Abstract
Context
Heart failure (HF)-specific health status (symptom burden, functional status, and health-related quality of life) is an important patient-reported outcome that is associated with palliative care needs, hospitalizations, and death.
Objectives
To identify potentially modifiable patient-reported factors that predict HF-specific health status over one year.
Methods
This was a prospective cohort study using data from the Patient-Centered Disease Management trial. Participants were identified using population-based sampling of all patients with an HF diagnosis at four VA Medical Centers. Patients were enrolled with reduced HF-specific health status (i.e., significant HF symptoms, limited functional status, and poor quality of life, defined by a Kansas City Cardiomyopathy Questionnaire [KCCQ] score <60). Patient-reported factors at baseline were chest pain, other noncardiac pain, dry mouth, numbness/tingling, constipation, nausea, cough, dizziness, depressive symptoms (Patient Health Questionnaire–9), and spiritual well-being (validated, single-item measure). Patients reported HF-specific health status (KCCQ) at 3, 6, and 12 months.
Results
Of 384 U.S. veterans, 42% screened positive for depression and 76% described burdensome physical symptoms at baseline. In bivariate analyses, all patient-reported factors were correlated with KCCQ score over one year. Multivariable mixed-effect modeling showed that baseline chest pain, numbness/tingling, depressive symptoms, and higher comorbidity count predicted HF-specific health status over the following year.
Conclusion
Burdensome physical and depressive symptoms independently predicted subsequent HF-specific health status in patients with symptomatic HF. Whether addressing these aspects of the patient experience can improve health status and well-being in symptomatic HF should be studied further.
Keywords: Heart failure, health status, symptoms, spirituality, depression
Introduction
Patients with heart failure (HF) experience reduced health status because of HF, including significant HF symptoms, limited functional status, and poor quality of life.1 Assessing HF-specific health status can help identify patients who have unmet palliative care needs, including opportunities to improve patient well-being and assist with prognostication related to hospitalization or death.2–4 HF-specific health status is an independent predictor of subsequent mortality, cardiovascular events, hospitalization, and costs of care.5 HF-specific health status is recognized by the American Heart Association as an important patient-reported outcome in HF.5 This is consistent with the National Academy of Medicine, the Patient-Centered Outcomes Research Institute, and the World Health Organization support for the measurement of health status as a key patient-reported outcome.6–8 Indeed, ongoing HF palliative care trials are using HF-specific health status as the primary outcome.9,10
Although HF-specific health status is a clinically relevant patient-reported outcome in patients with HF, a recent American Heart Association Scientific Statement emphasized a “surprising paucity of research on the determinants of health status out-comes.”5 Traditional cardiac disease severity indices (e.g., left ventricular ejection fraction, B-type natriuretic peptide, myocardial ischemia) are only weakly associated with HF-specific health status.11–13 Known determinants of HF-specific health status include patient characteristics such as gender and socioeconomic status, functional dependency, comorbid conditions, and psychological factors such as depression.14–16 However, many of the known determinants of HF-specific health status (e.g., age, gender, socioeconomic status, and functional dependency) are not easily modifiable. Furthermore, prior studies do not focus on patients with reduced HF-specific health status, a population of patients with HF who may benefit from palliative care. Finally, prior studies do not include a wide range of patient-reported factors. Studies that do include a range of patient-reported factors are cross-sectional.17 Identification of potentially modifiable patient-reported factors that are predictive of subsequent HF-specific health status over one year may lead to new targets for patient-centered treatment interventions.
In addition to depression, other potentially modifiable patient-reported factors of HF-specific health status include spiritual well-being and physical symptoms.1 Spiritual well-being is an important, potentially modifiable coping resource in patients with HF. Among 60 outpatients with HF, greater spiritual well-being, particularly having a sense of life's meaning or peace, was strongly associated with less depression.18 In small single-site studies, a diverse number of symptoms, such as pain, numbness/tingling, and dry mouth are reported by patients with HF.19 The patient experience of spiritual well-being and diverse symptoms has not been examined in a large, multisite study. It is unknown whether spiritual well-being or diverse symptoms predict HF-specific health status.
