment in CON (n=9); AMD+IPOC = CXCR4 antagonist AMD3100 treatment in IPOC (n=11); CF = coronary flow; CON = untreated control hearts (n=12); CXCR4 = chemokine receptor type 4; HR = heart rate; IPOC = ischemic postconditioning (n=8); LVDP = left ventricular developed pressure; RPP = rate-pressure product; +dP/dtmax = maximum first derivative of left ventricular pressure.
Infarct size
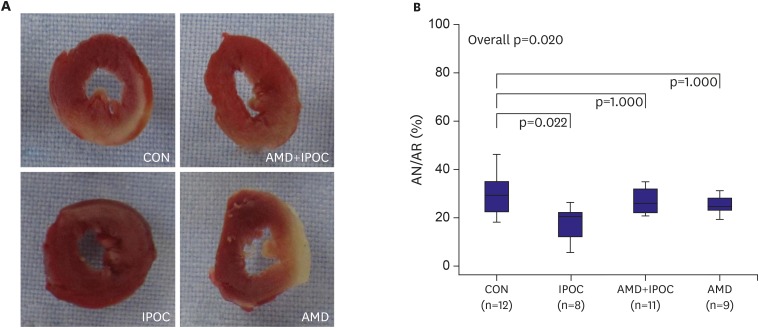
There were no significant differences between groups with respect to body weight, heart weight, LV volume, AR volume, and AR/LV (Table 2). The AR ranged from 54.5% to 61.7% of the LV volume, which indicates that an equivalent degree of regional ischemia was induced among the groups. AN/AR was significantly reduced in the IPOC group (17.8±7.0%) compared with the control group (29.3±8.4%, p=0.022; Figure 2). This infarct reducing effect of IPOC was completely countered by AMD3100 pretreatment (28.5±8.9%, p=0.045 vs. IPOC group). AMD3100 did not have effect on infarct size (25.5±7.7%, p=1.000 vs. CON group).
Table 2. Morphometric data.
| Group | BW (gm) | HW (gm) | LV volume (cm3) | AR volume (cm3) | AR/LV (%) |
|---|---|---|---|---|---|
| CON | 300.4±15.9 | 1.56±0.15 | 0.706±0.110 | 0.423±0.093 | 59.6±6.9 |
| IPOC | 297.5±21.4 | 1.58±0.17 | 0.736±0.134 | 0.401±0.090 | 54.5±5.1 |
| AMD+IPOC | 293.3±16.9 | 1.53±0.16 | 0.769±0.084 | 0.470±0.065 | 61.5±8.4 |
| AMD | 291.9±11.9 | 1.51±0.12 | 0.742±0.098 | 0.458±0.062 | 61.7±3.3 |
Data expressed as mean±standard deviation. There were no significant differences among groups.
AMD = AMD3100 treatment in CON (n=9); AMD+IPOC = CXCR4 antagonist AMD3100 treatment in IPOC (n=11); AR = area at risk; BW = body weight; CON = untreated control hearts (n=12); CXCR4 = chemokine receptor type 4; HW = heart weight; IPOC = ischemic postconditioning (n=8); LV = left ventricle.
Figure 2.
(A) Representative sequential LV slices from each group showing the AN (pale area) with TTC staining. (B) Percent of infarct area over AR.
All data are expressed as mean±standard deviation. There were significant differences between p=0.021 vs. CON group and p=0.045 vs. AMD+IPOC group.
AMD = AMD3100 treatment in CON (n=9); AMD+IPOC = CXCR4 antagonist AMD3100 treatment in IPOC (n=11); AN = area of necrosis; AR = area at risk; CON = untreated control hearts (n=12); IPOC = ischemic postconditioning (n=8); TTC = 2,3,5-triphenyltetrazolium chloride.
