Abstract
This review covers the use of plasma technology relevant to the preparation of dressings for wound healing. The current state of knowledge of plasma treatments that have potential to provide enhanced functional surfaces for rapid and effective healing is summarized. Dressings that are specialized to the needs of individual cases of chronic wounds such as diabetic ulcers are a special focus. A summary of the biology of wound healing and a discussion of the various types of plasmas that are suitable for the customizing of wound dressings are given. Plasma treatment allows the surface energy and air permeability of the dressing to be controlled, to ensure optimum interaction with the wound. Plasmas also provide control over the surface chemistry and in cases where the plasma creates energetic ion bombardment, activation with long-lived radicals that can bind therapeutic molecules covalently to the surface of the dressing. Therapeutic innovations enabled by plasma treatment include the attachment of microRNA or antimicrobial peptides. Bioactive molecules that promote subsequent cell adhesion and proliferation can also be bound, leading to the recruitment of cells to the dressing that may be stem cells or patient-derived cells. The presence of a communicating cell population expressing factors promotes healing.
Keywords: Plasma treatments, Surface modification, Wound dressing, Attachment and growth of cells, Immobilization of biomolecules
Introduction
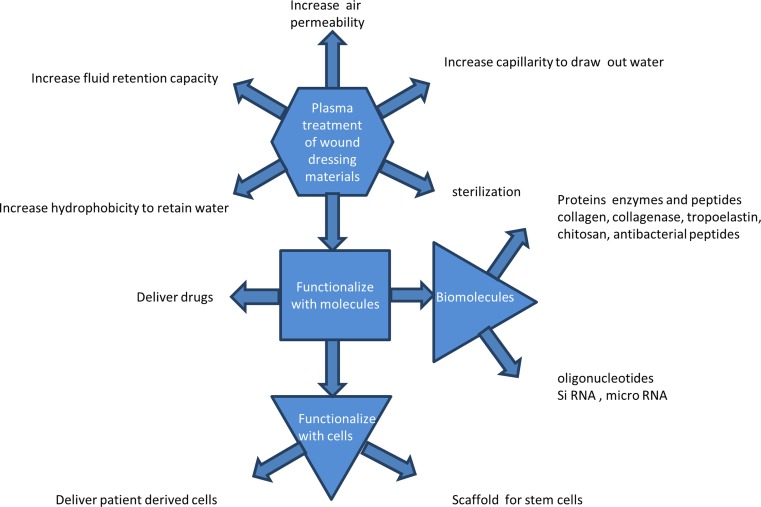
The use of plasmas (ionized gases) to modify the surfaces of materials used for medical applications is advancing rapidly. Plasma surface modification is an effective tool to improve the surface properties without affecting the bulk properties of a material and, in its most general form, can be applied to any material to modify mechanical properties, roughness, hydrophilicity, hydrophobicity and surface chemistry. It is highly efficient in its use of chemicals and, being a dry process, it avoids the formation of liquid waste, resulting in an environmentally friendly dry process which can be exploited to give specific modifications (Parvizi et al. 2007) of physical, chemical and biological properties. While plasmas have been found to be especially useful in the modification of polymers of both synthetic and biological origin for application in sterile packaging, drug packaging and medical devices (Oehr 2003), the application of plasmas to the production and improvement of wound dressings is still in its infancy.
Wound dressings are used in treating wounds to prevent infection and to accelerate healing. Dressings can be of natural or synthetic polymers in the form of fibers, colloids, gels, foam, gauze and films. Some wound dressings are required to have good wettability and moisture retention to manage wound exudates (Breitwieser et al. 2013), while others are required to assist in the retention of moisture in dry wounds. Dressings may also require mechanical strength for wound protection and controlled adhesion. Some examples of useful materials are natural and modified cellulose, alginates, chitosan, collagen and hydrogels (Gupta et al. 2010; Le et al. 1997). Cellulosic materials have been used as medical dressings for many different wounds because their micro-fibrillar structure has excellent moisture-retaining properties. These cellulose materials are subjected to pre-treatments such as bleaching, mercerization, and alkaline washing with chemicals to increase the moisture absorption capacity (Durso 1978; Fengel and Wegener 1984; Freytag and Donzé 1983; Lewin 1984). Unfortunately, some chemical treatments such as these cause deterioration in the mechanical properties of the fabric as well as causing ecological pollution (Vigo 1994).
Wound dressings are now tailored to suit a specific wound repairing process, to the extent that there may now be more than 1000 different types. This trend will continue as the best route to healing an individual patient’s wound which requires customized strategies. The challenge remains, however, of finding more effective strategies for serious and chronic wounds. To achieve this, dressings can be made up of a number of components, each of which is intended for a specific wound healing process. “Smart” wound dressings with customized functionalities find many applications, and industries are developing to supply innovative dressings. For example, PolyMem™ have developed a polyurethane-based multifunctional dressing, Transparency™ have developed superabsorbent polymers, Lubrizol™ have developed a range of products based on thermoplastic polyurethane, XTRASORB™ have developed a hydrogel colloidal sheet, and DermNet NZ™ have developed gauze-based dressings and paste bandages such as zinc paste bandages as wound dressings. These specialized products are already having an impact in treating wounds where healing is delayed, as, for example, in diabetic ulcers, and in the treatment of large wounds such as burns. Plasma processes are ideally suited to the customization process owing to their flexibility and diversity. As an example of a commercial product that employs a plasma process, MySkin™ is a 5-cm-diameter circular silicone disc with an acrylic acid plasma polymer surface which allows proliferation of autologous keratinocytes, with the aim of returning the patient’s own keratinocytes to the wound bed.
Several reviews and books exist on the plasma surface modification of textiles (Zille et al. 2015), polymers for biomedical applications (Gomathi et al. 2008), biodegradable polymers (Morent et al. 2011), polymeric biomaterials (Desmet et al. 2009), medical implants (Bilek and McKenzie 2010), biocompatibility and controlled drug release (Yoshida et al. 2013), biomolecule immobilization and cell colonization (Siow et al. 2006), and for the attachment of bioactive compounds (Goddard and Hotchkiss 2007). These reviews cover the various plasma treatment techniques for specific applications. A number of reviews have also been published on wound dressings and on wound-healing agents (Agrawal et al. 2014) and systems (Mayet et al. 2014). Wound-healing dressings and systems for drug delivery into wounds have been reviewed by Boateng et al. (2008). The authors give a good general introduction and cover desirable characteristics including physical and chemical characteristics of wound dressings. Their article reviews different types of wound dressings and novel materials for controlled drug delivery to heal different types of wounds.
There are currently no reviews that cover recent developments in the role of plasmas for carrying out modifications for wound dressings. There has been considerable progress in the use of plasmas to create energetic ion bombardment of the surface, and new knowledge has been gained concerning the attachment of molecules and cells to surfaces that have potential in wound healing. Here, we focus on the science and technology that underpins the plasma treatment of materials for wound dressings. Current methods of modification as well as the future challenges to develop eco-friendly and cost-effective techniques are reviewed and discussed.
A summary of the biology of wound healing
Skin is the largest organ in the human body, serving as a barrier against external stresses including pathogens. A wound is any break or a disruption of the skin by a thermal, chemical, mechanical or surgical injury. Healing of wounds is a natural and complex process which involves several overlapping phases. Depending on the healing time, wounds are classified as acute or chronic (Dreifke et al. 2015). Acute wounds that require intervention include mechanical abrasions, tear, surgical procedure, burns and chemical injuries. Chronic wounds include diabetic foot ulcers, pressure ulcers and leg ulcers (Boateng et al. 2008; Jáuregui et al. 2009). In general, acute wounds heal within 3 weeks while chronic wounds persist for more than 12 weeks and often reoccur. The healing of wounds is associated with factors specific to an individual’s age, obesity status, medication and overall health (Guo and DiPietro 2010).
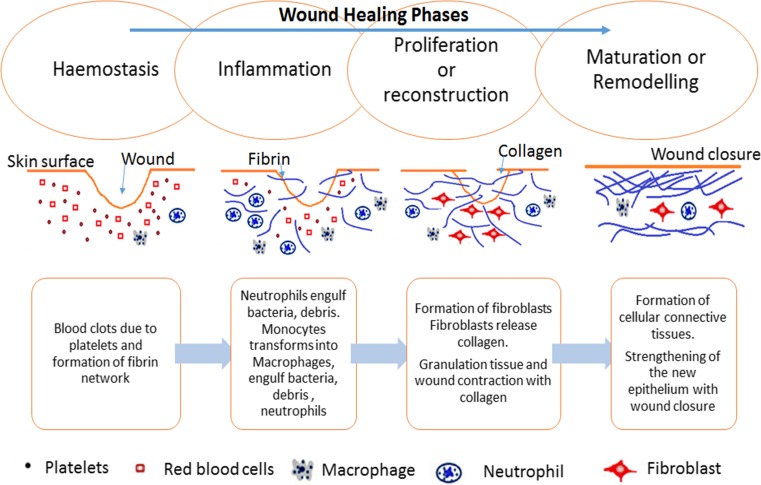
Wound healing involves complex biochemical and cellular processes, comprising the dynamic overlapping phases of hemostasis, inflammation, proliferation or reconstruction and maturation or remodeling (Boateng et al. 2008). Figure 1 shows a schematic representation of the phases of wound healing. In this review, a summary only of the biology of wound healing is presented, as a more detailed explanation is available elsewhere (Aukhil 2000). Hemostasis is the cessation of the flow of blood when there is an injury without disrupting the normal blood flow, and is commonly included as a component of the next, or inflammation, phase. Healing is initiated by the formation of a platelet plug and strengthening of the plug with the formation of a fibrin network. At the beginning of the inflammation phase, white blood cells migrate into the wound site. Within the first few days after the injury, the body attempts to remove or isolate any bacteria, damaged cells and debris present in the wound. White blood cells (neutrophils) migrate from the blood stream through surrounding tissue into the wound and engulf the bacteria. The migration begins at the capillaries, which dilate and allow blood cells to pass into the wound to form inflammation in the wound site, surrounded by blood plasma. After a few days, the neutrophils die off while white blood cells (monocytes) migrate from the blood to the wound to continue clean-up. The monocytes mature into macrophages and engulf the bacteria, debris and dead neutrophils. Macrophages play a key role in wound healing by attracting and stimulating other types of cells to accomplish reconstruction and to initiate the next phase, the proliferation or reconstruction phase. In this phase, granulation tissue consisting of fibroblasts invades the wound site and creates tough fibers of collagen to provide strength to the wound. Capillaries are also a part of the granulation tissue, bringing oxygen and nutrients to the cells in the wound, and carrying away many unwanted products during the healing process. Subsequently, cells in the upper layer, the epidermis, form a layer, and wound closure takes place while fibroblast cells transform into muscle cells to assist the contraction of the wound edges. The final phase of healing process is the maturation or remodeling phase which takes days to months to complete. During this phase, repairs carried out in the proliferation stage are made stronger by replacing the early collagen by a stronger form of collagen with a regular pattern. Finally, the wound contacts and is covered with collagen-rich scar tissue.
Fig. 1.
Schematic representation of the four phases of wound healing, showing the processes that occur and the cell types involved
Specialty fibers suitable for wound dressings
Wound dressings are available in various forms including gauze, films, hydrogels, hydrocolloids and fabric containing fibers. Among the fiber-containing types, nanofibrous materials are sometimes preferred. Nanofibers are mainly prepared by electrospinning using an electric field to assist the fiber-drawing process. After spinning, these fibers can further be modified to suit a particular application. Natural and synthetic bio-polymers are usually prepared as wound dressings by electrospinning because the small fiber diameters produced by this method provide a large surface area. Zahedi et al. (2010) have published a detailed review on the different types of wound dressing, with an emphasis on electrospun nanofibrous polymeric bandages. In this review, we focus on woven and non-woven fiber structures which have their surfaces modified using plasma-based techniques.
Electrospinning of nanofibres
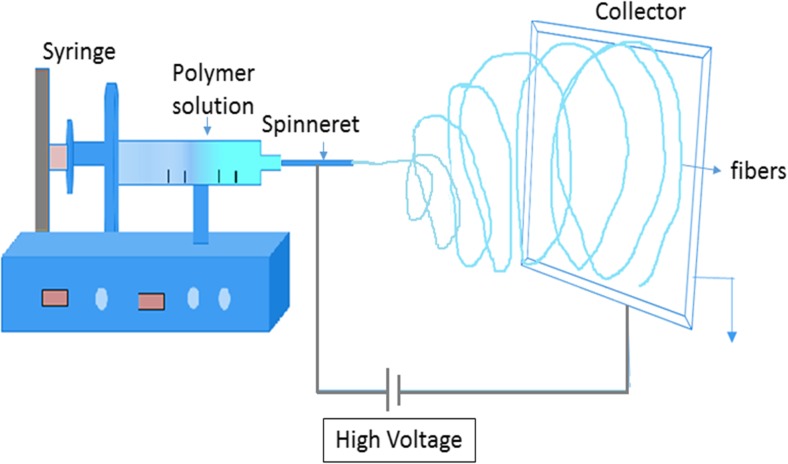
Electrospinning is a simple, effective and unique method which utilizes electrostatic forces for the fabrication of polymer fibers with diameters ranging from 2 nm to several micrometers. Nanofibers are produced by applying a high voltage between the tip of a syringe loaded with the desired polymer and the collector. Usually, the precursor of the polymer will be in the form of a sol–gel or melt which is allowed to flow at a low rate, forming a drop at the tip of the syringe. When the voltage is increased, the repulsive electrical forces distort the drop into a conical shape, known as a Taylor cone. As the voltage reaches a critical value, the electrical forces overcome the surface tension forces and a liquid jet begins to flow from the Taylor cone, which reaches the collector. During the travel of the fibrous jet, the solvent evaporates and a nanofiber is formed on the collector surface. Figure 2 shows a schematic diagram of the basic electrospinning process (Bhardwaj and Kundu 2010). The incorporation of drugs, protein, growth factors, DNA and RNA into electrospun polymeric nanofibers has been reviewed (Hu et al. 2014).
Fig. 2.
The electrospinning process as carried out in air using a high voltage to extract a nanofiber from a solution. The extracted jet dries in flight and is collected on a surface
Plasmas for treatment of surfaces
The fourth state of matter, plasma, consists of partially ionized, electrically neutral gas. Most plasmas contain neutral atoms as well as charged species, and are a source of photons emitted through recombination and excitation processes. Because of the presence of charged particles, plasmas are strongly responsive to electromagnetic fields. Plasmas can be readily produced in the laboratory, and can be used to modify a wide range of polymers, polymer fibers and textiles through bombardment by the ions, electrons and photons produced by the plasma.
