Abstract
The purpose of this study was to demonstrate the safety and technical feasibility of intracorporeal hand-sewn esophagojejunostomy after laparoscopic total gastrectomy. Laparoscopic total gastrectomy (LTG) is a technically challenging procedure, especially for esophagojejunal anastomosis (EJA). Various techniques have been described to overcome these difficulties using staplers with variable results. We report successfully performed complete intracorporeal hand-sewn EJA after LTG. The perioperative clinical data and short-term outcomes for 30 patients who underwent LTG using hand-sewn EJA for gastric cancer between 2013 and 2015 have been retrospectively reviewed. The mean age was 49.9 years; 64 % of patients were male and 36 % were female. The mean body mass index (kg/m2) was 22.4, and the mean American Society of Anesthesiologists (ASA) score was 1.4. Eleven patients had co-morbidities, and six patients had previous abdominal operations. The mean operative time, time for EJA, and blood loss was 136.9 min, 13.25 min, and 166 ml, respectively. The conversion rate was nil. The mean time for the first oral feeding and mean hospital stay was 8.3 and 9.8 days respectively. The postoperative complications were found in 16 % of patients with one case of 30-day mortality because of lobar pneumonia. There were three cases of anastomotic stenosis; however, no leakage was identified both clinically and radiologically. Complete intracorporeal hand-sewn EJA is a safe and feasible technique in the hands of experienced surgeons that can be considered as an alternative cost-effective method when performing LTG.
Keywords: Stomach cancer, Laparoscopy, Gastrectomy, Esophagojejunostomy
Introduction
Gastric cancer is the second most common cancer among men and third most among women worldwide [1]. In India, it is the second most common killer among men and women, with the highest incidence in southern states [2]. The prevalence is linked to dietary factors and low socioeconomic status [3].
Although there is controversy about the oncological safety of laparoscopy in early gastric cancer, it has been now adopted as the standard surgical option for early gastric cancer, especially in Japan [4] and Korea [5]. Laparoscopic distal gastrectomy (LDG) for gastric cancer has been accepted worldwide as a safe and feasible technique; however, laparoscopic total gastrectomy (LTG) for gastric cancer has not gotten such popularity because of technical difficulties, especially at the esophagojejunal anastomosis (EJA). These include the purse string suture and the difficulty of introducing the anvil of the circular stapler into the esophagus and the fear of postoperative complications especially anastomotic leak and stricture [6].
Esophagojejunostomy (EJA) can be done using either extracorporeal or intracorporeal techniques after LTG. Various techniques have been described to overcome these difficulties of EJA using intracorporeal anastomosis; however, none of them is easy to follow nor free of postoperative complications [7]. These techniques include a conventional anvil head method, an OrVil TM system method, a hemi-double stapling technique with the anvil head, and side-to-side esophagojejunostomy with a linear stapler [8].
Nagai et al. adopted a technique of the inverted T-shaped EJA anastomosis by linear stapler [7]. Inaba et al. used the overlap method with side-to-side EJA between the anterior wall of the ascending limb of the jejunum 7 cm distal to the stapler line and the left side of the esophageal stump using a linear stapler [9]. However, this technique is quite difficult and requires adequate length of esophagus for anastomosis, besides the reported rates of postoperative leaks and stasis.
Therefore, we are sharing our experience of treating 30 patients with gastric cancer who underwent laparoscopic total gastrectomy (LTG) at the Galaxy Care Laparoscopy Institute, Pune, India, using complete intracorporeal hand-sewn esophagojejunostomy, with perfect short-term outcomes, namely postoperative anastomotic leak (n = 0).
