Abstract
Epilepsy is a neurologic disorder affecting approximately 50 million people worldwide, or about 0.7% of the population [1]. Thus, the use of anticonvulsant drugs in the treatment of epilepsy is common and widespread. There are three generations of anticonvulsant drugs, categorized by the year in which they were developed and released. The aim of this review is to discuss the pharmacokinetics, drug-drug interactions, and adverse events of the third generation of anticonvulsant drugs. Where available, overdose data will be included. The pharmacokinetic properties of third-generation anticonvulsant drugs include relatively fewer drug-drug interactions, as well as several unique and life-threatening adverse events. Overdose data are limited, so thorough review of adverse events and knowledge of drug mechanism will guide expectant management of future overdose cases. Reporting of these cases as they occur will be necessary to further clarify toxicity of these drugs.
Keywords: Anticonvulsants, Poisoning, Drug overdose, Pharmacokinetics, Epilepsy
Introduction
Epilepsy is a common neurological disorder affecting millions of Americans. The International League Against Epilepsy (ILAE) defines epilepsy as two or more unprovoked seizures occurring more than 24 h apart. Epilepsy can also be diagnosed after one unprovoked seizure if additional historical or diagnostic testing data suggest that the risk of recurrent seizure is high. The estimated prevalence of epilepsy of all causes in the USA is 8.5 cases per 1000 people, or just less than 1% of the US population [2].
Seizures, as the clinical manifestations of epilepsy, are divided into two major categories, generalized and partial. Generalized seizures involve both cerebral hemispheres simultaneously whereas partial seizures have a specific focus. With partial seizures, the discharges may stay confined to a region such as the mesial temporal lobe or they may secondarily generalize. The term partial-onset refers to the origin of the discharges and does not distinguish between those that stay confined to one region versus those that secondarily generalize. Most seizures stop after 2–3 min; however, when the brain’s inhibitory mechanisms fail, seizures may be prolonged. After 5 min of seizure activity, the term status epilepticus is applied [3]. Status epilepticus can be convulsive or non-convulsive with several subcategories which are beyond the scope of this paper. Classification of seizures is important because certain anticonvulsant drugs (ACDs) can be beneficial or harmful depending on the seizure type.
Neurologists prescribe ACDs with the goal of seizure freedom for their epilepsy patients. However, seizure freedom with initial ACD monotherapy is achieved in only 47% of patients and nearly 30% of patients go on to develop drug-resistant epilepsy [4]. Drug-resistant epilepsy means that these patients are on multiple ACDs across different generations which increase the risk of drug-drug interactions and adverse effects (AEs).
There are currently three generations of ACDs. Generations of ACDs are not divided by structure or mechanism but only by the timeframe in which they were developed. The first-generation of ACDs include phenobarbital, primidone, phenytoin, ethosuximide, valproic acid, and carbamazepine. These drugs were developed as early as 1912 (phenobarbital) through the 1970s. Some well-known disadvantages of first-generation ACDs include zero-order kinetics seen with phenytoin, autoinduction seen with carbamazepine, high protein binding with phenytoin and valproic acid, metabolism through major cytochrome P450 enzymes, and anticonvulsant hypersensitivity syndrome. With these unfavorable characteristics in mind, newer ACDs have been developed.
The second-generation drugs include felbamate (Felbatol®), gabapentin (Neurontin®), lamotrigine (Lamictal®), levetiracetam (Keppra®), oxcarbazepine (Trileptal®), tiagabine (Gabitril®), topiramate (Topamax®), pregabalin (Lyrica ®), and zonisamide (Zonegran®). The first of the second-generation drugs, felbamate, was approved in 1993. Each of these drugs has unique benefit for epilepsy management that is beyond the scope of this review. The overall goal of developing newer ACDs is high efficacy and good tolerability. Favorable pharmacokinetics would include high oral availability, minimal protein binding, minimal or no metabolism through P450 enzymes, and renal elimination. As an example, a second-generation ACD with these properties is levetiracetam. Other second-generation ACDs do not have as favorable pharmacokinetics. Felbamate is an inhibitor of CYP2C19, CYP1A2, and beta-oxidation, thus inhibiting the metabolism of several other ACDs [5]. Other examples of limitations of second-generation ACDs include cognitive impairment with topiramate, Steven-Johnson Syndrome with lamotrigine, kidney stones with topiramate and zonisamide, encephalopathy and non-convulsive status epilepticus with tiagabine, to name a few.
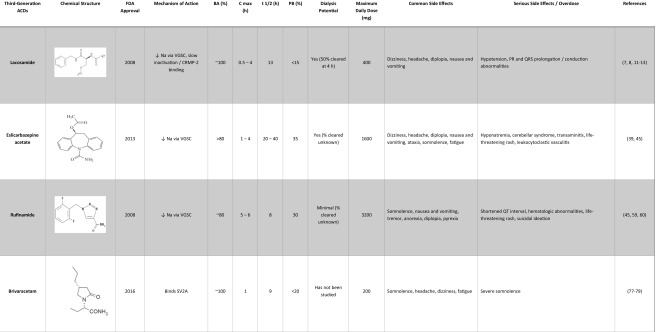
As regarded by most authors over time, the third generation of ACDs begins with the 2008 approval of lacosamide and includes lacosamide, eslicarbazepine acetate, rufinamide, brivaracetam, perampanel, vigabatrin, clobazam, and ezogabine. The label of third generation was retrospectively applied as the number of new medications grew. In this review, the pharmacokinetics, drug-drug interactions, and AEs of this third generation of ACDs will be discussed (Table 1).
Table 1.
Third-generation anticonvulsant drug summary
It is important to note that epilepsy is associated with psychiatric comorbidities including depression, anxiety, and psychosis. Suicide prevalence in epilepsy is double that of the general population (12%), and suicide attempts occur in 5–30% of patients with epilepsy [6]. All ACDs carry a warning for suicidality; however, approximately 80% of neurologists do not screen for depression in patients with epilepsy [6]. Knowing the pharmacology and AEs of ACDs is important in the context of this increased suicide rate, especially because overdose of prescribed medication is often a means of attempted suicide. In the setting of intentional overdose, as well as iatrogenic and unintentional overdose, medical toxicologists may be consulted for management. Although cases are limited, toxicity from overdose of third-generation AEDs will be highlighted in this review. Knowledge of the pharmacologic properties will also guide management of adverse events and overdose.
Literature Search
PubMed and EmBase searches (1970—December 2016) were performed combining drug names (e.g., “clobazam”) with phrases such as “randomized clinical trials,” “meta-analysis,” and “case reports” with the following keywords: “pharmacokinetics,” “metabolism,” “overdose,” “suicide,” “adverse events,” “poisoning,” “intoxication,” or “dialysis.” The prescribing information for each drug was also reviewed. Other relevant articles were found through the “Related Articles” feature on PubMeb. The authors also reviewed the references of several landmark studies to identify literature not identified through our PubMed search. In addition, meeting abstracts were reviewed for the North American Congress of Clinical Toxicology (Clinical Toxicology) and the American College of Medical Toxicology (Journal of Medical Toxicology) annual meetings. Searches yielded varying numbers of articles depending on the drug being searched. Of these, the authors found 498 articles that were relevant to our review of which many were eliminated due to redundancy or lack of direct relevance to overdose or adverse events related to the drugs included.
Third-Generation Anticonvulsant Drugs
Lacosamide
Lacosamide (Vimpat®) was approved by the FDA in 2008 as adjunctive therapy for partial-onset seizures in patients 17 years and older [7]. Later, lacosamide (LCM) was approved as a monotherapy for partial-onset seizures [8]. Dosage forms include oral and intravenous with a maximum daily dose of 400 mg, typically divided twice daily [8]. Off-label use is seen in status epilepticus [9, 10].
The mechanism of action of LCM is unique in that it enhances slow inactivation of voltage-gated sodium channels [11]. LCM may also mitigate neuronal rearrangement, a process theorized to be involved in the pathogenesis of epilepsy, via inhibition of collapsin response-mediated protein 2 (CRMP-2) [11]. The pharmacokinetic properties of LCM include 100% bioavailability, time to peak plasma concentration of 0.5–4 h, <15% protein bound, volume of distribution of 0.6 L/kg, and a half-life of 13 h [12]. Half-life may extend to 20 h in renal disease, and about 50% of LCM is cleared by 4 h of hemodialysis [12, 13]. Continuous renal replacement therapy and hemodialysis have each been reported to enhance elimination in a single overdose patient, although no drug concentrations were obtained [14, 15]. Forty percent is excreted unchanged in the urine while a component is metabolized in the liver through various cytochrome P450 enzymes [12]. Minimal drug-drug interactions have been reported [16–21].
AEs of oral LCM have been reported in randomized, double-blind, placebo-controlled clinical trials and are well known among experienced epileptologists. Common AEs include dizziness, headache, diplopia, nausea, and vomiting [22–25], a side effect profile similar to other sodium channel-affecting anticonvulsants. Dizziness was reported in approximately 30% of patients and was the primary reason for discontinuation from clinical trials [23]. Safety data on intravenous LCM is less robust but shows a similar side-effect profile and is largely dose dependent [26]. At a maximum FDA-approved dose of 400 mg daily, LCM prolongs the mean PR interval by 4.4 ms and has no effect on heart rate, QRS, or QTc intervals [27]. As doses exceed 400 mg daily, there is further prolongation in mean PR interval (6.6 ms at 600 mg daily) and minimal prolongation of mean QRS (2.3 ms at 600 mg daily) [27].
Clinical trials did not demonstrate a statistically significant increase in suicidal ideation or depression. However, there have been case reports demonstrating suicidal ideation and depression and one case of psychosis in the setting of LCM titration [28, 29]. There is one case report of status epilepticus in a patient with epilepsy who overdosed on lacosamide and lamotrigine. Peak plasma concentration of lacosamide occurred at 10 h post-ingestion and was 53.9 mg/L (approximate therapeutic range 6–18 mg/L) [30]. Status epilepticus has also been reported in overdose in epilepsy patients taking other anticonvulsants either therapeutically or as co-ingestants [15, 31]. Additional case reports have shown hepatotoxicity [32], pancreatitis [33], and lacosamide-induced valproate toxicity [34]. In the setting of concomitant sodium channel-blocking drugs, there has been reported sinus node dysfunction [35] and atrial fibrillation/flutter [36].