Irreversible myocardial damage
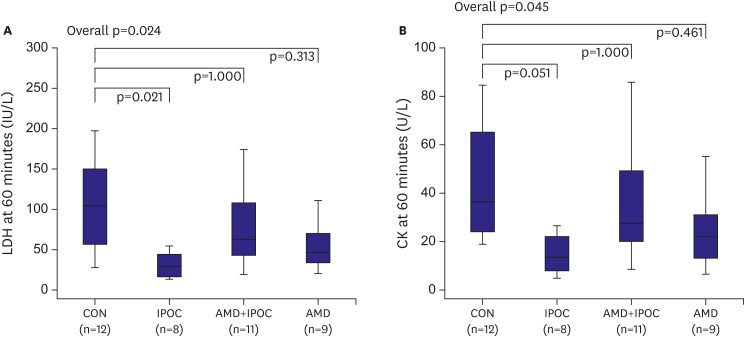
Consistent with the change in AN/AR, the LDH concentration in the IPOC group (31.5±15.5 IU/L) was decreased significantly compared to the CON group (101.9±60.3 IU/L, p=0.020). However, the level of LDH was completely reversed by pretreatment with AMD3100 (116.4±94.5 IU/L, p=0.047 vs. IPOC group; Figure 3). Similarly, the CK level was significantly lower in the IPOC group than in the CON group (44.8±24.4 U/L vs. 122.7±80.0 U/L, respectively, p=0.048), and these changes of CK were completely reversed by AMD3100 (152.7±116.4 U/L, p=0.042; Figure 3).
Figure 3.
Coronary effluent LDH and CK measured at 60 minutes after reperfusion.
Data are expressed as mean±standard deviation. p<0.050 vs. CON group.
AMD = AMD3100 treatment in CON (n=9); AMD+IPOC = CXCR4 antagonist AMD3100 treatment in IPOC (n=11); CK = creatine kinase; CON = untreated control hearts (n=12); IPOC = ischemic postconditioning (n=8); LDH = lactate dehydrogenase.
SDF-1α concentrations and confocal immunofluorescence microscopy
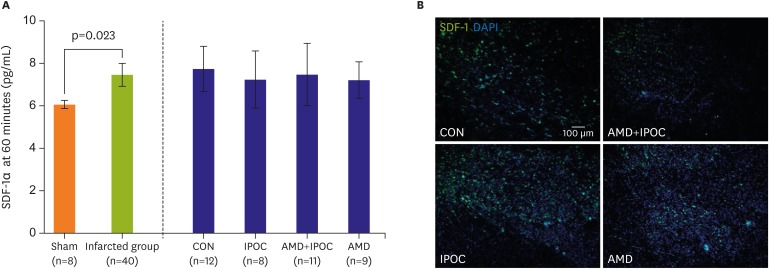
The following SDF-1α concentrations were used: 6.06±0.23 pg/mL in the control heart group, 7.76±1.66 pg/mL in the CON group, 7.25±1.61 pg/mL in the IPOC group, 7.49±2.17 pg/mL in the AMD+IPOC group, and 7.22±1.12 pg/mL in the AMD group. When we categorized these groups into a non-infarcted control heart group (n=8) versus infarcted groups (n=40), ischemia significantly induced myocardial SDF-1α production (6.06±0.23 pg/mL vs. 7.46±1.66 pg/mL, respectively, p=0.023). However, IPOC group did not additionally increase SDF-1α concentrations, compared with the other 3 infarcted groups (p=0.880). Interestingly, SDF-1α expression in the IPOC group was increased, compared with the control group or AMD group, based on confocal imaging. A reverse effect of AMD3100 was represented in the AMD+IPOC group by the decreased expression of SDF-1α/CXCR4 (Figure 4).
Figure 4.
Confocal image of representative immunofluorescent staining for SDF-1 (green), DAPI (blue, nuclear DNA), and merged image from isolated perfused rat hearts.
AMD = AMD3100 treatment in CON (n=9); AMD+IPOC = CXCR4 antagonist AMD3100 treatment in IPOC (n=11); CON = untreated control hearts (n=12); DAPI = 4',6-diamidino-2-phenylindole; IPOC = ischemic postconditioning (n=8); SDF-1 = stromal cell-derived factor-1.