Methods
Patients
A total of 30 patients underwent LTG at the Galaxy Care Laparoscopy Institute between January 2013 and January 2015, and data was retrospectively analyzed. Preoperative assessments were done using esophagogastroduodenoscopy, abdominal ultrasonography, and computed tomography. The indication for LTG at our institute is for patients with T1, 2, 3 N1, 2 gastric tumors. Patients with extensive peritoneal invasion, extensive esophageal invasion, or extensive lymph node metastasis or are medically unfit for major surgery were excluded. Patient characteristics including age, gender, BMI (kg/m2), ASA score, co-morbidities, and previous operation were recorded. Intra-operative characteristics were the operative time in minutes, the time for EJA, the estimated blood loss in milliliters, the extent of dissection, intra-operative complications, the conversion rate, and the intra-operative leak test at EJ anastomosis. The operative time was calculated from the insertion of the Verrus needle till closure of the minilaparotomy. The short-term surgical outcome in the form of postoperative complications and leak from the EJ, time of oral feeding, hospital stay, pathological data including the number of LN retrieved, tumor size and grade, surgical margins, final staging, and 30-day mortality were all recorded.
Surgical Procedure
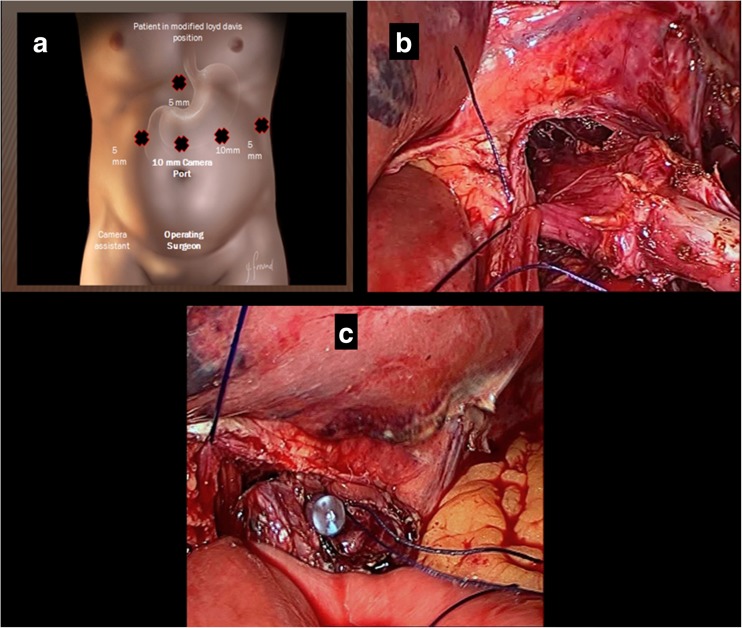
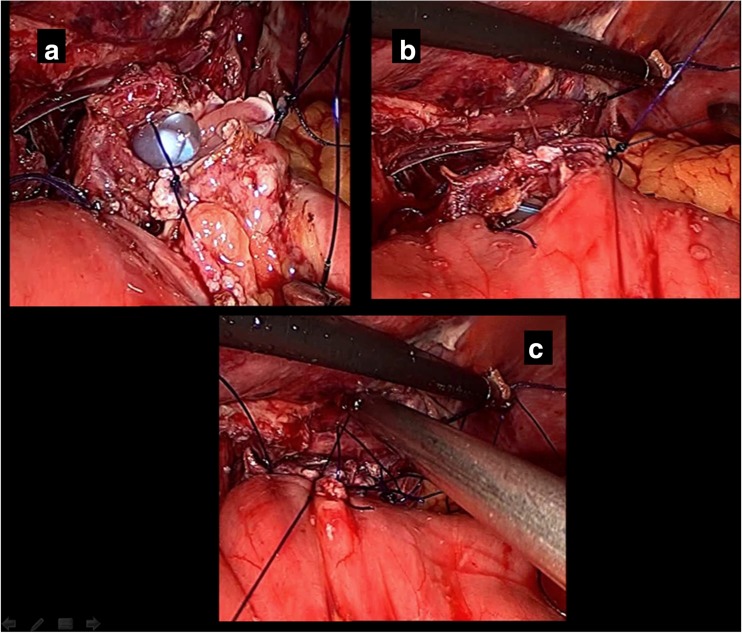
With the patient under general anesthesia and in the modified Lloyd Davis position, the surgeon stood between the patient’s legs and the first assistant stood on the patient’s right. Pneumoperitoneum was made using a Verrus needle through Palmer’s point. Ports were put as shown (Fig. 1a). After extensive dissection of the lymph nodes, the duodenal bulb was divided using an endostapler; the stomach was then reflected to dissect the posterior attachment removing the capsule of the pancreas, continuing dissection up to the gastroesophageal junction (GEJ). The dissection is then done on the left and right crura of the diaphragm freeing up the esophageal attachment up to 2 cm above the diaphragm. Two anchoring stitches are taken at the lateral sides of the esophagus to the corresponding crura of the diaphragm, leaving at least 2 cm distal for anastomosis with the jejunum, before cutting it to avoid retraction of the esophagus into the chest (Fig. 1b). After ensuring an adequate safety margin, the specimen is separated from the esophagus using a Harmonic device. The specimen is then secured in an Endobag. Our reconstruction of the gastrointestinal tract starts using a loop of jejunum 30 cm distal to the ligament of Treitz, passing in a retrocoloic fashion toward the proximal end of the esophagus to be attached. The loop of jejunum is then attached to the cut end of esophagus by another two anchoring stitches using 