Abstract
Cancer remains a leading causes of death worldwide and an elevated systemic inflammatory response (SIR) is associated with reduced survival in patients with operable cancer. This review aims to examine the evidence for the role of systemic inflammation based prognostic scores in patients with operable cancers. A wide-ranging literature review using targeted medical subject headings for human studies in English was carried out in the MEDLINE, EMBASE, and CDSR databases until the end of 2016. The SIR has independent prognostic value, across tumour types and geographical locations. In particular neutrophil lymphocyte ratio (NLR) (n = 158), platelet lymphocyte ratio (PLR) (n = 68), lymphocyte monocyte ratio (LMR) (n = 21) and Glasgow Prognostic Score/ modified Glasgow Prognostic Score (GPS/mGPS) (n = 60) were consistently validated. On meta-analysis there was a significant relationship between elevated NLR and overall survival (OS) (p < 0.00001)/ cancer specific survival (CSS) (p < 0.00001), between elevated LMR and OS (p < 0.00001)/CSS (p < 0.00001), and elevated PLR and OS (p < 0.00001)/CSS (p = 0.005). There was also a significant relationship between elevated GPS/mGPS and OS (p < 0.00001)/CSS (p < 0.00001). These results consolidate the prognostic value of the NLR, PLR, LMR and GPS/mGPS in patients with resectable cancers. This is particularly true for the NLR/GPS/mGPS which should form part of the routine preoperative and postoperative workup.
Introduction
Cancer remains one of the leading causes of mortality worldwide and is responsible for 8.8 million deaths per year1. Overall, it has been estimated that one in three people will develop cancer in their lifetime, and one in four will die from it2,3. Indeed, in the UK alone it is estimated that 150,000 people die because of cancer each year1,3. Such a large burden of disease accounts for a significant proportion of the healthcare budgets of the UK, US and worldwide medical care1,3,4.
Four cancers: lung, colorectal, breast and prostate, account for approximately half of all new cases and deaths2. For a range of solid organ malignancies including colorectal, lung, breast and prostate cancers, definitive local therapy in the form of surgical resection remains the cornerstone of treatment2.
The genetic composition of many different types of cancer has been widely reported, however there is also increasing evidence that the host inflammatory response plays an important role in the development and progression of cancer3,5–7. In 2010 Roxburgh and McMillan published the first comprehensive review of the role of the systemic inflammatory response in predicting survival in patients with primary operable cancer2. They identified 80 studies where the systemic inflammatory response was related to either overall, and cancer specific survival2. However the majority of studies used singular markers of the inflammatory response such as CRP, albumin neutrophil, lymphocyte and platelet counts, indeed just 18 studies reported combined prognostic scores to improve prediction of survival2. These included eight that reported the prognostic value of the GPS, and nine studies that reported the prognostic value of NLR. While these studies reported a significant relationship between the systemic inflammatory response and survival there were variable thresholds used for the single or combined markers resulting in considerable variability in the magnitude of the effect reported2.
However, since this review there has been a marked increase in the number of studies reporting the prognostic value of combined scoring systems based on the systemic inflammatory response. The majority reported have principally been ratios of components of the white cell count such as the neutrophil lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR), lymphocyte monocyte ratio (LMR) but also acute phase proteins such as C-reactive protein/albumin ratio (CAR). Another approach is to combine scores of the acute phase proteins such as GPS/mGPS3,8,9. The presence of an elevated systemic inflammatory response as shown by the presence of circulating white cells and acute phase proteins is an important unifying host characteristic in patients with cancer. The prognostic ability of the combined scores has been widely reported and there have been reviews of NLR9,10 and mGPS11 and in advanced cancer12. The present review is the first since 2010 to focus on primarily operable cancer and to include all recognised systemic inflammation based prognostic scores. This will rationalise the evidence for the role of systemic inflammation based prognostic scores in patients with primary operable cancers.
Methods
This systematic review and meta-analysis of published literature was undertaken according to a pre-defined protocol described in the PRISMA-P statement and in a similar fashion to that recently reported with advanced inoperable cancer12. The primary outcome was to assess the prognostic value of the validated combined scores of the systemic inflammatory response (NLR, PLR, LMR, GPS and mGPS) in patients with primary operable cancer.
This was carried out by a wide-ranging literature search to identify studies carried out up to December 2016. Medical subject heading (MeSH) terms(Cancer, GPS, Glasgow Prognostic Score, mGPS, modified Glasgow Prognostic Score, NLR, Neutrophil Lymphocyte Ratio, LMR, Leucocyte Monocyte Ratio, PLR, Platelet Lymphocyte Ratio), were used in the US National Library of Medicine (MEDLINE), the Excerpta Medica database (EMBASE) and the Cochrane Database of Systematic Reviews (CDSR) to identify articles.
On completion of the online search, the title and abstract of each identified study was examined for relevance. Studies not in cancer patients, studies not available in English and those published in abstract form only were excluded. Where there were multiple publications from the same cohort the most recent paper was included. Full texts were obtained for all studies deemed potentially relevant. Once further exclusions outlined below were carried out, the bibliographies of all included articles were subsequently hand searched to identify any additional studies.
Only articles that reported survival analysis and gave hazard ratios (HR) with associated confidence intervals were included in the final meta-analysis. Articles reporting survival analysis in relative risk (RR) and odds ratio (OR) were also included but not in the meta-analysis. Studies that did not follow the majority of other studies in terms of score or ratio direction interpretation were excluded from the final meta-analysis. Studies with patients who had chemotherapy and/or radiotherapy before or after surgery were also included.
Statistics
The HRs and 95% CIs were directly retrieved from the article. If several estimates were reported for the same marker, the multivariate estimate was used in preference to the univariate analysis. Data was assessed for heterogeneity using the I2 statistic and χ2 test interpreted using the guidance from the Cochrane Handbook for Systematic Reviews of Interventions13. The degrees of heterogeneity were defined as minimal between 0% and 30%, moderate between 30% and 50%, substantial between 50% and 80% and considerable between 80% and 100%. Given the likely differences in methodology of the studies included, meta-analysis was performed using the random- effects (DerSimonian – Laird method) model. The Z test was used to assess the overall impact of systemic inflammation based scores on overall and cancer specific survival. All P values were 2-sided and P < 0.05 were considered statistical significant. Evidence of publication bias was evaluated using visual inspection of funnel plots. All analyses were performed using Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014.
Results
Study selection process
The study selection process is summarised in Fig. 1. Initial search strategy identified 4780 articles whose titles and abstracts were reviewed. Articles were excluded if the treatment regime was chemotherapy/radiotherapy only (n = 659), where survival was not the primary outcome measure (n = 2811), full articles were not available (n = 372), and those that were a systematic review/meta-analysis (n = 374).
Figure 1.
PRISMA flowchart demonstrating study selection.