Several case studies involving excessive consumption of LCM highlight its effects on the cardiovascular system. In overdose, LCM has been associated with hypotension (blood pressure 60/30 mmHg) [31], prolonged PR interval (265 msec) [31], prolonged QRS interval (206 msec) [37], bi-bundle branch block [37], junctional rhythm [37], atrioventricular block [38], and death related to cardiac conduction abnormalities [37]. The patient who died ingested 7 g of LCM and had a serum concentration of 27.7 mcg/mL (approximate therapeutic range 6–18 mcg/mL) [37]. It should be noted that these cases also involve ingestion of other medications that may have contributed to conduction abnormalities.
Eslicarbazepine Acetate
Eslicarbazepine acetate (Aptiom®) was approved by the FDA in 2013 as adjunctive therapy for partial-onset seizure [39]. Later, it gained approval as monotherapy for partial-onset seizure [39]. Efficacy has also been seen in bipolar disorder; however, it is not approved for this condition [40, 41]. Eslicarbazepine acetate (ESL) is administered once daily with a maximum dose of 1600 mg [39]. There is no intravenous form of ESL.
ESL competitively binds to the inactive state of voltage-gated sodium channels to inhibit abnormal neuronal firing [42]. ESL was designed to lack toxic metabolites such as epoxides and avoid autoinduction like its related drug, carbamazepine [42]. ESL is metabolized to its active metabolite eslicarbazepine and minor metabolites, R-licarbazepine and oxcarbazepine, through CYP3A4 and CYP2C19 [43, 44]. The pharmacokinetic properties of ESL include >80% bioavailability, time to peak plasma concentration of 1–4 h, 35% protein bound, volume of distribution of 2.7 L/kg, and a half-life of 20–40 h [45]. Dose adjustments are needed in renal impairment, and hemodialysis successfully removes metabolites [46]. Dose adjustments are not needed in mild to moderate hepatic impairment [47]. Drug-drug interactions include moderately increasing phenytoin levels [48], significant (>50%) reduction in systemic simvastatin levels [49], and decreased bioavailability of topiramate [50]. Overall, ESL has few drug-drug interactions.
Clinical trials provided evidence for the efficacy and side effect profile of ESL. Common AEs are like other sodium channel-affecting ACDs and include dizziness, blurred and double vision, nausea and vomiting, ataxia, headache, and somnolence and fatigue [51]. Overall, AEs lessened when switched from carbamazepine and oxcarbazepine and were dose dependent [52–54]. Liver function tests were not affected by ESL [44], and although there was a small increase in PR interval, there were no significant changes in cardiac conduction up to 2400 mg [55].
Serious AEs (SAEs) are rare but have been reported in case studies and clinical trials. SAEs associated with ESL include hyponatremia [52, 56], cerebellar syndrome [51], and increased liver enzymes [51]. Dermatologic reactions have included erythema multiforme major [57], leukocytoclastic vasculitis [54], and toxic epidermal necrolysis [58]. There are no case reports of overdose.
Rufinamide
Rufinamide (Banzel®) was approved by the FDA in 2008 for adjunctive treatment for children 4 years and older and adults with Lennox-Gastaut syndrome, [59] which is a childhood epilepsy syndrome characterized by seizures, intellectual dysfunction, and particular EEG abnormalities. Off-label use is seen in partial-onset seizures and super-refractory tonic-status epilepticus. Dose form is oral with a maximum daily dose of 45 mg/kg/day or 3200 mg (whichever is less) in children and 3200 mg in adults, divided twice daily [59]. There is no intravenous form of rufinamide (RFM).
The exact mechanism of action of RFM is not known but likely causes prolongation of the inactivated state of voltage-gated sodium channels [60]. The pharmacokinetic properties of RFM include ~80% bioavailability, time to peak plasma concentration of 5–6 h, 30% protein bound, volume of distribution of 0.7–1.1 L/kg, and a half-life of 8 h [45]. RFM metabolism does not involve cytochrome P450 enzymes—rather, it is hydrolyzed by carboxylesterases to CGP 47292, an inactive metabolite which is excreted in the urine [61]. Renal impairment does not have a significant effect on RFM pharmacokinetics, and hemodialysis had minimal effect on plasma concentration at 3 h, but dose adjustments may be needed [61]. The effect of rufinamide on hepatic impairment is not well studied. RFM increases the clearance of lamotrigine and carbamazepine and decreases the clearance of phenytoin and phenobarbital [61]. Valproic acid increases the plasma concentration of RFM by 60–70% in children; therefore, RFM dose adjustments are required [61]. Conversely, enzyme-inducing ACDs like phenytoin and carbamazepine decrease RFM levels [45]. It would therefore be prudent for physicians to monitor drug concentrations in patients taking hepatically metabolized drugs to avoid toxicity and promote efficacy.
Several clinical trials have supported the efficacy of RFM and elaborated on potential AEs. Common AEs included somnolence, dizziness, fatigue, headache, vomiting, nausea, tremor, decreased appetite, diplopia, and pyrexia [62–69]. Elger et al. reported a patient developing leucopenia at a dose of 400 mg daily that resolved with discontinuation [70]. Likewise, Biton et al. reported two cases of lymphopenia (one patient with low lymphocyte count and one patient with low lymphocyte concentration) and one case of neutropenia [62]. SAEs reported in case studies include drug-reaction with eosinophilia and systemic symptoms (DRESS) [71], Stevens-Johnson syndrome [72], two cases of suicidal ideation in the setting of bipolar disorder [73], severe constipation [74], and agranulocytosis [75]. RFM does have the potential to shorten QT interval and is contraindicated in patients with Familial Short QT syndrome [76]. There is one report of a patient taking 7200 mg of RFM with no AEs [59]; otherwise, there are no reported cases of overdose.
Brivaracetam
Brivaracetam (Briviact®) was approved by the FDA in 2016 as adjunctive therapy for partial-onset seizure in patients 16 years and older [77]. Dosage forms include oral and intravenous with a maximum daily dose of 200 mg daily, divided twice daily [77]. Efficacy in status epilepticus is currently being studied.
Brivaracetam (BRV) binds selectively to synaptic vesicle protein 2A (SV2A) with greater affinity than levetiracetam, another well-known SV2A ligand [78]. The pharmacokinetic properties of BRV include high oral bioavailability, time to peak plasma concentration of ~1 h, <20% protein bound, volume of distribution of 0.5 L/kg, and a half-life of 9 h [77, 79]. Metabolism occurs by hydrolysis to its acid metabolite and, to a lesser extent, via hydroxylation involving hepatic CYP2C19 [80]. BRV has not shown any significant effect on CYP3A activity [81]. After BRV is broken down to its metabolic products, it is excreted in the urine. Some of the drug-drug interactions include decreased plasma concentration of BRV with co-administration of rifampin [82] and increased carbamazepine-epoxide [83] and phenytoin levels [77] when co-administered with carbamazepine and phenytoin, respectively.
Several clinical trials have supported the efficacy and defined the AEs of BRV. Common AEs include somnolence, headache, dizziness, and fatigue [84–90]. Clinical trials did not demonstrate an effect on cardiac function and did not demonstrate a statistically significant change in mood [79]. Two serious AEs were reported at a dose of 50 mg/day that may have related to BRV; this included a generalized tonic-clonic seizure and a psychotic disorder [89]. Doses up to 1400 mg (14 times the recommended single dose) have been used in healthy volunteers and caused severe reversible somnolence in one patient [91]. In healthy volunteers, the mean plasma concentration of BRV at 1400 mg was 41.28 mcg/mL compared to a mean plasma concentration of 4.24 mcg/mL at 150 mg (maximum BRV single dose 100 mg) [91]. There are no reported cases of overdose.
Perampanel
Perampanel (Fycompa®) was approved by the FDA in 2012 for partial-onset seizures and later gained approval as adjunctive treatment for primary generalized tonic-clonic seizures [92]. Dosage form is oral with a maximum dose of 12 mg, once daily [92]. There is no intravenous form of perampanel (PER).
PER is a non-competitive antagonist at the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor causing reduced AMPA receptor-mediated synaptic transmission [93]. Through this mechanism of action, PER is thought to reduce neuronal hyperexcitability and decrease seizure occurrence. The pharmacokinetic properties of PER include ~100% bioavailability, time to peak plasma concentration of 0.15–2 h, 95% protein bound, volume of distribution of ~80 L/kg, and half-life of 48–105 h [94, 95]. PER is metabolized in the liver by CYP3A4 to several inactive metabolites, and clearance is increased by CYP3A4 inducing ACDs such as carbamazepine and phenytoin [94]. PER is highly protein bound and has a low extraction ratio, so increasing free levels will cause a proportional increase in clearance [94]. Dose adjustments are needed for hepatic impairment, and PER is not recommended in those with severe renal impairment or on hemodialysis [92].
Efficacy and AEs of PER have been well established in clinical trials. Common AEs include dizziness, somnolence, headache, fatigue, and irritability [96–99]. There is one case report of DRESS associated with PER use [100]. PER has not been shown to affect QT interval [101] or overall cognition [102] at therapeutic doses.
Neurologists prescribing PER should be aware of potential psychiatric side effects [103]. PER carries a black box warning for several psychiatric AEs including homicidal ideation. Psychiatric AEs showing a higher incidence in the treatment group compared to placebo included aggression/hostility and irritability [96, 104, 105]. Other psychiatric AEs that possibly or probably relate to treatment included suicide ideation or suicide attempt [96, 106, 107], psychosis [106, 108], one case of schizophrenia [109], and one adolescent hospitalized for aggression [97]. Pooled data from three pivotal trials confirmed a higher rate of irritability and aggression compared to placebo but no increased risk of suicidal ideation [105]. This contrasts with two case series that report higher rates of suicidal ideation. Huber reported 3/23 patients (13%) and Coyle et al. reported 3/47 (6.4%) patients with suicidal ideation treated with PER [104, 110]. Further, Coyle et al. reported adverse behavioral effects as the most common reason for discontinuation [104].
