Abstract
Adhesion formation after abdominal and pelvic operations remains a challenging problem. Role of adjuvant barriers have been studied but there is no comparative study between liquid paraffin and hyaluronic acid as a barrier method. Hence, we planned to compare the effectiveness of 0.4 % hyaluronic acid and liquid paraffin in the prevention of postoperative intraperitoneal adhesions in rats. This prospective, randomized and controlled study was conducted in 60 adult Wistar albino rats. Surgical trauma by caecal abrasion and 1 g talcum powder was used in the rat model to induce adhesion formation. After trauma, 3 ml normal saline was instilled in the peritoneal cavity in control group (n = 20), 3 ml liquid paraffin was instilled in experimental group A (n = 20) and 3 ml 0.4 % hyaluronic acid was instilled in experimental group B (n = 20). Two weeks after laparotomy, repeat laparotomy was performed and the adhesions were scored according to Zuhlke classification. Liquid paraffin and hyaluronic acid both reduce the extent and grade of adhesions both macroscopically (p = 0.018, p = 0.017) and microscopically (p = 0.019, p = 0.019) respectively. Although there was significant reduction in adhesions by hyaluronic acid at certain specific sites as compared with liquid paraffin, its overall effectiveness in preventing postoperative intraperitoneal adhesions is not significantly different from liquid paraffin (p = 0.092, p = 0.193) respectively. The presence of liquid paraffin and hyaluronic acid in the peritoneal cavity reduce postoperative intraperitoneal adhesions significantly in rats. However, there is no overall significant difference in the effectiveness of two groups. Dosage and safety of these chemicals in human beings remains to be established.
Keywords: Adhesions, Postoperative, Intraperitoneal, Hyaluronic acid, Liquid paraffin
Introduction
Adhesion formation after abdominal and pelvic operations remains extremely common and is a source of considerable morbidity [1]. Adhesion formation is considered to be an inevitable result of surgical trauma to the peritoneal surface [2]. Trauma initiates an inflammatory reaction, resulting in an increase in vascular permeability and release of fibrin rich exudates [3]. If fibrinolysis is not effective enough, the result will be dense adhesion formation. Post surgical adhesions severely affect the quality of life of millions of people worldwide, causing small bowel obstruction [4], difficult re-operative surgery [5], chronic abdominal and pelvic pain and female infertility [1, 6].
A variety of clinical techniques and agents have been advocated for prevention of both primary and secondary postoperative adhesion formation. The main approaches in preventing adhesions include adjusting surgical techniques, limiting trauma to intra-abdominal structures and applying adjuvants to decrease adhesion formation [7]. A wide variety of barrier substances have been tested in adhesion prevention after open abdominal surgery. Hyaluronic acid is a naturally occurring glycosaminoglycan that is present in soft tissues of all vertebrates. Under aqueous physiological conditions, hyaluronic acid forms a highly viscous solution that appears to coat serosal surface. This property appears to provide a certain degree of protection against serosal desiccation and other type of tissue injury. It has been reported to inhibit the release of protease from peritoneal leukocytes and of oxygen radicals from macrophages and to scavenge free oxygen radicals [8]. It has also been reported to lower the plasminogen inhibitor activity [9]. Experimental and clinical data using various contents for hyaluronic acid solution have suggested that covering an operation with soluble hyaluronic acid would reduce postoperative adhesions [10, 11].
Liquid paraffin reduces postoperative adhesions presumably through forming a thin film over the raw area thus giving the raw area a chance to heal and meanwhile preventing gut adhesions to it [12]. Role of adjuvant barriers have been studied but there is no comparative study between liquid paraffin and hyaluronic acid as a barrier method. In this study, we have evaluated the effectiveness of hyaluronic acid and liquid paraffin in the prevention of postoperative intraperitoneal adhesions in rats.