Western immunoblots
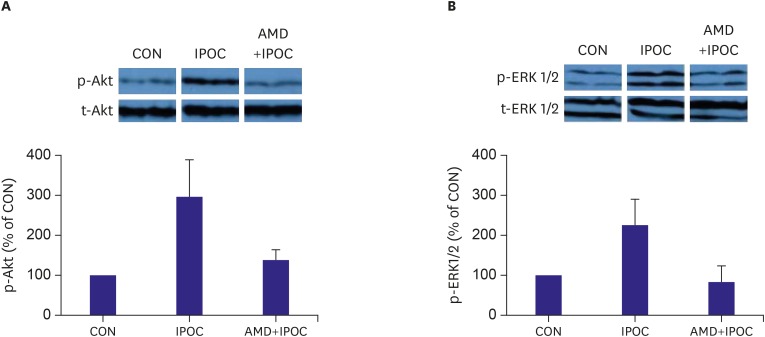
ERK1/2 (Thr202/Tyr204) and protein kinase B or Akt (Ser473) phosphorylation was measured in perfused rat hearts. IPOC significantly increased ERK1/2 phosphorylation (226.1±71.8%, p=0.010 vs. CON group) after reperfusion (Figure 5). The increased ERK1/2 phosphorylation in IPOC was attenuated by the CXCR4 antagonist AMD3100 (83.8±28.0%, p=0.020 vs. IPOC group). IPOC also significantly increased Akt phosphorylation (296.4±93.1%, p=0.020) after reperfusion, compared to control hearts. The increased Akt phosphorylation in IPOC was completely blocked by AMD3100 (138.3±28.5%, p=0.009 vs. IPOC group).
Figure 5.
(A) Representative western blotting for p-Akt and t-Akt intensity. (B) Representative western blotting for p-ERK1/2 and t-ERK1/2 intensity. Bar graph shows percentage change in ERK1/2 and Akt phosphorylation relative to CON group.
Increased phosphorylation of Akt and ERK1/2 in IPOC-induced hearts was totally blocked by AMD3100. Data are expressed as mean±standard deviation. p<0.050 vs. CON group.
AMD = AMD3100 treatment in CON (n=9); AMD+IPOC = CXCR4 antagonist AMD3100 treatment in IPOC (n=11); CON = untreated control hearts (n=12); IPOC = ischemic postconditioning (n=8); p-Akt = phospho-Akt; p-ERK1/2 = phospho-extracellular signal-regulated kinase 1/2; t-Akt = total-Akt; t-ERK1/2 = total-extracellular signal-regulated kinase 1/2.
DISCUSSION
In this study, we demonstrated that IPOC effectively reduced myocardial infarction and the levels of LDH and CK following I/R injury. This result is comparable with other studies performed in isolated rat hearts.2),5),12) Interestingly, the infarct limitation effect of IPOC was totally countered by AMD3100. In addition, the increasing effect in phosphorylation of ERK1/2 and Akt by IPOC was completely attenuated by AMD3100. Altogether, the results of this study strongly suggest, that the intracardiac protective effect of IPOC was modulated by the SDF-1α cognate receptor, CXCR4, in isolated rat hearts.
SDF-1α is the predominant isoform of SDF-1 and it is constitutively expressed in many tissues. CXCR4 is a member of the guanine nucleotide binding protein-coupled receptors (GPCRs) superfamily and a specific receptor for SDF-1α.13) SDF-1α plays an important role in organogenesis, cell proliferation, and in pathophysiological processes such as migration of bone marrow-derived stem cell and endothelial progenitor cells, which express CXCR4, into ischemic injury sites.8) It is well established that SDF-1α and CXCR4 expression increases under hypoxic conditions, such as myocardial infarction/injury.7),14) Additionally, recent evidence has indicated that the SDF-1α/CXCR4 axis has a direct and rapid cardioprotective effect from myocardial I/R injury. Segret et al.15) showed increased SDF-1α/CXCR4 expression in isolated cardiomyocytes and in the rat myocardial infarction model, based on immunohistochemistry results, and suggested that increased SDF-1α levels may directly contribute to cardiac function recovery via an autocrine and/or paracrine mechanism. Moreover, Hu et al.9) found that SDF-1α expression increased early in cardiomyocytes cultured under hypoxic conditions as well as in in vivo ischemic preconditioning. They also reported that exogenous administration of SDF-1α protects the myocardium from I/R injury and that administration of AMD3100 before SDF-1α treatment blocked the cardioprotective effect of SDF-1α. Huang et al.10) showed that exogenously administered SDF-1α improved post-ischemic myocardial function recovery and those effects were countered by administration of AMD3100 in acute myocardial I/R injury models with isolated mouse hearts. Our very recent data demonstrated that exogenously sustained SDF-1α targeting reperfusion also effectively reduced myocardial infarction in a dose-dependent manner in isolated rat hearts.11) Altogether, exogenous administration of SDF-1α to target both ischemia and reperfusion effectively protects after myocardial I/R injury.