3-0 PDS suture (Fig. 1c). After ensuring a good length of the loop in the supracolic compartment to avoid tension on the suture line, using 3-0 PDS suture, the anastomosis is carried out intracorporeally, starting first at the posterior wall of the esophagus on the left side, by one anchoring stitch. An enterotomy is made in the antimesenteric border of the jejunal loop, and then the rest of the posterior wall of the anastomosis is completed using interrupted 3-0 PDS stitches (Fig. 2a). Before starting the anterior layer, the anesthesiologist advances the NGT into the bowel lumen to guide our stitches. The anterior stitch line is then completed using the interrupted intracorporeal stitches of 3-0 PDS suture (Fig. 2b). The reconstruction of esophagojejunal anastomosis (EJA) is completed (Fig. 2c). We used to test for EJA competence intra-operatively using the NGT; the anesthesiologist injects 50 ml air during the wash, and if there is any bubbling, we may reinforce the area with another stitch until we make sure that there is no leak intra-operatively. Side-to-side jejunojejunostomy is then performed using an EndoGIA stapler. The specimen is then retrieved through 5-cm minilaparotomy at the supraumbilical port. A feeding jejunostomy tube is inserted as a routine and the procedure is then completed.
Fig. 1.
a Port positions in laparoscopic total gastrectomy. b Anchoring stitches to the diaphragm. c Jejunum anchored to the esophagus
Fig. 2.
a Posterior layer of EJ anastomosis. b Anterior layer of EJA started with Ryle’s tube across the anastomosis. c EJA construction is completed
Postoperative Management
All the patients were observed for signs of postoperative leak. Feeding started on the POD 2 through the jejunostomy tube. The NGT was left in place and a postoperative gastrograffin study was done in POD 7 for all the patients to exclude any kind of stasis, stenosis, or leak. If the dye study was negative for any leak, the NGT was removed and sips of water were started to initiate oral feeding and the patient was discharged on POD 8–10 after tolerating soft diet. The jejunostomy tube was removed after 6 weeks.
Results
A total of 30 patients underwent laparoscopic total gastrectomy (LTG) for gastric cancer for early and locally advanced gastric cancer (T1b, T2, T3).
Patient characteristics (Table 1): The mean age was 49.9 years (range 39–59); 11 (36 %) patients were female and 19 (64 %) were male. The average BMI was 22.4 (range 17.9–28). Eleven patients (36 %) had co-morbidities, five (16 %) had hypertension, four (13 %) had diabetes mellitus, one (3.3 %) had chronic obstructive pulmonary disease (COPD), and one (3.3 %) had hypothyroidism. There was one patient with multiple co-morbidities. The ASA score was average 1.4, and seven (23 %) patients had previous abdominal operations as illustrated in the table.
Table 1.