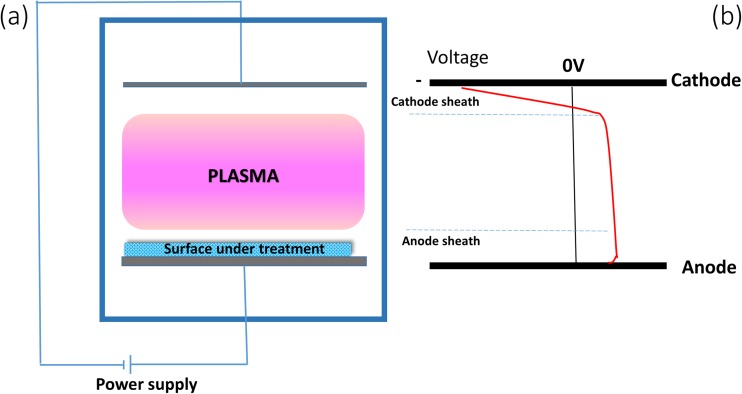
A plasma is created by application of electric fields to a working gas, often at reduced pressure, as shown in Fig. 3a. The plasma can be initiated by an external voltage applied to electrodes, or through the application of electromagnetic fields from an external source. The field may be constant or changing in magnitude and/or direction with audio, radio or microwave frequencies. The surface to be modified is placed so that ions, electrons, neutral species (reactive and non-reactive) and photons generated in the plasma bombard the surface. In plasma surface modification processes, the surfaces relevant to wound dressing applications are usually electrically insulating and, when placed in contact with the plasma, develop a negative self-bias with respect to the plasma, resulting from the surface charge by attachment of electrons. Electrons are the most mobile charged species in the plasma and develop negative charge on a nearby surface to allow equilibrium between charges flowing to and from the surface. This negative self-bias is usually of the order of a few volts to a few tens of volts and attracts ions to the surface, giving them kinetic energy of a few to a few tens of eV so that they bombard the surface, causing physical and chemical changes. The design of the plasma reactor for the treatment of insulating surfaces such as wound-dressing materials can take various forms, depending on the way in which the plasma is created by the application of electric fields, the composition of the precursor gases and the relationship of the surfaces to the applied electric fields. The different forms of plasma reactor differ in the type and energy of the species that are incident on the sample. A relatively recent innovation is the use of a plasma immersion ion implantation (PIII), a process that operates by creating more energetic ion impacts than previously available plasma processes.
Fig. 3.
Schematic diagram of an elementary glow discharge, in which a constant potential difference is applied between two electrodes. b The variation of potential between the electrodes. In this example, the sample under treatment is placed on the anode. The cathode has an associated region or cathode sheath across which a large fraction of the total applied potential difference appears
The type of plasma shown in Fig. 3 is known as a glow discharge, where electrodes play an important role in sustaining the plasma, which is not the case for some other types of discharge, such as an inductively coupled radiofrequency (RF) plasma where the applied electric potential that sustains the plasma is the result of a time-varying magnetic field. In the case of a direct current (DC) glow discharge plasma, the potential difference is constant in one direction but is not symmetrically arranged with respect to the electrodes and shows two different regions of spatially varying potential as shown in Fig. 3a. The region near the cathode is called the cathode sheath or cathode dark space where the electric field is very strong and across which the greater part of the applied potential difference appears. The ions are accelerated across this sheath and collide with the cathode to produce secondary electrons. The secondary electrons are in turn accelerated across the sheath away from the cathode surface to collide with neutral atoms, creating ionization which sustains the discharge. The cathode sheath region appears to be dark as the electron and ion densities are low and light emission from recombination and excitation is small. In the glow regions, collisions, excitation, ionization recombination and dissociation take place while the potential is changing only slowly with distance. The region adjacent to the anode is called the anode sheath, where the electrons travel towards the anode (Bogaerts et al. 2002). A detailed description of the fundamentals of plasma can be found elsewhere (Chapman 1980). The location of the sample to be treated in a plasma is important in determining the type of modification to which it is subjected. The sample receives maximum ion bombardment when the sample is placed near the cathode as it is exposed directly to energetic ions from the plasma. When the sample is placed on the anode, as shown in the Fig. 3a, the electron bombardment is relatively more intense. If large amounts of the cathode or anode surfaces are covered with insulating materials, the insulating surfaces charge up and the discharge may be extinguished. Applying a pulsed or alternating voltage to the electrodes enables the plasma process to be sustained.
Plasma surface modification has gained attention in the medical technology industry as a replacement for wet chemical, thermal and radiative processes (Chaivan et al. 2005; Guimond et al. 2010; McCord et al. 2003; Wang et al. 2008). When plasma impinges on the surface of the material, it modifies the surface properties to a depth in the range 5–50 nm without affecting the bulk properties (Abidi and Hequet 2004; Cai and Qiu 2006; Inbakumar et al. 2010; Kan et al. 2004; Vohrer et al. 1998). This ionized gas contains excited atoms, free radicals, electrons, ions and molecules that interact physically and chemically with the surface (Karahan and Özdogan 2008). These interactions can be exploited to improve surface energy, roughness, permeability, hydrophilicity, hydrophobicity, dyeability and adhesion to achieve the desired finishing processes (Chen 1991; Ferrerro and Bongiovanni 2006; Hocker 2002; Pane et al. 2001; Samanta et al. 2009). The advantages of plasma surface modification processes are cost-effectiveness, high efficiency, and speed of processing. The fact that it is a dry process is a further advantage as it avoids liquid waste that requires suitable disposal to avoid environmental damage (Wang et al. 2009).
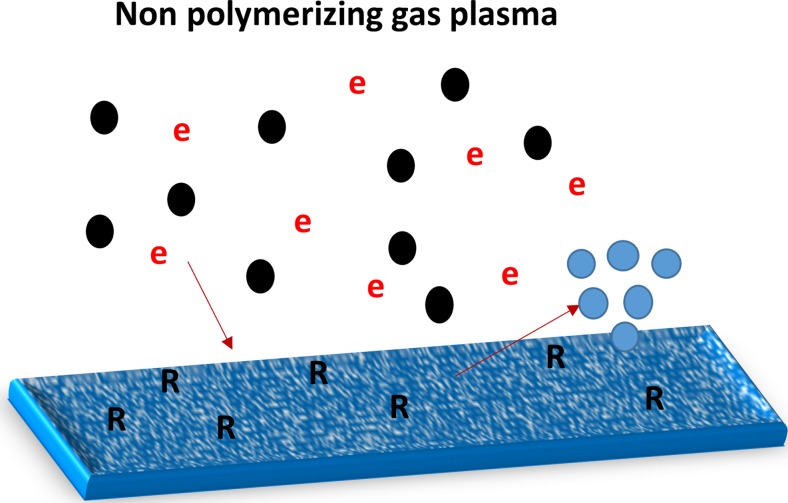
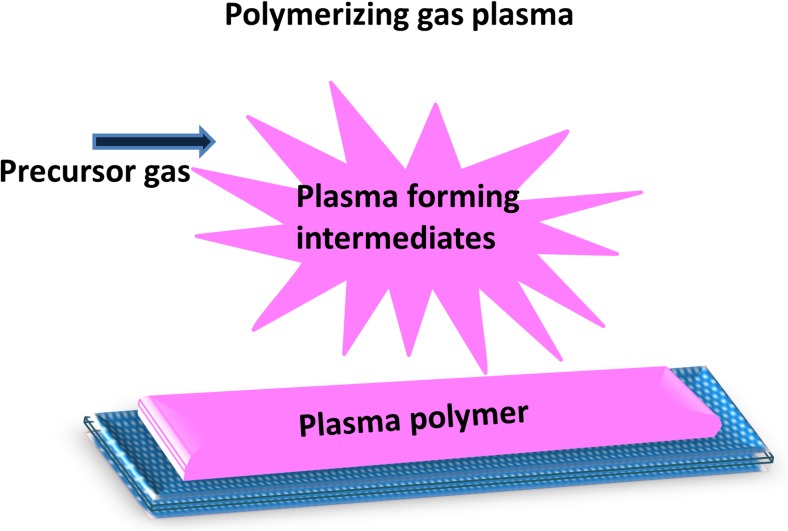
We now distinguish two types of plasma modifications according to the chemical composition of the plasma. These are non-polymerizing plasma which modifies only by bombardment, implantation and etching (Fig. 4) and polymerizing plasma treatments which produce chemical groups that can condense on a surface to form a coating (Fig. 5).
Fig. 4.
Schematic showing the plasma interaction with the surface of a non-polymerizing plasma consisting of ions (black) and electrons (e), causing atoms to be implanted, damage to be created that might be associated with radical formation in the surface (labeled R) and the surface to be etched, releasing atoms or molecules of surface material (blue)
Fig. 5.
Deposition of a thin coating of plasma polymer onto a surface by polymerizing plasma
Non polymerizing plasmas
Gases that result in non-polymerizing plasmas may nevertheless produce chemically active fragments that, while they do not aggregate on a surface to form a deposit, can take part in reactions with the surface, resulting in etching, cleaning, sterilization and activation of the surface (Fig. 4), resulting in turn in a change in surface characteristics. Gases that have been used to modify materials without producing a deposit on the surface include nitrogen, argon, oxygen and helium. The reactions between the gas phase species and the surface may result in the formation of functional groups, crosslinking, and a concentration of stable free radicals on the surface and subsurface. The usefulness of these changes in surface characteristics is dependent on various parameters such as treatment time, power and plasma composition. The stability of the treatments is dependent on the storage conditions such as temperature and atmosphere as well as the treatment parameters. The interaction of non- polymerizing plasma with the surface leads to various modifications such as physical, chemical, mechanical and antimicrobial properties as discussed later in “Property modifications induced by plasma”.
Polymerizing plasmas
These are types of plasma that convert a precursor gas to a thin film coating, as shown in Fig. 5. Collisions with electrons and ions in the plasma break down the precursor gas molecules to form reactive species that accumulate on a surface, forming a plasma polymer if the precursor gas contains some hydrocarbon component (Dowling 2014). The plasma polymer which is formed on the surface is different from conventional polymers as it contains crosslinked fragmented structures that are arranged in a disordered manner. The choice of precursor affects the composition and structure of the plasma-deposited coating. The degree to which the coating reflects the original molecular structure of the precursor depends on the plasma conditions. Energetic plasmas containing electrons or ions have sufficient energy to break the precursor molecules into smaller fragments before incorporation with the coating, while low-energy plasmas preserve the precursor structure to a variable extent. The thin polymer formed on the surface usually adheres well to the substrate material and the surface properties of the material are then changed to reflect those of the thin film coating. Usually, polymer films are chemically inert and resemble other strongly crosslinked polymers in being physically stable and mechanically tough (Morent et al. 2011). These polymer films find wide applications in different fields due to their surface characteristics which can be designed to suit the application. Free radicals are usually present on the surface of plasma polymers because of their disordered nature and can be utilized for specific functionalization or grafting purposes (Ershov et al. 2014) .
Plasma polymerization can utilize almost all organic, organo-metallic and heteroatomic organic compounds as precursors by breaking them down into reactive fragments. Only small quantities of precursor compounds are needed to treat large surface areas, making it a non-energy-intensive and economical treatment method.
These two types of plasma interactions with the surface of a material can be created using excitation methods such as DC, RF, microwave, dielectric barrier discharge (DBD), capacitive coupling and inductive coupling. These treatments are not limited to modifying a two-dimensional object such as a sheet. Recently, plasma immersion ion implantation techniques have been used to modify the three-dimensional surfaces with complex structures, as discussed in the next section.
Plasma immersion ion implantation
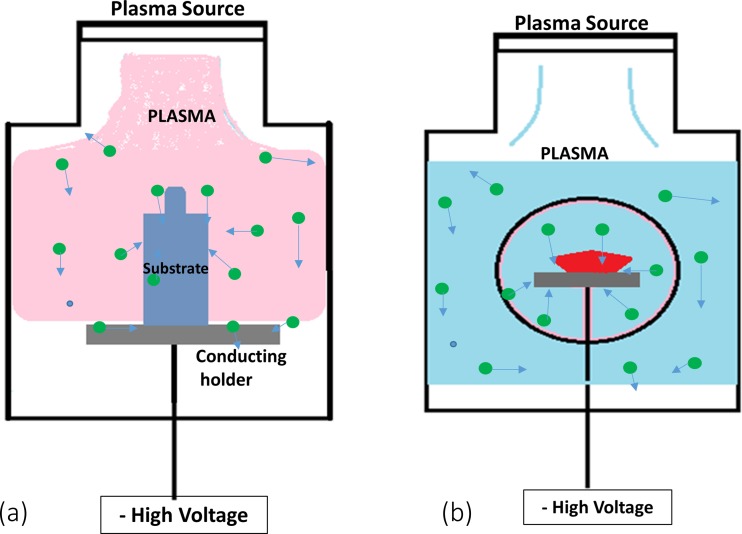
PIII processes impart energies of more than a few tens of eV to ions using applied electrical bias. In PIII, the object is placed in the plasma and a negative bias potential applied to an electrode placed behind or around the object. Referring to Fig. 3a, this can be done, for example, by placing an object on the cathode where it is subjected to bombardment by ions accelerated by the electric field in the cathode dark space. If the electrode is placed behind the object, there are some constraints on the thickness of the object to maintain the bombardment, while, if a cathodic electrode transparent to ions, such as a mesh, is placed around the object, many different shapes of object can be subjected to bombardment. PIII has been used to modify the surface of polymers for several engineering applications. Figure 6 shows a schematic diagram of how a material is treated using PIII.
Fig. 6.
Shows two ways of achieving PIII treatment of an object termed the substrate: a placement on a conductive holder, b surrounding the object to be treated with a mesh
In a PIII process, the object is subjected to higher energy ion impacts than possible using the self-bias that applies to conventional plasma treatments. For insulating objects such as polymers, ion implantation creates positive charge that limits the amount of ion bombardment that can be achieved unless neutralization is allowed for by pulsing or alternating the direction of the applied voltage. Ion energies of up to 20 keV are convenient using pulsed power supplies. As the ions implant into a polymer material, they break bonds and lead to the formation of crosslinks and to the formation of free radicals in the surface and in the subsurface layers. The unpaired electrons from the radicals form in an extended subsurface layer, but can propagate to the surface where they may facilitate the formation of new chemical bonds between the surface and any adsorbed species present on the surface. The radical groups formed in the PIII treatment have lifetimes ranging from minutes to years (Kosobrodova et al. 2012). Long-lasting radicals formed in PIII are able to retain the ability of treated polymers to form new covalent bonds for long periods. The hydrophilic character of the surface helps retain the functional conformation of any attached biomolecules for long periods (Kosobrodova et al. 2015). PIII methods are proven to operate in both non polymerizing plasma and polymerizing plasma interactions. Recently, polymerizing plasma using PIII is important in creating active binding surfaces suitable for biomedical applications, as discussed later.