This led to a review of the full text of 564 articles. A further 351 articles were excluded if progression free survival (PFS) was the only outcome measured (n = 112), if the treatment regime was chemotherapy/radiotherapy only (n = 58) and if survival was not expressed as HR/OR/RR (95% CI; n = 181). The remaining 213 articles, had their bibliographies reviewed in a systematic manner and this identified a further 31 articles to be included in the final analysis leading to final figure of 244 articles considered in the present systematic review and meta-analysis.
Studies of the prognostic value of Glasgow Prognostic Score (GPS) or modified Glasgow Prognostic Score (mGPS) in patients with primary operable cancer
Eighty articles with both overall survival (OS) and/or cancer specific survival (CSS) as their primary outcome measures were identified (Supplementary Table). This comprised data on 25,207 patients (9,361 deaths) reporting the significant prognostic value of GPS/mGPS in cohorts of patients with primary operable cancer (Supplementary Table). Seventy two studies were carried out in a retrospective manner while eight were prospective (Supplementary Table). Seventy two studies used multivariate and eight used univariate survival analysis (Supplementary Table).
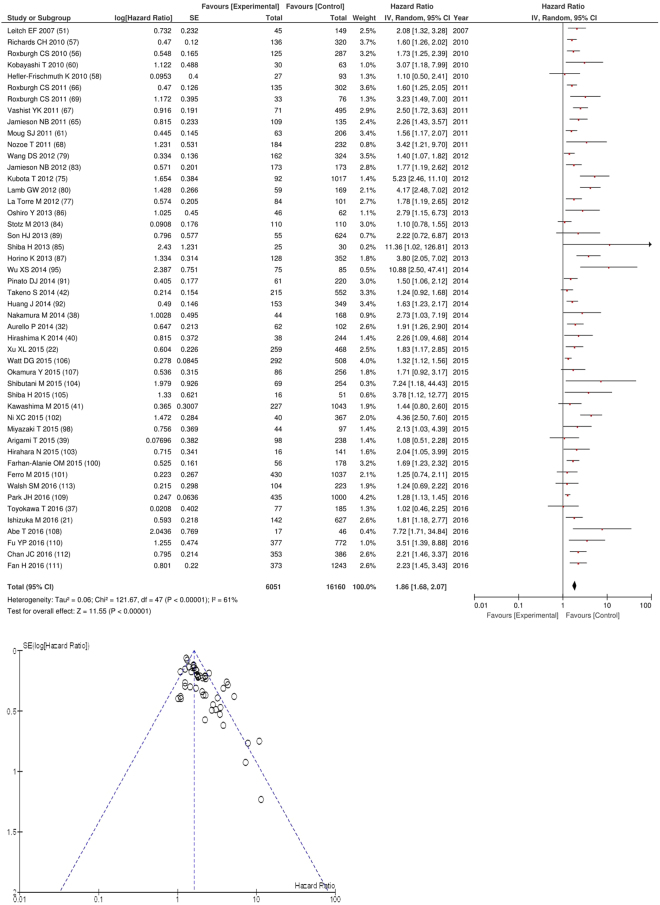
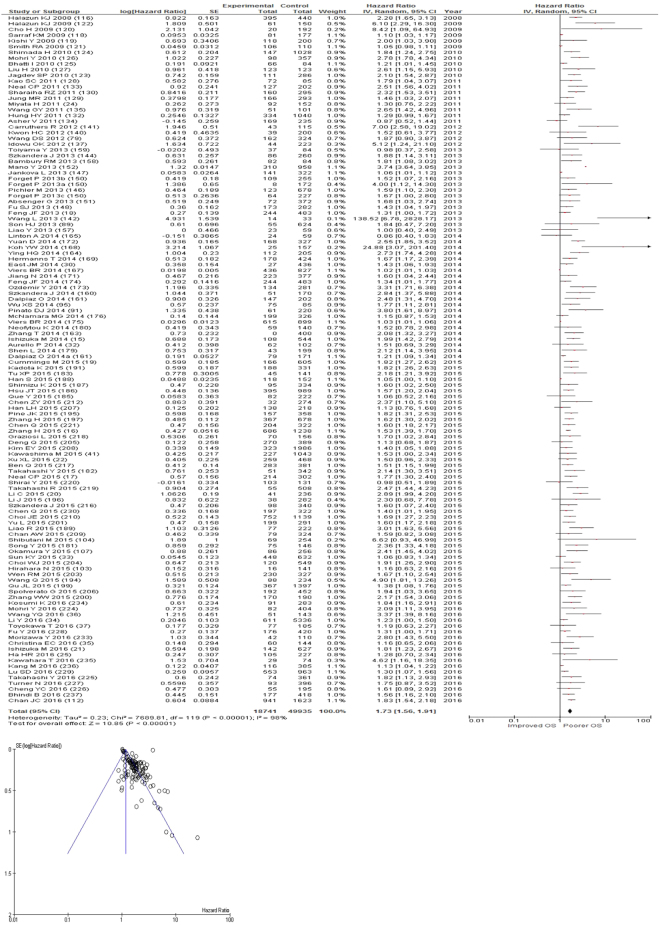
After exclusion forty eight studies examined the relationship with overall survival including 16,160 patients (6,051 deaths), as the primary outcome measure. On meta-analysis there was a significant association between GPS/mGPS and overall survival (HR 1.86 95% CI 1.68–2.07, p < 0.00001) with a substantial degree of heterogeneity (I2 = 61%, Fig. 2). These included studies on colorectal (n = 12), oesophageal (n = 7), liver (n = 6), gastric (n = 6), pancreatic (n = 5), lung (n = 4), gallbladder (n = 2), colorectal liver metastases (n = 1), renal (n = 1), bladder (n = 1), cholangiocarcinoma (n = 1), oral (n = 1) and vulval cancers (n = 1).
Figure 2.
Forrest and Funnel Plot of Studies investigating the prognostic value of GPS/mGPS in terms of OS in an unselected cohort of patients with operable cancer.
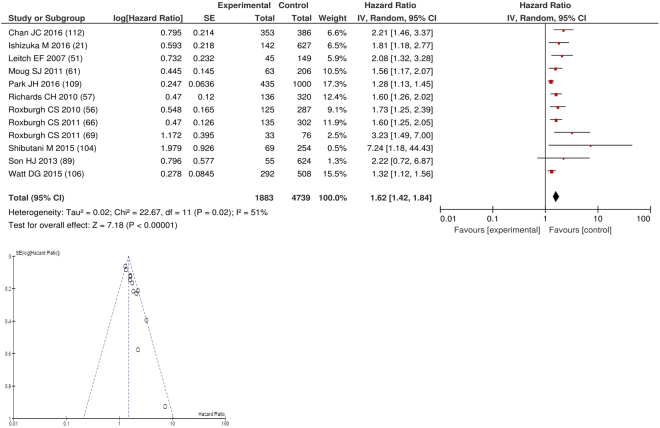
On meta-analysis of those studies carried out in colorectal cancer (n = 12), including 4,739 patients (1,883 deaths), there was a significant association between elevated GPS/mGPS and overall survival (HR: 1.62 95% CI 1.42–1.84, p < 0.00001) with a substantial degree of heterogeneity (I2 = 51%, Fig. 3). These included studies carried out in the UK (n = 8), Japan (n = 2), Korea (n = 1) and Australia (n = 1). The proportion of patients who had an elevated GPS/mGPS was 60% in Australia, 39% in Japan, 37% in the UK and 21% in Korea.