Patient characteristics
| Age | 49.9 years (39–59 years) |
| Gender | 19 male, 11 female |
| BMI (kg/m2) | 22.4 (17.9–28) |
| Co-morbidities | HTN, 5 (16 %) |
| DM type II, 4 (13 %) | |
| COPD, 1/20 (3.3 %) | |
| Hypothyroid, 1 (3.3 %) | |
| HTN, DM, 1/20 (3.3 %) | |
| ASA score | 1.4 (1–2) |
| Previous operations | Appendectomy, 3/30 |
| Lap. cholecystectomy, 2/30 | |
| C.S., 1/30 | |
| Tubal pregnancy, 2/30 | |
| Inguinal hernia repair, 1/30 |
The operative and postoperative outcome is summarized in Table 2. The type of dissection was D2 in 5 patients (16 %) and D1+β in 25 patients (84 %). There was no intra-operative mortality. The mean operative time was 136.9 min (range 115–190 min), and the estimated blood loss was 166 ml (range 130–190 ml). The mean time for esophagojejunal anastomosis (EJA) was 13.25 min (range 10–18 min). Five patients had intra-operative complications (16 %) as illustrated in Table 2. However, the conversion to open approach was zero. The intra-operative leak test was done for all patients which revealed intra-operative leak in one patient (3.3 %), for which reinforcement interrupted stitches were taken.
Table 2.
The operative and postoperative outcome
| The mean operative time (min) | 136.9 (115–190) |
| The time for EJA (min) | 13.25 (10–18) |
| The estimated blood loss (ml) | 166 (130–190) |
| Extent of LN dissection | D2, 5/30 (16 %) D1+β, 25/20 (84 %) |
| Conversion rate | 0 |
| Intra-operative competence test | 1/30 (3.3 %) corrected intra-operative |
| Complications (intra-operative) | Sp. capsule laceration, 2/30 (6.6 %) Liver tear, 1/30 (3.3 %) Duodenal stump laceration, 1/30 (3.3 %) Pancreatic tail injury, 1/30 (3.3 %) |
| Anastomotic leak (postoperative) | 0 |
| EJA stenosis | 3/30 (10 %) |
| Early postoperative complications | Wound infection, 4/30 (13.3 %) Atelectasis, 1/30 (3.3 %) Lobar pneumonia, 1/30 (3.3 %) Atelectasis + wound infection, 1/30 (3.3 %) |
| Time of first oral feeding (days) | 8.3 (7–11) |
| The mean hospital stay (days) | 9.8 (8–18) |
| 30-day mortality | 1/30 (3.3 %) |
| The mean follow-up period (months) | 10.9 (6–18) |
The mean follow-up duration was 10.9 months (range 6–18 months). The time for first oral feeding was 8.3 days (range 7–11 days), and the mean hospital stay was 9.8 days (range 8–18 days). In the early postoperative period, the complication rate was 20 % (6 patients) in the form of wound infection in 3 patients (10 %) and pulmonary complications in 3 patients (10 %). However, no anastomotic leak was found in any of the patients as confirmed by oral contrast study on POD 7. The number of patients who suffered from EJA stenosis was 3 cases (10 %) in the first 6 months and required balloon dilatation. All cases with postoperative morbidities were managed conservatively. There was no immediate postoperative mortality (30 days), apart from one patient with history of COPD who suffered from resistant left-sided lobar pneumonia, for which he had to be readmitted on day 28 and died of pulmonary complications.
On pathological evaluation (Table 3), the average tumor size was 4.8 cm (range 2.2–7.5 cm). The tumor location was midportion in 9 patients (30 %), proximal portion in 18 patients (60 %), and multicentric in 3 patients (10 %). The mean proximal margin achieved was 3.7 cm (range 1.8–6 cm). Tumor grades were grade IV adenocarcinoma in 5 patients (16 %), grade III adenocarcinoma in 9 patients (30 %), grade II adenocarcinoma in 9 patients (30 %), and grade I adenocarcinoma in 7 patients (24 %). The number of lymph nodes retrieved in the final pathology reports was 26.7 (range 16–35 LN). The average lymph node involved with metastasis was 4.3 (range 2–8 LN). The final clinical staging was stage 1B in 5 patients (16 %), stage IIA in 9 patients (30 %), stage IIB in 6 patients (20 %), stage IIIA in 7 patients (24 %), and stage IIIB in 3 patients (10 %).
Table 3.