Post-discharge plasma treatments
In the plasma processes mentioned so far, the materials to be treated are immersed in the plasma. If the materials for treatment are placed outside the plasma, and are subjected only to the products of the plasma process that are carried downstream in a gas flow, we refer to the process as a post-discharge plasma treatment. An example of such a treatment is the use of a microwave plasma to generate species from an Ar-CF4 plasma (Canal et al. 2009) that are used to treat materials some distance from the plasma source. Post-discharge treatments are attractive because the materials under treatment do not receive bombardment by energetic ions that, in high fluences, may cause excessive carbonization and possible embrittlement of fibers. Another example of a pos- discharge plasma treatment is the treatment of a surface using the flowing stream produced by a plasma torch that operates at atmospheric pressure (Encinas et al. 2010).
Plasma modification of internal surfaces of woven and non-woven fabrics
Wound dressing materials that consist of woven or non-woven fabrics and fibrous masses present a special challenge to plasma processing, as it is usually desired to treat the internal surfaces. Atmospheric pressure devices such as plasma torches are essentially post-discharge plasma treatments in the sense of the previous section, and can provide a solution whereby the species produced by the plasma are able to flow through the fibrous structure of the wound dressing, attaching to the fibers from the flowing gas. Another solution is to enable the plasma to enter the three-dimensional structure by providing appropriate plasma conditions. To provide these conditions, we draw upon the similarity parameter, the pressure distance product (Pd). It has been found that the behavior of plasmas is frequently similar at different pressures when examined on an appropriate spatial scale which scales inversely with the pressure. Such a principle is employed in packed bed plasma reactors where the dielectric discharge plasma created by pulsed or alternating fields can penetrate a packed bed of insulting beads provided that the pressure P and distance between the beads d is appropriate (De Geyter et al. 2006). Experimental observations in our laboratory shows that a plasma can penetrate a space between insulators in a DBD separated by 10 mm if the pressure is higher than 0.1 mbar in a dielectric barrier discharge operating at 3 kV. This implies that a plasma could penetrate and exist within a fibrous wound dressing fabric with a spacing between fibers of 10 μm if the pressure is 100 mbar. This is in fact the case, as shown by Poll et al. (2001), who studied the penetration of plasma into woven cotton textile fabrics and found that pressures of between 1 and 100 mbar gave optimum penetration of a plasma operating with the geometry of Fig. 3 at a frequency of 20 kHz in air, oxygen or hydrogen. There is considerable experience with the modification of textiles using plasmas. For example, nylon (Tseng et al. 2009) and PET (Yang and Gupta 2004) were made more hydrophilic by atmospheric plasma treatments. Low-pressure plasmas are also used for this purpose (Hochart et al. 2003). The book by Kan (2014) discusses the considerable literature on the topic of textiles by plasmas at atmospheric and reduced pressure plasmas.
Property modifications induced by plasmas
Examples of useful property modifications caused by plasma treatments are discussed in the following sections.
Surface energy modification
The polar component of the surface energy of a material determines the attractiveness of the surface to polar molecules such as water. Without modification, polymeric materials for the most part are not strongly hydrophilic, with water contact angles in the mid- to upper range between 0 and 90°. Features that are required in a wound dressing may include moisture retention, moisture repulsion, permeability and porosity as well as antimicrobial action, all of which are impacted upon by the water contact angle. If a wound dressing is required to have a delivery function, for example, a drug, biomolecules or cells to the site, then the water contact angle must then be refined for the individual case.
The surface energy, or interfacial energy, is usually characterized by the contact angle of water to determine the polar component of surface energy and the contact angle of a non-polar liquid to determine the non polar, or dispersive, component. Contact angles are usually measured using a goniometer by observing the profile of a liquid drop on the solid surface (Kwok and Neumann 1999). The basic equation for a liquid drop in contact with the solid surface is given by Young (1805) . The relationship between the contact angle and the interfacial surface energies is represented using Young’s equation (Kwok and Neumann 1999).
| 1 |
where γLG is the liquid–gas interfacial energy, θc is the equilibrium contact angle, γSG is the solid–gas interfacial energy, and γSL is the solid–liquid interfacial energy. The contact angle can be measured by static drop micro-observation or the dynamic testing method which uses Wilhelmy’s principle. The dynamic contact angle method is used to study the surface and interface tension between the solid, liduid and gas. It measures the wetting force to estimate the contact angle (Huang et al. 2006).
The roughness of a surface affects the apparent contact angle. The contact angle is also affected by chemical heterogeneity, the presence of polar groups, adsorbed layers, porosity, swelling, molecular orientation and yarn torsion (Maejima 1983; Perwuelz et al. 2001). Surfaces which have a water contact angle of less than 90° when smooth develop stronger hydrophilic behavior when rough, and surfaces that have a water contact angle of more than 90° when smooth develop stronger hydrophobic behavior when rough (Miwa et al. 2000).
The surface energy of materials can be increased or decreased with non-polymerizing plasma depending on the gas used to form the plasma. For example, plasmas containing gases such as CF4, Freon, SF6 and hexafluoroethane decrease the surface energy, making the surface water-repellent. Sigurdsson and Shishoo (1997) modified the surface of PE, PP and cellophane using CF4 plasma to produce surfaces with a low surface energy of 2–20 mJ/m2. Similarly, Hodak et al. (2008) and Chaivan et al. (2005) have used a plasma to increase the contact angle of silk in an hexafluoroethane atmosphere. The latter found that there was a significant increase in the contact angle of silk with a decrease in surface energy from the natural value of 95–20 mJ/m2.
Non-polymerizing plasma is widely used to modify the hydrophilicity of materials by introducing functional groups such as –COOH, −OH and NH2 (Lai et al. 2006; Yang et al. 2009). Recently, Zille et al. (2015) have extensively reviewed the use of plasmas for modification of textiles for mainly non-medical applications. The review includes a table of the effect of plasma treatments on contact angle for different polymeric fibers such as PET, PE, PP, polytetrafluoroethylene (PTFE), silk, polyamide, polyimide, nylon-cotton-elastane, wool, hemp, carbon fibers, aramid, leather and cellulose. Ferrerro and Bongiovanni (2006) have studied the effect of non-polymerizing RF air plasma on wettability, adhesion and the dyeing properties of a cellulose film. The wettability was tested for different polar liquids and was found to increase with plasma treatment.
Polymerizing plasma treatments have also been used to increase the hydrophobicity of materials using, in particular, polymerizing fluorocarbon gases like hexafluoropropylene (Li and Jinjin 2007), hexafluoroethane (Sun and Stylios 2006) and tetrafluoromethane (Sigurdsson and Shishoo 1997), which have all been used to increase the hydrophobicity of fabrics. Tsafack and Levalois-Grützmacher (2007) have introduced flame- and water-repellent properties to cotton fabrics by plasma-induced graft polymerization. They have found that multifunctional surfaces can be produced by plasma-induced graft polymerization. The review by Zille et al.(2015) discusses the increase in hydrophobicity using polymerizing plasma for different polymers, and includes a table listing the contact angles obtained using various monomer precursors. Plasma-polymerized surfaces have been used for cell attachment and proliferation for wound healing, as discussed later.
Huang et al. (2006) studied the surface roughness and the dynamic contact angle of plasma-treated polypropylene fibers. Polypropylene fibers were treated with oxygen and argon plasma at various powers. The surface roughness was analyzed using AFM and the dynamic contact angle was analyzed using Wilhelmy’s theory. The contact angle measurements revealed that the advancing and receding contact angle decreased considerably after oxygen or argon plasma treatment. AFM observations revealed that etching of the fibers after plasma treatment increases the surface roughness and concluded that the decrease in the receding angle was due to the surface roughness while the decrease in the advancing angle was due to the surface properties of the fibers. The surface roughness influences the wettability of the materials, is dependent on the intensity of plasma and increases with plasma intensity (Larsson and D’erand 2002), which is defined as the ratio of discharge power to gas flow rate. The increase in surface roughness increases the surface area of the material and is also useful in facilitating cell attachment (Govindarajan and Shandas 2014).
The surface properties of five different (high density PE, polystyrene, PTFE, PS, PP, PET) thermoplastic polymers treated with argon plasma have been compared by Řezníčkovác et al. (2011). The contact angle as well as the surface roughness were found to decrease with plasma treatment. It was found that PS has the highest concentration of oxidized structures when compared to other polymers and that is correlated with enhanced hydrophilicity. Similarly, PP and PET films were modified by DBD in air, argon and helium, and the variation in contact angle with the gas type was studied. It was found that air plasma is more effective than argon and helium plasma in introducing oxygen-containing functional groups onto the surface, significantly decreasing the contact angle (De Geyter et al. 2007).
Peršin et al. (2013) modified wound-dressing materials to increase the absorption of wound exudate and blood from the wound site. They have investigated the absorption properties of oxygen plasma-treated cellulose fabrics for saline solution, exudate and blood. The sorption dynamics on a macroscopic and microscopic scale were studied using the Wilhelmy balance pendent drop method and optical polarization microscopy. The aging effect of the treated samples was studied in air, nitrogen and argon atmospheres. The microscopic and macroscopic studies showed increased absorption rates and capacity for saline and exudate solutions. The aging effect was found to be small and was independent of the atmosphere of exposure.
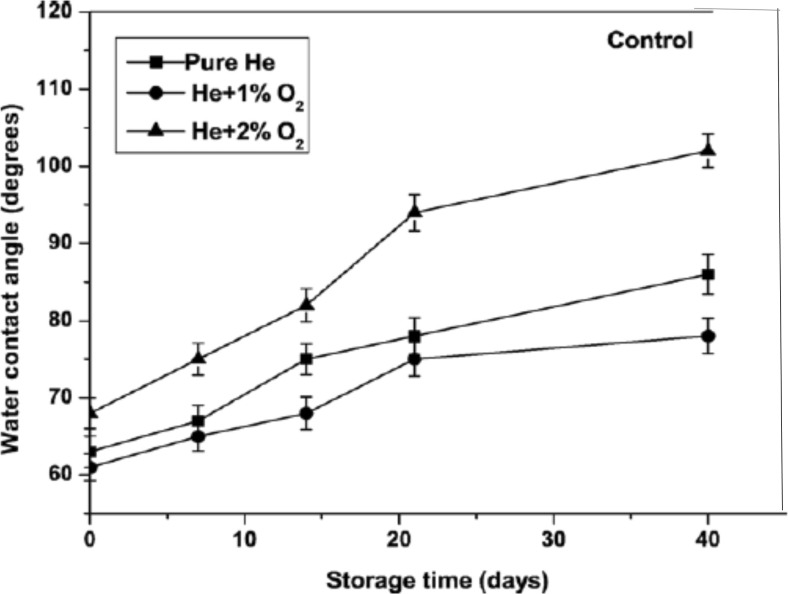
Pivec et al. (2014) describe the effect of a low-pressure oxygen plasma on a non-woven cellulose textile. They found that the plasma treatment decreased the contact angle to below 30° from the initial 90°. Similar reports (Nithya et al. 2015; Peršin et al. 2014b) have shown that oxygen plasma treatment of polymers results in a decrease in the contact angle. However, it is usually found that there is hydrophobic recovery after treatment of polymer films (Bodas and Khan-Malek 2007; Lawton et al. 2005), fabrics and foils (Hossain et al. 2006; Morent et al. 2010) in which the contact angle progressively increases again with time after treatment. Such an effect has also been reported in polyethylene fibers treated in a He/O2 plasma. The initial reduction in contact angle was followed by a recovery to a larger contact angles, as shown in Fig. 7. The fibers treated with He +2% O2 were found to be hydrophobic after 40 days of aging, while treatments with less oxygen in the plasma recovered more slowly. The hydrophobic recovery was attributed to the decrease in the surface oxygen concentration for the treated fibers with storage time.
Fig. 7.
The hydrophobic recovery shown as a plot of water contact angle as a function of storage time for polyethylene fibers treated in a He/O2 plasma (Ren et al. 2008)
Hydrophobic recovery after plasma treatment sets a limit to the shelf life of plasma modified dressings. In some special cases, strategies have been devised to slow down the process, as reported by Eddington et al. (2006). Samples of PDMS were thermally aged and cured before oxygen plasma treatment, and assessed for hydrophobic recovery rate over 14 days by contact angle measurement. The mechanism for the hydrophobic recovery was suggested to be the diffusion of low molecular weight polymer chains from the bulk of the plasma-treated PDMS to cover the unstable surface. Thermal aging was found to delay the hydrophobic recovery ,and the hydrophilic surfaces were stabilized if the samples were stored in nitrogen atmosphere.
Chemical property modifications
Modification of the chemical properties of a polymer surface by plasmas is facilitated by the formation of functional groups including radical-containing groups. The presence of unpaired electrons in radical groups is quantified by the method known as electron paramagnetic resonance or electron spin resonance (ESR) (Kosobrodova et al. 2012). Tasker et al. (1994) reported that significant free radical concentrations can be created on polymeric surfaces under cold plasma environments, regardless of the nature of the substrates or plasma gases. Ar, O2, N2, H2, CO, CH4 and CF4 plasma-generated relative free-radical intensities from cellulose fiber surfaces, such as cotton, linen, viscose rayon and polynosic fibers, and also from wool, silk, nylon 6 and poly(ethylene terephthalate) were measured by ESR. The results obtained revealed that the trapped free radicals can survive in the polymer matrix and that their concentrations can vary significantly with the nature of the fibers and the plasma gases. The order of free radical concentrations was established for various substrates and plasma gases in the order: cotton > linen > mercerized cotton > nylon 6 > polynosic fibers = viscose rayon, and CF4 > CO > H2 > Ar > CH4 > N2 = O2. It was also found that the longer the treatment time, the higher the free-radical concentrations. X-ray photoelectron spectroscopy (XPS) studies revealed that exposure to a CF4 discharge induces comparable relative surface atomic compositions regardless of the nature of the substrate. The stability of active free radicals trapped in cotton fibers after exposure to O2, CF4 and CO plasmas at different temperatures was investigated by Wakida et al. (1989). The activated fibers were conditioned at room temperature, and within a temperature range of 120–200 °C. The relative free-radical concentrations in the CF4 and CO plasma-treated fibers decreased markedly with an increase in temperature and exposure time to air, whereas free-radical intensities of oxygen plasma-treated substrates did not undergo substantial changes. Similar free-radical instabilities were observed on argon plasma-treated jute and cellulose by Sabharwal et al. (1993). Plasma immersion ion implantation may assist in the preservation of the hydrophilic modification by creating a deeper reservoir of radicals that may diffuse to the surface and maintain a lower contact angle (Bilek et al. 2011).
The surface chemistry changes of plasma-modified samples have been measured by techniques such as ATR-FTIR, XPS which measure the changes in the population of chemical groups, and chemical bonding states. Plasma treatment introduces not only free radicals but also functional groups, such as carboxyl and aldehyde groups (Nithya et al. 2011; Vosmanská et al. 2015), C = O (Guruvenketa et al. 2004; Lai et al. 2006), NH2 (Chevallier et al. 2001; Schroder et al. 2001) and fluorine (McCord et al. 2003; Woodward et al. 2003), containing functional groups depending on the type of working gas and subsequent environmental exposure that may be the result of the reaction of the radicals with surface-adsorbed gases. Plasma treatment of cotton fabric is characterized by the formation of carboxyl groups and free radicals on the fabric surface, making it more reactive by changing the surface chemistry and morphology (Chen 1991; Ward et al. 1979). These free radicals and the functional groups created using plasma can be utilized to incorporate specific functionality to the surface as discussed in later sections.