Figure 3.
Forrest and Funnel Plot of Studies investigating the prognostic value of GPS/mGPS in terms of OS in patients with operable colorectal cancer.
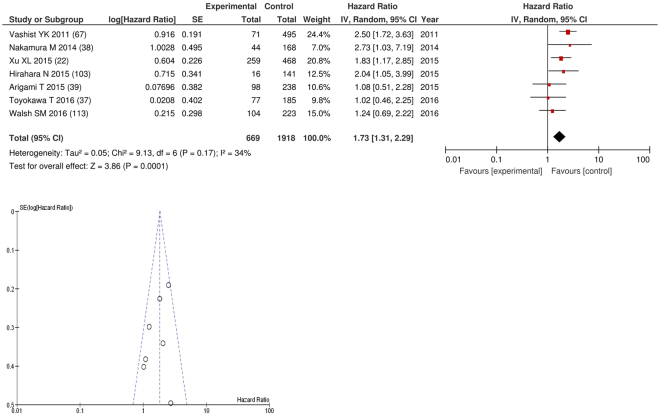
On meta-analysis of studies involving oesophageal cancer (n = 7), including 1,918 patients (669 deaths), there was a significant association between GPS/mGPS and overall survival (HR: 1.73 95% CI 1.31–2.29, p < 0.0001) with a minimal degree of heterogeneity (I2 = 34%, Fig. 4). These included studies carried out in Japan (n = 4), Germany (n = 1), China (n = 1) and Ireland (n = 1). The proportion of patients who had an elevated GPS/mGPS was 19% in Japan, 46% in Germany, 28% in China and 22% in Ireland.
Figure 4.
Forrest and Funnel Plot of Studies investigating the prognostic value of GPS/mGPS in terms of OS in patients with operable oesophageal cancer.
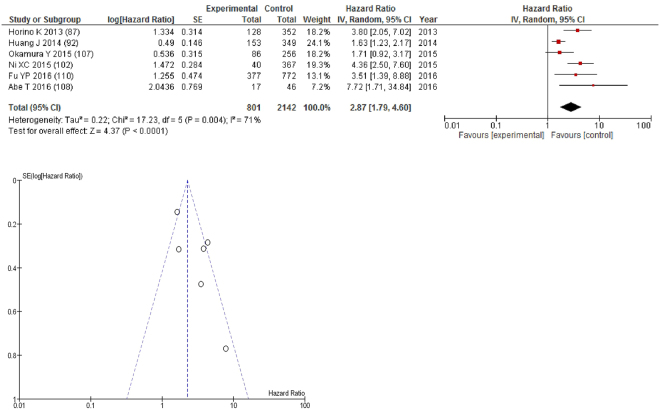
On meta-analysis of studies involving liver cancer (n = 6), including 2,142 patients (801 deaths), there was a significant association between GPS/mGPS and overall survival (HR: 2.87 95% CI 1.79–4.60, p < 0.0001) with a substantial degree of heterogeneity (I2 = 71%, Fig. 5). These included studies carried out in Japan (n = 3) and China (n = 3). The proportion of patients who had an elevated GPS/mGPS was 20% in Japan and 12% in China.
Figure 5.
Forrest and Funnel Plot of Studies investigating the prognostic value of GPS/mGPS in terms of OS in patients with operable liver cancer.
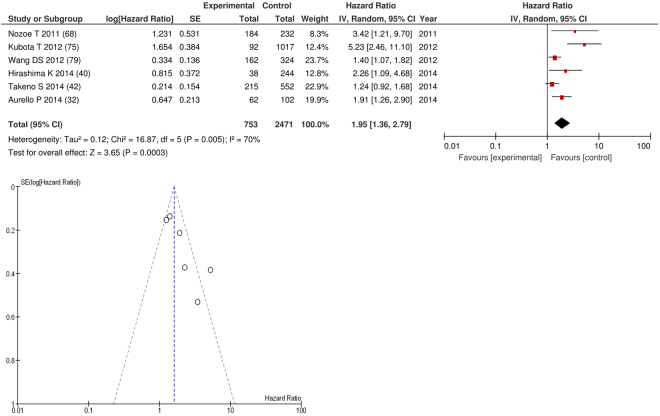
On meta-analysis of studies involving gastric cancer (n = 6), including 2,471 patients (753 deaths), there was a significant association between GPS/mGPS and overall survival (HR: 1.95 95% CI 1.36–2.79, p = 0.0003) with a substantial degree of heterogeneity (I2 = 70%, Fig. 6). These included studies carried out in Japan (n = 4), China (n = 1) and Italy (n = 1). The proportion of patients who had an elevated GPS/mGPS was, 30% in Japan, 23% in China and 52% in Italy.
Figure 6.
Forrest and Funnel Plot of Studies investigating the prognostic value of GPS/mGPS in terms of OS in patients with operable gastric cancer.
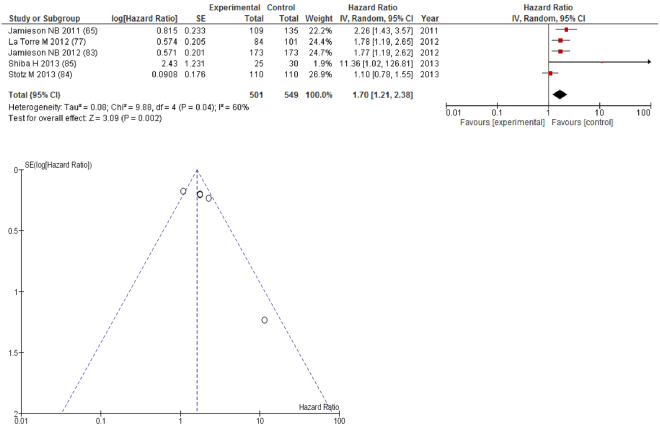
On meta-analysis those studies carried out in pancreatic cancer (n = 5), including 549 patients (501 deaths), there was a significant association between GPS/mGPS and overall survival (HR: 1.70 95% CI 1.21–2.38, p = 0.002) with a substantial degree of heterogeneity (I2 = 60%, Fig. 7). These included studies carried out in the UK (n = 2), Japan (n = 1), Italy (n = 1) and Austria (n = 1). The proportion of patients who had an elevated GPS/mGPS was 45% in the UK, 23% in Japan, 68% in Italy and 34% in Austria.