Pathological outcome
| Tumor size (cm) | 4.8 (2.2–7.5) |
| Tumor location | Proximal stomach, 18/30 (60 %) Midgastric, 9/30 (30 %) Multicentric, 3/30 (10 %) |
| The average proximal margin (cm) | 3.7 (1.8–6) |
| Tumor grade (adenocarcinoma) | Grade I, 7/30 (24 %) Grade II, 9/30 (30 %) Grade III, 9/30 (30 %) Grade IV, 5/20 (16 %); poorly differentiated and signet ring cell carcinoma |
| Depth of invasion | Submucosa, 5/30 (15 %) Muscularis propria, 9/30 (30 %) Subserosa, 13/30 (45 %) Invading serosa, 3/30 (10 %) |
| Lymph node yield | 26.7 (16–35) |
| The mean number of positive LNs | 4.3 (0–8) |
| Clinical staging | Stage IB, 5/30 (16 %) Stage IIA, 9/30 (30 %) Stage IIB, 6/30 (20 %) Stage IIIA, 7/30 (24 %) Stage IIIB, 3/30 (10 %) |
Discussion
Laparoscopy is an established surgical approach with the advantage of a more favorable clinical course compared to open surgery for treatment of gastrointestinal diseases, including early gastric cancer [10–13]. Moreover, low morbidity and mortality rates have been reported with even extensive laparoscopic lymphadenectomy in gastric cancer compared to the open approach [5, 14, 15]. Since the first report of laparoscopic distal gastrectomy (LDG) in 1991 by Kitano et al., LDG has been widely adopted as an established surgical option for early gastric cancer [14–16]. The number of laparoscopic total gastrectomies has been limited [17–20]. Performing esophagojejunostomy is considered the major technical obstacle during LTG [8, 21]. An extracorporeal approach could be reasonable [18, 20–22]. However, most surgeons previously performed extracorporeal esophagojejunostomy using an additional minilaparotomy and operating in a narrow space in patients with a large anteroposterior diameter is not always feasible, especially if the patient is obese and entails a risk of unnecessary tension at the EJ anastomosis. Inserting the anvil into the distal end of the esophagus could be quite challenging. Therefore, many experts have invented some modifications and techniques of intracorporeal EJA to overcome these challenges, and reported their feasibility and safety.
Takiguchi et al. described a semiautomatic suture device (Endostitch, Covidien) for conventional EJA with the anvil head into the distal esophagus and making the purse string suture around it [23]. Usui et al. introduced LTG using an endoscopic purse string suture instrument (Endo PSI) and a circular stapler [24]. However, these devices are not widely available. Besides, these circular-stapled methods are cumbersome and have disadvantages such as reestablishment of a pneumoperitoneum and placement of the minilaparotomy in the supraumbilical area for insertion of the circular-stapled instruments. Takiguchi et al. described one minor anastomotic leak, which was attributed to the nipping of the jejunal wall near the anastomotic portion which necessitated re-anastomosis. Usui et al. reported no morbidity nor mortality, but the mean operative time was quite long (301 min). Kinoshita et al. also described an intracorporeal hand-sewn purse string to attach the anvil into the esophagus [25].
Jeong and Park [26] introduced intracorporeal circular stapling EJ using a transorally inserted anvil (OrVil) in 16 consecutive patients with gastric cancer in 2008. They reported a mean operation time of 194 min (range 160–270 min), and one patient had an intra-abdominal abscess that required surgical drainage. Recently, it has been a common method of EJA after LTG; however, it does carry risks of injury to the pharynx down to the esophagus or anvil disconnection at areas of natural esophageal constriction that may require endoscopic removal [27]. Results have been variable with the use of OrVil after LTG [6, 8, 26–29].
Xie et al. demonstrated excellent surgical outcome in 28 patients who underwent LATG using the OrVil system. The mean operative time was 143 min, mean blood loss was 70 ml, and hospital stay was 9.6 days with no postoperative leak or stenosis. There were two cases of aspiration pneumonia which were treated conservatively [28]. Zuiki et al. also reported that the incidence of anastomotic leakage was 1.9 % and stenosis was 21.2 % in 52 patients who underwent LTG using OrVil [29].