Mechanical property modifications
The mechanical properties of a wound dressing determine whether the dressing is durable, stress-resistant, soft or elastic (Khan et al. 2000). Surface mechanical property changes have been measured using indentation equipment that measures the force displacement responses of sharp tips of known tip radius (Powles et al. 2007). Plasma modifications lead to a change in the surface property, leaving the bulk at depths greater than 40 nm unaltered. However, some deterioration of the bulk property of the polymer may takes place under extreme conditions of plasma treatment (Hwang et al. 2005). A physical modification like etching by a plasma may lead to changes in a polymeric material which affect the fabric hand value (Sun and Stylios 2005). Wong et al. (1999) found that the surface roughness increase due to plasma etching affects the low-stress mechanical property of the polymeric material. The tensile strength of the fabrics was reduced due to the plasma treatment conditions which affected the bulk molecular structure. However, there are also reports that showed an increase in the tensile strength of some fibers after plasma etching (Hwang et al. 2003; Qiu et al. 2002). The tensile strength and Young’s modulus of the treated fabrics were increased with plasma treatment. Chen et al. (2001) studied the structural and mechanical properties of UHMWPE by N+ ion implantation. The results revealed that the implanted UHMWPE was found to have higher hardness and Young’s modulus. Valenza et al. (2004) have studied the effect of ion implantation of various types of ions with a range of ion energies on UHMWPE, and observed that the irradiated polyethylene had graphite-like surface structures which showed improved mechanical resistance of the polymer surface.
Air permeability modifications
Air permeability is a property in addition to fluid absorption and moisture retention important to wound healing. However, excessive permeability to gases and water vapor dries out the wound, potentially leading to cell death and a negative impact on healing (Khan et al. 2000; Maver et al. 2015). Literature on the plasma treatment reports that the change in the air permeability and water vapor transmission is dependent on the plasma treatment conditions (Nema and Jhala 2015), substrate thickness and the change in the surface morphology (Kan and Yuen 2005; Yip et al. 2002). Plasma treatment increases the air permeability of fabrics by surface etching and consequent opening of the spaces between the fibers (Nithya et al. 2012; Pivec et al. 2014). Hwang et al. (2005) found that the surface properties such as surface roughness, frictional smoothness, and surface coefficient of friction increased with plasma exposure time. Conversely, several authors have reported a decrease in the air permeability of plasma-treated fabrics (Kan and Yuen 2005; Yip et al. 2002). However, as mentioned earlier, the change in the permeability depends on the plasma treatment conditions and the thickness of the materials and whether they are fabric, film or foam. An optimum gas and water vapor transmission rate to maintain a desired moist environment in a wound can potentially be achieved by tuning plasma treatment conditions.
Antimicrobial property modifications
Plasma surface modification may influence antimicrobial activity of a dressing. Some studies (Canal et al. 2009; Peršin et al. 2014a, c) have reported that gases like ammonia and Ar-CF4 show antimicrobial activity. An ammonia radiofrequency plasma treatment increased the hydrophilicity and was antimicrobial against two Gram-positive bacteria (i.e. S. aureus and E. faecalis) but not against two Gram-negative bacteria (E. coli and P. aeruginosa). Persin et al. (2014c) studied the surface chemical composition, wound-fluid handling properties and antimicrobial activity of ammonia plasma-treated viscose fibers in air and argon atmosphere. The surfaces were found to have good absorption of wound fluids and biostatic properties against skin wound pathogens, irrespective of storage gas, for 30 days. Further, these authors (Peršin et al. 2014a) have studied the effect of storage gases on the durability of ammonia plasma treatments on the biostatic activity and wound fluid absorption. Canal et al. (2009) have studied the antibacterial activity of wool, cotton and polyamide 6 fabrics treated with Ar-CF4 plasma post-discharge for wound dressing application. They observed that the effective bactericidal effect is due to the quantity of incorporated fluorine on the treated wool and polyamide 6 surface. However, cotton samples did not show antimicrobial activity due to the kind of bonding between the fluorine atoms and the surface. Functionalization of surfaces with antimicrobial molecules may further enhance activity against micro-organisms as discussed in the following section.
Biomolecular functionalization of the plasma-treated surfaces
There are two important reasons for providing a further functionalization of the surface of a wound dressing with molecules, even after a plasma treatment has been used. The first is to encourage the growth of the cells in the healing wound and the second is to discourage the growth of bacterial cells that may infect the wound. In this section, we will discuss the functionalization of the modified surface with biocompatible molecules and antimicrobial molecules.
Functionalization with molecules
Additional functionalization of the plasma-modified surface with molecules can be carried out to enhance the interface between healing tissues and the surface of wound dressing. Covalent binding of these molecules may be assisted by prior plasma processing. A summary of the types of molecules and cells that have been used for functionalizing polymeric materials together with the significance of the findings is presented in Table 1.
Table 1.
Functionalization of polymeric materials with molecules and cells
| Material | Structure | Preparation method | Treatment | Cell attachment | In vivo/in vitro | Significance to wound healing | Ref. |
|---|---|---|---|---|---|---|---|
| Polypropylene | Non-woven fabric | Taiwan Textile Research Institute | DC-pulsed plasma-grafting acrylic acid | Chemical grafting with chitosan and poly (N-isopropylacrylamide) | In vivo, Sprague Dawley rats | Increases antibacterial activity, with effective wound closure | Chen et al. 2012 |
| Polypropylene | Non-woven fabric | - | DC-pulsed plasma- grafting acrylic acid | Poly(N-isopropylacrylamide) | In vivo, Sprague Dawley rats | Temperature responsive and effective wound healing | Chen and Lee, 2008 |
| PVC, PP | Films | Plasma polymerization | Hydrocarbon plasma polymer, collagen I | Human keratinocytes | In vitro | Surface chemistry and cell transfer capability were unaffected by sterilization pocedures | Haddow et al. 2003 |
| Chitosan | Membranes, films | Chemical methods | Argon plasma | Human skin fibroblasts | In vitro | Improved surface hydrophilicity, cell attachment, migration and proliferation | Zhu et al. 2005 |
| Regenerated cellulose | Non-woven | KEMEX, The Netherlands | RF plasma (oxygen/ ammonium) treatment + alkaline treatment | Silver chloride nanoparticles | In vitro | Improved antimicrobial activity | Peršin et al. 2014a |
| Regenerated cellulose | Non-woven | – | RF oxygen plasma treatment + alkali treatment | Silver chloride nanoparticles | In vitro | Combination of all three treatments improved the characteristics | Pivec et al. 2014 |
| Viscose | Non-woven fabric | KEMEX, The Netherlands | Ammonia plasma | – | – | Improved absorption and the biostatic properties | Peršin et al. 2014b |
| Wool cotton polyamide6 | Fabric |
Dimtex S.A, Mas Molas S.A Flotats S.A. |
Ar-2%CF4 microwave –post-discharge plasma | – | – | Improved in the antimicrobial activity of fluorinated surface due to the quantity of F and the kind of bonding | Canal et al. 2009 |
| Cellulose | Non-woven | Holzbecher, Zl’ıˇc Czech republic | Argon plasma | Chitosan, AgCl | – | Improved hydrophilicity due to plasma in turn increased chitosan content and Agcl precipitation on the surface enhancing antimicrobial activity. | Vosmanská et al. 2015 |
| PVC | Film | Beijing Huaer Co., Ltd | Oxygen PIII | Triclosan and bronopol | - | Increased hydrophilicity and antimicrobial activity | Zhang et al. 2006 |
| Chitosan | Nanofibers | Electrospinning | Argon plasma | Silver nanoparticles | – | Increased antimicrobial activity | Annur et al. 2015 |
| Chitosan | Membranes | Solvent casting | Nitrogen and argon plasma | L929 cells | In vitro | Enhanced cell adhesion and proliferation | Luna et al. 2011 |
| Polystyrene | Dishes | Sterelin, UK | Isopropyl alcohol plasma | Human fibroblast cells | - | Increased hydrophilicity, cell attachment and proliferation | Mitchell et al. 2004 |
| Silicon wafers | Film | Plasma polymerization | Acetylene and N2/Ar pulsed RF plasma | Horseradish peroxide | - | Good mechanical properties, high protein activity for longer time | Yin et al. 2009 |
| Polyethylene | Film | - | N2 co-PIII | Silver | In vitro | Improved antimicrobial activity and biocompatibility. | Zhang et al. 2008 |
| Silk fibroin | Nanofibers | Electrospinning | Oxygen/methane plasma | Normal human epidermal keratinocytes and fibroblast | In vitro | Increased hydrophilicity, cell attachment and proliferation for oxygen plasma-treated samples | Jeong et al. 2009 |
| Silk fibroin | Nanofibrous scaffold | Electrospinning | Microwave argon plasma | Human articular chondrocytes | In vitro | Increased cell attachment and proliferation | Baek et al. 2008 |
| Silk fibroin and wool keratose | 3D nanofibrous scaffold | Electrospinning | Microwave-induced argon plasma | Human articular keratinocytes | In vitro | Increased hydrophilicity, cell attachment and proliferation | Cheon et al. 2010 |
| Chitosan | Membranes | Chemical methods | RF Ar and nitrogen plasma | F1 544 cells | In vitro | Increased surface roughness facilitating cell attachment and migration | Saranwong et al. 2012 |
| Polyethylene | Foil | Granitol Ltd.,Czech Republic | DC argon plasma | Grafted with biphenyldithiol, Au nanoparticles | In vitro | Increased cell adhesion | Kasálková et al. 2012 |
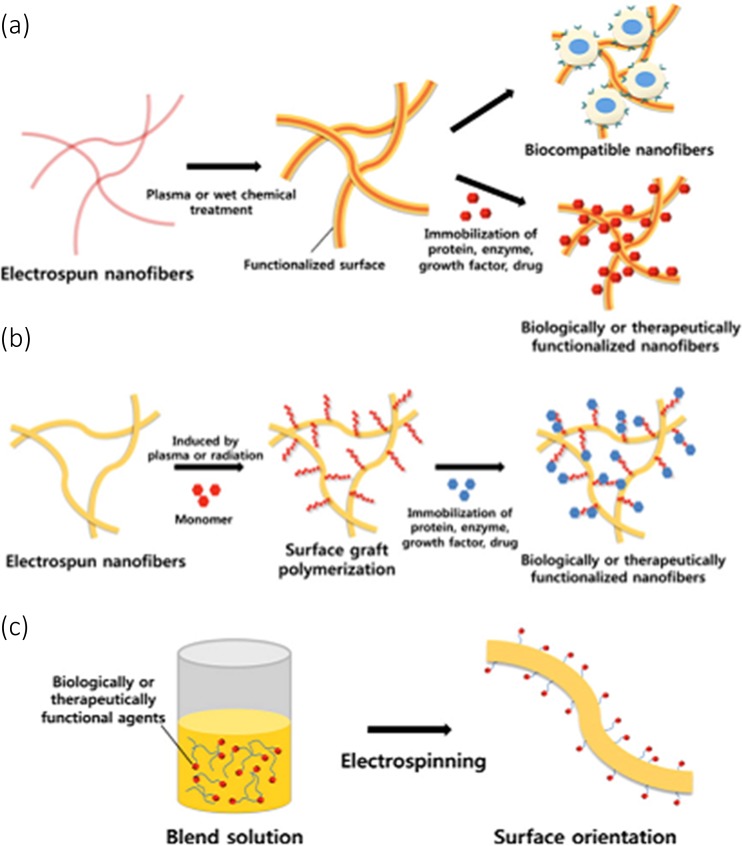
Some studies have reported the functionalizing of polymers with molecules, both with and without plasma treatment. Some strategies for functionalizing polymer fibers are shown in Fig. 8, following Yoo et al. (2009). Plasma treatment is part of the wider set of technologies for functionalizing the surfaces of polymer fibers. Wet chemical, surface graft polymerization and co-electrospinning surface modification techniques for electrospun nanofibers have been carried out, as shown in Fig. 8.
Fig. 8.
Schematic diagram showing strategies for functionalizing the surfaces of fibers: a Immobilizing biomolecules, b surface graft polymerization, c co-electrospinning (Yoo et al. 2009) (Permission requested but not yet received)
These methods have been used to attach various molecules to woven and non-woven wound dressings (Fig. 8). Molecules may be attached using physical adsorption or chemical bonding as for example, by a covalent bond. The strength of physical adsorption is primarily affected by surface energy. The modification of surface energy and its measurement by contact angle has been discussed in “Property modifications induced by plasma”. Covalent bonding is carried out using plasma grafting when a polymer is covalently bonded with another. Chen and Lee (2008) developed a polypropylene non-woven fabric wound dressing by grafting collagen and poly (N-isopropylacrylamide) chemically on plasma-induced graft polymerization of acrylic acid. The authors have studied the physical, chemical and microstructure of the modified non-woven fabrics and tested the samples for wound healing on Sprague Dawley (SD) rats. The results revealed that water-absorbing capacity was improved after plasma treatment and subsequent grafting, which gives the material a potential use as a wound dressing. The wound-healing test results showed a decrease in wound area to 5% in 17 days for bi-grafted polypropylene fabrics as compared to conventional gauze. Similarly, Chen et al. (2012) have modified polypropylene non-woven fabric using DC-pulsed oxygen plasma-induced graft polymerization of acrylic acid to improve hydrophilicity. Chitosan was grafted on to the polymerized surface and subsequently grafted with poly (N-isopropylacrylamide) chemically to obtain temperature responsive and easy strip-off characteristics from the wound. The samples were then analyzed in vitro for cytotoxicity, antibacterial activity and wound healing in SD rats. The results revealed that the water-absorbing capacity increased after plasma treatment and increased further when grafted with chitosan and poly (N-isopropylacrylamide) which in turn increased the absorbance of wound exudate. The antibacterial activity of the chitosan-grafted samples were found to increase to 99.7% and decreased slightly to 97.1% after grafting with poly (N-isopropylacrylamide). The wound test results revealed decreased wound area of 4.9% after 17 days for the polypropylene-bigrafted samples when compared to commercial gauze. Histological observations revealed no inflammation or infection in the wound site during the healing process.
Yin et al. (2009) investigated the immobilization of proteins on a range of surfaces by means of deposition from polymerizing plasmas containing acetylene and either nitrogen or argon or both. Plasma polymerization was carried out onto silicon wafers with a pulsed power supply coupled to a conductive sample holder. Horseradish peroxidase (HRP) was immobilized on the plasma-polymerized surfaces and tested for its activity. From the results, it was observed that the plasma polymer surfaces were smooth with good mechanical properties and had a high density of attachment sites for strong immobilization of proteins. They also observed that the protein activity of HRP was preserved for a longer time after immobilizing on the plasma polymer surfaces than when on the untreated surfaces.
