Abstract
Agrosilvopastoral and silvopastoral systems can increase carbon sequestration, offset greenhouse gas (GHG) emissions and reduce the carbon footprint generated by animal production. The objective of this study was to estimate GHG emissions, the tree and grass aboveground biomass production and carbon storage in different agrosilvopastoral and silvopastoral systems in southeastern Brazil. The number of trees required to offset these emissions were also estimated. The GHG emissions were calculated based on pre-farm (e.g. agrochemical production, storage, and transportation), and on-farm activities (e.g. fertilization and machinery operation). Aboveground tree grass biomass and carbon storage in all systems was estimated with allometric equations. GHG emissions from the agroforestry systems ranged from 2.81 to 7.98 t CO2e ha−1. Carbon storage in the aboveground trees and grass biomass were 54.6, 11.4, 25.7 and 5.9 t C ha−1, and 3.3, 3.6, 3.8 and 3.3 t C ha−1 for systems 1, 2, 3 and 4, respectively. The number of trees necessary to offset the emissions ranged from 17 to 44 trees ha−1, which was lower than the total planted in the systems. Agroforestry systems sequester CO2 from the atmosphere and can help the GHG emission-reduction policy of the Brazilian government.
Introduction
The Paris Agreement, adopted in the 21st session of the Conference of the Parties (COP 21) for the United Nations Framework Convention on Climate Change (UNFCCC), aims to maintain the global average temperature below 2 °C of pre-industrial levels1. The signatory countries stipulate their Intended Nationally Determined Contributions (INDCs), which are the main commitments and contributions of that country for the fulfillment of the agreement2,3.
The Brazilian INDC proposed to reduce the greenhouse gases (GHG) emission by 37% in 2025, based on 2005 levels4. Agriculture is the main emission source with enteric fermentation being responsible for 90% of CH4 and animal manure on pasture for 33% of N2O emissions in Brazil in 20145. The Brazilian government established a “low-carbon agriculture plan” to promote sustainable practices in agriculture by reducing greenhouse gas (GHG) emissions while maintaining profitability6.
This plan is based on practices such as restoration of degraded pastures, crop-livestock-forest integration, no-till farming, biological nitrogen fixation and forestry and agroforestry systems6. The agroforestry system is a land use management system combining trees and/or woody perennial plants, pasture and livestock benefiting from ecological and economic interactions between its component parts due to production diversification7. Food production8 and carbon sequestration by tree planting9 in these systems can help to reduce deforestation in tropical countries10,11.
Agrosilvopastoral and silvopastoral systems are agroforestry system types that can reduce and offset GHG emissions from the Brazilian agricultural sector, mainly using cattle and forest integration12–14. These systems lower animal emission levels12 by improving grass quality, which can reduce CH4 emissions from enteric fermentation15 and digestion efficiency16. Furthermore, these systems may mitigate GHG emissions by enhancing carbon sequestration through increasing above and belowground biomass17–19.
The objective of this study was to estimate GHG emissions, tree and grass aboveground biomass and carbon storage in silvopastoral and agrosilvopastoral systems in southeastern Brazil, and the number of trees required to offset these emissions.
Results
GHG emissions
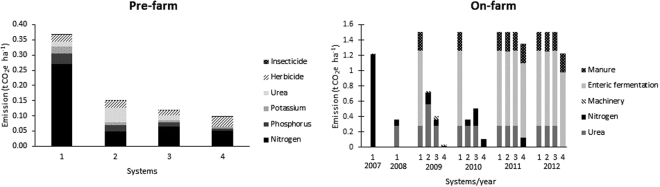
The pre-farm GHG emissions were 0.37, 0.15, 0.12 and 0.10 t CO2e ha-1 in systems 1, 2, 3 and 4, respectively. Nitrogen production was the main emission source for pre-farm activities (Fig. 1). On-farm GHG emissions were 7.61, 4.10, 3.92 and 2.71 t CO2e ha−1 in systems 1, 2, 3 and 4, respectively. Enteric fermentation and manure produced by livestock were the main emission sources for on-farm activity (Fig. 1). Total GHG emissions were 7.98, 4.25, 4.04 and 2.80 t CO2e ha−1, on systems 1, 2, 3 and 4, respectively (Table 1).
Figure 1.
Greenhouse gas emissions (t CO2e ha−1) during pre-farm and on-farm stages in system 1, 2, 3 and 4.
Table 1.
GHG emission, carbon stock, carbon balance, trees to offset, total trees, surplus trees in all systems.
| System | Emission (t CO2e ha−1) | Carbon Stock (t CO2e ha−1) | Balance (t CO2e ha−1) | Trees to offset | Total trees | Surplus Trees |
|---|---|---|---|---|---|---|
| 1 | 7.98 | 200.14 | 192.16 | 17 | 424 | 407 |
| 2 | 4.25 | 41.86 | 37.61 | 39 | 388 | 349 |
| 3 | 4.04 | 94.33 | 90.29 | 44 | 1031 | 987 |
| 4 | 2.81 | 21.78 | 18.97 | 35 | 267 | 232 |
Aboveground biomass and carbon storage
The equation m1 was the best to predict aboveground biomass and carbon storage in systems 1, 3 and 4 (Tables 2 and 3). These equations had the highest R 2 adj, and lower RMSE (%) than the m2. In system 2, the equation m1 was rejected due to the incoherence of the values for parameter b21, with negative values (Tables 2 and 3).