We verified the difference in SDF-1α protein levels between the non-infarcted control group and the four experimental infarcted groups. The IPOC did not increase SDF-1α protein levels, compared with the CON group at 60 minutes after IPOC. However, in confocal imaging, SDF-1α expression of the IPOC group increased, compared with the control group or AMD group and a reverse effect of AMD3100 was represented in the AMD+IPOC group by the decreased expression of SDF-1α/CXCR4 (Figure 4). In our study, IPOC significantly reduced infarct size as well as cardiac enzymes levels, and AMD3100 completely reversed the infarct-reducing effect of IPOC. There are several possibilities that could account for this result. First, there may be an undiscovered intermediate mechanism in the SDF-1α/CXCR4 axis for IPOC instead of the increasing release of SDF-1α, followed by CXCR4 activation. Second, sampling time for SDF-1α protein levels may have been too late, at 60 minutes after IPOC. Hu et al.'s study9) actually supports this hypothesis and showed that SDF-1α/CXCR4 pathway activation to modulate cardiomyocyte signaling in response to hypoxia/ischemia was very rapid and transient, occurring by 5 minutes and then disappearing by 30 minutes. Further research is warranted to address this possibility. Nonetheless, the present study reveals a crucial role for the SDF-1α/CXCR4 axis in mediating IPOC for cardioprotection against I/R injury.
In comparison, it is well-documented that IPOC mediates cardioprotection via activation of the reperfusion injury salvage kinase (RISK) pathways, such as ERK1/2 and phosphoinositide 3-kinase (PI3K)/Akt.16) Zhu et al.17) found that the IPOC mediates cardioprotection via activation of the PI3K/Akt pathway in the remodeled rat myocardium and Yang et al.18) found that activation of the ERK1/2 component is required to mediate the protection induced by IPOC. Our present study yielded the same result, namely that IPOC significantly increased both ERK1/2 and Akt phosphorylation. Interestingly, AMD3100 completely inhibited IPOC-induced ERK1/2 and Akt phosphorylation. Based on this result, we hypothesize that CXCR4 is an upstream key component of ERK1/2 activation and Akt signaling by IPOC. It is also well known that myocardial STAT3 is involved in the cardioprotective effects of the SDF-1α/CXCR4 axis, but we did not examine the role of myocardial STAT3, therefore further research is needed.10)
Jujo et al.19) investigated the effect of CXCR blockade after myocardial infarction in a mouse model, and reported that continuous infusion of AMD3100 worsened functional myocardial recovery and neovascularization after myocardial infarction, which was explained by AMD3100 blockade of bone marrow-derived endothelial progenitor cells that were incorporated in the heart. However, our study was performed in isolated working rat hearts, and the results indicated that the CXCR4 blocking effect of AMD3100 and the subsequent result in inhibiting the RISK pathway may need to be incorporated in the context of other studies to better understand this process. Further research is also warranted to investigate the mechanisms of these results.
GPCRs, which are introduced as cell surface proteins that convert extracellular stimuli into cellular signals, are solid established mediators in the IPOC pathway. Chen et al.20) and Methner et al.21) reported that the activation of GPCRs with ligands such as morphine or adenosine, effectively reduced infarct size at the time of reperfusion. Interestingly, there may be considerable cross-talk between GPCRs. For example, cardioprotection by the adenosine or opioid receptors may be countered by opioid or adenosine receptor antagonists.22) CXCR4 is a member of the GPCRs superfamily and it should be determined whether the role of CXCR4 in IPOC, as demonstrated in our study, is countered by other GPCRs antagonists in a future study.
In conclusion, our study was performed in an isolated heart model and demonstrates a new function for the SDF-1α/CXCR4 axis in IPOC. The SDF-1α/CXCR4 signaling may be directly involved in IPOC cardioprotection and this signaling pathway is coupled to downstream intracellular ERK1/2 and Akt signaling. We believe that these findings provide further insight into the underlying mechanisms and beneficial effects on IPOC against I/R injury.
Footnotes
Funding: This study was supported by the Research Institute for Convergence of Biomedical Science and Technology Grant (30-2013-000), Pusan National University Yangsan Hospital.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Kim JS, Jang YH, Kim JH, Ban CI, Ahn KH.
- Data curation: Kim JS, Jang YH.