Accordingly, the objectives of this study were to 1) describe the prevalence and burden of a diverse range of patient-reported factors including physical symptoms, depression, and spiritual well-being in a multisite sample of outpatients with HF and reduced HF-specific health status and 2) evaluate whether a diverse range of patient-reported factors were independently associated with HF-specific health status over one year. Among patients with HF and reduced HF-specific health status, identifying patient-reported factors at baseline that are associated with HF-specific health status over one year could inform novel patient-centered treatment interventions to improve this important patient-reported outcome.
Methods
Study Design and Participants
This secondary analysis of the Patient-Centered Disease Management (PCDM) trial aims to describe the prevalence of patient-reported factors (physical symptoms, depressive symptoms, and spiritual well-being) at baseline and determine whether these patient-reported factors are associated with HF-specific health status over one year. The PCDM trial enrolled outpatients with an HF diagnosis and reduced HF-specific health status; trial design has been previously described.20 All patients with a diagnosis of HF at four Veterans Affairs Medical Centers were screened for eligibility, starting in May 2009, with 12-month follow-up completed by June 2012. HF-specific health status was measured using the Kansas City Cardiomyopathy Questionnaire (KCCQ, scale range = 0–100).3 Patients with a KCCQ score of <60 had reduced HF-specific health status, indicating significant HF symptoms, limited functional status, and poor quality of life, were eligible to enroll in the PCDM trial, and provided written informed consent. Key exclusion criteria were as follows: severe cognitive or psychiatric impairment; current residence in a nursing home; irreversible, noncardiac medical conditions likely to affect six-month survival or ability to execute the study protocol (e.g., metastatic cancer); prior heart transplantation; and alcohol abuse as indicated by the Alcohol Use Disorders Identification Test score ≥7.21 Institutional review board approval was obtained at each study site.
Enrolled patients were randomized to receive usual care or the PCDM intervention, which included 1) collaborative care management by a team that included a nurse, cardiologist, internist, and psychiatrist, and who worked with patients and their primary care providers to provide guideline-concordant care management; 2) home telemonitoring and guided patient self-management support; and 3) screening and treatment for comorbid depression. Because the PCDM intervention did not influence HF-specific health status, this secondary analysis includes all trial participants, irrespective of randomization. Specifically, after one year, the KCCQ score increased by 13.5 points for both the intervention and control groups (P = 0.97).22
Study Outcome
The study outcome was HF-specific health status over one year using KCCQ overall score measured at 3, 6, and 12 months.20 The KCCQ is a valid and reliable 23-item, self-administered questionnaire with score ranges from 0 to 100, with higher scores reflecting better HF-specific health status. It is sensitive to clinical change, predicts hospitalization and mortality, and is a common primary patient-reported outcome of palliative care and disease management clinical trials for patients with HF.2,3,9,10,23 The KCCQ score was used for all analyses. Although it is likely that associations between baseline study variables and HF-specific health status over one year will be strongest for the earlier assessments (i.e., three and six months), to facilitate interpretation, the study outcome was based on a repeated-measures model where the relationship between baseline KCCQ score and the three subsequent measurements were assumed to be equal across all three follow-up time points.