Table 2.
Estimated regression coefficients and adjusted standard errors (±SE) coefficient of determination ( R 2 adj ), model bias (), and root mean square error (±RMSE) of aboveground biomass equations.
| System | Model | Parameter | Estimate | SE | R 2 adj(%) | (%) | RMSE (%) |
|---|---|---|---|---|---|---|---|
| 1 | m1 | b01 | 0.0204 | 0.0371 | 85.409 | −0.549 | 19.588 |
| b11 | 1.9908 | 0.6453 | |||||
| b21 | 1.0264 | 0.8239 | |||||
| m2 | b02 | 0.0208 | 0.0313 | 85.407 | −0.551 | 19.589 | |
| b12 | 1.0035 | 0.1581 | |||||
| 2 | m1 | b01 | 0.1563 | 0.1784 | 90.699 | −0.221 | 16.911 |
| b11 | 3.0839 | 1.0899 | |||||
| b21 | −0.7883 | 1.2929 | |||||
| m2 | b02 | 0.0601 | 0.0586 | 88.996 | 0.040 | 18.390 | |
| b12 | 0.8691 | 0.1163 | |||||
| 3 | m1 | b01 | 0.0737 | 0.1087 | 90.182 | −0.922 | 19.953 |
| b11 | 1.8942 | 0.5883 | |||||
| b21 | 0.6112 | 0.9028 | |||||
| m2 | b02 | 0.0534 | 0.0480 | 90.112 | −0.857 | 20.023 | |
| b12 | 0.8678 | 0.1086 | |||||
| 4 | m1 | b01 | 0.0895 | 0.0659 | 94.741 | −0.769 | 16.781 |
| b11 | 1.4018 | 0.6370 | |||||
| b21 | 1.0429 | 0.8270 | |||||
| B2 | b02 | 0.1027 | 0.0543 | 94.706 | −0.857 | 16.836 | |
| b12 | 0.7960 | 0.0621 |
Table 3.
Estimated regression coefficients and adjusted standard errors (±SE), adjusted coefficient of determination (R 2 adj), model bias (), and root mean square error (±RMSE) of carbon equations.
| System | Model | Parameter | Estimate | SE | R 2 adj(%) | (%) | RMSE (%) |
|---|---|---|---|---|---|---|---|
| 1 | m1 | b01 | 0.0108 | 0.0197 | 85.439 | −0.541 | 19.550 |
| b11 | 1.9977 | 0.6440 | |||||
| b21 | 1.0153 | 0.8218 | |||||
| m2 | b02 | 0.0110 | 0.0165 | 85.439 | −0.542 | 19.550 | |
| b12 | 1.0031 | 0.1578 | |||||
| 2 | m1 | b01 | 0.0842 | 0.0960 | 90.663 | −0.226 | 16.906 |
| b11 | 3.0889 | 1.0900 | |||||
| b21 | −0.8036 | 1.2927 | |||||
| m2 | b02 | 0.0322 | 0.0314 | 88.930 | 0.039 | 18.409 | |
| b12 | 0.8663 | 0.1162 | |||||
| 3 | m1 | b01 | 0.0382 | 0.0564 | 90.193 | −0.920 | 19.920 |
| b11 | 1.8823 | 0.5867 | |||||
| b21 | 0.6251 | 0.9013 | |||||
| m2 | b02 | 0.0282 | 0.0253 | 90.131 | −0.859 | 19.982 | |
| b12 | 0.8666 | 0.1083 | |||||
| 4 | m1 | b01 | 0.0475 | 0.0344 | 94.868 | −0.736 | 16.536 |
| b11 | 1.3937 | 0.6278 | |||||
| b21 | 1.0498 | 0.8151 | |||||
| m2 | b02 | 0.0547 | 0.0285 | 94.831 | −0.827 | 16.596 | |
| b12 | 0.7950 | 0.0612 |
The tree stems were responsible for 90, 70, 76 and 70% of the total aboveground biomass on systems 1, 2, 3 and 4, respectively. The branches were responsible for 4, 18, 14 and 15% and the leaves for 6, 12, 10 and 15% of the total aboveground biomass in systems 1, 2, 3 and 4, respectively. The leaves had the highest carbon content in all systems (57.0, 55.2, 56.0 and 56%), followed by the stem (52.4, 52.1, 52.2 and 52.3%), and the branches (52.1, 51.3, 50.5 and 52.3%). Carbon storage in trees and grass aboveground biomass was 54.58, 11.42, 25.73, 5.94 t C ha-1 and 3.28, 3.60, 3.77, 3.32 t C ha−1, for systems 1, 2, 3 and 4, respectively.