In a large study by Ito et al. comparing the surgical outcome of patients who underwent LTG using OrVil with those after open total gastrectomy and LATG, they demonstrated better rates of anastomotic leak and stenosis with the OrVil group; however, it was not significant [6].
In spite of the shortened operation time using the OrVil device, the digestive tract reconstruction-related complications still remain. Xie et al. compared the EJA complication rates of their cases with those in previous reported studies. It is obvious that reported leak and stenosis rates of end-to-side circular staple anastomosis with OrVil after LATG ranged from 0 to 16.7 % and from 0 to 33.3 %, respectively [28].
Shim et al. used the anterior wall of the stomach to introduce the anvil through gastrotomy after applying detachable silk suture attached to its head. A linear stapler was then applied to transect the esophagus just below the anvil to separate the stomach, while the suture was lifted up to keep the anvil head out of the linear stapler. After firing, the suture was pulled tight to show the center rod of the anvil, and a circular stapler is applied. The result was the hemi-double stapling technique with the anvil head. They described this technique in 14 patients [8]. Only a selected patient would be a candidate for this technique where the tumor is at least 2–3 cm below the esophagojejunal junction. The postoperative complication rate also was high with regard to the sample size of this group, 42.8 % with a leak and 7 % stenosis [8].
Various groups described the use of linear staplers for EJ anastomosis after LTG [7–9]. The common feature of all was that the distal esophagus should be extensively mobilized in order to apply the forks of the linear stapler. This carries the risk of serious mediastinal infection if the proximal end of the staple line retracted within the thoracic cavity [7].
Nagai et al. [7] used the linear stapler for EJ anastomosis in an inverted T-shaped fashion after rotating the esophagus clockwise 90°. Their mean operative time was quite long (368 min), estimated blood loss was 80.4 ± 115 ml, and mean hospital stay was 14.2 days. The rate of complications in the recent group was 17.5 % (10/57 patients); however, there was no leak and a case of stasis at EJA.
Inaba et al. described the overlap method using the linear stapler to perform EJA after LTG [9]. They performed the EJA after ensuring an adequate length of the esophagus to make end-to-end anastomosis with the jejunal loop. They reported a mean operative time of 373.4 min (215–663 min), estimated blood loss of 146.5–325.3 g, and mean hospital stay of 14.4 days. The morbidity rate was 24.5 %, with two cases of anastomotic leak at EJA (3.8 %), stasis in two patients (3.8 %), and pancreatic fistula in three patients (5.7 %); a leak from the duodenal stump led to intra-abdominal abscess.
Although all of the previous studies have shown reasonable outcome with regard to the safety of the procedure and reasonable feasibility doing it, they depend on a single surgeon or team experience and lack of comparable data serves as a limitation for all of them. Until now there is no consensus describing the best approach for laparoscopic EJA after LTG, and a widely accepted method for EJ reconstruction after LTG has not yet been developed.
To the best of our knowledge, there is no report describing complete intracorporeal hand-sewn esophagojejunostomy after LTG except for So and Park in 2011, who reported the procedure in six patients and demonstrated its safety and feasibility. However, the number of patients was very small, and the authors suggested validating the results on a larger scale is required [30].
Our technique has shown excellent surgical outcome in the early postoperative period with no leak. Although all of the patients did not have any complaints suggesting stenosis and absence of radiological evidence of stenosis in the first couple of months, three patients suffered from stenosis at EJA that required endoscopic dilatation within the first 6 months. The early postoperative complication rate was comparable with most of the previous studies. All were minor and treated conservatively. However, there was one case of mortality because of lobar pneumonia in a patient with COPD with impaired pulmonary function. The mean operative time of 136.9 min was short compared with most of the previous studies. The time of oral feeding was in the average common practice in the previous studies around the 8th day. However, the mean hospital stay in our study was shorter (9.8 days).