Patient-Reported Factors
The study predictor variables included potentially modifiable patient-reported factors (physical symptoms, depressive symptoms, and spiritual well-being) that were measured at baseline. We minimized instrument burden by avoiding duplication of symptom assessment for this study sample with reduced HF-specific health status. The physical symptoms were chest pain or pressure, other pain, dry mouth, numbness/tingling in hands or feet, constipation, nausea, cough, and dizziness. These physical symptoms were chosen because studies have shown these symptoms to be highly prevalent and/or distressing in HF,17,19,24 and they were not already included in the KCCQ or Patient Health Questionnaire–9 (PHQ-9). The symptoms assessed in the KCCQ are dyspnea, fatigue, and edema. Participants used a five-category Likert scale to rate how much each symptom bothered him/her over the past two weeks from “not at all” to “extremely bothersome.” Depressive symptoms were measured using the PHQ-9, a valid and reliable instrument that provides a continuous measure of depressive symptoms with a range of 0–27.25 Depressive symptom severity was categorized based on validated levels of PHQ-9 scores: minimal (0–4), mild (5–9), moderate (10–14), moderately severe (15–19), and severe (≥20).25 Because the PHQ-9 is a continuous measure (range 0–27), it was rescaled in the multivariable analyses to facilitate comparison with other variables, which were all five-point ordinal variables. Specifically, the original PHQ-9 score was divided by five so that one unit of change in the rescaled PHQ-9 variable was equivalent to a five-point change in the original PHQ-9 score. “A sense of peace” is a key component of spiritual well-being. The degree of feeling “at peace” was measured using a validated, single-item measure where participants responded on a five-category Likert scale to the statement, “I feel at peace.” Possible responses ranged from “completely at peace” to “not at all at peace.”26,27
Specific baseline demographic, clinical, and patient-reported factors (depressive symptoms, spiritual well-being, and physical symptoms) were selected a priori as potential factors associated with HF-specific health status. These factors were chosen because either previous studies or a theoretical rationale suggested they were associated with HF-specific health status. Demographic factors included age, gender, and race. Clinical factors were left ventricular ejection fraction class, presence of a biventricular pacemaker, and certain comorbid conditions. The presence of a biventricular pacemaker was included as a clinical factor because it can influence HF-specific health status.28 The number of comorbidities was based on the presence of the following conditions that are common or clinically relevant in patients with HF, collected by medical record review: coronary artery disease (including prior myocardial infarction, prior percutaneous intervention, or coronary artery bypass graft), diabetes, hypertension, transient ischemic attack/stroke, chronic obstructive pulmonary disease, atrial fibrillation, peripheral vascular disease, alcohol abuse, or other substance abuse. The comorbidity count variable ranged from 0 to 9.
Statistical Analysis
Summary statistics and measures of variability were compiled for all study variables. We examined the magnitude and patterns of missing data to check the sensitivity of the model to any missing data. Pearson correlation was calculated pairwise for each baseline variable and the study outcome of KCCQ score over one year.
To identify individual factors associated with KCCQ score over one year, we first used a group variable selection approach to determine whether groups of variables were associated with the outcome. The groups were 1) demographic factors (age, gender, race), 2) clinical factors (ejection fraction class, biventricular pacemaker, comorbidity count), and 3) patient-reported factors (eight physical symptoms, depressive symptoms, spiritual well-being). We tested each group for significance individually using an F test; then if the group was significant, used backward selection based on a P-value <0.05 to identify individual variables within each group for inclusion in the final model. Baseline KCCQ score was not included as a covariate to avoid collinearity with other measures and because the focus of this study was to identify potential modifiable patient-reported factors that may influence subsequent HF-specific health status. Modified R2 statistics appropriate for mixed-effects models were calculated for each chunk and overall in the final model.29 All multivariate modeling was conducted using SAS, version 9.3 (SAS Institute, Cary, NC). Graphics were generated using R (http://www.R-project.org/).
Regarding missing data, there were 36 (9.5%) patients with missing ejection fraction and five patients (1.3%) with missing PHQ-9 scores at baseline. Because clinical factors were not significant in the groupwise variable selection approach, ejection fraction did not warrant separate testing in the final multivariable model. Thus, because inclusion of the 36 patients with missing ejection fraction data did not measurably change the model results, the 36 patients were excluded from the final model. Patients with missing ejection fraction did not differ with respect to the study outcome, KCCQ score over one year. Because PHQ-9 score was an important predictor of health status and the number of patients missing baseline PHQ-9 scores was small, we excluded the five patients missing baseline PHQ-9 scores from the model. The total number of patients excluded from multivariate modeling due to missing data was 41 (10%).
Results
Study Participants
A total of 384 U.S. veterans completed baseline measures in the PCDM trial (Table 1). Participants had a mean age of 66 years and were mostly white men. The mean baseline KCCQ score was 39 (SD 14.0, range 0–60), indicating significant HF symptom burden, limited functional status, and poor quality of life in the study sample. Nearly half of the study sample had HF with normal ejection fraction (47%), whereas one-third had moderately or severely reduced ejection fraction (<40%). The baseline prevalence and severity of patient-reported factors, including physical symptoms, depressive symptoms, and degree of feeling “at peace” are shown in Figure 1. Seventy-six percent described at least one burdensome physical symptom at baseline (reported as a score of 4 “quite a bit” or 5 “extremely bothersome”), including noncardiac, other pain (46%), numbness/tingling (40%), dry mouth (31%), dizziness (17%), constipation (16%), and cough (15%). At baseline, 42% screened positive for depression based on PHQ-9 score ≥10. Nearly one-quarter of the study sample reported feeling a limited sense of peace (6% “not at all at peace,” 17% “a little at peace”).