A total of 17, 39, 44 and 35 trees ha−1 are necessary to offset all GHG emissions, which is equivalent to 4.0, 10.2, 3.4 and 13.1% of the total numbers of trees in systems 1, 2, 3 and 4, respectively (Table 1).
Discussion
The average annual GHG emissions ranged from 0.93 to 1.60 t CO2e ha−1 yr−1, which may be considered low when compared to other systems20,21, probably due to the use of no-till farming and the adoption of agroforestry systems with reduced machinery use, fuel inputs and CO2 emissions22. No-till farming in these systems may increase organic carbon and nitrogen content in the soil, and the microbial biomass, mitigating GHG emissions23–26. Usual management practices in agroforestry systems, such as no-till farming and optimal fertilization/manure regimes can increase carbon sequestration while reducing GHG emissions27. Such a combination provides additional environmental benefits such as soil erosion reduction and prevention28,29, more efficient water-use30, and improvement in biodiversity31.
The difference in the mean annual aboveground carbon increment (MAI-AGB) on the four systems indicates that the amount of this element sequestered may depend on tree species, age, geographic location, environmental factors, and system management32,33. System 1 presented the largest MAI-AGB (11.19 t ha−1 yr−1) due to its older age and the fertilization carried out to enhance maize production indirectly increasing tree biomass34. System 3 presented the second largest IMA due to its greater plant density (9 × 1 spacing), however competition between plants can negatively affect individual growth35,36 and may increase future mortality37. All systems were important in carbon sequestration and had environmental benefits such as soil fertility and water quality improvement and erosion reduction18,38–40. The estimated MAI-AGB found was higher than the 1.43 t ha−1 yr−1 in a silvopastoral system with 105 trees per hectare (eucalypt and acacia) in Minas Gerais, Brazil41. The MAI-AGB of 7.67 t ha−1 yr−1 of an agrosilvopastoral system with eucalyptus spaced 10 × 4 m and rice in Paracatu, Minas Gerais, Brazil42 was similar to that observed in the system 2 of this research.
The estimated aboveground grass carbon sequestration was similar to the 3.71 kg C ha−1 of an agrosilvopastoral system with eucalypt in Minas Gerais, Brazil42, and the 3.29 kg C ha−1 of a silvopastoral system with 200 pine trees ha−1 in São Paulo, Brazil43. These systems had a similar production due to the wide spacing of the trees, allowing sufficient radiation transmittance18 and improving the microclimate for the forage15,44. This shows that agroforestry systems are an alternative to recover degraded pasture land by improving chemical, physical and biological soil conditions and enhancing carbon sequestration12,18,45–47.
The number of trees required to offset GHG emissions was lower than that planted in the systems studied, demonstrating their great potential to sequester carbon and to reduce GHG emissions.12,48,49. Agroforestry systems are important for the “Low-Carbon Agriculture Plan” of the Brazilian government to achieve GHG emission-reduction targets. These systems decrease the pressure on forests48, and improve animal welfare and crop production12. Furthermore, the remaining sequestered carbon can be sold in voluntary markets with a higher price for technologies that bring social and environmental benefits including higher farmer income50.
The systems had a positive carbon balance and a tree surplus ranging from 232 to 987. The number of trees was higher than necessary to offset GHG emissions in all systems. Therefore, the agroforestry systems can effectively mitigate GHG emissions.
Methods
Study systems
The study was conducted in silvopastoral and agrosilvopastoral systems in Viçosa, Minas Gerais, Brazil. The climate in this region is humid subtropical with dry winters and hot summers, classified as Cwa (Köppen classification). The average annual temperature and rainfall are 19.4 °C and 1,200 mm, respectively. The soil is classified as red-yellow latosol and the topography ranges from strongly undulated to mountainous with an average altitude of 689.7 m.
The agrosilvopastoral systems were composed of maize (Zeya mays) and Eucalyptus saligna (system 1), and bean (Phaseolus vulgaris) and E. urophylla x E. grandis (system 2) during the first year, and the crops were replaced by pasture (Brachiaria decumbens) with livestock grazing in the second year (Table 4). The silvopastoral systems (3 and 4) had pasture (Brachiaria decumbens) + E. urophylla x E. grandis (Table 4). No-till farming was used in all systems. Beef cattle were reared in all systems (one animal/ha).
Table 4.
Study area characterization.
| System | Crop | Pasture | Planting | Area (ha) | Tree arrangements (m) |
|---|---|---|---|---|---|
| 1 | Maize | Brachiaria | Dec/2007 | 0.93 | 8 × 3 |
| 2 | Beans | Brachiaria | Dec/2009 | 0.72 | 8 × 3 |
| 3 | — | Brachiaria | Dec/2009 | 0.55 | 9 × 1 |
| 4 | — | Brachiaria | Nov/2009 | 3.48 | 12 × 3 |
System 1 was fertilized after soil analysis. In December 2007, a posthole digger machine was used and 0.2 kg of N-P-K (06-30-06) applied per tree hole. Additional fertilization of 0.16 kg of N-P-K (20-05-20) pit−1 was carried out three months after tree planting. Weeds and leaf-cutting ants were controlled before, during and after tree planting. Animal traction was used to apply 500 kg of N-P-K (08-24-12) ha−1 on maize before planting, and another 500 kg of N-P-K (30-00-10) ha−1 30 days later. The pasture received 100 kg of urea ha−1 year−1.