In the known literature, there are nine reported cases of PER overdose. Eight cases were reported in one of the clinical trials as unintentional with the highest dose being 36 mg. Reported symptoms were nausea, vomiting, somnolence, ataxia, and dysarthria [108]. One case report highlights a 34-year-old with tuberous sclerosis who intentionally ingested 204 mg of PER with subsequent dysarthria and fatigue followed by stupor, disorientation, and “misjudging situations” for 2 days [111]. Electroencephalogram showed generalized slowing without epileptiform abnormalities, and labs and ECG were unremarkable [111]. No case reports of overdose included plasma concentration of PER.
Vigabatrin
Vigabatrin (Sabril®) was approved by the FDA in 2009 as adjunct treatment in refractory complex partial seizures in patients 10 years and older and as monotherapy for infantile spasms [112]. Vigabatrin (VGB) use is restricted due to the risk of permanent vision loss through an unknown mechanism. Dose form is oral, and dosage depends on age and indication. The maximum daily dose for adults 17 years and older is 3000 mg, divided twice daily [112]. There is no intravenous form of VGB.
The proposed mechanism of VGB involves increasing the inhibitory neurotransmitter GABA in the CNS by irreversibly inhibiting GABA-transaminase [113]. The pharmacokinetic properties of VGB include >60% bioavailability, time to peak plasma concentration of ~2 h, 0% protein bound, volume of distribution of 1.1 L/kg, and a half-life of 5–10 h [45, 113]. The effects of VGB may outlast its half-life by up to 48 h due to the irreversible binding to GABA-transaminase [114]. VGB is a racemic mixture in which S-enantiomers are the only active component [114]. VGB is not metabolized by hepatic cytochrome 450 enzymes so it has few drug-drug interactions with the exception of lowering plasma phenytoin levels for unknown reasons [115, 116]. VGB is excreted by the kidneys, and therefore, dose adjustments are necessary in renal impairment and with geriatric patients.
A systematic review examined 11 randomized, double-blind, placebo-controlled trials for the treatment of drug-resistant partial epilepsy in adults. The most common AEs associated with VGB were fatigue, drowsiness, dizziness, depression, and ataxia [117]. VGB-associated concentric visual field loss is the most well-known AE and occurs in up to 30–45% of patients, is permanent, and is related to duration and dose of VGB [118–122]. Several studies have reported MRI signal abnormalities in the basal ganglia and brainstem in children with infantile spasms treated with VGB [123, 124]. Although a cause-and-effect relationship has not been established, there are case reports of movement disorders and/or encephalopathy associated with these MRI findings as well as encephalopathy without MRI findings [125–129]. There are reported cases of psychosis [130–132], fatal hepatitis [133], life-threatening encephalopathy [126], status epilepticus [134, 135], allergic vasculitis [136], sensorineural hearing loss [137], and suppression of liver function tests [138] associated with therapeutic doses of VGB.
In clinical trials and post-marketing surveillance, there have been reports of patients taking 3–90 g of VGB, usually with co-administration of other medications [112]. The most common SAEs were related to suppressed consciousness and included coma [112]. Cases of psychosis, respiratory compromise, bradycardia, hypotension, vertigo, and status epilepticus were seen [112]. There were no deaths reported and patients recovered with supportive care [112]. In a case report of overdose of an unknown amount of vigabatrin in an epilepsy patient, diplopia and visual disturbance were followed by unresponsiveness [139]. No plasma concentrations were included in these case reports. VGB does not appear to have a significant impact on cardiac conduction. Hemodialysis does increase the clearance of VGB by up to 60% and could potentially be helpful in overdose [140, 141].
Clobazam
Clobazam (Onfi®) was approved by the FDA in 2011 for adjunctive treatment in patients with Lennox-Gastaut syndrome age 2 and older [142]. Clobazam has been available in other nations since the 1970s. Off-label use is seen in generalized and partial-onset seizures. Dosage form is oral, and total dose depends on weight with 40 mg/day being the maximum dose for patients >30 kg [142]. There is no intravenous form of clobazam.
Clobazam is a benzodiazepine with nitrogen atoms in the 1 and 5 positions rather than 1,4-benzodiazepine which is the structure of all other benzodiazepines [143]. Clobazam binds to GABA-A receptors enhancing GABA binding to ultimately increase chloride conduction causing neuronal hyperpolarization. The pharmacokinetic properties of clobazam include >87% bioavailability, time to peak plasma concentration of 1–4 h, high protein binding, volume of distribution of 100 L, and a highly variable half-life [144]. In the liver, clobazam is metabolized primarily by CYP3A4 to produce N-desmethylclobazam, an active metabolite which is later metabolized by CYP2C19 [145]. Both clobazam and desmethylclobazam are active, but there are insufficient data on which is more potent. Dose adjustments are reasonable for hepatic impairment, but some pharmacokinetic data suggest that this may not be necessary [146]. Dose adjustments are not needed in mild to moderate renal impairment [142]. N-desmethylclobazam and its metabolites are excreted in the urine [145].
Jose de Leon et al. [145] expand on the complicated pharmacokinetics of clobazam and desmethylclobazam, including the wide variation in half-life. Mean half-life of clobazam is approximately 24 h whereas the half-life of desmethylclobazam is approximately 70–100 h depending on the study, but it may be drastically longer in patients with poor or no CYP2C19 activity and patients on CYP2C19 inhibitors such as felbamate [145]. This is further supported by a recent case of a 10-year-old girl with somnolence, enuresis, and elevated desmethylclobazam levels in the setting of a genetic mutation causing poor CYP2C19 activity [147]. Jose de Leon et al. [145] further highlights cases of possible competitive inhibition of CYP2C19 between clobazam and phenytoin leading to phenytoin toxicity [148]. Further drug-drug interactions may theoretically occur in the setting of drugs metabolized by CYP3A4 and has been seen in a patient taking etravirine who developed neurotoxicity [149], as well as in a patient taking carbamazepine [150].
Safety information regarding clobazam is discussed in dozens of clinical trials. The most common AEs reported include sedation/somnolence, mood and behavioral changes (aggression and psychomotor hyperexcitability), drooling, ataxia, and a higher incidence of upper respiratory tract infection and pyrexia in children [151–153]. One case report presents a 4-year-old with non-epileptic eye rolling, ataxia, and back arching after initiation of clobazam [154]. This patient had desmethylclobazam levels 5–7 times the upper limit of normal which suggested poor CYP2C19 activity. Other cases report toxic epidermal necrosis [155], gelastic seizures and sleep disturbances [156], and clobazam-induced systemic lupus erythematosus [157].
In a case series of 13 children and 31 adults (mean ingested dose of 37 mg in children and 382 mg in adults), no deaths were observed, and 75% of patients had mild effects on cognition without severe symptoms. The authors conclude that clobazam may have a more favorable profile in acute toxicity than the 1,4-benzodiazepines [158].
However, there are two reported cases of fatal overdose summarized in de Leon et al. [145]. One case involved suspected respiratory depression, and the toxicology report showed an elevated clobazam level (3900 ng/mL) but did not include desmethylclobazam levels [145, 159]. The second case involved a 70-year-old woman who was found dead with elevated levels of clobazam (720 ng/mL) and desmethylclobazam (36,000 ng/mL) [145, 160]. The therapeutic range of clobazam is wide and ranges between 30 and 400 ng/mL. Normal serum concentration for desmethylclobazam is 300–3000 ng/mL [145].
Flumazenil is a benzodiazepine antagonist that reverses the effects of benzodiazepines and theoretically could serve as an antidote in clobazam overdose. However, its use should be avoided because the sudden withdrawal of clobazam may cause life-threatening side effects, such as status epilepticus, especially in patients with epilepsy. AEs of clobazam may be higher in those who are poor metabolizers due to genetic and inhibitory effects on CYP2C19. The model of therapeutic drug monitoring proposed by de Leon et al. of checking desmethylclobazam/clobazam serum ratios is an effective means of detecting those who may develop high concentrations of desmethylclobazam due to poor CYP2C19 activity.
Ezogabine
Ezogabine (Potiga®) was approved by the FDA in 2011 as adjunctive treatment for patients with partial-onset seizures aged 18 years and older who have not responded to multiple alternative treatments [161]. Ezogabine (EZB) is dosed orally at a maximum daily dose of 1200 mg, divided three times daily [161]. There is no intravenous form of EZB.
The mechanism of action of EZB is unique in that EZB opens voltage-gated potassium (K+) channels which allow K+ across the cell membrane to decrease neuronal excitability [162]. An additional mechanism of action of EZB involves its effects on GABA-A receptors [163]. The pharmacokinetic properties of EZB include ~60% bioavailability, time to peak plasma concentration of 0.5–2.0 h, volume of distribution 2–3 L/kg, and a half-life of 6–8 h but extended in the elderly [164, 165]. Although EZB is 80% protein bound, there is no clinically significant evidence of competitive displacement leading to AEs [161]. Metabolism occurs in the liver by N-acetylation and then N-glucuronidation, and in the process, a less potent N-acetyl metabolite is formed [166]. Both EZB and its metabolites are excreted in the urine. Dose adjustments are recommended in geriatric patients, renal impairment, and moderate to severe hepatic impairment [161, 167]. Drug-drug interactions are minimal with other ACDs, because EZB is not metabolized by cytochrome P450 enzymes. Carbamazepine and phenytoin increase EZB clearance by 27 and 36%, respectively [168].