There are some advantages in our technique. It is very simple, safe, and cost-effective. Furthermore, excessive mobilization of the esophagus and Roux limb is not required. Also, it has the least tension and tissue injury of the anastomotic site than other stapled methods, besides the well-known advantages of laparoscopic surgery. This technique could be reproducible in the hands of laparoscopic surgeons with considerable experience of laparoscopic suturing techniques. Besides, putting anchoring stitches on the lateral esophageal wall guarantees the absence of tension on the anastomotic line and ensures a constant length of distal esophagus intra-abdominally, which in turn gives the operator enough space to work on using the needle holder.
Conclusion
Our data suggest that laparoscopic total gastrectomy for gastric cancer using intracorporeal laparoscopic hand-sewn esophagojejunostomy is safe and technically feasible in the hand of experienced laparoscopic surgeons. Therefore, it may be accepted as one method among various techniques described to perform esophagojejunostomy using complete laparoscopic approach. However, larger-scale randomized comparative studies should be conducted to validate its efficacy and safety.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Peter Boyle, Bernard Levin., editors. GLOBOCAN 2008, Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer. 2010. Available from: http://www.globocan.iarc.fr. World Cancer Report, 2008 IARC; 2008
- 2.Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R, Kumar R, Roy S, Suraweera W, Bray F, Mallath M, Singh PK, Sinha DN, Shet AS, Gelband H, Jha P. Million death study collaborators. Cancer mortality in India: a nationally representative survey. Lancet. 2012;379(9828):1807–1816. doi: 10.1016/S0140-6736(12)60358-4. [DOI] [PubMed] [Google Scholar]
- 3.Dikshit RP, Mathur G, Mhatre S, Yeole BB. Epidemiological review of gastric cancer in India. Indian J Med Paediatr Oncol. 2011;32(1):3–11. doi: 10.4103/0971-5851.81883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report—a phase III multicenter, prospective, randomized trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 6.Ito H, Inoue H, Odaka N, Satodate H, Onimaro M, et al. Evaluation of the safety and efficacy of esophagojejunostomy after totally laparoscopic total gastrectomy using a trans-orally inserted anvil: a single-center comparative study. Surg Endosc. 2014;28:1929–1935. doi: 10.1007/s00464-014-3417-x. [DOI] [PubMed] [Google Scholar]
- 7.Nagai E, Ohuchida K, Nakata K, Miyasaka Y, et al. Feasibility and safety of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy: inverted T-shaped anastomosis using linear staplers. Surgery. 2013;153:732–738. doi: 10.1016/j.surg.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Shim JH, Yoo HM, Oh SI, Nam MJ, et al. Various types of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy for gastric cancer. Gastric Cancer. 2013;16:420–427. doi: 10.1007/s10120-012-0207-9. [DOI] [PubMed] [Google Scholar]
- 9.Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg. 2010;211:e25–e29. doi: 10.1016/j.jamcollsurg.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:306–311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 11.Migoh S, Hasuda K, Nakashima K, Anai H. The benefit of laparoscopy- assisted distal gastrectomy compared with conventional open distal gastrectomy: a case-matched control study. Hepatogastroenterology. 2003;50:2251–2254. [PubMed] [Google Scholar]
- 12.Tanimura S, Higashino M, Fukunaga Y, et al. Respiratory function after laparoscopic distal gastrectomy, an index of minimally invasive surgery. World J Surg. 2006;30:1–5. doi: 10.1007/s00268-005-0115-9. [DOI] [PubMed] [Google Scholar]
- 13.Oki E, Sakaguchi Y, Ohgaki K, et al. Totally laparoscopic distal gastrectomy for elderly with gastric cancer. Fukuoka Acta Med. 2013;104(9):290–298. [PubMed] [Google Scholar]
