Abstract
The ExactVu™ Micro-Ultrasound system is a new high resolution imaging system for visualizing the prostate and has been FDA, CE, and Health Canada approved for visualization and biopsy of the prostate. The PRI-MUS™ (Prostate Risk Identification for Micro-Ultrasound) protocol has previously been demonstrated to correlate with risk of prostate cancer and severity of cancer. Here we present a case where a healthy 50 year old subject with no known risk factors volunteered to test the ExactVu system and was found to harbour multiple PRI-MUS 3–5 lesions. This prompted PSA testing, biopsy and eventual diagnosis of significant prostate cancer.
Keywords: Prostate cancer, Micro-ultrasound, Prostate biopsy, PRI-MUS, Biomarkers
Abbreviations
- PRI-MUS
Prostate Risk Identification for Micro-ultrasound
- PI-RADS
Prostate Imaging, Reporting, and Data System
- MpMRI
Multi-parametric Magnetic Resonance Imaging
1. Introduction
Prostate cancer screening is problematic due to the lack of a high sensitivity test to rule-in potential disease for further testing. Traditional screening makes use of digital rectal exam along with the PSA blood test, however there has been significant debate over what PSA threshold to use and when to begin testing.1 40–50% of men with PSA of >4ng/ml do not harbour cancer on systematic biopsy while up to 25% of men with PSA <4ng/ml may harbour high grade cancer if they undergo biopsy.2
Prostate biopsy is invasive with risk of sepsis and the potential for detecting indolent disease leading to psychological burden to the patient for which treatment may carry a higher risk of harm than the disease itself.
In other diseases, imaging is often used as part of screening to improve accuracy. The PROMIS trial recently suggested applying this same approach to the prostate using mpMRI.3 Here we present a case where a new imaging modality - - micro-ultrasound - - unexpectedly prompted further screening of a young age individual, and the observed lesions were eventually confirmed with a diagnosis of significant prostate cancer.
2. Case presentation
Due to our involvement in the Exact Imaging clinical trial, we were asked to perform an imaging session with the ExactVu micro-ultrasound device (Exact Imaging, Markham, Canada) to verify image quality shortly after Health Canada approval. The subject of this case was a volunteer from the company who agreed to be imaged as part of these tests.
The patient was a 50 year old gentleman with no family history and normal DRE. He had no history of PSA testing. Micro-ultrasound imaging revealed a 30 cc gland with a large PRI-MUS 4–5 lesion in left base-mid and PRI-MUS 3 lesions in the left apex and right base (see Fig. 1).4 These PRI-MUS scores are reflective of suspicious prostatic tissue.4 Based on these imaging results, the patient was recommended for PSA screening.
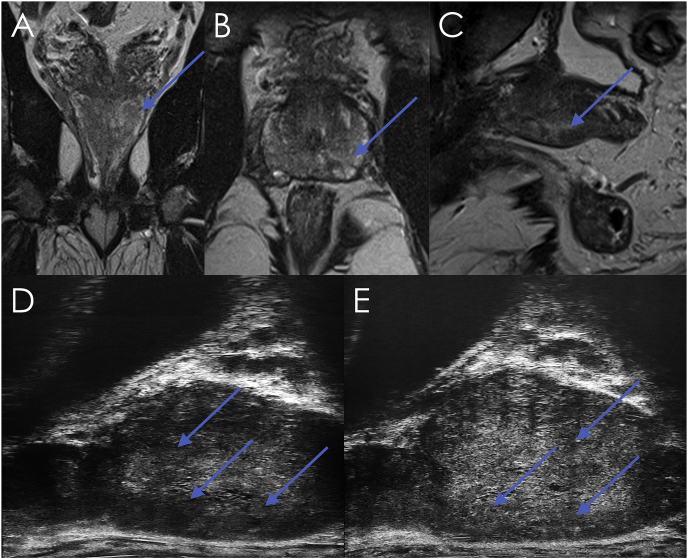
Fig. 1.