Figure 7.
Forrest and Funnel Plot of Studies investigating the prognostic value of GPS/mGPS in terms of OS in patients with operable pancreatic cancer.
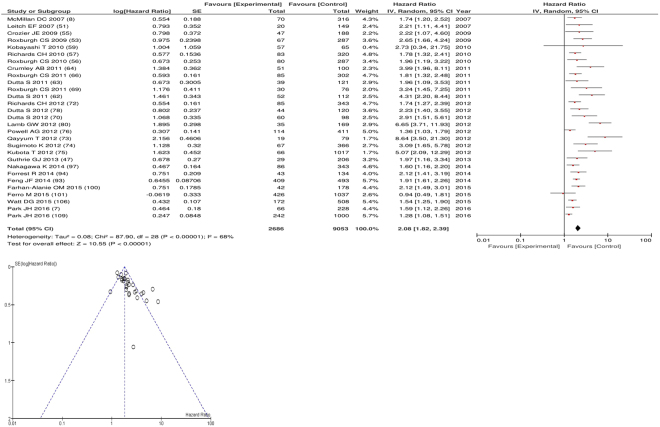
After exclusion twenty nine studies examined cancer specific survival (CSS) including 9,053 patients (2,686 deaths), as its primary outcome measure. On meta-analysis there was a significant association between GPS/mGPS and cancer specific survival (HR 2.08 95% CI 1.82–2.39, p < 0.00001) with a substantial degree of heterogeneity (I2 = 68%, Fig. 8). These included studies on colorectal (n = 16), oesophageal (n = 4), oesophago-gastric (n = 2), gastric (n = 2), renal cell (n = 2), colorectal liver metastases (n = 1), oral (n = 1) and bladder cancers (n = 1).
Figure 8.
Forrest and Funnel Plot of Studies investigating the prognostic value of GPS/mGPS in terms of CSS in an unselected cohort of patients with operable cancer.
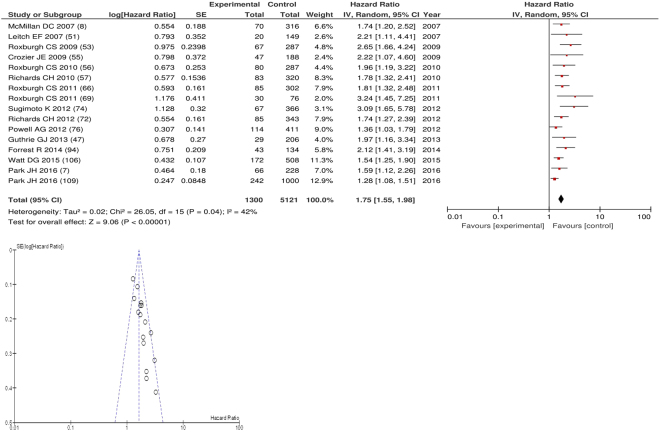
On meta-analysis of studies involving colorectal cancer (n = 16), including 5121 patients (1300 deaths), there was a significant association between GPS/mGPS and cancer specific survival (HR: 1.75 95% CI 1.55–1.98, p < 0.00001) with a moderate degree of heterogeneity (I2 = 42%, Fig. 9). These included studies carried out in the UK (n = 15) and Japan (n = 1). The proportion of patients who had an elevated GPS/mGPS was 39% in the UK and 8% in Japan.
Figure 9.
Forrest and Funnel Plot of Studies investigating the prognostic value of GPS/mGPS in terms of CSS in patients with operable colorectal cancer.
Studies of the prognostic value of Neutrophil Lymphocyte Ratio (NLR) in patients with primary operable cancer
One hundred and fifty eight articles with both overall survival (OS) and/or cancer specific survival (CSS) as their primary outcome measures were identified (Supplementary Table). This comprised data on 63,837 patients (22,681 deaths) reporting the significant prognostic value of NLR in cohorts of patients with primary operable cancer. All one hundred and fifty eight studies were carried out in a retrospective manner (Supplementary Table). One hundred and twenty eight studies used multivariate and thirty used univariate survival analysis (Supplementary Table).
After exclusion one hundred and nineteen studies examined the relationship with overall survival including 49,664 patients (18,542 deaths), as the primary outcome measure. On meta-analysis there was a significant association between NLR and overall survival (HR 1.73 95% CI 1.56–1.91, p < 0.00001) with a considerable degree of heterogeneity (I2 = 98%, Fig. 10). The most common NLR threshold examined was ≥5 (n = 29). Other thresholds were ≥3 (n = 9), ≥2.5 (n = 7), NLR as continuous variable (n = 7), ≥4 (n = 7) and ≥2 (n = 5). Other thresholds were used in <5 studies and thus, meta-analysis was not carried out (n = 55).
Figure 10.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR in terms of OS in an unselected cohort of patients with operable cancer.
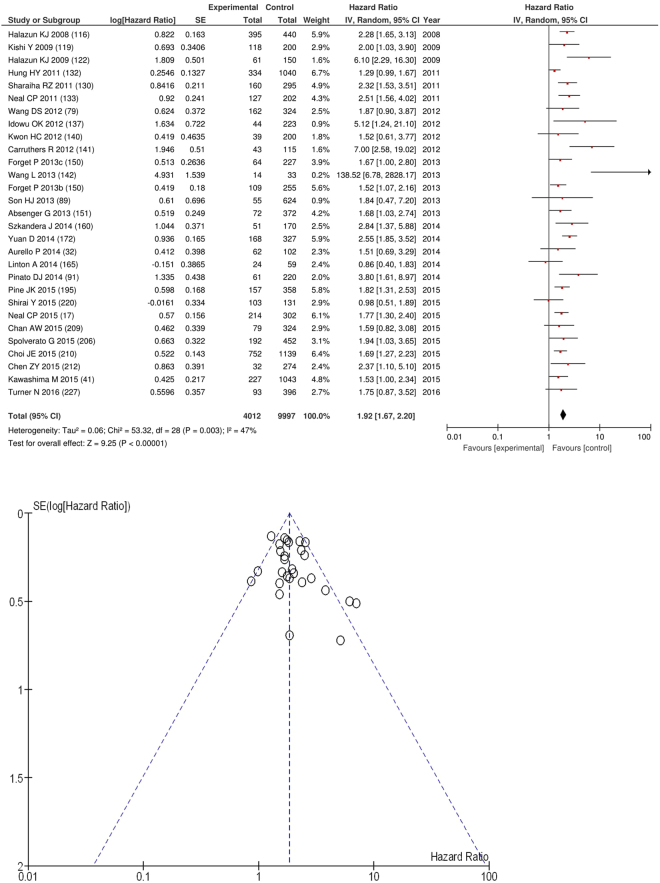
On meta-analysis of those studies with a threshold of ≥5 (n = 29), including 9,997 patients (4,012 deaths) there was a significant association between elevated NLR and overall survival (HR: 1.92 95% CI 1.67–2.20, p < 0.00001) with a moderate degree of heterogeneity (I2 = 47%, Fig. 11). These included colorectal (n = 8), lung (n = 4), colorectal liver metastases (n = 4), oesophageal (n = 3), gastric (n = 2), soft tissue sarcoma (n = 2), liver (n = 2), pancreatic (n = 1), renal (n = 1), pleural mesothelioma (n = 1) and hepato-pancreatico-biliary cancers (n = 1).