Systems 2 and 3, implemented in December 2009, received the same treatment as the system 1 for eucalypt planting and weed/leaf-cutter ant control. In system 2, the bean crop received 250 kg of N-P-K (08-28-16) ha−1 and 200 kg urea ha−1 as top-dressing fertilization.
The eucalypt trees in system 4 were planted, in November 2009, using a posthole digger machine with 0.2 kg N-P-K (06-30-06) applied per hole. Additional fertilization with 0.05 kg N-P-K (20-05-20) plant−1, 0.1 kg of N-P-K (20-05-20) plant−1, 0.15 kg and 0.1 kg KCl plant−1 were undertaken at 60, 120, 300 and 550 days after tree planting, respectively. Weed and leafcutter ants were controlled before, during and after tree planting. The pasture received 100 kg of N-P-K (20-05-20) ha−1 one year after eucalypt planting.
GHG emissions
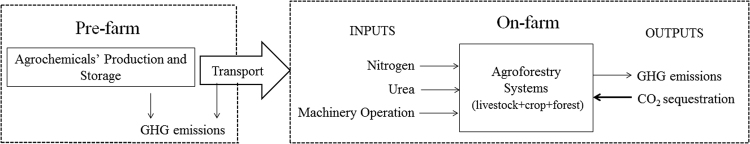
GHG emission calculations per system were based on pre-farm activities, such as production, storage, and transportation of agrochemicals, and on-farm activities such as fertilization and machinery use (Fig. 2). The data were estimated from personal interviews with farmers. They were asked to report on the use of machine fuel, agrochemicals and estimated crop yield.
Figure 2.
GHG inventory for the agroforestry systems, for pre-farm and on-farm stages.
Pre-farm emissions were calculated using emission factors (Table 5)51 and the following equation: emAgr = agrochemical *EF*(44/12), EmAgr = annual emissions resulting from production, packaging, storage and distribution of agrochemicals, kg CO2 year−1; agrochemical = agrochemical applied, kg year−1; EF = emission factor, kg carbon equivalent kg−1; 44/12 = C to CO2 conversion factor.
Table 5.
Carbon emissions (mean ± SD) for the production, transportation, storage, and transfer of agrochemicals. Values according to a previous study51.
| Agrochemicals | Carbon emission (kg C kg substance−1) |
|---|---|
| Nitrogen fertilizer | 1.30 ± 0.30 |
| Phosphorus fertilizer | 0.20 ± 0.06 |
| Potassium fertilizer | 0.15 ± 0.06 |
| Urea | 0.16 ± 0.11 |
| Herbicide | 6.30 ± 2.70 |
| Insecticide | 5.10 ± 3.00 |
| Fungicide | 3.90 ± 2.20 |
On-farm emissions were calculated based on the “Guidelines for National Greenhouse Gas Inventories”52. GHG sources included nitrogen fertilization, farm machinery, enteric fermentation, and manure management.
Input emissions from synthetic fertilizers were calculated via two pathways: direct and indirect. The direct emissions refer to mineral fertilizer applications52. Direct emissions are the product of the nitrogen applied by the emission factor (0.01)52 using the 44/28 factor to convert N2 to N2O, and N2O global warming potential (298 units of CO2e)53. The equation used to estimate direct emissions was: EmDiF = FSN/FRP*EF1*(44/28)*GWP; EmDiF = direct CO2e emissions from N inputs to managed soils, kg CO2 ha−1; FSN = annual amount of synthetic fertilizer N applied to soils, kg N ha−1; FPRP = annual amount of dung and urine N deposited on soils, kg N−1; EF1 = emission factor developed for N2O emissions from synthetic fertilizer, kg N2O–N (kg N)−1; 44/28 = N2 to N2O conversion factor; GWP = global warming potential.
Indirect emissions result from volatilization, atmospheric deposition of NH3 and NOx, and nitrogen leaching and runoff from the fertilizers54,55. Indirect emissions were calculated using annual amount of fertilizer N applied to soils and the nitrogen fraction lost by volatilization, leaching and/or runoff56. The emission factor was 0.01 for volatilization and 0.0075 for leaching/runoff. The nitrogen fraction lost due to volatilization and leaching/runoff was fixed as 0.1 and 0.2, respectively52. The equation used to estimate indirect on-farm N2O emissions per system was EmLnL = FSN*FracLEACH-(H)*EF3*(44/28)*GWP, where EmLnL = amount of CO2e produced from additions to managed soils, kg CO2 ha−1; FSN = amount of synthetic fertilizer N applied to soils, kg N ha−1; EF3 = emission factor for N2O emissions from N leaching and runoff, kg N2O–N (kg N leached and runoff)−1; FracLEACH-(H) = fraction of all N added to/mineralized in managed soils in regions where leaching/runoff occurs that is lost through leaching and runoff, kg N (kg of N additions)−1; GWP = global warming potential.