The efficacy of EZB has been established through clinical trials. The major AEs in clinical trials were dizziness, somnolence, confusion, ataxia, dysarthria, paresthesia, amnesia, blurred vision, nausea, and urinary tract infection [169, 170]. Psychiatric AEs included confusional state (9%), hallucination (2%), and psychosis (1%) [161]. Anxiety and disorientation has been reported as well [169]. Porter et al. [170] reported single cases of suicidal ideation, abnormal thinking, psychosis, nausea, and vertigo that were considered serious but provided no further information. EZB has been shown to cause urinary retention via its effect on potassium channels in the bladder [171] and has been associated with a mean QT prolongation of 7.7 ms [161]. There is a black box warning for retinal abnormalities and potential vision loss [172]. With long-term use, a blue-gray discoloration of the skin, lip, and sclera have been observed [172]. EZB can also cause a sense of euphoria and has potential for abuse, making it a controlled substance [173].
Cases of overdose are limited. Per the prescribing information [161], there were cases of patients taking over 2500 mg which caused aggression and agitation. The prescribing information [161] further mentions two healthy volunteers who experienced cardiac arrhythmia (asystole or ventricular tachycardia) 3 h after a 900 mg dose of EZB. Plasma concentrations of EZB were not included.
Conclusion
This review introduces the pharmacokinetic properties, overdose reports, and adverse event profiles of third-generation ACDs, which have some therapeutic advantages relative to traditional (first- and second-generation) ACDs. The pharmacokinetics properties of third-generation ACDs all share good bioavailability. Plasma protein binding is relatively low except with clobazam, ezogabine, and perampanel. Many of these ACDs are not metabolized via major cytochrome P450 enzymes except clobazam, perampanel, and eslicarbazepine acetate. Overall, these properties appear to lead to less drug-drug interactions. Although these properties are relatively more favorable, knowledge of the pharmacokinetic properties should guide the clinician in managing SAEs and overdose.
Several life-threatening AEs were also discussed throughout this review including cases of overdose. The data are limited due to the short duration these drugs have been available for clinical use. Most of the AEs caused by third-generation ACDs are dose-related. One may then extrapolate that in cases of overdose, similar AEs may occur but further down the spectrum of severity. The limited data on AEs and overdose underscores the importance of further reporting to prepare clinicians in managing the toxicity of third-generation ACDs.
Compliance with Ethical Standards
Conflict of Interest
None.
Sources of Funding
None.
Conflict Delineations
None.
Footnotes
Dr. Tormoehlen presented a version of this paper as part of a lecture presentation at the ACMT Annual Scientific Meeting – “Targets and Toxicities of Newer Anticonvulsants.”
References
- 1.World Health Organization. Epilepsy Fact Sheet [updated February 2017. Available from: http://www.who.int/mediacentre/factsheets/fs999/en/.
- 2.Helmers SL, Thurman DJ, Durgin TL, Pai AK, Faught E. Descriptive epidemiology of epilepsy in the U.S. population: a different approach. Epilepsia. 2015;56(6):942–948. doi: 10.1111/epi.13001. [DOI] [PubMed] [Google Scholar]
- 3.Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus—report of the ILAE task force on classification of status Epilepticus. Epilepsia. 2015;56(10):1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Khalil BW. Antiepileptic drugs. Continuum (Minneapolis, Minn). 2016;22(1 Epilepsy):132–56. [DOI] [PubMed]
- 6.Sirven JI. Management of epilepsy comorbidities. Continuum (Minneapolis, Minn). 2016;22(1 Epilepsy):191–203. [DOI] [PubMed]
- 7.LACOSAMIDE oral solution, CV [PDF]. 20016 [updated 04/2016. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204839s000lbl.pdf.
- 8.Vimpat (Lacosamide). In: Pharma U, editor. 2014.
- 9.Hofler J, Trinka E. Lacosamide as a new treatment option in status epilepticus. Epilepsia. 2013;54(3):393–404. doi: 10.1111/epi.12058. [DOI] [PubMed] [Google Scholar]
- 10.Kellinghaus C, Berning S, Immisch I, Larch J, Rosenow F, Rossetti AO, et al. Intravenous lacosamide for treatment of status epilepticus. Acta Neurol Scand. 2011;123(2):137–141. doi: 10.1111/j.1600-0404.2010.01423.x. [DOI] [PubMed] [Google Scholar]
- 11.Beyreuther BK, Freitag J, Heers C, Krebsfanger N, Scharfenecker U, Stohr T. Lacosamide: a review of preclinical properties. CNS drug reviews. 2007;13(1):21–42. doi: 10.1111/j.1527-3458.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cawello W. Clinical pharmacokinetic and pharmacodynamic profile of lacosamide. Clin Pharmacokinet. 2015;54(9):901–914. doi: 10.1007/s40262-015-0276-0. [DOI] [PubMed] [Google Scholar]
- 13.Cawello W, Fuhr U, Hering U, Maatouk H, Halabi A. Impact of impaired renal function on the pharmacokinetics of the antiepileptic drug lacosamide. Clin Pharmacokinet. 2013;52(10):897–906. doi: 10.1007/s40262-013-0080-7. [DOI] [PubMed] [Google Scholar]
- 14.Boyd M, Proschek C, Kazzi Z. 2014 annual meeting of the north American congress of clinical toxicology (NACCT). Abstract: massive lacosamide overdose treated with continuous renal replacement therapy. Clin Toxicol. 2014;52(7):757–758. [Google Scholar]
- 15.Charlton N, Holstege C. Abstracts of the XXVIII international congress of the European Association of Poison Centres and Clinical Toxicologists, May 6–9, 2008, Seville, Spain. Abstract: lacosamide overdose induced status epilepticus treated with hemodialysis. Clin Toxicol. 2008;46(5):351–421. doi: 10.1080/15563650802071703. [DOI] [PubMed] [Google Scholar]
- 16.Cawello W, Bonn R. No pharmacokinetic interaction between lacosamide and valproic acid in healthy volunteers. J Clin Pharmacol. 2012;52(11):1739–1748. doi: 10.1177/0091270011426875. [DOI] [PubMed] [Google Scholar]
- 17.Cawello W, Mueller-Voessing C, Andreas JO. Effect of lacosamide on the steady-state pharmacokinetics of digoxin: results from a phase I, multiple-dose, double-blind, randomised, placebo-controlled, crossover trial. Clin Drug Investig. 2014;34(5):327–334. doi: 10.1007/s40261-014-0180-7. [DOI] [PubMed] [Google Scholar]
- 18.Cawello W, Mueller-Voessing C, Fichtner A. Pharmacokinetics of lacosamide and omeprazole coadministration in healthy volunteers: results from a phase I, randomized, crossover trial. Clin Drug Investig. 2014;34(5):317–325. doi: 10.1007/s40261-014-0177-2. [DOI] [PubMed] [Google Scholar]
- 19.Cawello W, Nickel B, Eggert-Formella A. No pharmacokinetic interaction between lacosamide and carbamazepine in healthy volunteers. J Clin Pharmacol. 2010;50(4):459–471. doi: 10.1177/0091270009347675. [DOI] [PubMed] [Google Scholar]
- 20.Cawello W, Rosenkranz B, Schmid B, Wierich W. Pharmacodynamic and pharmacokinetic evaluation of coadministration of lacosamide and an oral contraceptive (levonorgestrel plus ethinylestradiol) in healthy female volunteers. Epilepsia. 2013;54(3):530–536. doi: 10.1111/epi.12085. [DOI] [PubMed] [Google Scholar]
- 21.Stockis A, van Lier JJ, Cawello W, Kumke T, Eckhardt K. Lack of effect of lacosamide on the pharmacokinetic and pharmacodynamic profiles of warfarin. Epilepsia. 2013;54(7):1161–1166. doi: 10.1111/epi.12192. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48(7):1308–1317. doi: 10.1111/j.1528-1167.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 23.Biton V, Gil-Nagel A, Isojarvi J, Doty P, Hebert D, Fountain NB. Safety and tolerability of lacosamide as adjunctive therapy for adults with partial-onset seizures: analysis of data pooled from three randomized, double-blind, placebo-controlled clinical trials. Epilepsy & behavior : E&B. 2015;52(Pt A):119–127. doi: 10.1016/j.yebeh.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Halasz P, Kalviainen R, Mazurkiewicz-Beldzinska M, Rosenow F, Doty P, Hebert D, et al. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50(3):443–453. doi: 10.1111/j.1528-1167.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 25.Husain A, Chung S, Faught E, Isojarvi J, McShea C, Doty P. Long-term safety and efficacy in patients with uncontrolled partial-onset seizures treated with adjunctive lacosamide: results from a phase III open-label extension trial. Epilepsia. 2012;53(3):521–528. doi: 10.1111/j.1528-1167.2012.03407.x. [DOI] [PubMed] [Google Scholar]
- 26.Fountain NB, Krauss G, Isojarvi J, Dilley D, Doty P, Rudd GD. Safety and tolerability of adjunctive lacosamide intravenous loading dose in lacosamide-naive patients with partial-onset seizures. Epilepsia. 2013;54(1):58–65. doi: 10.1111/j.1528-1167.2012.03543.x. [DOI] [PubMed] [Google Scholar]
- 27.Rudd GD, Haverkamp W, Mason JW, Wenger T, Jay G, Hebert D, et al. Lacosamide cardiac safety: clinical trials in patients with partial-onset seizures. Acta Neurol Scand. 2015;132(5):355–363. doi: 10.1111/ane.12414. [DOI] [PubMed] [Google Scholar]
- 28.Kellinghaus C. Reversible suicidal ideation after exposure to lacosamide. Seizure. 2013;22(4):318–319. doi: 10.1016/j.seizure.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Pinkhasov A, Lam T, Hayes D, Friedman M, Singh D, Cohen H. Lacosamide induced psychosis: case report, review of differential diagnosis and relevant pharmacokinetics. Clin Neuropharmacol. 2015;38(5):198–200. doi: 10.1097/WNF.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 30.Deslandes G, Bouquié R, Lorber J, Bruneau C, Gregoire M, Grison-Hernando H, et al. Status epilepticus following self-poisoning of lacosamide and lamotrigine: a case report with follow-up of drug serum concentrations. Toxicologie Analytique et Clinique. 2015;27(2):88–90. doi: 10.1016/j.toxac.2015.02.002. [DOI] [Google Scholar]
- 31.Bauer S, David Rudd G, Mylius V, Hamer HM, Rosenow F. Lacosamide intoxication in attempted suicide. Epilepsy Behavior. 2010;17(4):549–551. doi: 10.1016/j.yebeh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Sunwoo JS, Byun JI, Lee SK. A case of lacosamide-induced hepatotoxicity. Int J Clin Pharmacol Ther. 2015;53(6):471–473. doi: 10.5414/CP202282. [DOI] [PubMed] [Google Scholar]
- 33.del Val AA, Ble Caso M, Higon Ballester MD, Ortuno Cortes JA. Lacosamide-induced acute pancreatitis with positive rechallenge test. J Clin Gastroenterol. 2014;48(7):651. doi: 10.1097/MCG.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 34.Jones GL, Popli GS, Silvia MT. Lacosamide-induced valproic acid toxicity. Pediatr Neurol. 2013;48(4):308–310. doi: 10.1016/j.pediatrneurol.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 35.Chinnasami S, Rathore C, Duncan JS. Sinus node dysfunction: an adverse effect of lacosamide. Epilepsia. 2013;54(6):e90–e93. doi: 10.1111/epi.12108. [DOI] [PubMed] [Google Scholar]
- 36.Degiorgio CM. Atrial flutter/atrial fibrillation associated with lacosamide for partial seizures. Epilepsy Behav. 2010;18(3):322–324. doi: 10.1016/j.yebeh.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 37.Malissin I, Baud FJ, Deveaux M, Champion S, Deye N, Megarbane B. Fatal lacosamide poisoning in relation to cardiac conduction impairment and cardiovascular failure. Clinical toxicology (Philadelphia, Pa) 2013;51(4):381–382. doi: 10.3109/15563650.2013.778993. [DOI] [PubMed] [Google Scholar]
- 38.Krause LU, Brodowski KO, Kellinghaus C. Atrioventricular block following lacosamide intoxication. Epilepsy Behav. 2011;20(4):725–727. doi: 10.1016/j.yebeh.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 39.APTIOM® (eslicarbazepine acetate) tablet Marlborough, MA 01752 USA Sunovion Pharmaceuticals Inc; 2015 [Available from: http://www.aptiom.com/Aptiom-Prescribing-Information.pdf.