A different approach has been adopted by de Jesus Martínez-Gómez et al. (2010) to immobilize α-Chymotrypsin on oxygen or argon plasma-treated polypropylene surfaces. The immobilization was carried out on the in situ gas phase derivatised spacer chain molecules synthesized from ethylene diamine, oxalyl chloride, and glutaraldehyde after plasma treatment. The free radicals which are produced by plasma treatment interact with the derivatised molecules to form stable compounds on the surface. The in situ derivatization was found to be advantageous, as the authors were able to avoid the formation of undesired chemical compounds on the surface which block the free radicals. The activity of such immobilized enzymes was found to be high on the oxygen plasma-modified polypropylene surface. These biomolecules which are functionalized on the modified surfaces are proven to last for longer times and can further facilitate the attachment and growth of cells to accelerate healing in chronic wounds as discussed in “Attachment and growth of cells on plasma treated surfaces”.
Antimicrobial functionalization
Functionalization of polymeric wound dressing materials with antimicrobial agents is desirable to avoid infections. This functionalization of the surface can be achieved using biological or inorganic antimicrobial agents. Dastjerdi and Montazer (2010) have reviewed the various approaches to the antimicrobial modification of textiles with a focus on inorganic nanostructured antimicrobial agents. Some approaches include cytotoxic agents such as silver and other molecules and agents that disrupt the ability of bacteria to form a biofilm (this approach uses peptides and other biomolecules). Silver has been used for a long time as an antimicrobial agent from simple wound dressings to medical implants. Several studies have been carried out using silver compounds on plasma-treated surfaces for antimicrobial applications. Peršin et al. (2014b) have followed two different procedures for improving antimicrobial activity and also hydrophilicity. The authors have modified the non-woven viscose fibers by two procedures. The first is with alkaline or oxygen plasma treatment and the second with ammonia plasma treatment to improve the hydrophilicity followed by the incorporation of silver chloride nanoparticles by a sol–gel method. A three-step modification of cellulose for antibacterial wound dressings was studied by Vosmanská et al. (2015). The authors modified the surface of cellulose with plasma, which increased the amount of chitosan impregnated and in turn the precipitation of silver chloride. The combination of the chitosan impregnation and silver chloride precipitation increased the antibacterial activity more than these treatments individually applied. Pivec et al. (2014) modified viscose non-woven fabrics to increase the absorption capacity and antimicrobial activity. Silver nanoparticle, alkali and oxygen plasma treatments were carried out for viscose fabrics. The results revealed that the combination of all the treatments modified the surface of fabrics and could impart many of the desired characteristics of a wound dressing. Silver nanoparticle coatings provided antimicrobial activity for the fabrics and alkali treatment increased their breaking force and air permeability. Plasma treatment was effective in increasing the water retention capacity and air permeability of the viscose non-woven fabrics, both desirable characteristics of a wound dressing.
There are more examples of how a plasma can work synergistically with conventional methods. Annur et al. (2015) prepared electrospun nanofibers chitosan/PEO acetic acid solutions that were preloaded with silver nitrate. The nanofibers were subjected to argon plasma treatment resulting in the decomposition of silver nitrate to silver nanoparticles immobilized on the surface. The antimicrobial activity against E. coli increased with plasma treatment at different concentrations of silver nitrate on the electrospun nanofiber. Zhang et al. (2008) employed the PIII method to implant silver on polyethylene to improve its antibacterial property as well as biocompatibility. They have also used N2 co-PIII treatment to prolong the antibacterial effects but had little effect on the Ag distribution near the surface. The silver impregnation using PIII treatment showed antimicrobial activity with biocompatibility and further enhanced the cell attachment.
As mentioned earlier, there are also certain organic and inorganic molecules which show antimicrobial activity when functionalized with plasma-treated surface. Yao et al. (2008) modified electrospun polyurethane nanofibers with poly(4-vinyl-N-hexyl pyridinium bromide) involving glow discharge plasma pretreatment. Subsequently, the samples were UV-induced graft-copolymerized followed by quaternization of the grafted pyridine groups with hexylbromide. Polyurethane fibrous membranes were treated with glow discharge argon plasma at a power of 35 W and frequency of 40 kHz. The mechanical properties of tensile strength, elongation at breakage and Young’s moduli were all assessed. It was found that there was a decrease in the tensile strength and elongation at breakage with no significant change in the Young’s modulus. The antimicrobial activity of the modified nanofibers against E. coli and S. aureus were effective, preventing biofilm formation on the modified surface, attributed to the presence of quaternary ammonium groups. The nature of the bacterial action is hypothesized to be that the immobilized moieties disrupt the integrity of the cytoplasmic membrane to cause cell death, similar to the mechanism of free biocides.
Nithya et al. (2012) reported the synergistic effect of plasma and cellulase enzyme treatment of cotton fabrics for enhanced hydrophilicity and antimicrobial activity. The authors carried out combinations of enzyme and plasma treatments for enhancing the hydrophilicity, which helps in the absorption of natural antimicrobial agents extracted from the leaves of the neem tree. The synergistic treatments were found to have improved hydrophilicity and antimicrobial activity. Enzyme-pretreated plasma samples were reported to have maximum antimicrobial activity against bacteria and fungi and have found applications as a medical textile. Similar studies have been carried out by other authors employing natural antimicrobial agents (Anitha et al. 2015; Vaideki et al. 2008).
Zhang et al. (2006) modified medical-grade PVC with oxygen plasma treatment to improve the antibacterial performance. These authors used PIII to coat PVC with triclosan and bronopal to enhance its antimicrobial properties. The results revealed that the triclosan- and bronopal-treated samples showed antibacterial activity but was found to decrease with time. Furanones are effective antimicrobial agents which can inhibit the growth of both Gram-positive and Gram-negative bacteria. Plasma polymerization has been used by Hume et al. (2004) as a step in the covalent binding of a furanone to a polymer surface which can be used as catheters. These researchers found that the furanone compound attached covalently maintained the stability and activity even after the sterilization process. These furanones can also be used in wound dressings as an antimicrobial agent. A variety of antimicrobial agents have been exploited, but challenges still exist to prolong the antimicrobial activity in plasma-treated surfaces especially for medical applications.
Attachment and growth of cells on plasma treated surfaces
Recently, the wound care industry has focused on developing wound healing using molecular and cellular therapies. A detailed review on the molecular and cellular therapies for wound care using functional biomaterials was published by Piraino and Selimovic (2015). Healing of wounds depends on various growth factors and interactions between cells as well as with cells and matrixes. The therapeutic agents depend on the type of wound and the tissue property to accelerate the healing process. As cell attachment is influenced by surface energy, surface chemistry and surface mechanical properties, plasma treatments are used to modify these properties in wound-dressing materials. In this section, we discuss the attachment and proliferation of cells on plasma-treated surfaces and on plasma-functionalized surfaces with bio-molecules specific to wound healing.
Bioactive molecules to promote cell adhesion and proliferation
Plasma surface modification enables the introduction of diverse functional groups on the polymer surface which have significant effects on the attachment and growth of cells. Alternatively, linker bioactive molecules can also be immobilized on the plasma-functionalized surface to promote the attachment and spreading of cells onto the surface of wound-dressing material. For example, immobilization of proteins such as laminin, collagen, gelatin tropoelastin and fibronectin on the plasm- treated polymer surface enhances cell adhesion and proliferation (Baek et al. 2008; He et al. 2005, 2006; Koh et al. 2008).
Plasma polymers can play an important role in wound healing when they are tailored with specific surface properties to enhance cell adhesion and proliferation. Haddow et al. (1999) compared the proliferation and growth of human keratinocytes on plasma copolymers and self-assembled monolayers. Cell attachment and subsequent proliferation was studied on plasma copolymers of acrylic acid/1, 7 octadiene with tissue culture poly (styrene) (TCPS), a hydrocarbon PP, collagen I, a methyl-terminated SAM, and a pure gold surface as reference surfaces. Plasma copolymerization was carried out in RF plasma. Plasma copolymer surfaces with a low concentration of carboxylic acid were found to attach, proliferate and promote the growth of human keratinocytes when compared to hydrocarbon surfaces. DNA assays confirmed that the number of cells on the acid-containing surfaces were higher than the collagen I surfaces. Mitchell et al. (2004) studied the deposition of films using isopropyl alcohol plasma for cell attachment and proliferation of human fibroblast cells in the absence of carboxylic acid groups. Isopropyl alcohol plasma incorporated C-OR and carbonyl groups increased the oxygen species in a polystyrene surface, which increased the hydrophilicity with no significant changes in the surface roughness. Cell-patterning experiments revealed that cell attachment occurred on the hydrophilic surfaces and was found to extend to hydrophobic surfaces, showing the ability of 1BR.3 N cells to facilitate the attachment and proliferation.
Nanofibrous materials acting as a scaffold for the attachment and controlled delivery of cells to wound sites benefit from plasma treatments. Plasma-modified nanofibrous surface were found to be more beneficial in cell behavior than untreated ones. Nanofibers made of synthetic biopolymers such as poly(glycolic acid), poly(l-lactic acid), or poly(lactic-co-glycolic acid) were modified by oxygen plasma with in situ polymerization of acrylic acid for surface activation introducing carboxylic acid (Park et al. 2007). The study revealed plasma modification facilitates the fibroblast adhesion and proliferation without affecting the bulk and mechanical properties. Similarly, studies on plasma-modified, silk and chitosan nanofibrous materials for cell attachment and proliferation have been reported, as these materials find application in wound healing. Jeong et al. (2009) treated silk fibroin nanofibers in oxygen and methane plasmas to increase their hydrophilicity and to study the cellular activity of normal human epidermal keratinocytes (NHEK) and fibroblast (NHEF). Contact measurements showed that the hydrophilicity of oxygen plasma-treated silk fibroins increased but methane plasma-treated nanofibers showed hydrophobic behavior. Cell attachment and spreading were analyzed for treated and untreated samples in vitro. The results revealed that the oxygen plasma-treated silk fibroin nanofibers showed good cell adhesion, spreading and activity for both NHEK and NHEF. Microwave-induced argon plasma was used by Baek et al. (2008) to treat the surface of silk fibroin. Nanofibrous silk fibroin scaffolds were treated with 2.45GHz, waveguide-based microwave plasma with argon as a working gas. Cell behavior studies revealed that the cell attachment and proliferation onto the scaffolds were significantly higher than for untreated samples. The density of attachment of human articular chondrocytes was significantly increased by the plasma treatment. The chondrocytes formed a single layer coverage and their natural shape was preserved. Similarly, Cheon et al. (2010) blended silk fibroin and wool keratose into a three-dimensional nanofibrous scaffold by electrospinning. The blended scaffolds were then treated with microwave-induced argon plasma to improve the attachment and proliferation of neonatal human articular keratinocytes. The scaffolds were treated with a waveguide-based microwave-induced argon plasma at a frequency of 2.45 GHz for 12.4 s at atmospheric pressure. The water contact angle was found to decrease after plasma treatment. The formation of nanoscale texture by argon plasma treatment has facilitated the increase in hydrophilicity, cell attachment, growth and proliferation.
Saranwong et al. (2012) modified the chitosan with argon and nitrogen PIII for human skin fibroblast F1 544 cell attachment. The authors found that the argon plasma treatment showed enhanced cell attachment while nitrogen plasma had an inhibiting effect. It is reported that the argon plasma increased the surface roughness by providing the cells with a large surface area to attach and to migrate. Zhu et al. (2005) studied the effect of RF argon plasma treatment on human skin-derived fibroblasts on chitosan membranes. The plasma treatment was carried out at a power of 200 W for a period ranging from 30 s to 10 min. The argon plasma treatment caused the chitosan surface to become bipolar. that is, having acidic and basic regions which enhanced the adsorption of proteins like fibronectin and vitronectin from serum proteins. They have observed that the increase in surface energy, polarity and hydrophilicity of the surface-promoted cell attachment. SEM and MTT assay tests revealed that there is an optimum range of acidity and basicity of the surface that encouraged the cells to attach and proliferate. Silva et al. (2008) carried out characterization and preliminary cell studies on RF plasma-treated chitosan membranes. The results revealed that the surface roughness and surface energy increased due to plasma etching. The cell viability of chitosan membranes increased for plasma-treated samples when compared to untreated ones. The authors proposed that the plasma treatment could facilitate cell attachment and proliferation which can be exploited in wound dressings and tissue engineering applications. As an extension of the work, Luna et al. (2011) modified the chitosan-based membrane using argon and nitrogen RF plasma in optimized conditions to study the surface roughness, hydrophilicity, hydrophobicity and cytotoxicity. In vitro cell studies were carried out to study the cell attachment and proliferation of L929 cells on plasma-treated chitosan membranes. The results revealed that plasma treatment improved the cell adhesion and proliferation due to the presence of oxygen and nitrogen groups on the treated surface when compared to the untreated membranes. Zhou et al. (2008) prepared a biocompatible composite nanofibrous membrane by electrospinning carboxymethyl chitosan/polyvinyl alcohol aqueous solution. In vitro studies were carried out using mouse fibroblasts L929 and indirect cytotoxic assessment were carried out on the electrospun mat for L929 cells. It was evident from the results that the electrospun mats showed no cytotoxicity for the growth of cells and the cells were found to attach and proliferate on the mat.
Plasma-treated polymers were grafted with metallic nanoparticles to study the cell behavior. Švorčík et al. (2009) studied the attractiveness of Ar plasma-treated polyethylene grafted with Au nanoparticles on the adhesion and growth of vascular smooth muscle cells (VSMC) and mouse embryonic 3 T3 fibroblasts. The DC glow discharge plasma was carried out on the high-density polyethylene samples and grafted with Au nanoparticles. The Au nanoparticles were found up to a depth of 100 nm from the surface of the samples, with a change in the surface roughness and hydrophilicity. The contact angle was found to decrease and then increase with plasma exposure time, it was observed that the hydrophilicity increased for an exposure time of 50 s for gold-grafted samples and then became hydrophobic when the exposure time increased. They have also observed that the surface roughness decreased for plasma-treated samples after exposing it to water. The Au-grafted plasma-treated samples significantly increased the attractiveness of VSMC and NIH 3 T3 fibroblasts. As an extension of the work, Kasálková et al. (2012) stabilized the gold nanoparticles on the DC argon plasma-treated polyethylene by grafting with biphenyldithiol. When biphenyldithiol is grafted onto the plasma-treated surface, cell adhesion was increased and proliferation also increased but not by as much for plasma treatment alone. When plasma-treated samples were grafted with biphenyldithiol and Au nanoparticles. the surface electrical charge was modified, as evident from the zeta potential. The presence of biphenyldithiol and gold nanoparticles influenced the adhesion and proliferation of VSMC.