Comparative MRI and Micro-ultrasound images of index lesion. A) Coronal T2 MRI. B) Axial T2 MRI. C) Sagittal T2 MRI. D) Parasagittal micro-ultrasound of left lateral edge of prostate. E) Parasagittal micro-ultrasound of left medial edge of lesion. The Micro-ultrasound images show mottled tissue consistent with PRI-MUS grade 4, along with suspicious shadowing consistent with PRI-MUS grade 5. Suspicious findings in all images are marked with arrows.
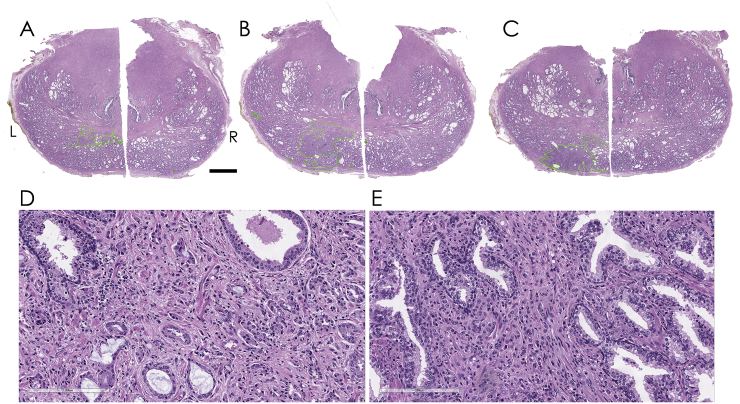
PSA was 4.1ng/mL, and subsequent mpMRI (unblinded to the micro-ultrasound images) revealed a PI-RADS 3 lesion in the left base-mid. A fusion biopsy was performed with 11 systematic samples plus an additional 5 cores targeted at the PI-RADS 3 lesion in the left base-mid. The index lesion was found to be GS 7 = 4 + 3 tissue in 2 of the 3 targeted left base samples (2% and 30%) and in 2/2 targeted left mid samples (30% and 50% involvement). The remaining systematic samples identified either benign tissue (8 samples) or clinically insignificant low volume GS 6 disease (3 samples, up to 5% involvement). Full pathology and imaging results are presented in Table 1. The prostate was removed using an open radical prostatectomy approach and sectioned for detailed histological analysis (Fig. 2) This analysis upgraded the diagnosis due to the presence of a small area of pattern 5 carcinoma (Fig. 2E) on the left side. The left apex was further involved by a small Gleason score 6 (3 + 3) adenocarcinoma. The right side of the prostate was found to contain a small Gleason score 7 (3 + 4) carcinoma with mucinous features, though it is not clear whether such a small focus could have contributed to the equivocal micro-ultrasound findings in that area.
Table 1.
Pathology and Imaging results.
| Sample | MpMRI PI-RADS |
Micro-ultrasound PRI-MUS | Number of Cores | Biopsy Pathology |
|---|---|---|---|---|
| R Lat Base | 2 | 3 | 1 | Benign |
| R Lat Mid | 1 | 2 | 1 | Benign |
| R Lat Apex | 2 | 2 | 1 | Benign |
| R Med Base | 2 | 3 | 1 | Benign |
| R Med Mid | 2 | 2 | 1 | Benign |
| R Med Apex | 2 | 2 | 1 | Benign |
| L Med Apex | 2 | 3 | 1 | GS 6 = 3 + 3, 5% |
| L Lat Base | 1 | 4 | 1 | GS 6 = 3 + 3, 5% perineural invasion |
| L Lat Mid | 1 | 5 | 1 | GS 6 = 3 + 3, 1–2% |
| L Lat Apex | 2 | 2 | 1 | Benign |
| L Base MRI | 3 | 4 | 3 | GS 7 = 3 + 4, 2/3 cores 30% and 2% of positive cores |
| L Mid MRI | 3 | 5 | 2 | GS 7 = 4 + 3, 2/2 cores 50% and 30% |
| Central/Midgland base MRI | 1 | 2 | 2 | Benign |
Fig. 2.