- 40.Grunze H, Kotlik E, Costa R, Nunes T, Falcao A, Almeida L, et al. Assessment of the efficacy and safety of eslicarbazepine acetate in acute mania and prevention of recurrence: experience from multicentre, double-blind, randomised phase II clinical studies in patients with bipolar disorder I. J Affect Disord. 2015;174:70–82. doi: 10.1016/j.jad.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Nath K, Bhattacharya A, Praharaj SK. Eslicarbazepine acetate in the management of refractory bipolar disorder. Clin Neuropharmacol. 2012;35(6):295. doi: 10.1097/WNF.0b013e318271220b. [DOI] [PubMed] [Google Scholar]
- 42.Almeida L, Falcao A, Maia J, Mazur D, Gellert M, Soares-da-Silva P. Single-dose and steady-state pharmacokinetics of eslicarbazepine acetate (BIA 2-093) in healthy elderly and young subjects. J Clin Pharmacol. 2005;45(9):1062–1066. doi: 10.1177/0091270005279364. [DOI] [PubMed] [Google Scholar]
- 43.Almeida L, Minciu I, Nunes T, Butoianu N, Falcao A, Magureanu SA, et al. Pharmacokinetics, efficacy, and tolerability of eslicarbazepine acetate in children and adolescents with epilepsy. J Clin Pharmacol. 2008;48(8):966–977. doi: 10.1177/0091270008319706. [DOI] [PubMed] [Google Scholar]
- 44.Ley M, Principe A, Jimenez-Conde J, Rocamora R. Assessing long-term effects of eslicarbazepine acetate on lipid metabolism profile, sodium values and liver function tests. Epilepsy Res. 2015;115:147–152. doi: 10.1016/j.eplepsyres.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Jacob S, Nair AB. An updated overview on therapeutic drug monitoring of recent antiepileptic drugs. Drugs R&D. 2016;16(4):303–316. doi: 10.1007/s40268-016-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maia J, Almeida L, Falcao A, Soares E, Mota F, Potgieter MA, et al. Effect of renal impairment on the pharmacokinetics of eslicarbazepine acetate. Int J Clin Pharmacol Ther. 2008;46(3):119–130. doi: 10.5414/CPP46119. [DOI] [PubMed] [Google Scholar]
- 47.Almeida L, Potgieter JH, Maia J, Potgieter MA, Mota F, Soares-da-Silva P. Pharmacokinetics of eslicarbazepine acetate in patients with moderate hepatic impairment. Eur J Clin Pharmacol. 2008;64(3):267–273. doi: 10.1007/s00228-007-0414-1. [DOI] [PubMed] [Google Scholar]
- 48.Zaccara G, Perucca E. Interactions between antiepileptic drugs, and between antiepileptic drugs and other drugs. Epileptic Disord. 2014;16(4):409–431. doi: 10.1684/epd.2014.0714. [DOI] [PubMed] [Google Scholar]
- 49.Falcao A, Pinto R, Nunes T, Soares-da-Silva P. Effect of repeated administration of eslicarbazepine acetate on the pharmacokinetics of simvastatin in healthy subjects. Epilepsy Res. 2013;106(1–2):244–249. doi: 10.1016/j.eplepsyres.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Nunes T, Sicard E, Almeida L, Falcao A, Rocha JF, Brunet JS, et al. Pharmacokinetic interaction study between eslicarbazepine acetate and topiramate in healthy subjects. Curr Med Res Opin. 2010;26(6):1355–1362. doi: 10.1185/03007991003740861. [DOI] [PubMed] [Google Scholar]
- 51.Verrotti A, Loiacono G, Rossi A, Zaccara G. Eslicarbazepine acetate: an update on efficacy and safety in epilepsy. Epilepsy Res. 2014;108(1):1–10. doi: 10.1016/j.eplepsyres.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Villanueva V, Serratosa JM, Guillamon E, Garces M, Giraldez BG, Toledo M, et al. Long-term safety and efficacy of eslicarbazepine acetate in patients with focal seizures: results of the 1-year ESLIBASE retrospective study. Epilepsy Res. 2014;108(7):1243–1252. doi: 10.1016/j.eplepsyres.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Zaccara G, Giovannelli F, Maratea D, Fadda V, Verrotti A. Neurological adverse events of new generation sodium blocker antiepileptic drugs. Meta-analysis of randomized, double-blinded studies with eslicarbazepine acetate, lacosamide and oxcarbazepine. Seizure. 2013;22(7):528–536. doi: 10.1016/j.seizure.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Sperling MR, Abou-Khalil B, Harvey J, Rogin JB, Biraben A, Galimberti CA, et al. Eslicarbazepine acetate as adjunctive therapy in patients with uncontrolled partial-onset seizures: results of a phase III, double-blind, randomized, placebo-controlled trial. Epilepsia. 2015;56(2):244–253. doi: 10.1111/epi.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaz-Da-Silva M, Nunes T, Almeida L, Gutierrez MJ, Litwin JS, Soares-Da-Silva P. Evaluation of Eslicarbazepine acetate on cardiac repolarization in a thorough QT/QTc study. J Clin Pharmacol. 2012;52(2):222–233. doi: 10.1177/0091270010391789. [DOI] [PubMed] [Google Scholar]
- 56.Gupta DK, Bhoi SK, Kalita J, Misra UK. Hyponatremia following esclicarbazepine therapy. Seizure. 2015;29:11–14. doi: 10.1016/j.seizure.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Massot A, Gimenez-Arnau A. Cutaneous adverse drug reaction type erythema multiforme major induced by eslicarbazepine. J Pharmacol Pharmacother. 2014;5(4):271–274. doi: 10.4103/0976-500X.142456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alcantara-Reifs CM, Salido-Vallejo R, Garnacho-Saucedo G, de la Corte-Sanchez S, Velez G-NA. Eslicarbazepine-induced toxic epidermal necrolysis. Epilepsia. 2016;57(5):854–855. doi: 10.1111/epi.13363. [DOI] [PubMed] [Google Scholar]
- 59.BANZEL® (rufinamide) 2010 [updated October 2010. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021911s005lbl.pdf.