Instead of attaching the cells directly on to the plasma-modified surface, a bed of biomolecules may be first applied to facilitate attachment and spreading. For example, PIII plasma pretreatment could be used to covalently attach protein to the delivery surface. These types of treatments can be exploited in the wound dressings to accelerate healing with specific cell delivery to the wound site. Bax et al. (2011b) have studied the covalent binding of tropoelastin to the PIII-treated PTFE. The PIII-treated covalent binding of proteins was found to exhibit cell activity for a long storage period (Bax et al. 2011a). This work shows how important it is to correctly orient covalently bound molecules on a surface. If a molecule such as tropoelastin is covalently bound with its cell-binding domain tethered to the surface, steric hindrance will prevent it being recognized by cells. Bax et al. (2012) also investigated the covalent binding of the proteins, tropoelastin and collagen I on PIII-modified surfaces of the electrically conducting polymer polypyrrole without using chemical linker molecules. The authors observed that the proteins were successfully able to bind covalently with a PIII-treated surface. A BSA (bovine serum albumin) blocker was used which promoted the attachment and spreading of cells. PIII-treated samples were found to regulate the surface retention of ECM (Extra Cellular Matrix) proteins.
Cell delivery to wounds
New delivery methods use functionalized surfaces to carry cells including stem cells for wound healing, especially diabetic ulcers. There are several papers reporting cell delivery to the wound sites (Machula et al. 2014; Rho et al. 2006); however, they have not deployed plasma-treated surfaces which makes it out of the scope of this review. As the use of plasma technology in wound dressings is in its infancy, the cell delivery to wounds from plasma-functionalized dressings have only been tested by a research group, as detailed below.
Haddow et al. (2003) were the first to investigate the use of plasma-polymerized surfaces which can attach and proliferate human keratinocytes, and showed that cells may be transferred to a wound bed model. Further, the authors have examined the functions of the surface after ethylene oxide sterilization. Acrylic acid and 1, 7-octadiene were polymerized on the surfaces of polyhydroxybutyrate followed by collagen I coating. The cell transfer from plasma-polymerized surfaces to de-epidermalized dermis was found to be higher for the carboxylic acid-containing (21%) surfaces. Later, Haddow et al. (2006) have developed a cell therapy product with past decades research contributions for non-healing diabetic foot ulcers. The research group has investigated cell attachment studies over a range of plasma polymers, and subsequently selected an acrylic acid plasma polymer surface which can proliferate and can be transferred to a wound bed model. The study found that 20.6% acid polymer surface was effective in delivering the cells to the wound which forms the active part of the product. They have transferred their technology from the laboratory by developing the product named MySkin™ for the treatment of diabetic foot ulcers, venous leg ulcers, burns and surgical wounds.
The use of adult mesenchymal stem cells (MSC), embryonic stem cells and induced pluripotent stem cells are being considered for delivery by scaffold-based systems that could be incorporated into wound dressings. Of these, MSCs are especially promising as an additive to wound dressings, since the therapeutic benefit appears to arise from the transmission of mediators into the wound rather than the direct injection of cells into the site. Therefore, the presence of a population of healthy stem cells incorporated into the wound dressing may give benefit. A review of this field has recently appeared (Duscher et al. 2016). Wound dressings that may also be described as bio-scaffolds made from materials such as collagen and hyaluronic acid incorporated into a hydrogel have proven effective (Rustad et al. 2012). An alternative to a hydrogel may be a plasma-treated polymeric fiber to which such molecules could be covalently bound to the surfaces of fibers within wound dressings. Machula et al. (2014) were the first to report the use of a tropoelastin-based biomaterial as a vehicle for the delivery of adipose-derived stem cells to treat dermal wounds. In their study, the authors have electrospun the recombinant human tropoelastin and crosslinked it with glutaraldehyde and, after sterilization, seeded it with adipose-derived stem cells. The authors were able to observe rapid proliferation of the cells on the scaffold-secreting extra-cellular matrix protein covering the entire scaffold. The stem cell-seeded tropoelastin scaffold when used on splinted wounds was found to have greater rates of closure and restoration of normal epithelium.
Future developments
Functionalization with antimicrobial peptides
There are many peptides known to have antimicrobial action, created by interaction with the membranes of bacteria-causing lysis. A review on the principles of action of various types of antimicrobial peptides has been given by Koczulla and Bals (2003). Plasmas can be used to assist in the preparation of the surface of a wound dressing so that it has suitable properties for binding and delivering peptides. Antimicrobial peptides are especially useful in treating polymicrobial infections which occur in diabetic wounds. Peptides are likely to become increasingly important in the treatment of infected wounds where there is resistance to, or a reluctance to use, conventional antibiotics.
Antimicrobial peptides act through several possible mechanisms, including prevention of initial attachment, membrane perturbation and the blocking of quorum-sensing molecules. Quorum sensing is used by bacteria to coordinate the growth of biofilms and in developing antibiotic resistance. The cathelicidin family of antimicrobial peptides inhibits the protease cathespin-L (Duplantier and van Hoek 2013) and is especially promising for treating polymicrobial infections. Since biofilm formation is significant in diabetic wounds, strategies to minimize biofilm formation should be considered as an important part of approaches to dressings for these wounds. New approaches for treating staphyloccocal biofilm infections have been reviewed by Kiedrowski and Horswill (2011). The authors have discussed in detail the molecule inhibitors like DNA, RNA and protein synthesis which are effective against staphylococci. Many new approaches to treat biofilms like enzyme treatment, quorum sensing, immunotherapy or using small molecules are also discussed. As an example of a quorum-sensing inhibitor, the RNA III inhibiting peptide has been shown to be effective in the treatment (Balaban et al. 2007) and prevention of biofilm infections (Balaban et al. 2005). Antimicrobial peptides have been found to be useful antimicrobial agents in treating wounds. One of these, a synthetic peptide D2A21, has been found to be effective in treating infected burns and did not have any deleterious effect on keratinocyte growth (Chalekson et al. 2003).
RNA delivery
MicroRNA and siRNA (small interfering RNA) are small noncoding RNA molecules with therapeutic potential for wounds that are resistant to conventional healing approaches (Banerjee and Sen 2015). MicroRNA is known to play a significant role in various phases of wound healing. siRNA have been used to turn off gene expression pathways in targeted molecules and have been used in cancer therapies, but not as yet for wound healing to any extent. However, there is potential for the treatment of wounds using siRNA where there is suspected involvement of cancer cells. The expression levels and types of microRNA are found to be different for normal and chronic wounds. Lai and Siu (2014) have reviewed the different patterns of microRNAs used for the treatment of chronic wounds. Madhyastha et al. (2012) have observed a significant increase in the rate of migration of fibroblasts to the wound site in diabetic wounds when a specific microRNA sequence is delivered. The authors have reported that the microRNAs exhibited dysregulation in diabetic wounds. A number of microRNA sequences were found to regulate the wound-healing process as detailed in the review by Fahs et al. (2015). There is a rapid development in the use of microRNA targeted therapies in wound healing. Plasma-modified surfaces have been shown to be capable of binding single- and double-stranded DNA and DNA sequences without affecting the ability of the sequences where single-standed bind to their complementary sequence (Tran et al. 2016). Plasma-treated dressings, therefore, have the potential in incorporating and transferring microRNA to the wound site.
Plasma treatment as a platform technology for customized wound dressings
The role of plasma treatment as an enabling technology for a powerful set of patient-specific wound healing strategies is illustrated as a flow chart in Fig. 9. The stages of customization begin with the plasma treatment of the basic dressing fabric, assumed to consist of some form of polymeric fibrous material. The plasma treatment is specified by the degree of penetration of the material by plasma, the treatment intensity and the chemical changes induced in the surface by implantation, etching and polymerization. The most penetrating treatments are achieved by pressure optimization to create plasma inside the network of void spaces between the fibers. The extent of implantation, etching and polymerization is determined by the chemical composition of the plasma and the energetics and type of bombardment, determined by the biasing level and polarity. The dressing may be ready for use at this stage, having been customized for a high level of sterilization or its ability to absorb water from or to encourage its retention in the wound, or to increase or decrease the exchange of air by opening or closing void spaces by etching or deposition, respectively. Alternatively, the dressing can pass to the second stage where the surface chemistry changes induced by the plasma are used to attach molecules to the surface. The attaching of molecules is assisted by the hydrophilicity or hydrophobicity increases or the provision of reactive radical groups or other functional groups such as amine, amide or carboxyl. The molecules can be drugs or therapeutic biomolecules, consisting of polypeptide or nucleobase sequences, selected according to the intended therapy. Some therapies are delivered by living cell populations which may be encouraged to attach and/or proliferate by the choice of molecule that has been made.
Fig. 9.
Flow chart to illustrate how plasma treatment can act as a platform technology for customizing wound healing to the specific needs of the patient
Conclusions
Wound healing is one of the major challenges in the medical industry. Dressings accelerate healing by controlling the moisture, chemical and biological environment of the wound. Plasma surface treatments have been proven as a means of controlling the wettability, water and air permeability and antimicrobial action of wound-dressing materials. Plasma treatments have emerged as an effective tool for not only modifying the surfaces of the dressings but also for incorporating molecules and cells to give specific assistance to the healing process. The provision of suitable proteins on the surfaces of dressings has been shown to assist in the healing of wounds. Plasma activation offers the opportunity for covalent binding of the proteins. Research on cell attachment and the proliferation on plasma functionalized surfaces has shown a strong growth in the medical field and has given rise to opportunities for wound healing. New approaches for healing chronic wounds are being developed, such as the delivery of stem cells and SiRNA and targeting mRNA molecules to the wound site. These approaches would benefit from the ability of plasma treatments to improve the binding of the healing agent to the surface of the dressing. Several promising strategies involving plasma techniques need to be developed further to produce novel wound-dressing materials which can deliver molecules and drugs such as microRNA and antimicrobial peptides to the wound environment with a potential to heal chronic and even cancerous wounds.
Compliance with ethical standards
Conflicts of interest
Nithya Eswaramoorthy declares that she has no conflicts of interest. David R. McKenzie declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Contributor Information
Nithya Eswaramoorthy, Phone: +61469 740 041, Email: nithya.eswaramoorthy@sydney.edu.au.
David R. McKenzie, Email: david.mckenzie@sydney.edu.au
References
- Abidi N, Hequet E. Cotton fabric graft copolymerization using microwave plasma. I. Universal attenuated Total reflectance-FTIR study. J Appl Polym Sci. 2004;93:145–154. [Google Scholar]
- Agrawal P, Soni S, Mittal G, Bhatnagar A. Role of polymeric biomaterials as wound healing agents. Int J Lower Extrem Wounds. 2014;13:180–190. doi: 10.1177/1534734614544523. [DOI] [PubMed] [Google Scholar]
- Anitha S, Vaideki K, Jayakumar S, Rajendran R. Enhancement of antimicrobial efficacy of neem oil vapour treated cotton fabric by plasma pretreatment. Mater Technol Adv Perform Mater. 2015;30:368–377. [Google Scholar]
- Annur D, Wang Z, Liao J, Kuo C. Plasma-synthesized silver nanoparticles on electrospun chitosan nanofiber surfaces for antibacterial applications. Biomacromolecules. 2015;16:3248–3255. doi: 10.1021/acs.biomac.5b00920. [DOI] [PubMed] [Google Scholar]
- Aukhil I. Biology of wound healing. Periodontology. 2000;22:44–50. doi: 10.1034/j.1600-0757.2000.2220104.x. [DOI] [PubMed] [Google Scholar]
- Baek HS, Park YH, Ki CS, Park JC, Rah DK. Enhanced chondrogenic responses of articular chondrocytes onto porous silk fibroin scaffolds treated with microwave-induced argon plasma. Surf Coat Technol. 2008;202:5794–5797. [Google Scholar]
- Balaban N, Stoodley P, Fux CA, Wilson S, Costerton J, Dell'Acqua G. Prevention of staphylococcal biofilm-associated infections by the quorum sensing inhibitor RIP. Clin Orthop Relat Res. 2005;437:48–54. doi: 10.1097/01.blo.0000175889.82865.67. [DOI] [PubMed] [Google Scholar]
- Balaban N, et al. Treatment of Staphylococcus Aureus biofilm infection by the quorum-sensing inhibitor R. Antimicrob Agents Chemother. 2007;51:2226–2229. doi: 10.1128/AAC.01097-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee J, Sen CK. microRNA and wound healing. Adv Exp Med Biol. 2015;888:291–305. doi: 10.1007/978-3-319-22671-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax DV, McKenzie DR, Bilek MM, Weiss AS. Directed cell attachment by tropoelastin on masked plasma immersion ion implantation treated PTFE. Biomaterials. 2011;32:6710–6718. doi: 10.1016/j.biomaterials.2011.05.060. [DOI] [PubMed] [Google Scholar]