Histology images from transverse sections taken from the radical prostatectomy specimen. A-C) Slices shown from mid-base region with adenocarcinoma areas traced in green. Scale bar represents 5mm. D-E) 20x magnification of Gleason pattern 4 (D) and single file pattern 5 carcinoma (E) of the lesion identified in (B).
3. Discussion
The ExactVu system is a real-time micro-ultrasound system capable of providing 300% higher resolution (down to 70 μm) compared to conventional trans-rectal ultrasound. A recent publication on the PRI-MUS protocol,4 developed to aid interpretation of these micro-ultrasound images, demonstrated encouraging levels of sensitivity for detection of clinically significant cancer. Here, we present the case of a younger aged man whose micro-ultrasound imaging prompted clinical follow up leading to early detection of a significant cancer.
Much like the 2012 report from the United States Preventative Services Task Force, the Canadian Task Force on Preventative Health Care5 has issued a strong recommendation that men under 55 should not be screened for prostate cancer using PSA. In this case, early image-based screening and follow-up led to a radical prostatectomy with nerve sparing approach on the right side which may not have been possible if detection had been delayed an additional 5 years.
Several imaging and non-imaging techniques have been suggested to provide the high sensitivity needed for prostate cancer screening, from the basic DRE through PSA and associated derivative compounds, more advanced liquid biomarkers, MRI and PET. In this case, had PSA testing been performed it would have likely prompted biopsy, however without the associated imaging it is not clear whether the significant lesions would have been found. While the biopsy was performed using MRI fusion, it is important to note that the index lesion was compared before biopsy to ensure that it matched the lesions found originally on micro-ultrasound. All suspicious regions identified in micro-ultrasound were correlated and found with mpMRI. Lower-risk PRI-MUS 3 lesions were visualized using micro-ultrasound on the right side but were not targeted, however a small spot of significant cancer in this region was noted on the radical prostatectomy specimen.
4. Conclusion
This case study adds to the debate that additional information provided by imaging - - such as mpMRI or micro-ultrasound - - may help with screening protocols by ensuring that all men with prostate cancer are offered biopsy in a timely manner, while reducing the number of men without clinically significant cancer that are required to undergo the procedure. Due to its ease of use, real-time nature, cost-effectiveness comparable to conventional ultrasound, and its comprehensive risk identification protocol (PRI-MUS), micro-ultrasound has the potential to be a powerful screening and targeting tool for urologists. Until this approach is validated, however, we must be clear that there is a risk of over-detection when sending men to biopsy based on these imaging tests, as well as risk of missed or delayed detection when imaging is used to delay biopsy.
Consent
The subject provided written consent to publish this case study.
Conflicts of interest
Dr. Ghai has received honoraria for speaking engagements from Exact Imaging unrelated to the current work. Dr. Van der Kwast has no conflicts to disclose.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eucr.2017.11.013.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Cary K.C., Cooperberg M.R. Biomarkers in prostate cancer surveillance and screening: past, present, and future. Ther Adv Urol. 2013;5(6):318–329. doi: 10.1177/1756287213495915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson I., Pauler D. Prevalence of prostate cancer among men with a prostate-specific antigen level <= 4.0 ng per milliliter. N Engl J Med. 2004:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed H.U., El-Shater Bosaily A., Brown L.C. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;6736(16):1–8. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 4.Ghai S., Eure G., Fradet V. Assessing cancer risk on novel 29 MHz micro-ultrasound images of the prostate: creation of the micro-ultrasound protocol for prostate risk identification. J Urol. 2016;196(2):562–569. doi: 10.1016/j.juro.2015.12.093. [DOI] [PubMed] [Google Scholar]
- 5.Bell N., Gorber S.C., Shane A. Recommendations on screening for prostate cancer with the prostate-specific antigen test. Can Med Assoc J. 2014;186(16):1225–1234. doi: 10.1503/cmaj.140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.