- 60.French JA, Gazzola DM. New generation antiepileptic drugs: what do they offer in terms of improved tolerability and safety? Ther Adv Drug Saf. 2011;2(4):141–158. doi: 10.1177/2042098611411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perucca E, Cloyd J, Critchley D, Fuseau E. Rufinamide: clinical pharmacokinetics and concentration-response relationships in patients with epilepsy. Epilepsia. 2008;49(7):1123–1141. doi: 10.1111/j.1528-1167.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- 62.Biton V, Krauss G, Vasquez-Santana B, Bibbiani F, Mann A, Perdomo C, et al. A randomized, double-blind, placebo-controlled, parallel-group study of rufinamide as adjunctive therapy for refractory partial-onset seizures. Epilepsia. 2011;52(2):234–242. doi: 10.1111/j.1528-1167.2010.02729.x. [DOI] [PubMed] [Google Scholar]
- 63.Brodie MJ, Rosenfeld WE, Vazquez B, Sachdeo R, Perdomo C, Mann A, et al. Rufinamide for the adjunctive treatment of partial seizures in adults and adolescents: a randomized placebo-controlled trial. Epilepsia. 2009;50(8):1899–1909. doi: 10.1111/j.1528-1167.2009.02160.x. [DOI] [PubMed] [Google Scholar]
- 64.Coppola G, Grosso S, Franzoni E, Veggiotti P, Zamponi N, Parisi P, et al. Rufinamide in refractory childhood epileptic encephalopathies other than Lennox-Gastaut syndrome. Eur J Neurol. 2011;18(2):246–251. doi: 10.1111/j.1468-1331.2010.03113.x. [DOI] [PubMed] [Google Scholar]
- 65.Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Rufinamide for generalized seizures associated with Lennox-Gastaut syndrome. Neurology. 2008;70(21):1950–1958. doi: 10.1212/01.wnl.0000303813.95800.0d. [DOI] [PubMed] [Google Scholar]
- 66.Kluger G, Glauser T, Krauss G, Seeruthun R, Perdomo C, Arroyo S. Adjunctive rufinamide in Lennox-Gastaut syndrome: a long-term, open-label extension study. Acta Neurol Scand. 2010;122(3):202–208. doi: 10.1111/j.1600-0404.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- 67.Ohtsuka Y, Yoshinaga H, Shirasaka Y, Takayama R, Takano H, Iyoda K. Rufinamide as an adjunctive therapy for Lennox-Gastaut syndrome: a randomized double-blind placebo-controlled trial in Japan. Epilepsy Res. 2014;108(9):1627–1636. doi: 10.1016/j.eplepsyres.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 68.Palhagen S, Canger R, Henriksen O, van Parys JA, Riviere ME, Karolchyk MA. Rufinamide: a double-blind, placebo-controlled proof of principle trial in patients with epilepsy. Epilepsy Res. 2001;43(2):115–124. doi: 10.1016/S0920-1211(00)00185-6. [DOI] [PubMed] [Google Scholar]
- 69.Alsaad AM, Koren G. Exposure to rufinamide and risks of CNS adverse events in drug-resistant epilepsy: a meta-analysis of randomized, placebo-controlled trials. Br J Clin Pharmacol. 78(6):1264–71. [DOI] [PMC free article] [PubMed]
- 70.Elger CE, Stefan H, Mann A, Narurkar M, Sun Y, Perdomo C. A 24-week multicenter, randomized, double-blind, parallel-group, dose-ranging study of rufinamide in adults and adolescents with inadequately controlled partial seizures. Epilepsy Res. 2010;88(2–3):255–263. doi: 10.1016/j.eplepsyres.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 71.Shahbaz S, Sivamani RK, Konia T, Burrall B. A case of drug rash with eosinophilia and systemic symptoms (DRESS) related to rufinamide. Dermatol Online J. 2013;19(4):4. [PubMed] [Google Scholar]
- 72.Chambel M, Mascarenhas MI, Regala J, Gouveia C, Prates S. Clinical Stevens-Johnson syndrome and rufinamide: a clinical case. Allergol Immunopathol. 2013;41(1):68–69. doi: 10.1016/j.aller.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Kaufman KR, Struck PJ. Activation of suicidal ideation with adjunctive rufinamide in bipolar disorder. Epilepsy Behav. 2011;20(2):386–389. doi: 10.1016/j.yebeh.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Drake K, Labiner DM. Severe constipation associated with the use of rufinamide (Banzel) in an adolescent. Epilepsy Behav. 2010;18(1–2):132. doi: 10.1016/j.yebeh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Ide M, Kato T, Nakata M, Saito K, Yoshida T, Awaya T, et al. A granulocytosis associated with rufinamide: a case report. Brain Dev. 2015;37(8):825–828. doi: 10.1016/j.braindev.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 76.Schimpf R, Veltmann C, Papavassiliu T, Rudic B, Goksu T, Kuschyk J, et al. Drug-induced QT-interval shortening following antiepileptic treatment with oral rufinamide. Heart Rhythm. 2012;9(5):776–781. doi: 10.1016/j.hrthm.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.BRIVIACT Smyrna, GA 30080: UCB; 2016 [updated 02/2016. highlights of prescribing information ]. Available from: www.accessdata.fda.gov/drugsatfda.
- 78.Gillard M, Fuks B, Leclercq K, Matagne A. Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anti-convulsant properties. Eur J Pharmacol. 2011;664(1–3):36–44. doi: 10.1016/j.ejphar.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 79.Ferlazzo E, Russo E, Mumoli L, Sueri C, Gasparini S, Palleria C, et al. Profile of brivaracetam and its potential in the treatment of epilepsy. Neuropsychiatr Dis Treat. 2015;11:2967–2973. doi: 10.2147/NDT.S60849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stockis A, Watanabe S, Rouits E, Matsuguma K, Irie S. Brivaracetam single and multiple rising oral dose study in healthy Japanese participants: influence of CYP2C19 genotype. Drug Metab Pharmacokinetics. 2014;29(5):394–399. doi: 10.2133/dmpk.DMPK-14-RG-010. [DOI] [PubMed] [Google Scholar]
- 81.Stockis A, Watanabe S, Scheen AJ. Effect of brivaracetam on CYP3A activity, measured by oral midazolam. J Clin Pharmacol. 2015;55(5):543–548. doi: 10.1002/jcph.446. [DOI] [PubMed] [Google Scholar]
- 82.Stockis A, Watanabe S, Scheen AJ, Tytgat D, Gerin B, Rosa M, et al. Effect of rifampin on the disposition of brivaracetam in human subjects: further insights into brivaracetam hydrolysis. Drug Metab Disposition. 2016;44(6):792–799. doi: 10.1124/dmd.115.069161. [DOI] [PubMed] [Google Scholar]
- 83.Stockis A, Chanteux H, Rosa M, Rolan P. Brivaracetam and carbamazepine interaction in healthy subjects and in vitro. Epilepsy Res. 2015;113:19–27. doi: 10.1016/j.eplepsyres.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Biton V, Berkovic SF, Abou-Khalil B, Sperling MR, Johnson ME, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia. 2014;55(1):57–66. doi: 10.1111/epi.12433. [DOI] [PubMed] [Google Scholar]
- 85.French JA, Costantini C, Brodsky A, von Rosenstiel P. Adjunctive brivaracetam for refractory partial-onset seizures: a randomized, controlled trial. Neurology. 2010;75(6):519–525. doi: 10.1212/WNL.0b013e3181ec7f7f. [DOI] [PubMed] [Google Scholar]
- 86.Kalviainen R, Genton P, Andermann E, Andermann F, Magaudda A, Frucht SJ, et al. Brivaracetam in Unverricht-Lundborg disease (EPM1): results from two randomized, double-blind, placebo-controlled studies. Epilepsia. 2016;57(2):210–221. doi: 10.1111/epi.13275. [DOI] [PubMed] [Google Scholar]
- 87.Klein P, Schiemann J, Sperling MR, Whitesides J, Liang W, Stalvey T, et al. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia. 2015;56(12):1890–1898. doi: 10.1111/epi.13212. [DOI] [PubMed] [Google Scholar]
- 88.Kwan P, Trinka E, Van Paesschen W, Rektor I, Johnson ME, Lu S. Adjunctive brivaracetam for uncontrolled focal and generalized epilepsies: results of a phase III, double-blind, randomized, placebo-controlled, flexible-dose trial. Epilepsia. 2014;55(1):38–46. doi: 10.1111/epi.12391. [DOI] [PubMed] [Google Scholar]
- 89.Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double-blind, randomized, placebo-controlled trial. Epilepsia. 2014;55(1):47–56. doi: 10.1111/epi.12432. [DOI] [PubMed] [Google Scholar]
- 90.Van Paesschen W, Hirsch E, Johnson M, Falter U, von Rosenstiel P. Efficacy and tolerability of adjunctive brivaracetam in adults with uncontrolled partial-onset seizures: a phase IIb, randomized, controlled trial. Epilepsia. 2013;54(1):89–97. doi: 10.1111/j.1528-1167.2012.03598.x. [DOI] [PubMed] [Google Scholar]
- 91.Sargentini-Maier ML, Rolan P, Connell J, Tytgat D, Jacobs T, Pigeolet E, et al. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after single increasing oral doses in healthy males. Br J Clin Pharmacol. 2007;63(6):680–688. doi: 10.1111/j.1365-2125.2006.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.FYCOMPA® (perampanel) tablets Woodcliff Lake, NJ 07677: Eisai Inc; 2015 [Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/202834s005lbl.pdf.