Table 1. Study Participant Characteristics (N = 384).
| Characteristic | N (%) or Median [IQR] |
|---|---|
| Age | 66 [61–75] |
| Male | 371 (96) |
| Race, white | 314 (81) |
| Baseline KCCQ score | 39 [27–48] |
| Ejection fraction class | |
| Normal (≥50%) | 146 (47) |
| Mildly reduced (40%–49%) | 64 (21) |
| Moderately reduced (30%–39%) | 68 (22) |
| Severely reduced (<30%) | 30 (10) |
| Biventricular pacemaker | 21 (5.5) |
| Comorbidity count | 3 [2–4] |
| Hypertension | 317 (83) |
| Coronary artery disease | 207 (54) |
| Diabetes | 192 (50) |
| Atrial fibrillation | 143 (37) |
| COPD | 116 (30) |
| Alcohol abuse | 60 (16) |
| Peripheral vascular disease | 48 (13) |
| TIA or stroke | 33 (8.6) |
| Other substance abuse | 28 (7.3) |
IQR = interquartile range; KCCQ = Kansas City Cardiomyopathy Questionnaire; COPD = chronic obstructive pulmonary disease; TIA = transient ischemic attack.
Fig. 1.
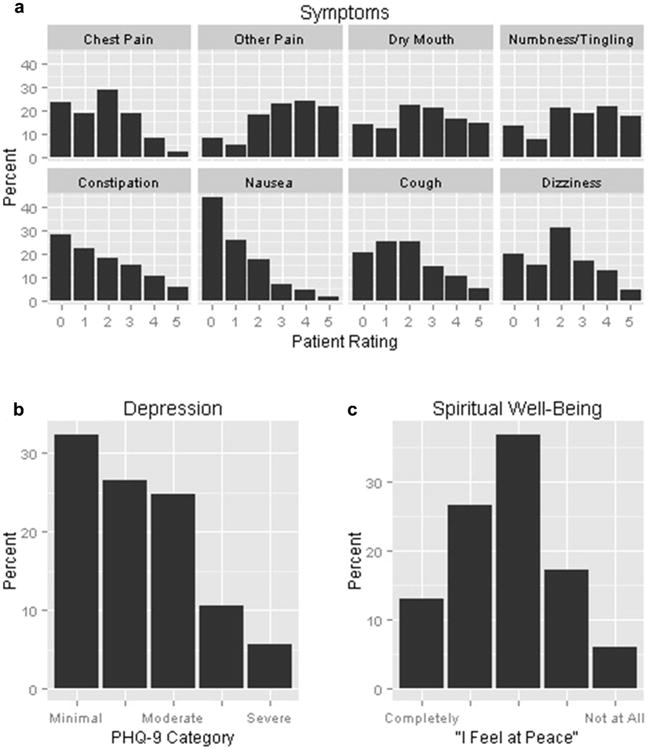
Baseline prevalence of patient-reported physical symptoms, depression, and spiritual well-being in 384 patients with heart failure. a) Symptoms based on how bothersome each symptom was: 0—dsymptom not present; 1—dsymptom not at all bothersome; 5—dsymptom extremely bothersome. b) Depression based on PHQ-9 category: minimal (0—4), mild (5—9), moderate (10—14), moderately severe (15—19), severe (≥20). c) Spiritual well-being based on “I feel at peace,” from completely to not at all on five-point Likert scale. PHQ-9 = Patient Health Questionnairee—9.