Figure 11.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR ≥5 in terms of OS in an unselected cohort of patients with operable cancer.
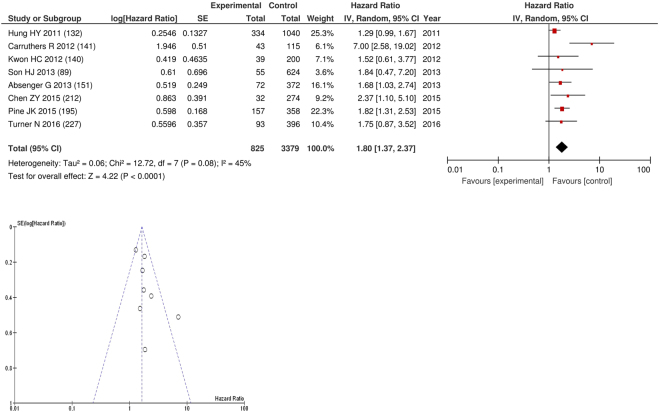
On meta-analysis of those studies with a threshold of ≥5 and colorectal cancer (n = 8), including 3,379 patients (825 deaths)there was a significant association between an NLR ≥5 and overall survival (HR: 1.80 95% CI 1.37–2.37, p < 0.0001) with moderate heterogeneity (I2 = 45%, Fig. 12). In these eight studies, there was a variation in their geographical locations including the UK (n = 2), Korea (n = 2), Taiwan (n = 1), Austria (n = 1), US (n = 1) and Australia (n = 1). The proportion of patients who had an NLR ≥5 with colorectal cancer was 25% in the UK, 5% in Korea, 25% in Taiwan, 11% in US and 30% in Australia. 29% in Korea and 20% in Japan. No country had more than 4 studies and therefore no further meta-analysis was carried out.
Figure 12.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR ≥5 in terms of OS in patients with operable colorectal cancer.
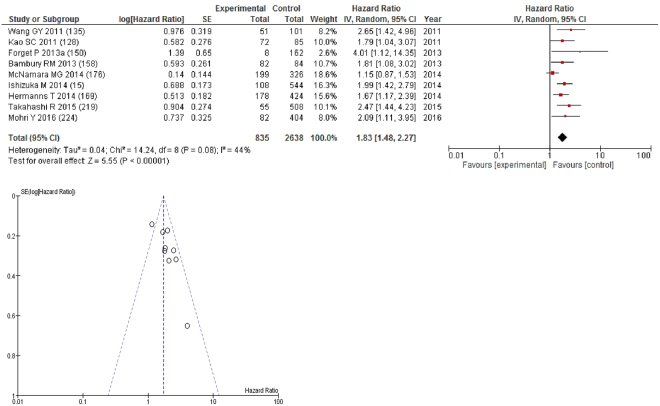
On meta-analysis of those studies with a threshold of ≥3 (n = 9), including 2,638 patients (835 deaths) there was a significant association between elevated NLR and overall survival (HR: 1.83 95% CI 1.48–2.27, p < 0.00001) with a moderate degree of heterogeneity (I2 = 44%, Fig. 13). These included gastric (n = 2), liver (n = 1), biliary tract (n = 1), bladder (n = 1), breast (n = 1), colorectal (n = 1), pleural mesothelioma (n = 1) and endometrial cancers (n = 1). In these nine studies, there was a variation in their geographical locations including Japan (n = 4), Canada (n = 2), China (n = 1), Belgium (n = 1) and Australia (n = 1). The proportion of patients who had an NLR ≥3 was 28% in Japan, 47% in Canada, 33% in China, 31% in Belgium and 52% in Australia. No tumour site had more than four studies and therefore no further meta-analysis was carried out.
Figure 13.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR ≥3 in terms of OS in an unselected cohort of patients with operable cancer.
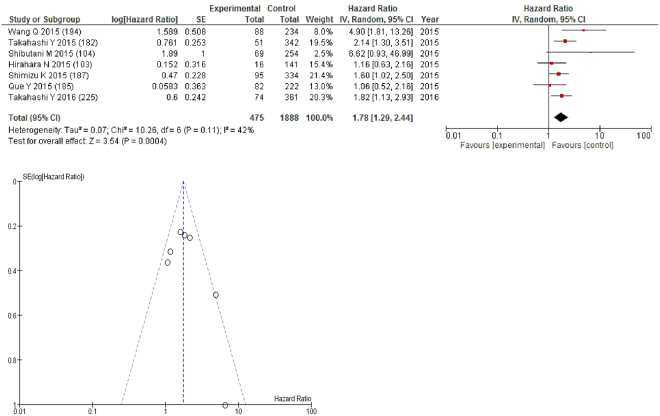
On meta-analysis of those studies with a threshold of ≥2.5 (n = 7), including 1,888 patients (475 deaths) there was a significant association between elevated NLR and overall survival (HR: 1.78 95% CI 1.29–2.44, p = 0.0004) with a moderate degree of heterogeneity (I2 = 42%, Fig. 14). These included lung (n = 3), oesophageal (n = 1), colorectal (n = 1), soft tissue sarcoma (n = 1) and liver cancers (n = 1). In these seven studies, there was a variation in their geographical locations including Japan (n = 5), China (n = 1) and US (n = 1). The proportion of patients who had an NLR ≥2.5 was 30% in Japan, 28% in China and 50% in US. No tumour site had more than four studies and therefore no further meta-analysis was carried out.
Figure 14.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR ≥2.5 in terms of OS in an unselected cohort of patients with operable cancer.