NO2 emissions from urea were calculated with the same equations used for the other nitrogen fertilizers. CO2 emissions were the product of the urea applied to the soil by its emission factor, 0.2052. The equation used to estimate on-farm CO2 emissions was EmUrea = M*EF4, where EmUrea = amount of CO2e produced from urea application, t CO2 ha−1; M = amount of urea applied to soils, t N ha−1; EF4 = emission factor for applied urea, t of C (ton of urea)−1.
CO2 emissions from agricultural machinery were those generated by fuel consumption during eucalypt planting due to its emission factor (EF5), 2.327 kg CO2 −1 52. The equation used in each system was EmD = F*EF5, where EmD = amount of CO2e produced from fuel consumed, kg CO2 ha−1; F = fuel consumed, L ha−1; EF5 = emission factor, kg C (L fuel)−1.
The CH4 emissions by enteric fermentation from cattle were calculated using the factor of 39 kg CH4 year−1 animal unit−1 57. The equation used was: EmFE = N* EF6* GWP, where EmFE = emissions from enteric fermentation, kg CO2 ha−1; N = number of animals, head ha−1; EF6 = emission factor for enteric fermentation (kg CH4) head−1; GWP = CH4 global warming potential. N2O emissions due to manure deposition were calculated with the same equations as those for nitrogen fertilizer.
Carbon storage in aboveground biomass
Ten pasture grass samples (1 m2) between tree rows were collected, per season, from June 2012 to October 2013. Their fresh weight was obtained and the fresh:dry weight ratio calculated with 25 g from each sample. These samples were dried at approximately 65 °C in an oven until weight stabilization.
The diameter at breast height (DBH), total height, and commercial height (stem height up to 3-cm diameter) of trees per system were measured between July and August 2012. Trees were grouped into DBH classes, and three individuals per class were selected and felled to determine their total volume, biomass and carbon levels in their stem, branches and leaves.
The trees selected were cut at ground level, and the stem diameters measured at 0.3, 0.7, and 1.3 m from their base, and thereafter at every 2 m until the diameter reached 3 cm. The volume of these stem sections was calculated using the Smalian’s formula58. The stems per sample were weighed and 2.5 cm thick stem discs were collected at the base, 25, 50, 75, and 100% of the commercial height to calculate the aboveground biomass. An additional stem disc was cut at breast height (1.3 m). The branches and stem discs were dried at 103 ± 2 °C until dry weight stabilization was reached. The leaf and branch weights per tree sampled were recorded. Fresh leaf and branch samples were weighed in the field, stored in bags and sent to the laboratory to determine their dry/fresh weight ratio59. Leaf and branch samples were dried at 65 ± 2 °C until dry weight stabilization.
The stem, leaf and branch carbon content was determined with a LECO TruSpec Micro CHN analyzer (LECO Corp., St. Joseph, MI). The carbon stock was obtained by multiplying the aboveground biomass by the carbon content.
Field data was fitted to allometric equations60,61 to estimate the tree aboveground biomass, and carbon (stem + branches + leaves) per system as: Y1 = β01*DBHβ11*H β21*ε1; Y2 = β02*(DBH2*H) β12*ε2, whereYj the biomass or carbon stock (kg) of the jth model; H total height (m); β0i, β1i, and β2i the parameters of the jth model and εi:the random errors.
All statistical analyses were performed with R statistical software62. The best equations were based on the criteria: parameter significance (p < 0.05) by Wald test; coherence of the sign associated with a specific parameter; goodness of fit statistics: R2 adj = 1 − [(n − p − 1)/(n − p)] * (1 − R2); R2 = 1 − [Σ(y − )2/Σ(y − )2); RMES% = (100/) * ; = (100/) * (Σ(y − )/n), where, R2 is the empirical determination coefficient or model efficiency; R 2 adj, an empirical adjusted determination coefficient; %, a relative bias; RMSE%, the root square error in percentage; n, the observation number; p, the number of explanatory variables; , the mean of dependent variable (volume, biomass and carbon); yi, the ith observed value; and , the ith value of the dependent variable.
Acknowledgements
We acknowledge the financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, productivity grants), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Ph.D. scholarship, Grant No. BEX 10570/12-8) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, research funding). Thanks also to farmers Francisco de Freitas and Lino Roberto Ferreira for allowing us to work inside their properties. We also thank Lucas Arthur, Breno Loureiro, Gabriel Barros, Henrique Colares, Paulo Villanova, Bruno Schettini, Samuel José and Mateus Castro for laboratory and fieldwork. Dr. Phillip John Villani (The University of Melbourne, Australia) revised and corrected the English language used in this manuscript.