Factors Associated With HF-Specific Health Status Over One Year
In bivariate analyses, the baseline patient-reported factors, including diverse physical symptoms, depressive symptoms, limited sense of peace, and comorbidity count, were correlated with KCCQ score over one year (Table 2), whereas age, gender, race, ejection fraction class, and biventricular pacemaker were not (data not shown). In the final multivariate mixed-effect model, chest pain and numbness/tingling, higher depressive symptoms, and higher comorbidity count were associated with KCCQ score over one year (Table 3), whereas age, gender, race, ejection fraction class, presence of biventricular pacemaker, and spiritual well-being were not. The proportion of variance in KCCQ score over one year explained by baseline burdensome physical symptoms (chest pain and numbness/tingling), depressive symptoms, and co-morbidity count was 21%.
Table 2. Baseline Patient-Reported Factors Associated With HF-Specific Health Status Over One Year: Bivariate Analysis.
| Variables | KCCQ Score | |
|---|---|---|
|
| ||
| Correlation | P-value | |
| Physical symptom | ||
| Chest pain | −0.26 | <0.001 |
| Dizziness | −0.26 | <0.001 |
| Numbness/tingling | −0.25 | <0.001 |
| Dry mouth | −0.23 | <0.001 |
| Other pain | −0.20 | <0.001 |
| Nausea | −0.15 | 0.004 |
| Cough | −0.15 | 0.004 |
| Constipation | −0.10 | 0.045 |
| Depressive symptoms | −0.38 | <0.001 |
| Lower spiritual well-being | −0.27 | <0.001 |
| Comorbidity count | −0.15 | 0.004 |
HF = heart failure; KCCQ = Kansas City Cardiomyopathy Questionnaire.
Table 3. Multivariable Analysis of Patient-Reported Factors Associated With HF-Specific Health Status Over One Year.
| Variables | Adjusted R2a | KCCQ Score | ||
|---|---|---|---|---|
|
| ||||
| Estimate | Standard Error | P-value | ||
| Overall model | 0.21 | <0.001 | ||
| Physical symptoms | ||||
| Chest pain | 0.05 | −2.02 | 0.70 | 0.004 |
| Numbness/tingling | −1.15 | 0.57 | 0.045 | |
| Depressive symptomsb | 0.09 | −4.84 | 0.83 | <0.001 |
| Comorbidity count | 0.02 | −1.55 | 0.67 | 0.022 |
HF = heart failure; KCCQ = Kansas City Cardiomyopathy Questionnaire; PHQ-9 = Patient Health Questionnairee−9.
The individual adjusted R-squared values do not add up to the overall model R-squared. This is an expected result of correlation between the factors.
Depressive symptoms estimate is per five-point increase in PHQ-9 score.
Discussion
In this longitudinal sample of community-dwelling HF patients with reduced HF-specific health status, several patient-reported factors were associated with HF-specific health status over one year, including burdensome physical symptoms, depressive symptoms, and comorbidity burden. Taken together, physical symptoms (chest pain and numbness/tingling), depressive symptoms, and comorbid conditions accounted for 21% of the variance in HF-specific health status over one year. These findings imply the need for increased emphasis on assessing patient-reported factors in HF, as these factors are strongly associated with HF-specific health status and may be important targets for patient-centered treatment interventions designed to improve the experience of living with symptomatic HF.
This is the first prospective study to understand determinants of HF-specific health status using a comprehensive range of patient-reported factors, demographic characteristics, and clinical variables. Up to this point, the majority of known determinants of HF-specific health status, including gender and socio-economic status, functional dependency, and comorbid conditions, are difficult to alter.3 This study identified new and potentially modifiable, patient-reported factors that are highly prevalent in patients with symptomatic HF. Chest pain and numbness/tingling were burdensome physical symptoms that were associated with reduced HF-specific health status, even when accounting for depressive symptoms and comorbidity burden. Numbness and tingling may not be a patient-reported symptom commonly considered to be relevant to HF-specific health status in general clinical practice, and this finding emphasizes the need for thorough assessment of patient concerns.