- Formal analysis: Kim JS, Chun KJ.
- Funding acquisition: Kim JS, Chun KJ.
- Investigation: Kim JS, Hwang SA, Chun KJ.
- Methodology: Kim JS, Jang YH, Hwang SA, Lee SR.
- Project administration: Kim JS, Jang YH, Kim JH, Chun KJ.
- Resources: Kim JS, Jang YH, Hwang SA.
- Software: Kim JS, Chun KJ.
- Supervision: Kim JH, Park YH, Kim J, Lee SR, Ban CI, Ahn KH.
- Validation: Lee SR.
- Visualization: Kim JS, Hwang SA.
- Writing - original draft: Kim JS, Jang YH, Chun KJ.
- Writing - review & editing: Kim JS, Jang YH, Xu Z, Chun KJ.
References
- 1.Kin H, Zhao ZQ, Sun HY, et al. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 3.Darling CE, Jiang R, Maynard M, Whittaker P, Vinten-Johansen J, Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am J Physiol Heart Circ Physiol. 2005;289:H1618–H1626. doi: 10.1152/ajpheart.00055.2005. [DOI] [PubMed] [Google Scholar]
- 4.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Jang Y, Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Postconditioning prevents reperfusion injury by activating delta-opioid receptors. Anesthesiology. 2008;108:243–250. doi: 10.1097/01.anes.0000299437.93898.4a. [DOI] [PubMed] [Google Scholar]
- 6.Mykytenko J, Reeves JG, Kin H, et al. Persistent beneficial effect of postconditioning against infarct size: role of mitochondrial K(ATP) channels during reperfusion. Basic Res Cardiol. 2008;103:472–484. doi: 10.1007/s00395-008-0731-2. [DOI] [PubMed] [Google Scholar]
- 7.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 8.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 9.Hu X, Dai S, Wu WJ, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Gu H, Zhang W, Manukyan MC, Shou W, Wang M. SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;301:H1496–H1505. doi: 10.1152/ajpheart.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang YH, Kim JH, Ban C, et al. Stromal cell derived factor-1 (SDF-1) targeting reperfusion reduces myocardial infarction in isolated rat hearts. Cardiovasc Ther. 2012;30:264–272. doi: 10.1111/j.1755-5922.2011.00301.x. [DOI] [PubMed] [Google Scholar]
- 12.van Vuuren D, Genis A, Genade S, Lochner A. Postconditioning the isolated working rat heart. Cardiovasc Drugs Ther. 2008;22:391–397. doi: 10.1007/s10557-008-6119-6. [DOI] [PubMed] [Google Scholar]
- 13.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 14.Wei YJ, Tang Y, Li J, et al. Cloning and expression pattern of dog SDF-1 and the implications of altered expression of SDF-1 in ischemic myocardium. Cytokine. 2007;40:52–59. doi: 10.1016/j.cyto.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Segret A, Rücker-Martin C, Pavoine C, et al. Structural localization and expression of CXCL12 and CXCR4 in rat heart and isolated cardiac myocytes. J Histochem Cytochem. 2007;55:141–150. doi: 10.1369/jhc.6A7050.2006. [DOI] [PubMed] [Google Scholar]
- 16.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Zhu M, Feng J, Lucchinetti E, et al. Ischemic postconditioning protects remodeled myocardium via the PI3K-PKB/Akt reperfusion injury salvage kinase pathway. Cardiovasc Res. 2006;72:152–162. doi: 10.1016/j.cardiores.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 19.Jujo K, Hamada H, Iwakura A, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci USA. 2010;107:11008–11013. doi: 10.1073/pnas.0914248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Li T, Zhang B. Morphine postconditioning protects against reperfusion injury in the isolated rat hearts. J Surg Res. 2008;145:287–294. doi: 10.1016/j.jss.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Methner C, Schmidt K, Cohen MV, Downey JM, Krieg T. Both A2a and A2b adenosine receptors at reperfusion are necessary to reduce infarct size in mouse hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1262–H1264. doi: 10.1152/ajpheart.00181.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peart JN, Gross GJ. Adenosine and opioid receptor-mediated cardioprotection in the rat: evidence for cross-talk between receptors. Am J Physiol Heart Circ Physiol. 2003;285:H81–H89. doi: 10.1152/ajpheart.00985.2002. [DOI] [PubMed] [Google Scholar]