Author Contributions
C.M.M.E.T.; L.A.G.J.; S.N.O.N. and L.R.F. conceived the study, C.M.M.E.T.; L.A.G.J., F.C.N. and S.N.O.N. conducted the experiment, C.M.M.E.T.; C.W.F.; F.C.N. and C.P.B.S. performed analyses, C.M.M.E.T. wrote the first draft of the manuscript, and L.A.G.J.; S.N.O.N.; C.W.F.; F.C.N.; C.P.B.S; J.C.Z. and P.G.L. contributed substantially to write the final version of the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNFCCC. Adoption of the Paris Agreement. (2015).
- 2.Rose A, Wei D, Miller N, Vandyck T. Equity, Emissions Allowance Trading and the Paris Agreement on Climate Change. Econ. Disasters Clim. Change. 2017;1:203–232. doi: 10.1007/s41885-017-0012-3. [DOI] [Google Scholar]
- 3.Rogelj J, et al. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature. 2016;534:631–639. doi: 10.1038/nature18307. [DOI] [PubMed] [Google Scholar]
- 4.Brazil. Intended nationally determined contribution towards achieving the objective of the United Nations Framework Convention on climate change. (2015).
- 5.MCTI. Estimativas anuais de emissões de gases de efeito estufa. (2016).
- 6.Brazil. Plano Setorial de Mitigação e de Adaptação às Mudanças Climáticas para a Consolidação de uma Economia de Baixa Emissão de Carbono na Agricultura. (2011).
- 7.Nair, P. K. R. An introduction to agroforestry. (Kluwer Academic Publishers, 1993).
- 8.Reed J, et al. Trees for life: The ecosystem service contribution of trees to food production and livelihoods in the tropics. For. Policy Econ. 2017;84:62–71. doi: 10.1016/j.forpol.2017.01.012. [DOI] [Google Scholar]
- 9.Zomer RJ, et al. Global Tree Cover and Biomass Carbon on Agricultural Land: The contribution of agroforestry to global and national carbon budgets. Sci. Rep. 2016;6:29987. doi: 10.1038/srep29987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oelbermann M, Paul Voroney R, Gordon AM. Carbon sequestration in tropical and temperate agroforestry systems: a review with examples from Costa Rica and southern Canada. Agric. Ecosyst. Environ. 2004;104:359–377. doi: 10.1016/j.agee.2004.04.001. [DOI] [Google Scholar]
- 11.Ibrahim, M., Guerra, L., Casasola, F. & Neely, C. Importance of silvopastoral systems for mitigation of climate change and harnessing of environmental benefits. in Grassland carbon sequestration: Management, policy and economics. Proceedings of the workshop on the role of grassland carbon sequestration in the mitigation of climate change. (eds Abberton, M., Conant, R. & Batello, C.) 189–196 (Food and Agriculture Organization of the United Nations, 2010).
- 12.Nair, P. K. R., Tonucci, R. G., Garcia, R. & Nair, V. D. Silvopasture and carbon sequestration with special reference to the Brazilian Savanna (Cerrado). in Carbon sequestration potential of agroforestry systems (eds Kumar, B. M. & Nair, P. K. R.) 145–162 (Springer Netherlands, 2011).
- 13.Balbino LC, Cordeiro LAM, Martínez GB. Contributions of the crop-livestock-forest integration systems (iLPF) for a low carbon emission agriculture. Rev. Bras. Geogr. Física. 2012;4:1163–1175. [Google Scholar]
- 14.Peters, M. et al. Tropical Forage-based Systems to Mitigate Greenhouse Gas Emissions. in Eco-Efficiency:From Visionto Reality (eds. Hershey, C. H. & Paul Neate) 171–190 (Centro Internacional de Agricultura Tropical (CIAT), 2013).
- 15.Bernardi RE, de Jonge IK, Holmgren M. Trees improve forage quality and abundance in South American subtropical grasslands. Agric. Ecosyst. Environ. 2016;232:227–231. doi: 10.1016/j.agee.2016.08.003. [DOI] [Google Scholar]
- 16.Thornton PK, Herrero M. Potential for reduced methane and carbon dioxide emissions from livestock and pasture management in the tropics. Proc. Natl. Acad. Sci. USA. 2010;107:19667–19672. doi: 10.1073/pnas.0912890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dube F, et al. Productivity and carbon storage in silvopastoral systems with Pinus ponderosa and Trifolium spp., plantations and pasture on an Andisol in Patagonia, Chile. Agrofor. Syst. 2012;86:113–128. doi: 10.1007/s10457-011-9471-7. [DOI] [Google Scholar]
- 18.Paula RR, et al. Eucalypt growth in monoculture and silvopastoral systems with varied tree initial densities and spatial arrangements. Agrofor. Syst. 2013;87:1295–1307. doi: 10.1007/s10457-013-9638-5. [DOI] [Google Scholar]
- 19.Nair, P. K. R. Climate change mitigation: A low-hanging fruit of agroforestry. in Agroforestry - The future of global landUse (eds Nair, P. K. R. & Garrity, D.) 31–67 (Springer Netherlands, 2012).