Material and Methods
A total of 60 healthy adult Wistar albino rats weighing between 200–250 g were used. The animals were provided food and water ad libitum. The study was approved by the Institute Animal Ethics Committee. They were divided into three groups of 20 rats each. Overnight fasting rats were anesthetized using 60 mg/kg of ketamine which was intraperitoneally.
Sterile surgical techniques were observed throughout the experimental work. Abdominal wall was shaved and surgical field was prepared with 5 % antiseptic povidone iodine solution. A midline incision sufficient to expose the intestine was made. The caecum was identified and 20 strokes were made on it with sterile gauze piece, to the extent of causing hyperemia but no bleeding, to cause aseptic inflammation and thus promote adhesions. The caecum was replaced in its anatomical position and 1 g of talcum powder was put into peritoneal cavity as adhesion inducing agent. Thereafter, various solutions were instilled into peritoneal cavity commensurating with the group prior to closure; the control group (C), 3 ml of normal saline was instilled. In experimental group (A), 3 ml liquid paraffin was instilled. In the second experimental group (B), 3 ml of 0.4 % hyaluronic acid was instilled.
All solutions as well as the talcum powder were sterilized. The abdomen was closed with a 4-0 nylon suture in single layer using continuous interlocking stitches. The animals were allowed to resume their normal diet from first postoperative day till the 14th postoperative day. Thereafter, they were sacrificed after administration of ketamine given intraperitoneally. The abdominal cavity was inspected through a U shaped incision of the anterior abdominal wall that was retracted caudally, providing maximum exposure.
Adhesions were scored according to macroscopic classification and microscopic (histological) classification developed by Zuhlke et al (1990) and subsequently used by Luijendijk et al (1996) [13, 14] as described in Table 1.
Table 1.
Macroscopic and microscopic classification according to Zuhlke
| Macroscopic classification according to Zuhlke | |
| I. | Flimsy and easy to separate by blunt dissection. |
| II. | Blunt dissection possible, partly sharp dissection necessary, beginning vascularization. |
| III. | Lysis possible by sharp dissection only, clear vascularization. |
| IV. | Lysis possible by sharp dissection only, organs strongly attached with severe adhesions, damage of organs hardly preventable. |
| Microscopic (histological) classification according to Zuhlke | |
| I | Loose connective tissue; cell rich, old and new fibrin, fine reticulin fibers. |
| II | Connective tissue with cells and capillaries, few collagen fibers. |
| III | Connective tissue more firm, fewer cells, more vessels, few elastic and smooth muscle fibers. |
| IV | Old firm granulation tissue, cell poor, serosal layers hardly distinguishable. |
Evaluation
The extent of adhesions in the peritoneum between the various viscera, intestinal loops and to the suture line was noted and evaluated as adhesion score and adhesion score index. A standard computer program G STAT was used. Analysis of comparison between two groups was done using Student t test. p value < 0.05 was considered as statistically significant.