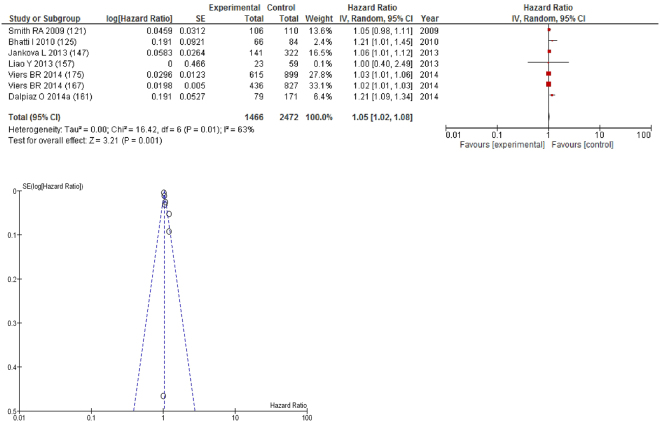
On meta-analysis those studies with NLR as continuous variable (n = 7), including 2,472 patients (1,466 deaths) there was a moderate association between elevated NLR and overall survival (HR: 1.05 95% CI 1.02–1.08, p = 0.001) with a substantial degree of heterogeneity (I2 = 63%, Fig. 15). These included pancreatic (n = 2), renal (n = 2), colorectal (n = 1), lung (n = 1) and bladder cancers (n = 1). In these seven studies, there was a variation in their geographical locations including the UK (n = 2), US (n = 2), China (n = 1), Austria (n = 1) and Australia (n = 1). No tumour site had more than four studies and therefore no further meta-analysis was carried out.
Figure 15.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR as a continuous variable in terms of OS in an unselected cohort of patients with operable cancer.
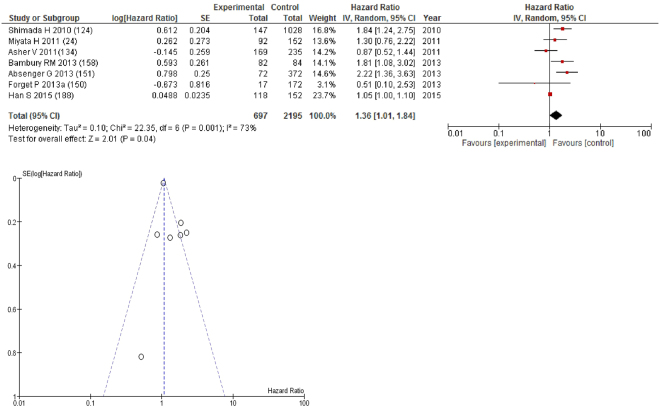
On meta-analysis those studies with a threshold of ≥4 (n = 7), including 2,195 patients (697 deaths) there was a significant association between elevated NLR and overall survival (HR: 1.36 95% CI 1.01–1.84, p = 0.04) with a substantial degree of heterogeneity (I2 = 73%, Fig. 16). These included glioblastoma (n = 2), gastric (n = 1), oesophageal (n = 1), ovarian (n = 1), breast (n = 1) and colon cancers (n = 1). In these seven studies, there was a variation in their geographical locations including Japan (n = 2), China (n = 1), the UK (n = 1), Belgium (n = 1), Austria (n = 1) and Ireland (n = 1). The proportion of patients who had an NLR ≥4 was 15% in Japan, 32% in China, 22% in Belgium and 36% in Ireland. No tumour site had more than four studies and therefore no further meta-analysis was carried out.
Figure 16.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR ≥4 in terms of OS in an unselected cohort of patients with operable cancer.
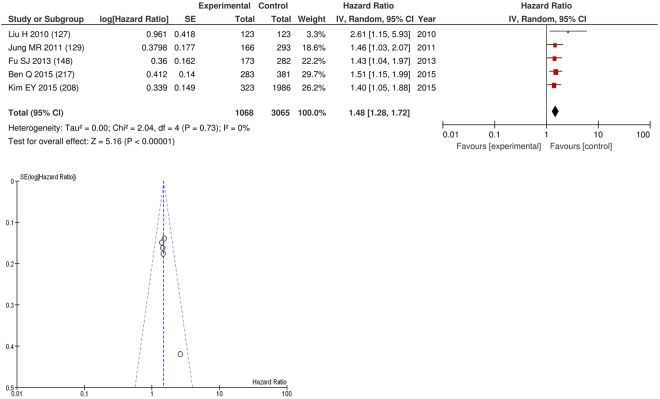
On meta-analysis those studies with a threshold of ≥2 (n = 5), including 3,065 patients (1,068 deaths) there was a significant association between elevated NLR and overall survival (HR: 1.48 95% CI 1.28–1.72, p < 0.00001) with minimal heterogeneity (I2 = 0%, Fig. 17). These cancers included gastric (n = 2), colorectal (n = 1), liver (n = 1) and pancreatic (n = 1). In these five studies, there was a variation in their geographical locations including China (n = 3) and Korea (n = 2). The proportion of patients who had an NLR ≥2 was 60% in China and 39% in Korea. No tumour site had more than four studies and therefore no further meta-analysis was carried out.
Figure 17.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR ≥2 in terms of OS in an unselected cohort of patients with operable cancer.
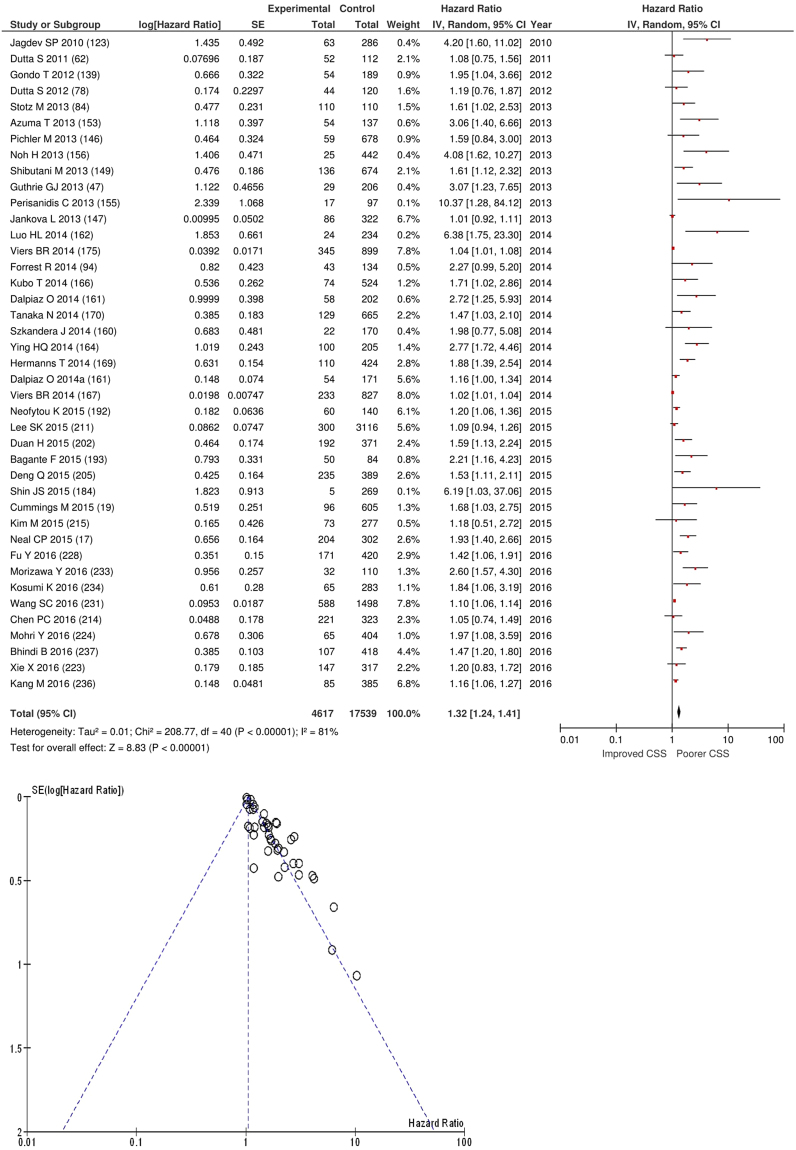
After exclusion forty one studies examined the relationship with cancer specific survival including 17,539 patients (4,617 deaths), as its primary outcome measure. On meta-analysis there was a significant association between NLR and cancer specific survival (HR 1.32 95% CI 1.24–1.41, p < 0.00001) with a considerable degree of heterogeneity (I2 = 81%, Fig. 18). The most common NLR thresholds used was ≥5 (n = 7), ≥3 (n = 6) and NLR as continuous variable (n = 5). Other thresholds did not have more than four studies and therefore meta-analysis was not carried out (n = 19).