- 93.Ceolin L, Bortolotto ZA, Bannister N, Collingridge GL, Lodge D, Volianskis A. A novel anti-epileptic agent, perampanel, selectively inhibits AMPA receptor-mediated synaptic transmission in the hippocampus. Neurochem Int. 2012;61(4):517–522. doi: 10.1016/j.neuint.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 94.Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X) Epilepsy Res. 2010;92(2–3):89–124. doi: 10.1016/j.eplepsyres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 95.Patsalos PN. The clinical pharmacology profile of the new antiepileptic drug perampanel: a novel noncompetitive AMPA receptor antagonist. Epilepsia. 2015;56(1):12–27. doi: 10.1111/epi.12865. [DOI] [PubMed] [Google Scholar]
- 96.French JA, Krauss GL, Wechsler RT, Wang XF, DiVentura B, Brandt C, et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy a randomized trial. Neurology. 2015;85(11):950–957. doi: 10.1212/WNL.0000000000001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lagae L, Villanueva V, Meador KJ, Bagul M, Laurenza A, Kumar D, et al. Adjunctive perampanel in adolescents with inadequately controlled partial-onset seizures: a randomized study evaluating behavior, efficacy, and safety. Epilepsia. 2016;57(7):1120–1129. doi: 10.1111/epi.13417. [DOI] [PubMed] [Google Scholar]
- 98.Rohracher A, Brigo F, Hofler J, Kalss G, Neuray C, Dobesberger J, et al. Perampanel for the treatment of primary generalized tonic-clonic seizures in idiopathic generalized epilepsy. Expert Opin Pharmacother. 2016;17(10):1403–1411. doi: 10.1080/14656566.2016.1195810. [DOI] [PubMed] [Google Scholar]
- 99.Strzelczyk A, Willems LM, Willig S, Rosenow F, Bauer S. Perampanel in the treatment of focal and idiopathic generalized epilepsies and of status epilepticus. Expert Rev Clin Pharmacol. 2015;8(6):733–740. doi: 10.1586/17512433.2015.1091303. [DOI] [PubMed] [Google Scholar]
- 100.Shimabukuro K, Gibbon F, Kerstetter J, Tinsley C, Ashwal S. DRESS associated with perampanel administration in a child with drug-resistant epilepsy. Neurology. 2014;83(23):2188. doi: 10.1212/WNL.0000000000001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang H, Laurenza A, Williams B, Patten A, Hussein Z, Ferry J. Lack of effect of perampanel on QT interval duration: results from a thorough QT analysis and pooled partial seizure phase III clinical trials. Epilepsy Res. 2015;114:122–130. doi: 10.1016/j.eplepsyres.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 102.Meador KJ, Yang H, Pina-Garza JE, Laurenza A, Kumar D, Wesnes KA. Cognitive effects of adjunctive perampanel for partial-onset seizures: a randomized trial. Epilepsia. 2016;57(2):243–251. doi: 10.1111/epi.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ettinger AB, LoPresti A, Yang H, Williams B, Zhou S, Fain R, et al. Psychiatric and behavioral adverse events in randomized clinical studies of the noncompetitive AMPA receptor antagonist perampanel. Epilepsia. 2015;56(8):1252–1263. doi: 10.1111/epi.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coyle H, Clough P, Cooper P, Mohanraj R. Clinical experience with perampanel: focus on psychiatric adverse effects. Epilepsy Behav. 2014;41:193–196. doi: 10.1016/j.yebeh.2014.09.072. [DOI] [PubMed] [Google Scholar]
- 105.Rugg-Gunn F. Adverse effects and safety profile of perampanel: a review of pooled data. Epilepsia. 2014;55(Suppl 1):13–15. doi: 10.1111/epi.12504. [DOI] [PubMed] [Google Scholar]
- 106.Krauss GL, Perucca E, Ben-Menachem E, Kwan P, Shih JJ, Squillacote D, et al. Perampanel, a selective, noncompetitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist, as adjunctive therapy for refractory partial-onset seizures: interim results from phase III, extension study 307. Epilepsia. 2013;54(1):126–134. doi: 10.1111/j.1528-1167.2012.03648.x. [DOI] [PubMed] [Google Scholar]
- 107.Krauss GL, Serratosa JM, Villanueva V, Endziniene M, Hong Z, French J, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78(18):1408–1415. doi: 10.1212/WNL.0b013e318254473a. [DOI] [PubMed] [Google Scholar]
- 108.French JA, Krauss GL, Steinhoff BJ, Squillacote D, Yang H, Kumar D, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54(1):117–125. doi: 10.1111/j.1528-1167.2012.03638.x. [DOI] [PubMed] [Google Scholar]
- 109.Rektor I, Krauss GL, Bar M, Biton V, Klapper JA, Vaiciene-Magistris N, et al. Perampanel study 207: long-term open-label evaluation in patients with epilepsy. Acta Neurol Scand. 2012;126(4):263–269. doi: 10.1111/ane.12001. [DOI] [PubMed] [Google Scholar]
- 110.Huber B. Increased risk of suicidality on perampanel (Fycompa(R))? Epilepsy Behav. 2014;31:71–72. doi: 10.1016/j.yebeh.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 111.Hoppner AC, Fauser S, Kerling F. Clinical course of intoxication with the new anticonvulsant drug perampanel. Epileptic Disord. 2013;15(3):362–364. doi: 10.1684/epd.2013.0598. [DOI] [PubMed] [Google Scholar]
- 112.SABRIL® (vigabatrin) [PDF]. 2016 [updated June 2016. Available from: http://www.lundbeck.com/upload/us/files/pdf/Products/Sabril_PI_US_EN.pdf.
- 113.Schechter PJ. Clinical pharmacology of vigabatrin. Br J Clin Pharmacol. 1989;27(Suppl 1):19s–22s. doi: 10.1111/j.1365-2125.1989.tb03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.French JA. Vigabatrin Epilepsia. 1999;40(Suppl 5):S11–S16. doi: 10.1111/j.1528-1157.1999.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 115.Bartoli A, Gatti G, Cipolla G, Barzaghi N, Veliz G, Fattore C, et al. A double-blind, placebo-controlled study on the effect of vigabatrin on in vivo parameters of hepatic microsomal enzyme induction and on the kinetics of steroid oral contraceptives in healthy female volunteers. Epilepsia. 1997;38(6):702–707. doi: 10.1111/j.1528-1157.1997.tb01240.x. [DOI] [PubMed] [Google Scholar]
- 116.Rimmer EM, Richens A. Interaction between vigabatrin and phenytoin. British journal of clinical pharmacology. 1989;27(Suppl 1):27s–33s. doi: 10.1111/j.1365-2125.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hemming K, Maguire MJ, Hutton JL, Marson AG. Vigabatrin for refractory partial epilepsy. The Cochrane database of systematic reviews. 2013(1):Cd007302. [DOI] [PubMed]
- 118.Clayton LM, Stern WM, Newman WD, Sander JW, Acheson J, Sisodiya SM. Evolution of visual field loss over ten years in individuals taking vigabatrin. Epilepsy Res. 2013;105(3):262–271. doi: 10.1016/j.eplepsyres.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 119.Eke T, Talbot JF, Lawden MC. Severe persistent visual field constriction associated with vigabatrin. BMJ (Clinical research ed) 1997;314(7075):180–181. doi: 10.1136/bmj.314.7075.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kalviainen R, Nousiainen I. Visual field defects with vigabatrin: epidemiology and therapeutic implications. CNS drugs. 2001;15(3):217–230. doi: 10.2165/00023210-200115030-00005. [DOI] [PubMed] [Google Scholar]
- 121.Krauss GL, Johnson MA, Miller NR. Vigabatrin-associated retinal cone system dysfunction: electroretinogram and ophthalmologic findings. Neurology. 1998;50(3):614–618. doi: 10.1212/WNL.50.3.614. [DOI] [PubMed] [Google Scholar]
- 122.Nicolson A, Leach JP, Chadwick DW, Smith DF. The legacy of vigabatrin in a regional epilepsy clinic. J Neurol Neurosurg Psychiatry. 2002;73(3):327–329. doi: 10.1136/jnnp.73.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Milh M, Villeneuve N, Chapon F, Pineau S, Lamoureux S, Livet MO, et al. Transient brain magnetic resonance imaging hyperintensity in basal ganglia and brain stem of epileptic infants treated with vigabatrin. J Child Neurol. 2009;24(3):305–315. doi: 10.1177/0883073808324219. [DOI] [PubMed] [Google Scholar]
- 124.Wheless JW, Carmant L, Bebin M, Conry JA, Chiron C, Elterman RD, et al. Magnetic resonance imaging abnormalities associated with vigabatrin in patients with epilepsy. Epilepsia. 2009;50(2):195–205. doi: 10.1111/j.1528-1167.2008.01896.x. [DOI] [PubMed] [Google Scholar]
- 125.Fong CY, Osborne JP, Edwards SW, Hemingway C, Hancock E, Johnson AL, et al. An investigation into the relationship between vigabatrin, movement disorders, and brain magnetic resonance imaging abnormalities in children with infantile spasms. Dev Med Child Neurol. 2013;55(9):862–867. doi: 10.1111/dmcn.12188. [DOI] [PubMed] [Google Scholar]
- 126.Hernandez Vega Y, Kaliakatsos M, UK-I JM, Lascelles K, Lim M. Reversible vigabatrin-induced life-threatening encephalopathy. JAMA Neurol. 2014;71(1):108–109. doi: 10.1001/jamaneurol.2013.1858. [DOI] [PubMed] [Google Scholar]
- 127.Schonstedt V, Stecher X, Venegas V, Silva C. Vigabatrin-induced MRI changes associated with extrapyramidal symptoms in a child with infantile spasms. Neuroradiol J. 2015;28(5):515–518. doi: 10.1177/1971400915598082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haas-Lude K, Wolff M, Riethmuller J, Niemann G, Krageloh-Mann I. Acute encephalopathy associated with vigabatrin in a six-month-old girl. Epilepsia. 2000;41(5):628–630. doi: 10.1111/j.1528-1157.2000.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 129.Sharief MK, Sander JW, Shorvon SD. Acute encephalopathy with vigabatrin. Lancet (London, England). 1993;342(8871):–619. [DOI] [PubMed]
- 130.Canovas Martinez A, Ordovas Baines JP, Beltran Marques M, Escriva Aparisi A, Delgado CF. Vigabatrin-associated reversible acute psychosis in a child. Ann Pharmacother. 1995;29(11):1115–1117. doi: 10.1177/106002809502901108. [DOI] [PubMed] [Google Scholar]
- 131.Levinson DF, Devinsky O. Psychiatric adverse events during vigabatrin therapy. Neurology. 1999;53(7):1503–1511. doi: 10.1212/WNL.53.7.1503. [DOI] [PubMed] [Google Scholar]
- 132.Xavier M, Bento MS, Pereira DP, De Almeida JM. Acute psychotic disorder associated with vigabatrin. Acta medica portuguesa. 2000;13(3):111–114. [PubMed] [Google Scholar]
- 133.Kellermann K, Soditt V, Rambeck B, Klinge O. Fatal hepatotoxicity in a child treated with vigabatrin. Acta Neurol Scand. 1996;93(5):380–381. doi: 10.1111/j.1600-0404.1996.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 134.de Krom MC, Verduin N, Visser E, Kleijer M, Scholtes F, De Groen JH. Status epilepticus during vigabatrin treatment: a report of three cases. Seizure. 1995;4(2):159–162. doi: 10.1016/S1059-1311(95)80099-9. [DOI] [PubMed] [Google Scholar]
- 135.Garcia Pastor A, Garcia-Zarza E, Peraita AR. Acute encephalopathy and myoclonic status induced by vigabatrin monotherapy. Neurologia (Barcelona, Spain) 2000;15(8):Ͱ–4. [PubMed] [Google Scholar]
- 136.Dieterle L, Becker EW, Berg PA, Berkenfeld R, Reinshagen G. Allergic vasculitis caused by Vigabatrin. Nervenarzt. 1994;65(2):122–124. [PubMed] [Google Scholar]
- 137.Papadeas E, Polychronopoulos P, Papathanasopoulos P, Frimas C, Pharmakakis N, Paschalis C. Sensorineural hearing loss: a reversible effect of vigabatrin. Neurology. 2003;61(7):1020–1021. doi: 10.1212/01.WNL.0000082396.01692.53. [DOI] [PubMed] [Google Scholar]
- 138.Williams A, Sekaninova S, Coakley J. Suppression of elevated alanine aminotransferase activity in liver disease by vigabatrin. J Paediatr Child Health. 1998;34(4):395–397. doi: 10.1046/j.1440-1754.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- 139.Karabiyik L. Acute vigabatrin poisoning in a patient with epilepsy. J Appl Res. 2003;3(2):156–158. [Google Scholar]
- 140.Bachmann D, Ritz R, Wad N, Haefeli WE. Vigabatrin dosing during haemodialysis. Seizure. 1996;5(3):239–242. doi: 10.1016/S1059-1311(96)80043-4. [DOI] [PubMed] [Google Scholar]
- 141.Jacqz-Aigrain E, Guillonneau M, Rey E, Macher MA, Montes C, Chiron C, et al. Pharmacokinetics of the S(+) and R(−) enantiomers of vigabatrin during chronic dosing in a patient with renal failure. Br J Clin Pharmacol. 1997;44(2):183–185. doi: 10.1046/j.1365-2125.1997.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.ONFI® (clobazam) [PDF]. [updated 11/2013. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202067s002,203993s002lbl.pdf.