- 14.Kim MC, Kim HH, Jung GJ. Surgical outcome of laparoscopy-assisted gastrectomy with extraperigastric lymph node dissection for gastric cancer. Eur J Surg Oncol. 2005;31:401–405. doi: 10.1016/j.ejso.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Noshiro H, Nagai E, Shimizu S, Uchiyama A, Tanaka M. Laparoscopically assisted distal gastrectomy with standard radical lymph node dissection for gastric cancer. Surg Endosc. 2005;19:1592–1596. doi: 10.1007/s00464-005-0175-9. [DOI] [PubMed] [Google Scholar]
- 16.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 17.Uyama I, Sugioka A, Matsui H, et al. Laparoscopic pancreas preserving total gastrectomy for proximal gastric cancer. A case and technical report. Surg Endosc. 2001;15:217–218. doi: 10.1007/BF03036283. [DOI] [PubMed] [Google Scholar]
- 18.Jeong GA, Cho GS, Kim HH, et al. Laparoscopy-assisted total gastrectomy for gastric cancer: a multicenter retrospective analysis. Surgery. 2009;146:469–474. doi: 10.1016/j.surg.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Shinohara T, Kanaya S, Taniguchi K, et al. Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg. 2009;144:1138–1142. doi: 10.1001/archsurg.2009.223. [DOI] [PubMed] [Google Scholar]
- 20.Usui S, Yoshida T, Ito K, et al. Laparoscopy-assisted total gastrectomy for early gastric cancer: comparison with conventional open total gastrectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:309–314. doi: 10.1097/01.sle.0000191589.84485.4a. [DOI] [PubMed] [Google Scholar]
- 21.Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, Cho SJ, Lee JY, et al. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol. 2009;100:392–395. doi: 10.1002/jso.21345. [DOI] [PubMed] [Google Scholar]
- 22.Kim SG, Lee YJ, Ha WS, et al. LATG with extracorporeal esophagojejunostomy: is this minimal invasive surgery for gastric cancer? J Laparoendosc Adv SurgTech A. 2008;18:572–578. doi: 10.1089/lap.2007.0106. [DOI] [PubMed] [Google Scholar]
- 23.Takiguchi S, Sekimoto M, Fujiwara Y, Miyata H, Yasuda T, Doki Y, et al. A simple technique for performing laparoscopic purse-string suturing during circular stapling anastomosis. Surg Today. 2005;35:896–899. doi: 10.1007/s00595-005-3030-7. [DOI] [PubMed] [Google Scholar]
- 24.Usui S, Nagai K, Hiranuma S, Takiguchi N, Matsumoto A, Sanada K. Laparoscopy-assisted esophagoenteral anastomosis using endoscopic purse-string suture instrument “Endo-PSI (II)” and circular stapler. Gastric Cancer. 2008;11:233–237. doi: 10.1007/s10120-008-0481-8. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita T, Oshiro T, Ito K, Shibasaki H, Okazumi S, Katoh R. Intracorporeal circular-stapled esophagojejunostomy using hand-sewn purse-string suture after laparoscopic total gastrectomy. Surg Endosc. 2010;24:2908–2912. doi: 10.1007/s00464-010-1041-y. [DOI] [PubMed] [Google Scholar]
- 26.Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc. 2009;23:2624–2630. doi: 10.1007/s00464-009-0461-z. [DOI] [PubMed] [Google Scholar]
- 27.Jung UJ, Kim DJ, Lee JH, W K. Safety of intracorporeal circular stapling esophagojejunostomy using trans-orally inserted anvil (OrVil™) following laparoscopic total or proximal gastrectomy—comparison with transcorporeal anastomosis. World J Surg Oncol. 2013;11:209. doi: 10.1186/1477-7819-11-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie JW, Huang CM, Zheng CH, et al. A safe anastomotic technique of using the transorally inserted anvil (OrVil™) in Roux-en-Y reconstruction after laparoscopy-assisted total gastrectomy for proximal malignant tumors of the stomach. World J Surg Oncol. 2013;11:256. doi: 10.1186/1477-7819-11-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuiki T, Hosoya Y, Kaneda Y, Kurashina K, Saito S, Ui T, et al. Stenosis after use of the double-stapling technique for reconstruction after laparoscopy-assisted total gastrectomy. Surg Endosc. 2013;27:3683–3689. doi: 10.1007/s00464-013-2945-0. [DOI] [PubMed] [Google Scholar]
- 30.So KO, Park JM. Totally laparoscopic total gastrectomy using intracorporeally hand-sewn esophagojejunostomy. J Gastric Cancer. 2011;11(4):206–2011. doi: 10.5230/jgc.2011.11.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]