In this large, multisite sample of patients with reduced HF-specific health status, the high prevalence of diverse physical symptoms reported to be burdensome at baseline was not surprising, and included noncardiac pain, numbness/tingling, dry mouth, dizziness, constipation, and cough. These findings are similar to other studies, including one study that found over 50% of outpatients with HF reported shortness of breath, lack of energy, pain, feeling drowsy, or dry mouth.19 The prevalence of pain in patients with HF is high. Studies have found 55%–84% of patients with HF have noncardiac pain.30,31 The presence of these symptoms suggests the importance of going beyond the common symptoms of dyspnea, edema, and fatigue and focusing on additional targets to improve the care of patients with symptomatic HF. These symptoms may be a result of comorbid conditions, cardiac or noncardiac medications, or HF itself. However, whether addressing the diverse physical symptoms can improve HF-specific health status over time needs to be investigated, especially because many patient-reported symptoms were not associated with HF-specific health status over one year. Additionally, the impact of medications on patient-reported symptoms and HF-specific health status warrants further study, specifically considering the number, type, efficacy, and side effects of medications.
Similar to other studies, we found that high depressive symptoms in patients with HF were prevalent and strongly associated with reduced HF-specific health status over one year.15,16 Although efforts to improve depression in HF using antidepressants have been disappointing, depression remains an important treatment target.16,32 To improve HF-specific health status, a combined approach of addressing depression, HF-related symptoms, and other concomitant symptoms that may be overlooked (e.g., numbness/tingling) may be necessary to impact health status. Future research can determine the effectiveness of interventions that are tailored to the individual's physical and affective symptoms.
Our results found that the single-item measure of spiritual well-being was associated with HF-specific health status over one year in bivariate but not multivariate analysis. A sense of peace is an important component of spiritual well-being, and the single-item measure (“I feel at peace”) has been validated in HF patients to be strongly correlated with emotional and spiritual well-being.27 The single-item measure may have had limited explanatory power when controlling for depressive symptoms. A more comprehensive measure of spiritual well-being, such as the Functional Assessment of Chronic Illness Therapy Spiritual Well-Being Scale, could be considered in future studies.33 The spiritual needs of patients with HF have been documented, including the inverse relationship between spiritual well-being, particularly meaning/peace, and depression18,34 A prior study found that patients with HF said that their spiritual concerns could have been addressed by having someone to talk to, supporting their informal (or family) caregivers, and medical staff showing sensitivity and taking care to foster hope.35 Future intervention studies will help clinicians caring for HF patients understand the extent to which spiritual well-being is associated with HF-specific health status over one year and how it can be modified to improve how the patient feels.
Although burdensome physical symptoms, depressive symptoms, and comorbid conditions accounted for 21% of the variance in KCCQ score over one year in an adjusted analysis, the majority of the variance in HF-specific health status was not explained by the demographic, clinical, or patient-reported factors assessed in this study. The existence of unexplained variance is common in the health status literature, and the amount of variance in HF-specific health status explained by the patient-reported factors identified in this study is consistent with the amount of variance explained in other studies. For example, one study explained 39% of the variance in HF-health status, although this study included patient functional level as a predictor, which is already a component of HF-specific health status.15 In a study of hospitalized patients with HF, low admission KCCQ score, high B-type natriuretic peptide, hyponatremia, tachycardia, hypotension, absence of β-blocker therapy, and history of diabetes mellitus and arrhythmia were independent predictors of reduced HF-specific health status or death over the next six months.36 However, that study did not include a comprehensive assessment of patient-reported factors and did not calculate the amount of variance explained. Further research is warranted to explore the potentially significant impact of other relevant factors on HF-specific health status, such as self-management ability, medications, care-giver availability and capability, and social and financial resources.
This study has several limitations. Because the study sample was primarily Caucasian men, typical of the U.S. veteran population, the results may not be generalizable to other health systems or more diverse racial or ethnic HF populations. Similarly, because of the limited number of women in the study sample, we did not perform a gender-specific analysis, and the results may have limited generalizability to female HF populations. Although these race and gender limitations exist, the PCDM trial attempted to limit biases because of referral patterns or patient selection by using a population-based, case-finding method using the VA electronic health record to identify veterans with a diagnosis of HF.20
Another limitation is that patient-reported symptoms were correlated; this may have led to a reduced ability to identify individual predictive symptoms. Although a comprehensive range of patient-reported symptoms were assessed at baseline, the focus was on symptom burden and prevalence rather than symptom severity or frequency. Depressive symptoms as measured with the PHQ-9 do include physical symptoms such as fatigue and sleep disturbance, so there is a limitation in drawing a sharp distinction between physical symptoms and depression. Finally, the patient-reported factors were measured at baseline only. It is possible that different associations might have been found if patient-reported factors were measured over time.