Results and Analysis
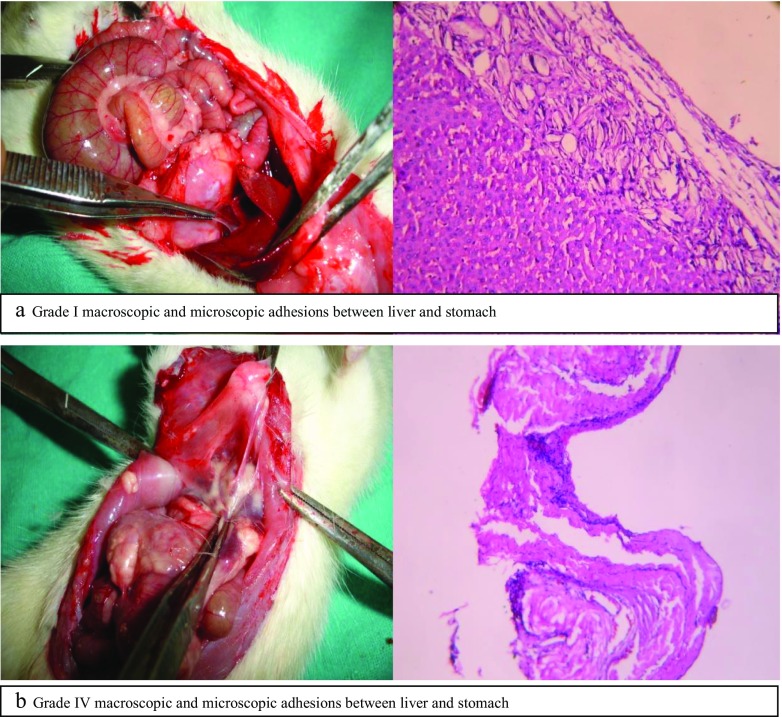
In all the groups, adhesions were noted at certain anatomical sites and different grades of adhesions have been described. In control group, 14 out of 20 rats survived at end of 2 weeks. This can be attributed to the learning curve of our experiment. The remaining 14 underwent a second laparotomy 2 weeks after the first operation. In experimental group A, 15 rats out of 20 survived at end of 2 weeks. Liquid paraffin was seen floating freely in the peritoneal cavity. In experimental group B, 16 rats survived at end of 2 weeks out of 20 rats. They were operated after 2 weeks and findings at second laparotomy with different grade of adhesions have been tabulated in Tables 2 and 3. Grade I and IV of macroscopic and microscopic adhesions have been shown in Fig. 1 (a and b).
Table 2.
Combined observation of the grade of adhesions of all groups (macroscopically)
| Site | Control group C | Experimental group A | Experimental group B |
|---|---|---|---|
| Liver and diaphragm | Grade III and IV | Grade I and II | Grade 0 and I |
| Liver and omentum | Grade II and III | Grade II | Grade I |
| Liver and stomach | Grade III and IV | Grade I | Grade I |
| Caecum and abdominal wall | Grade II and III | Grade 0 and I | Grade 0 and I |
| Caecum and bands | Grade II | Grade 0 | Grade 0 |
| Interloop adhesions | Grade I | Grade 0 and I | Grade 0 and I |
| Talc deposits in pelvis | Present | Present | Present |
Table 3.
Combined observation of the grade of adhesions of all groups (microscopically)
| Site | Control group C | Experimental group A | Experimental group B |
|---|---|---|---|
| Liver and diaphragm | Grade II and III | Grade I | Grade 0 and I |
| Liver and omentum | Grade II and III | Grade I | Grade I |
| Liver and stomach | Grade II | Grade I | Grade I |
| Caecum and abdominal wall | Grade II | Grade 0 and I | Grade 0 and I |
| Caecum and bands | Grade II | Grade 0 | Grade 0 |
| Interloop adhesions | Grade I | Grade 0 and I | Grade 0 and I |
| Talc deposits in pelvis | Present | Present | Present |
Fig. 1.
Grade I macroscopic and microscopic adhesions between liver and stomach
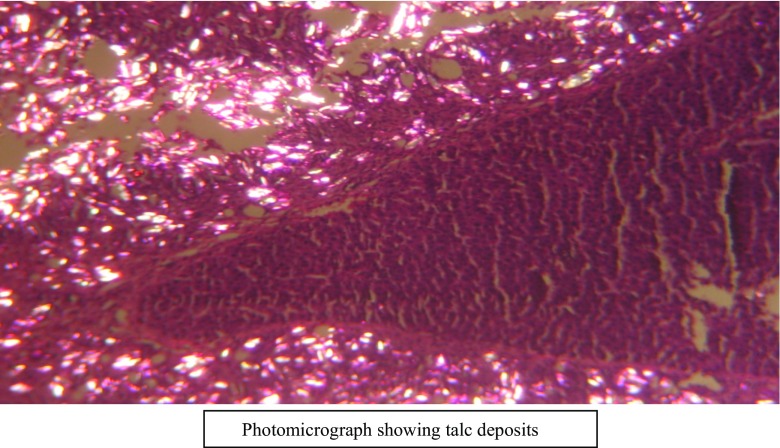
There was significant difference statistically in the reduction of intraperitoneal adhesions in rats by liquid paraffin and hyaluronic acid as compared to controls. This is supported by macroscopic (p = 0.018, p = 0.017) as well as microscopic observations (p = 0.019, p = 0.019), respectively. Talc deposits were seen in pelvis in all rats confirmed microscopically as shown in Fig. 2.