- 20.Browne NA, Eckard RJ, Behrendt R, Kingwell RS. A comparative analysis of on-farm greenhouse gas emissions from agricultural enterprises in south eastern Australia. Anim. Feed Sci. Technol. 2011;166–167:641–652. doi: 10.1016/j.anifeedsci.2011.04.045. [DOI] [Google Scholar]
- 21.Pradhan P, Reusser DE, Kropp JP. Embodied Greenhouse Gas Emissions in Diets. Plos One. 2013;8:e62228. doi: 10.1371/journal.pone.0062228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stavi I, Lal R. Agroforestry and biochar to offset climate change: a review. Agron. Sustain. Dev. 2013;33:81–96. doi: 10.1007/s13593-012-0081-1. [DOI] [Google Scholar]
- 23.West TO, Post WM. Soil organic carbon sequestration rates by tillage and crop rotation. Soil Sci. Soc. Am. J. 2002;66:1930–1946. doi: 10.2136/sssaj2002.1930. [DOI] [Google Scholar]
- 24.Six J, et al. The potential to mitigate global warming with no-tillage management is only realized when practised in the long term. Glob. Change Biol. 2004;10:155–160. doi: 10.1111/j.1529-8817.2003.00730.x. [DOI] [Google Scholar]
- 25.Thomas GA, Dalal RC, Standley J. No-till effects on organic matter, pH, cation exchange capacity and nutrient distribution in a Luvisol in the semi-arid subtropics. Soil Tillage Res. 2007;94:295–304. doi: 10.1016/j.still.2006.08.005. [DOI] [Google Scholar]
- 26.Varvel GE, Wilhelm WW. No-tillage increases soil profile carbon and nitrogen under long-term rainfed cropping systems. Soil Tillage Res. 2011;114:28–36. doi: 10.1016/j.still.2011.03.005. [DOI] [Google Scholar]
- 27.Killham K. Integrated soil management – moving towards globally sustainable agriculture. J. Agric. Sci. 2011;149:29–36. doi: 10.1017/S0021859610000845. [DOI] [Google Scholar]
- 28.Souza de., et al. Protective shade, tree diversity and soil properties in coffee agroforestry systems in the Atlantic Rainforest biome. Agric. Ecosyst. Environ. 2012;146:179–196. doi: 10.1016/j.agee.2011.11.007. [DOI] [Google Scholar]
- 29.Delgado MEM. & Canters, F. Modeling the impacts of agroforestry systems on the spatial patterns of soil erosion risk in three catchments of Claveria, the Philippines. Agrofor. Syst. 2012;85:411–423. doi: 10.1007/s10457-011-9442-z. [DOI] [Google Scholar]
- 30.Norwood CA. Water use and yield of limited-irrigated and dryland corn. Soil Sci. Soc. Am. J. 2000;64:365–370. doi: 10.2136/sssaj2000.641365x. [DOI] [Google Scholar]
- 31.Laclau J-P, et al. Mixing Eucalyptus and Acacia trees leads to fine root over-yielding and vertical segregation between species. Oecologia. 2013;172:903–913. doi: 10.1007/s00442-012-2526-2. [DOI] [PubMed] [Google Scholar]
- 32.Albrecht A, Kandji ST. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003;99:15–27. doi: 10.1016/S0167-8809(03)00138-5. [DOI] [Google Scholar]
- 33.Jose S. Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor. Syst. 2009;76:1–10. doi: 10.1007/s10457-009-9229-7. [DOI] [Google Scholar]
- 34.Mosquera-Losada, M. R., Freese, D. & Rigueiro-Rodríguez, A. Carbon sequestration in european agroforestry systems. in Carbon sequestration potential of agroforestry systems 43–59 (Springer, Dordrecht, 2011).
- 35.Leite HG, Nogueira GS, Moreira AM. Effect of spacing and age on stand variables of Pinus Taeda L. Rev. Árvore. 2006;30:603–612. doi: 10.1590/S0100-67622006000400013. [DOI] [Google Scholar]
- 36.Bernardo AL, Reis MGF, Reis GG, Harrison RB, Firme DJ. Effect of spacing on growth and biomass distribution in Eucalyptus camaldulensis, E. pellita and E. urophylla plantations in southeastern Brazil. For. Ecol. Manag. 1998;104:1–13. doi: 10.1016/S0378-1127(97)00199-0. [DOI] [Google Scholar]
- 37.Harrington TB, Harrington CA, DeBell DS. Effects of planting spacing and site quality on 25-year growth and mortality relationships of Douglas-fir (Pseudotsuga menziesii var. menziesii) For. Ecol. Manag. 2009;258:18–25. doi: 10.1016/j.foreco.2009.03.039. [DOI] [Google Scholar]
- 38.Oliveira Neto, S. N. et al. Sistema agrossilvipastoril: Integração lavoura, pecuária e floresta. (Sociedade de Investigações Florestais, 2010).