- Bax DV, Wang Y, Li Z, Maitz PKM, McKenzie DR, Bilek MM, Weiss AS. Binding of the cell adhesive protein tropoelastin to PTFE through plasma immersion ion implantation treatment. Biomaterials. 2011;32:5100–5111. doi: 10.1016/j.biomaterials.2011.03.079. [DOI] [PubMed] [Google Scholar]
- Bax DV, et al. Cell patterning via linker-free protein functionalization of an organic conducting polymer (polypyrrole) electrode. Acta Biomater. 2012;8:2538–2548. doi: 10.1016/j.actbio.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28:325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Bilek MM, McKenzie DR. Plasma modified surfaces for ovalent immobilization of functional biomolecules in the absence of chemical linkers: towards better biosensors and a new generation of medical implants. Biophys Rev. 2010;2:55–65. doi: 10.1007/s12551-010-0028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilek MM, et al. Free radical functionalization of surfaces to prevent adverse responses to biomedical devices. Proc Natl Acad Sci U S A. 2011;108:14405–14410. doi: 10.1073/pnas.1103277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97:2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- Bodas D, Khan-Malek D. Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma treatment- an SEM investigation. Sensors Actuators B. 2007;123:368–373. [Google Scholar]
- Bogaerts A, Neyts E, Gijbels R, van der Mullen J. Gas discharge plasmas and their applications. Spectrochim Acta B. 2002;57:609–658. doi: 10.1016/S0584-8547(01)00406-2. [DOI] [Google Scholar]
- Breitwieser D, et al. In situ preparation of silver nanocomposites on cellulosic fibers – microwave vs. conventional heating. Carbohydr Polym. 2013;94:677–686. doi: 10.1016/j.carbpol.2013.01.077. [DOI] [PubMed] [Google Scholar]
- Cai Z, Qiu Y. The mechanism of air/oxygen/helium atmospheric plasma action on PVA. J Appl Polym Sci. 2006;99:2233–2237. [Google Scholar]
- Canal C, Gaboriau F, Villeger S, Cvelbar U, Ricard A. Studies on antibacterial dressings obtained by fluorinated post-discharge plasma. Int J Pharm. 2009;367:155–161. doi: 10.1016/j.ijpharm.2008.09.038. [DOI] [PubMed] [Google Scholar]
- Chaivan P, Pasaja N, Boonyawan D, Suanpoot P, Vilaithong T. Low- temperature plasma treatment for hydrophobicity improvement of silk. Surf Coat Technol. 2005;193:356–360. [Google Scholar]
- Chalekson C, Neumeister M, Jaynes J. Treatment of infected wounds with the antimicrobial peptide D2A21. J Trauma Inj Infect Crit Care. 2003;54:770–774. doi: 10.1097/01.TA.0000047047.79701.6D. [DOI] [PubMed] [Google Scholar]
- Chapman B (1980) Glow discharge processes: sputtering and plasma etching. Wiley, New York
- Chen J. Free radicals of fibers treated with low temperature plasma. J Appl Polym Sci. 1991;42:2035–2037. [Google Scholar]
- Chen J-P, Lee W-L. Collagen-grafted temperature-responsive nonwoven fabric for wound dressing. Appl Surf Sci. 2008;255:412–415. [Google Scholar]
- Chen JS, et al. Structural and mechanical properties of nitrogen ion implanted ultra high molecular weight polyethylene. Surf Coat Technol. 2001;138:33–38. [Google Scholar]
- Chen J-P, Kuo C-Y, Lee W-L. Thermo-responsive wound dressings by grafting chitosan and poly(N-isopropylacrylamide) to plasma-induced graft polymerization modified non-woven fabrics. Appl Surf Sci. 2012;262:95–101. [Google Scholar]
- Cheon YW, et al. Enhanced Chondrogenic responses of human articular chondrocytes onto silk fibroin/wool Keratose scaffolds treated with microwave-induced argon plasma. Artif Organs. 2010;34:384–392. doi: 10.1111/j.1525-1594.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- Chevallier P, et al. Ammonia RF-plasma on PTFE surfaces: chemical characterization of the species created on the surface by vapour- phase chemical derivatization. J Phys Chem B. 2001;105:12490–12497. [Google Scholar]
- Dastjerdi R, Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids Surf B. 2010;79:5–18. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- De Geyter N, Morent R, Leys C. Pentration of a dieelctric barrier discharge plasma into textile structures at medium pressure. Plasma Sources Sci Technol. 2006;15:78. [Google Scholar]
- De Geyter N, Morent R, Leys C, Gengembre L, Payen E. Treatment of polymer films with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf Coat Technol. 2007;201:7066–7075. [Google Scholar]
- de Jesus Martínez-Gómez A, Manolache SO, Young RA, Denes FS. Surface functionalization and biomolecule immobilization using plasma-generated free radicals on polypropylene. Polym Bull. 2010;65:293–308. [Google Scholar]
- Desmet T, Morent R, Geyter ND, Leys C, Schacht E, Dubruel P. Nonthermal plasma technology as a versatileStrategy for polymeric biomaterials surface modification: a review. Biomacromolecules. 2009;10:2351–2378. doi: 10.1021/bm900186s. [DOI] [PubMed] [Google Scholar]
- Dowling DP. Surface processing using cold atmospheric pressure plasmas. In: Cameron D, editor. Comprehensive materials processing. Oxford: Elsevier; 2014. pp. 171–185. [Google Scholar]
- Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C. 2015;48:651–662. doi: 10.1016/j.msec.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplantier AJ, van Hoek ML. The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds. Front Immunol. 2013;4:1–14. doi: 10.3389/fimmu.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durso DF. Chemical modification of cellulose – a historical review. Modified Cellulosics. New York: Academic; 1978. [Google Scholar]
- Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, Gurtner GC. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology. 2016;62:216–225. doi: 10.1159/000381877. [DOI] [PubMed] [Google Scholar]
- Eddington DT, Puccinelli JP, Beebe DJ. Thermal aging and reduced hydrophobic recovery of polydimethylsiloxane. Sensors Actuators B. 2006;114:170–172. [Google Scholar]
- Encinas N, Díaz-Benito B, Abenojar J, Martínez MA. Extreme durability of wettability changes on polyolefin surfaces by atmospheric pressure plasma torch. Surf Coat Technol. 2010;205:396–402. doi: 10.1016/j.surfcoat.2010.06.069. [DOI] [Google Scholar]
- Ershov S, et al. Free radical generation and concentration in a plasma polymer: the effect of aromaticity. ACS Appl Mater Interfaces. 2014;6:12395–12405. doi: 10.1021/am502255p. [DOI] [PubMed] [Google Scholar]
- Fahs F, Bi X, Yu F-S, Zhou L, Mi Q-S. New insights into microRNAs in skin wound healing. IUBMB. 2015;67:889–896. doi: 10.1002/iub.1449. [DOI] [PubMed] [Google Scholar]
- Fengel D, Wegener G. Wood, chemistry, ultrastructure, reactions. Reactions in alkaline medium. Berlin: Walter de Gruyter; 1984. [Google Scholar]
- Ferrerro F, Bongiovanni R. Improving the surface properties of cellophane by air plasma treatment. Surf Coat Technol. 2006;200:4770–4776. [Google Scholar]
- Freytag R, Donzé JJ. Handbook of fiber science and technology: volume I. Chemical processing of fibers and fabrics, fundamentals and preparation, part a vol 1. Alkali treatment of cellulose fibres. New York: Marcel Deckker; 1983. [Google Scholar]
- Goddard JM, Hotchkiss JH. Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci. 2007;32:698–725. [Google Scholar]
- Gomathi N, Sureshkumar A, Neogi S. RF plasma treated polymers for biomedical applications. Curr Sci. 2008;94:1478–1486. [Google Scholar]
- Govindarajan T, Shandas R. A survey of surface modification techniques for next-generation shape memory polymer stent devices. Polymer. 2014;6:2309–2331. [Google Scholar]
- Guimond S, Hanselmann B, Amberg M, Hegemann D. Plasma functionalization of textiles: specifics and possibilities. Pure Appl Chem. 2010;82:1239–1245. [Google Scholar]
- Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B, Agarwal R, Alam MS. Textile based smart wound dressing. Indian J Fibers Text Res. 2010;35:174–187. [Google Scholar]
- Guruvenketa S, Mohan Rao G, Komath M, Raichur AM. Plasma surface modification of polystyrene and polyethylene. Appl Surf Sci. 2004;236:278–284. [Google Scholar]
- Haddow DB, France RM, Short RD, MacNeil S, Dawson RA, Leggett GJ, Cooper E. Comparison of proliferation and growth of human keratinocytes on plasma copolymers of acrylic acid/1,7-octadiene and self-assembled monolayers. J Biomed Mater Res. 1999;47:379–387. doi: 10.1002/(sici)1097-4636(19991205)47:3<379::aid-jbm13>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Haddow DB, Steele DA, Short RD, Dawson RA, MacNeil S. Plasma-polymerized surfaces for culture of human keratinocytes and transfer of cells to an in vitro wound-bed model. J Biomed Mater Res A. 2003;64:80–87. doi: 10.1002/jbm.a.10356. [DOI] [PubMed] [Google Scholar]
- Haddow DB, MacNeil S, Short RD. A cell therapy for chronic wounds based upon a plasma polymer delivery surface. Plasma Process Polym. 2006;3:419–430. [Google Scholar]
- He W, Ma Z, Yong T, Teo WE, Ramakrishna S. Fabrication of collagen-coated biodegradable polymer nanofiber mesh and its potential for endothelial cells growth. Biomaterials. 2005;26:7606–7615. doi: 10.1016/j.biomaterials.2005.05.049. [DOI] [PubMed] [Google Scholar]
- He W, Yong T, Ma ZW, Inai R, Teo WE, Ramakrishna S. Biodegradable polymer nanofiber mesh to maintain functions of endothelial cells. Tissue Eng. 2006;12:2457–2466. doi: 10.1089/ten.2006.12.2457. [DOI] [PubMed] [Google Scholar]
- Hochart F, De Jaeger R, Levalois-Grützmacher J. Graft-polymerization of a hydrophobic monomer onto PAN textile by low-pressure plasma treatments. Surf Coat Technol. 2003;165:201–210. [Google Scholar]
- Hocker H. Plasma treatment of textile fibers. Pure Appl Chem. 2002;74:423–427. [Google Scholar]
- Hodak SK, Supasai T, Paosawatyanyong B, Kamlangkla K, Pavarajarn V. Enhancement of the hydrophobicity of silk fabrics by SF6 plasma. Appl Surf Sci. 2008;254:4744–4749. [Google Scholar]
- Hossain MM, Hegemann D, Herrmann AS, Chabrecek P. Contact angle determination on plasma-treated poly(ethylene terephthalate) fabrics and foils. J Appl Polym Sci. 2006;102:1452–1458. [Google Scholar]
- Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release. 2014;185:12–21. doi: 10.1016/j.jconrel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Huang F, Wei Q, Wang X, Xu W. Dynamic contact angles and morphology of PP fibres treated with plasma. Polym Test. 2006;25:22–27. [Google Scholar]
- Hume EBH, et al. The control of staphylococcus epidermis biofilm formation and in vivo infection rates by covalently bound furanones. Biomaterials. 2004;25:5023–5030. doi: 10.1016/j.biomaterials.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Hwang Y, et al. The effects of atmospheric pressure helium plasma treatment on adhesion and mechanical properties of aramid fibers. J Adhes Sci Technol. 2003;17:847–860. [Google Scholar]
- Hwang YJ, Mccord MG, An JS, Kang BC, Park SW. Effects of helium atmospheric pressure plasma treatment on low-stress mechanical properties of polypropylene nonwoven fabrics. Text Res J. 2005;75:771–778. [Google Scholar]
- Inbakumar S, Morent R, De Geyter N, Desmet T, Anukaliani A, Dubruel P, Leys C. Chemical and physical analysis of cotton fabrics plasma- treated with a low pressure glow discharge. Cellulose. 2010;17:417–426. [Google Scholar]
- Jáuregui KMG, Cabrera JCC, Ceniceros EPS, Hernández JLM, Ilyina A. A new formulated stable papain-pectin aerosol spray for skin wound healing. Biotechnol Bioprocess Eng. 2009;14:450–456. [Google Scholar]
- Jeong L, et al. Plasma-treated silk fibroin nanofibers for skin regeneration. Int J Biol Macromol. 2009;44:222–228. doi: 10.1016/j.ijbiomac.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Kan Chi-wai. A Novel Green Treatment for Textiles. 2014. [Google Scholar]
- Kan CW, Yuen CWM. Effect of low temperature plasma treatment on wool fabric properties. Fibers Polym. 2005;6:169–173. doi: 10.1007/bf02875610. [DOI] [Google Scholar]
- Kan CW, Chan K, Yuen CWM. Surface characterization of low temperature plasma treated wool fiber. Fiber Polym. 2004;5:52–58. [Google Scholar]
- Karahan HA, Özdogan E. Improvements of surface functionality of cotton fibres by atmospheric plasma treatment. Fiber Polym. 2008;9:21–26. [Google Scholar]
- Kasálková NS, Slepička P, Kolská Z, Sajdl P, Bačáková L, Rimpelová S, Švorčík V. Cell adhesion and proliferation on polyethylene grafted with AU nanoparticles. Nucl Inst Methods Phys Res B. 2012;272:391–395. [Google Scholar]
- Khan TA, Peh KK, Ch’ng HS. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J Pharm Pharm Sci. 2000;3:303–311. [PubMed] [Google Scholar]
- Kiedrowski MR, Horswill AR. New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci. 2011;1241:104–121. doi: 10.1111/j.1749-6632.2011.06281.x. [DOI] [PubMed] [Google Scholar]
- Koczulla A, Bals R. Antimicrobial peptides: current status and theraputic. Potential Drugs. 2003;63:389–406. doi: 10.2165/00003495-200363040-00005. [DOI] [PubMed] [Google Scholar]
- Koh HS, Yong T, Chan CK, Ramakrishna S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials. 2008;29:3574–3582. doi: 10.1016/j.biomaterials.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Kosobrodova EA, Kondyurin AV, Fisher K, Moeller W, McKenzie DR, Bilek MMM. Free radical kinetics in a plasma immersion ion implanted polystyrene: theory and experiment. Nucl Inst Methods Phys Res B. 2012;280:26–35. [Google Scholar]
- Kosobrodova E, Jones RT, Kondyurin A, Chrzanowski W, Pigram PJ, McKenzie DR, Bilek MMM. Orientation and conformation of anti-CD34 antibody immobilised on untreated and plasma treated polycarbonate. Acta Biomater. 2015;19:128–137. doi: 10.1016/j.actbio.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Kwok DY, Neumann AW. Contact angle measurement and contact angle interpretation. Adv Colloid Interf Sci. 1999;81:167–249. doi: 10.1016/S0001-8686(98)00087-6. [DOI] [Google Scholar]
- Lai W-F, Siu PM. MicroRNAs as regulators of cutaneous wound healing. J Biosci. 2014;39:519–524. doi: 10.1007/s12038-014-9421-4. [DOI] [PubMed] [Google Scholar]
- Lai J, et al. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl Surf Sci. 2006;252:3375–3379. doi: 10.1016/j.apsusc.2005.05.038. [DOI] [Google Scholar]
- Larsson A, D’erand H. Stability of polycarbonate and polystyrene surfaces after Hydrophilization with high intensity oxygen RF plasma. J Colloid Interface Sci. 2002;246:214–221. doi: 10.1006/jcis.2001.8032. [DOI] [PubMed] [Google Scholar]