Fig. 2.
Photomicrograph showing talc deposits
While reduction in adhesions by hyaluronic acid at certain specific sites was significant statistically as compared with liquid paraffin, its overall effectiveness in preventing postoperative adhesions is not statistically significant when compared with liquid paraffin both macroscopically and microscopically (p = 0.092, p = 0.193).
Both macroscopic and microscopic grading yielded similar conclusions in all three groups. Within each group, there was no significant difference statistically between the two grading methods. Thus macroscopic and microscopic grading complements each other.
Discussion
So far, talcum powder has been widely used substance in the animal experiments as an inducing agent for adhesion formation [15]. The grading of adhesion has also been described previously in several studies [16, 17]. Swada et al used 3 ml of hyaluronic acid in different concentrations and found that lower concentration of hyaluronic acid was more effective as compared to higher concentrations of hyaluronic acid and normal saline in mice [18]. We noted adhesions at certain anatomical sites as described by Ahuja et al (2002) and in his study no statistical difference in the extent of adhesions at various sites have been found between the two groups operated at 2 and 3 weeks after the first operation. However, mortality was more at 3 weeks. They concluded that waiting for long time does not change the extent of adhesions. There is simply an increase in mortality, which decreases the validity of observations [17].
In the above study, there is gross reduction in extent and grade of adhesion formation both macroscopically and microscopically at all individual sites as compared to control group. No caecal and peritoneal bands were observed in liquid paraffin group. Shafik A et al (2002) described that liquid paraffin reduces postoperative adhesions presumably through forming a thin film over the raw area thus giving the raw area a chance to heal and meanwhile preventing gut adhesions to it [12]. The evidence of the absorption of liquid paraffin and its appearance in the blood chemically is not as conclusive as desired. The statement that the liquid paraffin is absorbed from the peritoneal cavity through lymphatics is purely an assumption, however probable it may be [19]. This is an added advantage of its very slow absorption from peritoneal cavity. There is not enough evidence to support the relation of histopathological findings to liquid paraffin absorption and deposition in various animal tissues and formation of lipoid granuloma in humans [20, 21].
Thus Liquid paraffin was recommended for use in preventing postoperative adhesions but consistency of liquid paraffin towards the action has not been demonstrated in clinical settings [22]. Ahuja et al (2002) proved the effect of liquid paraffin in adhesion prevention and reported a significant decrease in extent and grade of intraperitoneal adhesions [17].
Similarly 0.4 % hyaluronic acid led to gross reduction in the extent and grades of adhesion formation both macroscopically and microscopically at all individual sites as compared to control group. Urman et al (1991) studied the impact of hyaluronic acid solution in preventing intraperitoneal adhesions and found that pretreatment with hyaluronic acid was associated with a significant reduction in postoperative adhesions [23]. The effectiveness of inhibiting serosal tissue damage and preventing surgical adhesions by precoating tissues with dilute solutions of hyaluronic acid was evaluated in a rat caecal abrasion model by Burn et al (1995). In this study, the percentage of animals with no caecal adhesions increased from 11 % in the phosphate buffered saline group to 50 % in the 0.4 % HA treatment group. In a separate histological study employing 150 rats, HA solution significantly inhibited serosal tissue damage and ameliorated the inflammatory response due to abrasion and desiccation compared to that with no coating or precoating with buffered saline [24].