- 39.Blanco-Canqui, H. & Lal, R. Agroforestry. in Principles of soil conservation and management 259–283 (Springer Netherlands, 2010).
- 40.Silva GL, Lima HV, Campanha MM, Gilkes RJ, Oliveira TS. Soil physical quality of Luvisols under agroforestry, natural vegetation and conventional crop management systems in the Brazilian semi-arid region. Geoderma. 2011;167–168:61–70. doi: 10.1016/j.geoderma.2011.09.009. [DOI] [Google Scholar]
- 41.Müller MD, Fernandes EN, de Castro CRT, Paciullo DSC, de Alves FF. Estimativa de acúmulo de biomassa e carbono em sistema agrossilvipastoril na Zona da Mata Mineira. Pesqui. Florest. Bras. 2009;60:11–17. [Google Scholar]
- 42.Tsukamoto Filho A, et al. L. da. Fixação de carbono em um sistema agrissilvipastoril com eucalipto na região do cerrado de Minas Gerais. Rev. Agrossilvicultura. 2004;1:29–41. [Google Scholar]
- 43.Gutmanis, D. Estoque de carbono e dinâmica ecofisiológica em Sistemas Silvipastoris. (Universidade Estadual Paulista, 2004).
- 44.Santos D, de C, et al. Forage dry mass accumulation and structural characteristics of Piatã grass in silvopastoral systems in the Brazilian savannah. Agric. Ecosyst. Environ. 2016;233:16–24. doi: 10.1016/j.agee.2016.08.026. [DOI] [Google Scholar]
- 45.Macedo MCM. Crop and livestock integration: the state of the art and the near future. Rev. Bras. Zootec. 2009;38:133–146. doi: 10.1590/S1516-35982009001300015. [DOI] [Google Scholar]
- 46.Tonucci RG, Nair PKR, Nair VD, Garcia R, Bernardino FS. Soil carbon storage in silvopasture and related land-use systems in the Brazilian Cerrado. J. Environ. Qual. 2011;40:833. doi: 10.2134/jeq2010.0162. [DOI] [PubMed] [Google Scholar]
- 47.de Freitas ECS, et al. Litter fall and nutriente deposition on soil in an agrosilvopastoral system with eucalypt and acacia. Rev. Árvore. 2013;37:409–417. doi: 10.1590/S0100-67622013000300004. [DOI] [Google Scholar]
- 48.Montagnini F, Nair PKR. Carbon sequestration: An underexploited environmental benefit of agroforestry systems. Agrofor. Syst. 2004;61–62:281–295. [Google Scholar]
- 49.Schoeneberger MM. Agroforestry: working trees for sequestering carbon on agricultural lands. Agrofor. Syst. 2009;75:27–37. doi: 10.1007/s10457-008-9123-8. [DOI] [Google Scholar]
- 50.Lee J, Ingalls M, Erickson JD, Wollenberg E. Bridging organizations in agricultural carbon markets and poverty alleviation: An analysis of pro-Poor carbon market projects in East Africa. Glob. Environ. Change. 2016;39:98–107. doi: 10.1016/j.gloenvcha.2016.04.015. [DOI] [Google Scholar]
- 51.Lal R. Soil carbon sequestration impacts on global climate change and food security. Science. 2004;304:1623–1627. doi: 10.1126/science.1097396. [DOI] [PubMed] [Google Scholar]
- 52.IPCC. 2006IPCC Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme. (IGES, 2006).
- 53.IPCC. Climate change2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. (Cambridge University Press, 2007).
- 54.Mosier A, et al. Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle. Nutr. Cycl. Agroecosystems. 1998;52:225–248. doi: 10.1023/A:1009740530221. [DOI] [Google Scholar]
- 55.Bouwman AF, Boumans LJM, Batjes NH. Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Glob. Biogeochem. Cycles. 2002;16:6–1–6–13. [Google Scholar]
- 56.Jones CD, Fraisse CW, Ozores-Hampton M. Quantification of greenhouse gas emissions from open field-grown Florida tomato production. Agric. Syst. 2012;113:64–72. doi: 10.1016/j.agsy.2012.07.007. [DOI] [Google Scholar]
- 57.Esteves, S. N. et al. Estimativas da emissão de metano por bovinos criados em sistema de integração lavoura-pecuária em São Carlos, SP. 7 (Embrapa Pecuária Sudeste, 2010).
- 58.Loetsch, F. & Haller, K. E. Forest inventory. (BLV Verlagsgesellschaft, 1973).
- 59.Vital, B. R. Métodos de determinação da densidade da madeira. 21 (Sociedade de Investigações Florestais, 1984).
- 60.Schumacher FX, Hall F. dos S. Logarithmic expression of timber-tree volume. J. Agric. Res. 1933;47:719–734. [Google Scholar]
- 61.Spurr, S. H. Forestry inventory. (Ronald Press, 1952).
- 62.R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2013).