- 143.Sankar R. GABA(a) receptor physiology and its relationship to the mechanism of action of the 1,5-benzodiazepine clobazam. CNS drugs. 2012;26(3):229–244. doi: 10.2165/11599020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 144.Rupp W, Badian M, Christ O, Hajdu P, Kulkarni RD, Taeuber K, et al. Pharmacokinetics of single and multiple doses of clobazam in humans. Br J Clin Pharmacol. 1979;7(Suppl 1):51s–57s. doi: 10.1111/j.1365-2125.1979.tb04665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de Leon J, Spina E, Diaz FJ. Clobazam therapeutic drug monitoring: a comprehensive review of the literature with proposals to improve future studies. Ther Drug Monit. 2013;35(1):30–47. doi: 10.1097/FTD.0b013e31827ada88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tolbert D, Bekersky I, Chu HM, Ette EI. An integrative population pharmacokinetics approach to the characterization of the effect of hepatic impairment on clobazam pharmacokinetics. J Clin Pharmacol. 2016;56(2):213–222. doi: 10.1002/jcph.586. [DOI] [PubMed] [Google Scholar]
- 147.Parmeggiani A, Posar A, Sangiorgi S, Giovanardi-Rossi P. Unusual side-effects due to clobazam: a case report with genetic study of CYP2C19. Brain Dev. 2004;26(1):63–66. doi: 10.1016/S0387-7604(03)00074-3. [DOI] [PubMed] [Google Scholar]
- 148.Zifkin B, Sherwin A, Andermann F. Phenytoin toxicity due to interaction with clobazam. Neurology. 1991;41(2 ( Pt 1):313–314. doi: 10.1212/WNL.41.2_Part_1.313. [DOI] [PubMed] [Google Scholar]
- 149.Naccarato M, Yoong D, Kovacs C, Gough K. A case of a potential drug interaction between clobazam and etravirine-based antiretroviral therapy. Antivir Ther. 2012;17(3):589–592. doi: 10.3851/IMP1953. [DOI] [PubMed] [Google Scholar]
- 150.Genton P, Nguyen VH, Mesdjian E. Carbamazepine intoxication with negative myoclonus after the addition of clobazam. Epilepsia. 1998;39(10):1115–1118. doi: 10.1111/j.1528-1157.1998.tb01299.x. [DOI] [PubMed] [Google Scholar]
- 151.Faulkner MA. Comprehensive overview: efficacy, tolerability, and cost-effectiveness of clobazam in Lennox-Gastaut syndrome. Ther Clin Risk Manag. 2015;11:905–914. doi: 10.2147/TCRM.S55930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Klehm J, Thome-Souza S, Sanchez Fernandez I, Bergin AM, Bolton J, Harini C, et al. Clobazam: effect on frequency of seizures and safety profile in different subgroups of children with epilepsy. Pediatr Neurol. 2014;51(1):60–66. doi: 10.1016/j.pediatrneurol.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 153.Ng YT, Conry J, Mitchell WG, Buchhalter J, Isojarvi J, Lee D, et al. Clobazam is equally safe and efficacious for seizures associated with Lennox-Gastaut syndrome across different age groups: post hoc analyses of short- and long-term clinical trial results. Epilepsy Behav. 2015;46:221–226. doi: 10.1016/j.yebeh.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 154.Aylett SE, Cross H, Berry D. Eye rolling as a manifestation of clobazam toxicity in a child with epilepsy. Dev Med Child Neurol. 2006;48(7):612–615. doi: 10.1017/S0012162206001289. [DOI] [PubMed] [Google Scholar]
- 155.Dang CD, Beets-Shay L, Kahn EC. Toxic epidermal necrolysis triggered by clobazam: a case report in a 13-year-old girl. Pediatr Dermatol. 2015;32(3):e102–e103. doi: 10.1111/pde.12527. [DOI] [PubMed] [Google Scholar]
- 156.Iwasaki T, Miura H, Sunaoshi W, Hosoda N, Takei K, Katayama F. A case of intractable epilepsy showing frequent gelastic seizures by administration of clobazam. No to hattatsu Brain Dev. 2003;35(5):406–410. [PubMed] [Google Scholar]
- 157.Caramaschi P, Biasi D, Carletto A, Manzo T, Bambara LM. Clobazam-induced systemic lupus erythematosus. Clin Rheumatol. 1995;14(1):116. doi: 10.1007/BF02208098. [DOI] [PubMed] [Google Scholar]
- 158.Rauber-Luethy C, Gross S, Hofer KE, Hoffmann-Walbeck P, Prasa D, Farber E, et al. Annual Meeting of the North American Congress of Clinical Toxicology (NACCT). Abstract: Favorable acute toxicity profile of clobazam in overdose. Clin Toxicol. 2013;51(7):575–724.
- 159.Proenca P, Teixeira H, Pinheiro J, Marques EP, Vieira DN. Forensic intoxication with clobazam: HPLC/DAD/MSD analysis. Forensic Sci Int. 2004;143(2–3):205–209. doi: 10.1016/j.forsciint.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 160.Pok PR, Mauras M, De Saint Leger MN, Kuhlmann E, Charpenel-Durat C, Navarette C, et al. Blood concentrations of clobazam and norclobazam in a lethal case involving clobazam, meprobamate and clorazepate. Legal medicine (Tokyo, Japan) 2010;12(6):300–304. doi: 10.1016/j.legalmed.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 161.POTIGA (ezogabine) [PDF]. 2011 [updated 05/2016. 2016]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022345s011lbl.pdf.
- 162.Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 2012;53(3):412–424. doi: 10.1111/j.1528-1167.2011.03365.x. [DOI] [PubMed] [Google Scholar]
- 163.Treven M, Koenig X, Assadpour E, Gantumur E, Meyer C, Hilber K, et al. The anticonvulsant retigabine is a subtype selective modulator of GABAA receptors. Epilepsia. 2015;56(4):647–657. doi: 10.1111/epi.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ferron GM, Paul J, Fruncillo R, Richards L, Knebel N, Getsy J, et al. Multiple-dose, linear, dose-proportional pharmacokinetics of retigabine in healthy volunteers. J Clin Pharmacol. 2002;42(2):175–182. doi: 10.1177/00912700222011210. [DOI] [PubMed] [Google Scholar]
- 165.Tompson DJ, Crean CS. Clinical pharmacokinetics of retigabine/ezogabine. Curr Clin Pharmacol. 2013;8(4):319–331. doi: 10.2174/15748847113089990053. [DOI] [PubMed] [Google Scholar]
- 166.Amabile CM, Vasudevan A. Ezogabine: a novel antiepileptic for adjunctive treatment of partial-onset seizures. Pharmacotherapy. 2013;33(2):187–194. doi: 10.1002/phar.1185. [DOI] [PubMed] [Google Scholar]
- 167.Tompson DJ, Buraglio M, Bullman J, Crean CS, Rayner K. Effect of hemodialysis on pharmacokinetics of ezogabine/retigabine and its N-acetyl metabolite in patients with end stage renal disease. Curr Clin Pharmacol. 2014;9(4):319–325. doi: 10.2174/157488470904141105142635. [DOI] [PubMed] [Google Scholar]
- 168.Tompson DJ, Crean CS. The interaction potential of retigabine (ezogabine) with other antiepileptic drugs. Curr Clin Pharmacol. 2014;9(2):148–156. doi: 10.2174/1574884708666131111192311. [DOI] [PubMed] [Google Scholar]
- 169.French JA, Abou-Khalil BW, Leroy RF, Yacubian EM, Shin P, Hall S, et al. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology. 2011;76(18):1555–1563. doi: 10.1212/WNL.0b013e3182194bd3. [DOI] [PubMed] [Google Scholar]
- 170.Porter RJ, Partiot A, Sachdeo R, Nohria V, Alves WM. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68(15):1197–1204. doi: 10.1212/01.wnl.0000259034.45049.00. [DOI] [PubMed] [Google Scholar]
- 171.Brickel N, Gandhi P, VanLandingham K, Hammond J, DeRossett S. The urinary safety profile and secondary renal effects of retigabine (ezogabine): a first-in-class antiepileptic drug that targets KCNQ (K(v)7) potassium channels. Epilepsia. 2012;53(4):606–612. doi: 10.1111/j.1528-1167.2012.03441.x. [DOI] [PubMed] [Google Scholar]
- 172.Ezogabine (Potiga) toxicity. The Medical letter on drugs and therapeutics. 2013;55(1430):96. [PubMed]
- 173.Schedules of controlled substances: placement of ezogabine into Schedule V. Final rule. Fed Regist 2011;76(241):77895–99. [PubMed]