Currently a wide range of barrier material in liquid, low viscosity and solid formulations are clinically used to prevent postsurgical adhesion formation [25, 26]. Comparing liquid paraffin and hyaluronic acid group, the overall reduction in adhesions in hyaluronic acid group is not significant statistically as compared to liquid paraffin. This means that liquid paraffin is virtually and equally effective in reducing the grade and severity of postoperative intraperitoneal adhesions in rats.
Conclusion
Liquid paraffin and hyaluronic acid are both equally effective in preventing postoperative intraperitoneal adhesions in rats. Further studies are required to compare the efficacy and biocompatibility on a histological and biochemical level to determine dosage and safety in human beings which remains to be established.
Compliance with Ethical Standards
The study was approved by the Institute Animal Ethics Committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Parker MC. Epidemiology of adhesions: the burden. Hosp Med. 2004;65:330–6. doi: 10.12968/hosp.2004.65.6.13729. [DOI] [PubMed] [Google Scholar]
- 2.Bolan GM, Weigel RJ. Formation and prevention of postoperative abdominal adhesions. J Surg Res. 2006;132:553–8. doi: 10.1016/j.jss.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Rout UK, Diamond MP. Role of plasminogen activators during healing after uterine serosal lesioning in the rat. Fertil Steril. 2003;79:138–45. doi: 10.1016/S0015-0282(02)04569-7. [DOI] [PubMed] [Google Scholar]
- 4.Kossi J, Salminen P, Rantala A, Laato M. Population-based study of the surgical workload and economic impact of bowel obstruction caused by postoperative adhesions. Br J Surg. 2003;90:1441–4. doi: 10.1002/bjs.4272. [DOI] [PubMed] [Google Scholar]
- 5.Beck DE, Ferguson MA, Opelka FG, Fleshman JW, Gervaz P, Wexner SD. Effect of previous surgery on abdominal opening time. Dis Colon Rectum. 2000;43:1297–9. doi: 10.1007/BF02236862. [DOI] [PubMed] [Google Scholar]
- 6.Monk BJ, Berman ML, Montz FJ. Adhesions after extensive gynecologic surgery: clinical significance, etiology and prevention. Am J Obstet Gynecol. 1994;170:1396–03. doi: 10.1016/S0002-9378(13)90479-8. [DOI] [PubMed] [Google Scholar]
- 7.Kamel RM. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150:111–8. doi: 10.1016/j.ejogrb.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Greenwald RA, May WW. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980;223:455–61. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- 9.Wiegel PH, Fuller GM, LeBoeut RD. A model for the role of hyaluronic acid and fibrin in early events during the inflammatory response and wound healing. J Theor Biol. 1986;119:219–26. doi: 10.1016/S0022-5193(86)80076-5. [DOI] [PubMed] [Google Scholar]
- 10.Ustun C, Kocak I, Akapolat I. Effect of seprafilm (sodium hyluronate-based bioresorbable), Sepracoat (0.4% hyaluronic acid) and Ringer’s lactate on prevention of postsurgical adhesion formation in rat models. J Obstet Gynaecol. 2000;20:78–80. doi: 10.1080/01443610063543. [DOI] [PubMed] [Google Scholar]
- 11.Carta G, Cerrone L, Iovenitti P. Postoperative adhesion prevention in gynecologic surgery with hyaluronic acid. Clin Exp Obstet Gynecol. 2004;31:39–41. [PubMed] [Google Scholar]
- 12.Shafik A, El-Sibai O, Shafik AA. A novel method for preventing postoperative adhesions in rat models. J Gynaecol Surg. 2002;18:65–8. doi: 10.1089/104240602760172891. [DOI] [Google Scholar]