In conclusion, in this population-based sample of individuals with HF and reduced HF-specific health status, depression, chest pain, numbness/tingling, and comorbidity burden were associated with HF-specific health status over the subsequent one year. For clinicians caring for patients with symptomatic HF, these patient-reported factors may represent opportunities to improve health status.
Acknowledgments
The Patient-Centered Disease Management trial was funded by the Department of Veterans Affairs HSR&D grant IIR 06–068. Dr. Bekelman was funded by the Department of Veterans Affairs (HSR&D CDA 08–022). After approval of funding, the funding organizations did not participate in the study design and conduct; collection, management, analysis, or interpretation of data; or article preparation or decision to submit for publication.
Disclaimer: The views in this article are those of the authors and do not necessarily reflect the views of the Department of Veterans Affairs.
Footnotes
Disclosure: None of the authors has any conflicts to disclose.
Clinical Trial Registration Information: Patient-Centered Disease Management for Heart Failure Trial; Unique identifier: NCT00461513;
References
- 1.Bekelman DB, Hutt E, Masoudi FA, Kutner JS, Rumsfeld JS. Defining the role of palliative care in older adults with heart failure. Int J Cardiol. 2008;125:183–190. doi: 10.1016/j.ijcard.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Spertus JA, Jones PG, et al. Consortium COR. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47:752–756. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 4.Bekelman DB, Rumsfeld JS, Havranek EP, et al. Symptom burden, depression, and spiritual well-being: a comparison of heart failure and advanced cancer patients. J Gen Intern Med. 2009;24:592–598. doi: 10.1007/s11606-009-0931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rumsfeld JS, Alexander KP, Goff DC, et al. American Heart Association Council on Quality of Care and Outcomes Research CoCaSN, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Stroke Council. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association Circulation. 2013;127:2233–2249. doi: 10.1161/CIR.0b013e3182949a2e. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine, Committee on Quality Health Care in America. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academies Press; 2001. [Google Scholar]
- 7.Patient-Centered Outcomes Research Institute. Patient-Centered outcomes research. [Accessed November 6, 2012]; Available at: http://www.pcori.org/what-we-do/pcor/
- 8.World Health Organization. WHO definition of health. Preamble to the Constitution of the World Health Organization as adopted by the International Health Conference; New York. June 19–22, 1946; signed on July 22, 1946, by the representatives of 61 States (Official Records of the World Health Organization, No. 2, p. 100) and entered into force on April 7, 1948. [Google Scholar]
- 9.Clinicaltrials.gov. Collaborative Care to Alleviate Symptoms and Adjust to Illness in Chronic Heart Failure (CASA) Trial. [Accessed July 24, 2015]; Available at: https://clinicaltrials.gov/ct2/show/NCT01739686.
- 10.Mentz RJ, Tulsky JA, Granger BB, et al. The palliative care in heart failure trial: rationale and design. Am Heart J. 2014;168:645–651.e641. doi: 10.1016/j.ahj.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients' perceptions of the effects of heart failure on their quality of life. J Card Fail. 2006;12:87–92. doi: 10.1016/j.cardfail.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Luther SA, McCullough PA, Havranek EP, et al. Consortium COR. The relationship between B-type natriuretic peptide and health status in patients with heart failure. J Card Fail. 2005;11:414–421. doi: 10.1016/j.cardfail.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Gorkin L, Follick MJ, Geltman E, et al. Quality of life among patients post-myocardial infarction at baseline in the Survival and Ventricular Enlargement (SAVE) trial. Qual Life Res. 1994;3:111–119. doi: 10.1007/BF00435254. [DOI] [PubMed] [Google Scholar]