Figure 18.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR in terms of CSS in an unselected cohort of patients with operable cancer.
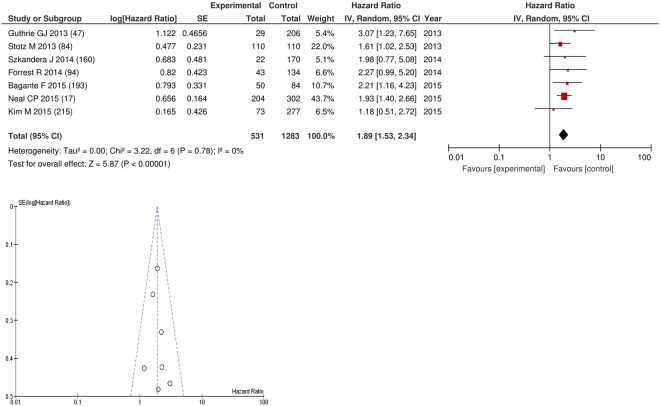
On meta-analysis those studies with a threshold of ≥5 (n = 7), including 1,283 patients (531 deaths) there was a significant association between elevated NLR and cancer specific survival (HR: 1.89 95% CI 1.53–2.34, p < 0.00001) with minimal heterogeneity (I2 = 0%, Fig. 19). These included colorectal (n = 2), liver only colorectal metastases (n = 1) and soft tissue sarcoma (n = 1), adrenal (n = 1), pancreatic (n = 1) and renal cancers (n = 1). In these seven studies, there was a variation in their geographical locations including the UK (n = 3), Austria (n = 2), US (n = 1) and South Korea (n = 1). The proportion of patients who had an NLR ≥5 was 19% in the UK, 35% in US and 7% in South Korea. No tumour site had more than four studies and therefore no further meta-analysis was carried out.
Figure 19.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR ≥5 in terms of CSS in an unselected cohort of patients with operable cancer.
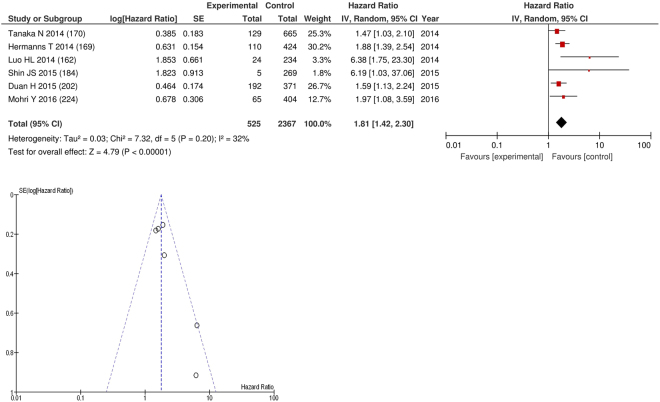
On meta-analysis those studies with a threshold of ≥3 (n = 6), including 2,367 patients (525 deaths) there was a significant association between elevated NLR and cancer specific survival (HR: 1.81 95% CI 1.42–2.30, p < 0.00001) with a moderate degree of heterogeneity (I2 = 32%, Fig. 20). These included renal (n = 2), bladder (n = 1), colorectal (n = 1), oesophageal (n = 1) and gastric cancers (n = 1). In these six studies, there was a variation in their geographical locations including Japan (n = 2), Korea (n = 1), China (n = 1), Taiwan (n = 1) and Canada (n = 1). The proportion of patients who had an NLR ≥3 was 25% in Japan, 20% in Korea, 20% in China, 40% in Taiwan and 51% in Canada. No tumour site had more than four studies and therefore no further meta-analysis was carried out.
Figure 20.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR ≥3 in terms of CSS in an unselected cohort of patients with operable cancer.
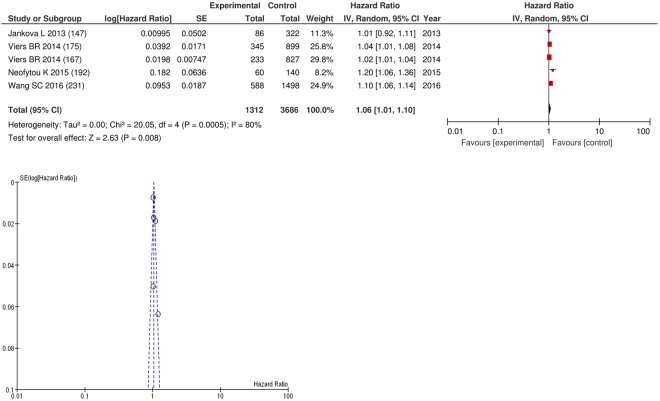
On meta-analysis those studies with NLR as continuous variable (n = 5), including 3,686 patients (1,312 deaths) there was a significant association between elevated NLR and cancer specific survival (HR: 1.06 95% CI 1.01–1.10, p = 0.008) with a substantial degree of heterogeneity (I2 = 80%, Fig. 21). These included renal (n = 1), bladder (n = 1), colorectal (n = 1), liver only colorectal metastases (n = 1) and gastric cancers (n = 1). In these six studies, there was a variation in their geographical locations including the US (n = 3), the UK (n = 1) and Australia (n = 1). No tumour site had more than four studies and therefore no further meta-analysis was carried out.
Figure 21.
Forrest and Funnel Plot of Studies investigating the prognostic value of NLR as a continuous variable in terms of CSS in an unselected cohort of patients with operable cancer.