- 13.Zuhlke HV, Lorenz EMP, Straub EM, Savvas V. Pathophysiologic und Klassifikation von Adhasionen. Langenbecks Arch Chir Suppl II Verch Detsch Ges Chir. 1990;345:1009–16. [PubMed] [Google Scholar]
- 14.Luijendijk RW, de lange DC, Wauters CC, Hop WC, Duron JJ, Pailler JL, et al. Foreign material in post-operative adhesions. Ann Surg. 1996;223:242–8. doi: 10.1097/00000658-199603000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YL, Pan CE, Yang PL, Tian Y, Pei SW, Dong M. Effects of antiadhesion preparation on free fibrinogen and fibrin degrading products in abdominal exudates of rabbits postoperatively. World J Gastroenterol. 2004;10:2762–6. doi: 10.3748/wjg.v10.i18.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rijhwani A, Sen S, Gunasekaran S, Ponnaiya J, Balasubramanian KA, Mammen KE. Allopurinol reduces the severity of peritoneal adhesions in mice. J Pediatr Surg. 1995;30:533–7. doi: 10.1016/0022-3468(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 17.Ahuja M, Singh VP, Kumar A. Prevention of postoperative intraperitoneal adhesions—an experimental study in rats. J Indian Assoc Pediatr Surg. 2002;7:10–15. [Google Scholar]
- 18.Sawada T, Hasegawa K, Tsukada K, Kawakami S. Adhesion preventive effect of hyaluronic acid after intraperitoneal surgery in mice. Hum Reprod. 1999;14:1470–2. doi: 10.1093/humrep/14.6.1470. [DOI] [PubMed] [Google Scholar]
- 19.Gideon Wells H and Lafayetle Mendel B. On absorption from the peritoneal cavity. American Journal of Physiology, 1907; 18: 150. Reprint. London: Forgotten Books, 2013. 158-9.
- 20.Cruickshank B, Thomas JM. Mineral oil (follicular) lipidosis: II. Histologic studies of spleen, liver, lymph nodes, and bone marrow. Hum Pathol. 1983;15:731–737. doi: 10.1016/S0046-8177(84)80163-X. [DOI] [PubMed] [Google Scholar]
- 21.Wanless IR, Geddie WR. Mineral oil lipogranulomata in liver and spleen. A study of 465 autopsies. Arch Pathol Lab Med. 1985;109:283–286. [PubMed] [Google Scholar]
- 22.Soules MR, Dennis L, Bosarge A, Moore DE. The prevention of postoperative pelvic adhesions: an animal study comparing barrier methods with dextran 70. Am J Obstet Gynecol. 1982;143:829–34. doi: 10.1016/0002-9378(82)90018-7. [DOI] [PubMed] [Google Scholar]
- 23.Urman B, Gomel V, Jetha N. Effect of hyaluronic acid on postoperative intraperitoneal adhesion formation in the rat model. Fertil Steril. 1991;56:563–7. doi: 10.1016/S0015-0282(16)54558-0. [DOI] [PubMed] [Google Scholar]
- 24.Burns JW, Skinner K, Colt J, Sheidlin A, Bronson R, Yaacobi Y, et al. Prevention of tissue injury and postsurgical adhesions by precoating tissues with hyaluronic acid solutions. J Surg Res. 1995;59:644–52. doi: 10.1006/jsre.1995.1218. [DOI] [PubMed] [Google Scholar]
- 25.Brochhuasen C, Schmitt VH, Rajab TK, Plank CN, Kramer B, Wallweiner M, et al. Intraperitoneal adhesions-an ongoing challenge between biomedical engineering and the life sciences. J Biomed Mater Res A. 2011;98:143–56. doi: 10.1002/jbm.a.33083. [DOI] [PubMed] [Google Scholar]
- 26.Rajab TK, Wallweiner M, Planck C, Brochhausen C, Kraemer B, Wallwiener CW. A direct comparison of seprafilm, adept, intercoat and spraygel for adhesion prophylaxis. J Surg Res. 2010;161:264–9. doi: 10.1016/j.jss.2008.11.839. [DOI] [PubMed] [Google Scholar]