- 14.Janssen DJ, Franssen FM, Wouters EF, Schols JM, Spruit MA. Impaired health status and care dependency in patients with advanced COPD or chronic heart failure. Qual Life Res. 2011;20:1679–1688. doi: 10.1007/s11136-011-9892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dekker RL, Lennie TA, Albert NM, et al. Depressive symptom trajectory predicts 1-year health-related quality of life in patients with heart failure. J Card Fail. 2011;17:755–763. doi: 10.1016/j.cardfail.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rumsfeld JS, Havranek E, Masoudi FA, et al. Consortium COR. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure. J Am Coll Cardiol. 2003;42:1811–1817. doi: 10.1016/j.jacc.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008;35:594–603. doi: 10.1016/j.jpainsymman.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bekelman DB, Dy SM, Becker DM, et al. Spiritual well-being and depression in patients with heart failure. J Gen Intern Med. 2007;22:470–77. doi: 10.1007/s11606-006-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13:643–648. doi: 10.1016/j.cardfail.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Bekelman DB, Plomondon ME, Sullivan MD, et al. Patient-centered disease management (PCDM) for heart failure: study protocol for a randomised controlled trial. BMC Cardiovasc Disord. 2013;13:49. doi: 10.1186/1471-2261-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 22.Bekelman DB, Plomondon ME, Carey EP, et al. Primary results of the Patient-Centered Disease Management (PCDM) for Heart Failure Study: a randomized clinical trial. JAMA Intern Med. 2015;175:725–732. doi: 10.1001/jamainternmed.2015.0315. [DOI] [PubMed] [Google Scholar]
- 23.Spertus J, Peterson E, Conard MW, et al. Consortium COR. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Janssen DJ, Spruit MA, Uszko-Lencer NH, Schols JM, Wouters EF. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med. 2011;14:735–743. doi: 10.1089/jpm.2010.0479. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinhauser KE, Clipp EC, Bosworth HB, et al. Measuring quality of life at the end of life: validation of the QUAL-E. Palliat Support Care. 2004;2:3–14. doi: 10.1017/s1478951504040027. [DOI] [PubMed] [Google Scholar]
- 27.Steinhauser KE, Voils CI, Clipp EC, et al. “Are you at peace?”: one item to probe spiritual concerns at the end of life. Arch Intern Med. 2006;166:101–105. doi: 10.1001/archinte.166.1.101. [DOI] [PubMed] [Google Scholar]
- 28.Versteeg H, van 't Sant J, Cramer MJ, et al. Discrepancy between echocardiographic and patient-reported health status response to cardiac resynchronization therapy: results of the PSYHEART-CRT study. Eur J Heart Fail. 2014;16:227–234. doi: 10.1002/ejhf.38. [DOI] [PubMed] [Google Scholar]
- 29.Edwards LJ, Muller KE, Wolfinger RD, Qaqish BF, Schabenberger O. An R2 statistic for fixed effects in the linear mixed model. Stat Med. 2008;27:6137–6157. doi: 10.1002/sim.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goebel JR, Doering LV, Shugarman LR, et al. Heart failure: the hidden problem of pain. J Pain Symptom Manage. 2009;38:698–707. doi: 10.1016/j.jpainsymman.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodlin SJ, Wingate S, Albert NM, et al. PAIN-HF Investigators. Investigating pain in heart failure patients: the pain assessment, incidence, and nature in heart failure (PAIN-HF) study. J Card Fail. 2012;18:776–783. doi: 10.1016/j.cardfail.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor CM, Jiang W, Kuchibhatla M, et al. S-C Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692–699. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bekelman DB, Parry C, Curlin FA, et al. A comparison of two spirituality instruments and their relationship with depression and quality of life in chronic heart failure. J Pain Symptom Manage. 2010;39:515–526. doi: 10.1016/j.jpainsymman.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray SA, Kendall M, Boyd K, Worth A, Benton TF. Exploring the spiritual needs of people dying of lung cancer or heart failure: a prospective qualitative interview study of patients and their carers. Palliat Med. 2004;18:39–45. doi: 10.1191/0269216304pm837oa. [DOI] [PubMed] [Google Scholar]
- 35.Ross L, Austin J. Spiritual needs and spiritual support preferences of people with end-stage heart failure and their carers: implications for nurse managers. J Nurs Manag. 2015;23:87–95. doi: 10.1111/jonm.12087. [DOI] [PubMed] [Google Scholar]
- 36.Allen LA, Gheorghiade M, Reid KJ, et al. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ Cardiovasc Qual Outcomes. 2011;4:389–398. doi: 10.1161/CIRCOUTCOMES.110.958009. [DOI] [PMC free article] [PubMed] [Google Scholar]