- Lawton RA, Price CR, Runge AF, Doherty WJ, Saavedra SS. Air plasma treatment of submicron thick PDMS polymer films: effect of oxidation time and storage conditions. Colloids Surf A Physicochem Eng Asp. 2005;253:213–215. [Google Scholar]
- Le Y, Anand SC, Horrocks AR (1997) Recent developments in fibres and materials for wound management. Indian J Fibre Text Res 22:337–347
- Lewin M. Handbook of fiber science and technology: chemical processing of fibers and fabrics, fundamentals and preparation, part B vol 1. Bleaching of cellulosic and synthetic fabrics. New York: Marcel Deckker; 1984. [Google Scholar]
- Li S, Jinjin D. Improvement of hydrophobic properties of silk and cotton by hexafluoropropene plasma treatment. Appl Surf Sci. 2007;253:5051–5055. [Google Scholar]
- Luna SM, Silva SS, Gomes ME, Mano JF, Reis RL. Cell adhesion and proliferation onto chitosan-based membranes treated by plasma surface modification. J Biomater Appl. 2011;26:101–116. doi: 10.1177/0885328210362924. [DOI] [PubMed] [Google Scholar]
- Machula H, Ensley B, Kellar R. Electospun Tropoelastin for delivery of Theraputic adipose derived stem cells to full thickness dermal wounds. Adv Wound Care. 2014;3:367–375. doi: 10.1089/wound.2013.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. MicroRNA signature in diabetic wound healing: promotive role of miR-21 in fibroblast migration. Int Wound J. 2012;9:355–361. doi: 10.1111/j.1742-481X.2011.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima M. Applying capillarity to estimation of space structure of fabrics. Text Res J. 1983;53:427–434. [Google Scholar]
- Maver T, Hribernik S, Mohan T, Smrke DM, Maver U, Stana-Kleinschek K. Functional wound dressing materials with highly tunable drug release properties. RSC Adv. 2015;5:77873–77884. doi: 10.1039/C5RA11972C. [DOI] [Google Scholar]
- Mayet N, Choonara YE, Kumar P, Tomar LK, Tyagi C, Du Toit LC, Pillay V. A comprehensive review of advanced biopolymeric wound healing systems. J Pharm Sci. 2014;103:2211–2230. doi: 10.1002/jps.24068. [DOI] [PubMed] [Google Scholar]
- McCord MG, Hwang YJ, Qiu Y, Hughes LK, Bourham MA. Surface analysis of cotton fabrics fluorinated in radio-frequency plasma. J Appl Polym Sci. 2003;88:2038–2047. [Google Scholar]
- McCord MG, Hwang YJ, Qui Y, Hughes LK, Bourham MA. Surface analysis of cotton fabrics fluorinated in radio frequency plasma. J Appl Polym Sci. 2003;88:2038–2047. [Google Scholar]
- Mitchell SA, Davidson MR, Emmison N, Bradley RH. Isopropyl alcohol plasma modification of polystyrene surfaces to influence cell attachment behaviour. Surf Sci. 2004;561:110–120. [Google Scholar]
- Miwa M, Nakajima A, Fujishima A, Hashimoto K, Watanabe T. Effects of the surface roughness on sliding angles of water droplets on Superhydrophobic surfaces. Langmuir. 2000;16:5754–5760. [Google Scholar]
- Morent R, De Geyter N, Trentesaux M, Gengembre L, Dubruel P, Leys C, Payen E. Influence of discharge atmosphere on the ageing behaviour of plasma-treated Polylactic acid. Plasma Chem Plasma Process. 2010;30:525–536. [Google Scholar]
- Morent R, De Geyter N, Desmet T, Dubruel P, Leys C. Plasma surface modification of biodegradable polymers: a review. Plasma Process Polym. 2011;8:171–190. [Google Scholar]
- Nema SK, Jhala PB (2015) Plasma technologies for textile and apparel. WPI India, New Delhi
- Nithya E, Radhai R, Rajendran R, Shalini S, Rajendran V, Jayakumar S. Synergetic effect of DC air plasma and cellulase enzyme treatment on the hydrophilicity of cotton fabrics. Carbohydr Polym. 2011;83:1652–1658. [Google Scholar]
- Nithya E, Radhai R, Rajendran R, Jayakumar S, Vaideki K. Enhancement of antimicrobial property of cotton fabric using plasma and enzyme pretreatments. Carbohydr Polym. 2012;88:986–991. [Google Scholar]
- Nithya E, Radhai R, Prasanna S, Vaideki K, Rajendran R, Jayakumar S. Physico-chemical analysis of oxygen plasma and enzyme treated cotton fabrics international journal of innovative research in science. Eng Technol. 2015;4:1333–1341. [Google Scholar]
- Oehr C. Plasma surface modification of polymers for biomedical use. Nucl Inst Methods Phys Res B. 2003;208:40–47. [Google Scholar]
- Pane S, Tedesco R, Greger R. Acrilic fabrics treated with plasma for outdoor application. J Ind Text. 2001;3:135–145. [Google Scholar]
- Park K, Ju YM, Son JS, Ahn KD, Han DK. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblast. J Biomater Sci Polym Ed. 2007;18:369–382. doi: 10.1163/156856207780424997. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Antoci V, Hickok NJ, Shapiro IM. Selfprotective smart orthopedic implants. Exp Rev Med Devices. 2007;4:55–64. doi: 10.1586/17434440.4.1.55. [DOI] [PubMed] [Google Scholar]
- Peršin Z, Devetak M, Drevenšek-Olenik I, Vesel A, Mozetič M, Stana-Kleinschek K. The study of plasma's modifiation effects in viscose used ass an absorbent for wound -relevant fluids. Carbohydr Polym. 2013;97:143–151. doi: 10.1016/j.carbpol.2013.04.045. [DOI] [PubMed] [Google Scholar]
- Peršin Z, Kleinschek KS, Mozetič M. The effects of storage gases on the durability of ammonia plasma effects with respect to wound fluid absorption and the biostatic activity of viscose non-wovens. Text Res J. 2014;84:751–763. [Google Scholar]
- Peršin Z, Maver U, Pivec T, Maver T, Vesel A, Mozetič M, Stana-Kleinschek K. Novel cellulose based materialas for safe and efficient wound treatment. Carbohydr Polym. 2014;100:55–64. doi: 10.1016/j.carbpol.2013.03.082. [DOI] [PubMed] [Google Scholar]
- Peršin Z, Zaplotnik R, Kleinschek KS. Ammonia plasma treated as a method promoting simultaneous hydrophilicity and antimicrobial activity of viscose wound dressing. Text Res J. 2014;84:140–156. [Google Scholar]
- Perwuelz A, Casetta M, Caze C. Liquid organisation during capillary rise in yarns—influence of yarn torsion. Polym Test. 2001;20:553–561. [Google Scholar]
- Piraino F, Selimovic S. A current view of functional biomaterials for wound care, molecular and cellular therapies. Biomed Res Int. 2015;2015:10. doi: 10.1155/2015/403801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivec T, et al. Modification of cellulose non-woven substrates for preparation of modern wound dressings. Text Res J. 2014;84:96–112. [Google Scholar]
- Poll H, Schladitz U, Schreiter S. Penetration of plasma effects into textile structures. Surf Coat Technol. 2001;142:489–493. [Google Scholar]
- Powles RC, McKenzie DR, Meure SJ, Swain MV, James NL. Nanoindentation response of PEEK modified by mesh-assisted plasma immersion ion implantation. Surf Coat Technol. 2007;201:7961–7969. [Google Scholar]
- Qiu Y, Hwang Y, Zhang C, McCord M. The effect of atmospheric pressure oxygen-helium plasma treatment on surface and mechanical properties of ultrahigh modulus polyethylene fiber. J Adhes Sci Technol. 2002;16:449–458. [Google Scholar]
- Ren Yu, Wang Chunxia, Qiu Yiping. Aging of surface properties of ultra high modulus polyethylene fibers treated with He/O2 atmospheric pressure plasma jet. Surface and Coatings Technology. 2008;202(12):2670–2676. [Google Scholar]
- Řezníčkovác A, Kolská Z, Hnatowicz V, Stopka P, Švorčík V. Comparison of glow argon plasma-induced surface changes of thermoplastic polymers. Nucl Inst Methods Phys Res B. 2011;269:83–88. [Google Scholar]
- Rho KS, et al. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27:1452–1461. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Rustad KC, et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012;33:80–90. doi: 10.1016/j.biomaterials.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharwal HS, Denes F, Nielsen L, Young RA. Free-radical formation in jute from argon plasma treatment. J Agric Food Chem. 1993;41:2202–2207. [Google Scholar]
- Samanta KK, Jassal M, Agrawal AK. Improvement in water and oil absorbency of textile substrate by atmospheric pressure cold plasma treatment. Surf Coat Technol. 2009;203:1336–1342. [Google Scholar]
- Saranwong N, Inthanon K, Wongkham W, Wanichapichart P, Suwannakachorn D, Yu LD. Surface and protein analyses of normal human cell attachment on PIII-modified chitosan membranes. Nucl Inst Methods Phys Res B. 2012;272:386–390. [Google Scholar]
- Schroder K, Meyer-Plath A, Keller D, Besch W, Babucke G, Ohl A. Plasma- induced surface functionalization of polymeric biomaterials in ammonis plasma. Contrib Plasma Physics. 2001;41:562–572. [Google Scholar]
- Sigurdsson S, Shishoo R. Surface properties of polymers treated withTetrafluoromethane plasma. J Appl Polym Sci. 1997;66:1591–1601. [Google Scholar]
- Silva SS, Luna SM, Gomes ME, Benesch J, Pashkuleva I, Mano JF, Reis RL. Plasma surface modification of chitosan membranes: characterization and preliminary cell response studies. Macromol Biosci. 2008;8:568–576. doi: 10.1002/mabi.200700264. [DOI] [PubMed] [Google Scholar]
- Siow KS, Britcher L, Kumar S, Griesser HJ. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization-a review. Plasma Process Polym. 2006;2:392–418. [Google Scholar]
- Sun D, Stylios GK. Investigating the plasma modification of natural fiber fabrics–the effect on fabric surface and mechanical properties. Text Res J. 2005;75:639–644. [Google Scholar]
- Sun D, Stylios GK. Fabric surface properties affected by low temperature plasma treatment. J Mater Process Technol. 2006;173:172–177. [Google Scholar]
- Švorčík V, et al. Cytocompatibility of Ar+ plasma treated and au nanoparticle-grafted PE nuclear instruments and methods in physics. Res Sect B. 2009;267:1904–1910. [Google Scholar]
- Tasker S, Badyal JPS, Backson SCE, Richards RW. Hydroxyl accessibility in celluloses. Polym J. 1994;35:4717–4721. [Google Scholar]
- Tran CTH, Craggs M, Smith LM, Stanley K, Kondyurin A, Bilek MM, McKenzie DR. Covalent linker-free immobilization of conjugatable oligonucleotides on polypropylene surfaces. RSC Adv. 2016;6:83328–83336. [Google Scholar]
- Tsafack MJ, Levalois-Grützmacher J. Towards multifunctional surfaces using the plasma-induced graft-polymerization (PIGP) process: flame and waterproof cotton textiles. Surf Coat Technol. 2007;201:5789–5795. [Google Scholar]
- Tseng H-J, Hsu S-h WM-W, Hsueh T-H, Tu P-C. Nylon textiles grafted with chitosan by open air plasma and their antimicrobial effect. Fibers Polym. 2009;10:53–59. [Google Scholar]
- Vaideki K, Jayakumar S, Rajendran R, Thilagavathi G. Investigation on the effect of RF air plasma and neem leaf extract treatment on the surface modification and antimicrobial activity of cotton fabric. Appl Surf Sci. 2008;254:2472–2478. doi: 10.1016/j.apsusc.2007.09.088. [DOI] [Google Scholar]
- Valenza A, Visco AM, Torrisi L, Campo N. Characterization of ultra-high-molecular-weight polyethylene (UHMWPE) modified by ion implantation. Polymer. 2004;45:1707–1715. [Google Scholar]
- Vigo TL. Textile processing and properties: dyeing, finishing, and performance. Amsterdam: Elsevier; 1994. [Google Scholar]
- Vohrer U, Muller M, Oehr C. Glow discharge treatment for the modification of textiles. Surf Coat Technol. 1998;98:1128–1131. [Google Scholar]
- Vosmanská V, Kolářová K, Rimpelová S, Kolská Z, Švorčík V. Antibacterial wound dressing: plasma treatment effect on chitosan impregnation and in situ synthesis of silver chloride on cellulose surface. RSC Advances. 2015;5:17690–17699. [Google Scholar]
- Wakida T, Takeda K, Tanaka I, Takagishi T. Free radicals in cellulose fibres treated with low temperature plasma. Text Res J. 1989;59:49–53. [Google Scholar]
- Wang Y, Zhao Y, Deng Y. Effect of enzymatic treatment on cotton fiber dissolution in NaOH/urea solution at cold temperature. Carbohydr Polym. 2008;72:178–184. [Google Scholar]
- Wang Q, Fan XR, Cui L, Wang P, Wu J, Chen J. Plasma – aided cotton bioscouring: dielectric barrier discharge versus low pressure oxygen plasma. Plasma Chem Plasma Process. 2009;29:399–409. [Google Scholar]
- Ward TL, Jung H, Hinojosa O, Benerito RR. Characterization and use of radio frequency plasma-activated natural polymers. J Appl Polym Sci. 1979;23:1987–2003. [Google Scholar]
- Wong K, Tao X, Yuen C, Yeung K. Low temperature plasma treatment of linen. Text Res J. 1999;69:846–855. [Google Scholar]
- Woodward I, Schofield W, Roucoules V, Badyal J. Super-hydrophobic surfaces produced by plasma fluorination of polybutadiene films. Langmuir. 2003;19:3432–3438. [Google Scholar]
- Yang S, Gupta M. Surface modification of polyethyleneterephthalate by an atmospheric-pressure plasma source. Surf Coat Technol. 2004;187:172–176. [Google Scholar]
- Yang L, Chen J, Guo Y, Zhang Z. Surface modification of a biomedical polyethylene terephthalate (PET) by air plasma. Appl Surf Sci. 2009;255:4446–4451. doi: 10.1016/j.apsusc.2008.11.048. [DOI] [Google Scholar]
- Yao C, Li X, Neoh KG, Shi Z, Kang ET. Surface modification and antibacterial activity of electrospun polyurethane fibrous membranes with quaternary ammonium moieties. J Membr Sci. 2008;320:259–267. [Google Scholar]
- Yin Y, Nosworthy N, Youssef H, Gong B, Bilek M, McKenzie D. Acetylene plasma coated surfaces for covalent immobilization of proteins. Thin Solid Films. 2009;517:5343–5346. [Google Scholar]
- Yip J, Chan K, Sin KM, Lau KS. Low temperature plasma-treated nylon fabrics. J Mater Process Technol. 2002;123:5–12. doi: 10.1016/S0924-0136(02)00024-9. [DOI] [Google Scholar]
- Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2009;61:1033–1042. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Hagiwara K, Hasebe T, Hotta A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf Coat Technol. 2013;233:99–107. [Google Scholar]
- Young T (1805) Philos Trans R Soc Lond A 95:65–75
- Zahedi P, Rezaeian I, Ranaei-Siadat S-O, Jafari S-H, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Technol. 2010;21:77–95. [Google Scholar]
- Zhang W, et al. Plasma surface modification of poly vinyl chloride for improvement of antibacterial properties. Biomaterials. 2006;27:44–51. doi: 10.1016/j.biomaterials.2005.05.067. [DOI] [PubMed] [Google Scholar]
- Zhang W, Luo Y, Wang H, Jiang J, Pu S, Chu PK. Ag and ag/N2 plasma modification of polyethylene for the enhancement of antibacterial properties and cell growth/proliferation. Acta Biomater. 2008;4:2028–2036. doi: 10.1016/j.actbio.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yang D, Chen X, Xu Q, Lu F, Nie J. Electrospun water-soluble Carboxyethyl chitosan/poly(vinyl alcohol) Nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules. 2008;9:349–354. doi: 10.1021/bm7009015. [DOI] [PubMed] [Google Scholar]
- Zhu X, Chian KS, Chan‐Park MBE, Lee ST. Effect of argon- plasma treatment on proliferation of human-skin derived fibroblast on chitosan membrane in vitro. J Biomed Mater Res A. 2005;73A:264–274. doi: 10.1002/jbm.a.30211. [DOI] [PubMed] [Google Scholar]
- Zille A, Oliveira FR, Souto AP. Plasma treatment in textile industry. Plasma Process Polym. 2015;12:98–131. [Google Scholar]