Studies of the prognostic value of platelet lymphocyte ratio (PLR) in patients with primary operable cancer
Sixty eight articles with both OS and/or CSS as their primary outcome measures were identified (Supplementary Table). This comprised data on 29,273 patients (10,729 deaths) reporting the significant prognostic value of PLR in cohorts of patients with primary operable cancer (Supplementary Table). All sixty eight studies were conducted in a retrospective manner. Forty three studies were conducted in a multivariate and twenty five in a univariate manner (Supplementary Table).
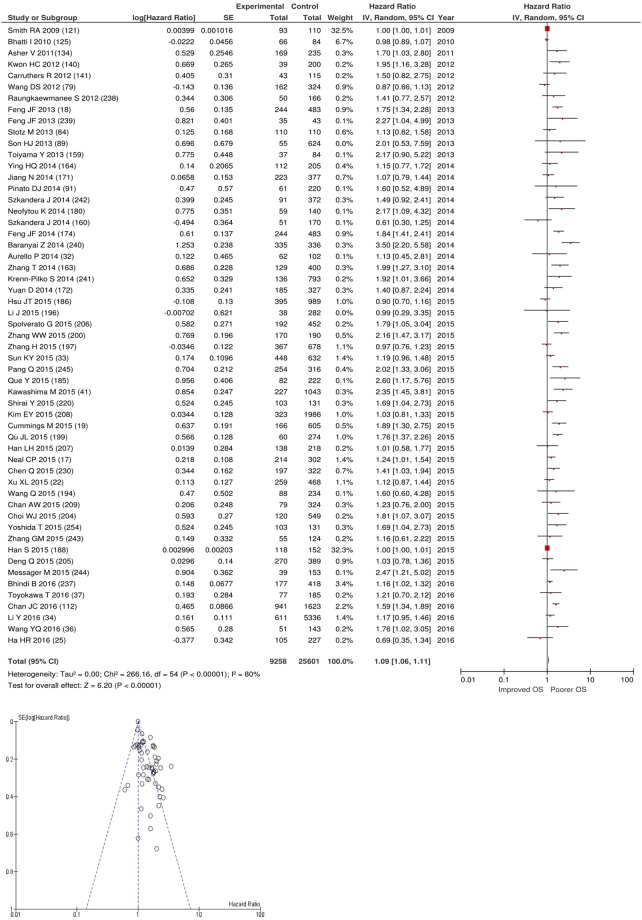
After exclusions fifty five studies examined the relationship with overall survival including 25,601 patients (9,258 deaths), as the primary outcome measure. On meta-analysis there was a significant association between an elevated PLR and overall survival (HR 1.09 95% CI 1.06–1.11, p < 0.00001) with a substantial degree of heterogeneity (I2 = 80%, Fig. 22). The most common PLR thresholds examined were ≥300 (n = 10) and ≥150 (n = 7). Other thresholds did not have more than four studies and therefore meta-analysis was not carried out (n = 58).
Figure 22.
Forrest and Funnel Plot of Studies investigating the prognostic value of PLR in terms of OS in an unselected cohort of patients with operable cancer.
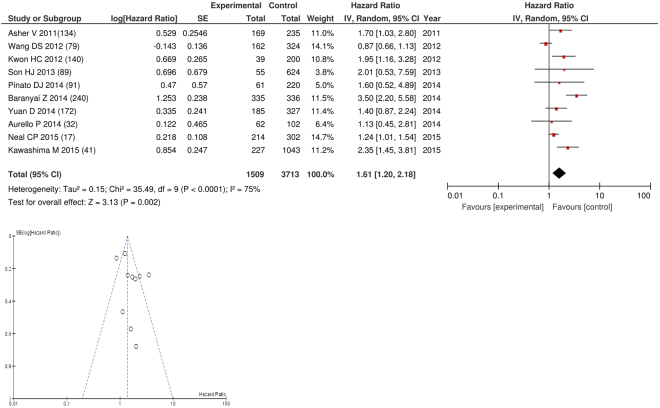
On meta-analysis those studies with a threshold of ≥300 (n = 10), including 3,713 patients (HR: 1.61 95% CI 1.20–2.18, p = 0.002) with a substantial degree of heterogeneity (I2 = 75%, Fig. 23). These included colorectal (n = 3), lung (n = 2), gastric (n = 2), colorectal liver metastases (n = 1), oesophageal (n = 1) and ovarian cancers (n = 1). In these ten studies, there was a variation in their geographical locations including the UK (n = 3), Korea (n = 2), China (n = 2), Hungary (n = 1), Italy (n = 1) and Japan (n = 1). The proportion of patients who had a PLR ≥300 was 20% in the UK, 4% in Korea, 10% in China, 13% in Italy and 5% in Japan. No tumour site had more than four studies and therefore no further meta-analysis was carried out.
Figure 23.
Forrest and Funnel Plot of Studies investigating the prognostic value of PLR ≥300 in terms of OS in an unselected cohort of patients with operable cancer.
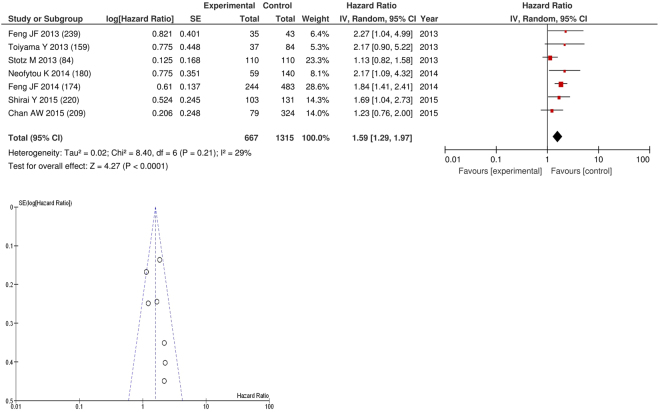
On meta-analysis those studies with a threshold of ≥150 (n = 7), including 1,315 patients (667 deaths) there was a significant association between elevated PLR and overall survival (HR: 1.59 95% CI 1.29–1.97, p < 0.0001) with a minimal degree of heterogeneity (I2 = 29%, Fig. 24). These included oesophageal (n = 2), pancreatic (n = 2), liver (n = 1), colorectal liver metastases (n = 1) and colorectal cancers (n = 1). In these seven studies, there was a variation in their geographical locations including China (n = 2), Japan (n = 2), the UK (n = 1), Hong Kong (n = 1) and Australia (n = 1). The proportion of patients who had a PLR ≥150 was 43% in China, 49% in Japan, 41% in the UK, 27% in Hong Kong and 75% in Australia. No tumour site had more than four studies and therefore no further meta-analysis was carried out.
Figure 24.
Forrest and Funnel Plot of Studies investigating the prognostic value of PLR ≥150 in terms of OS in an unselected cohort of patients with operable cancer.
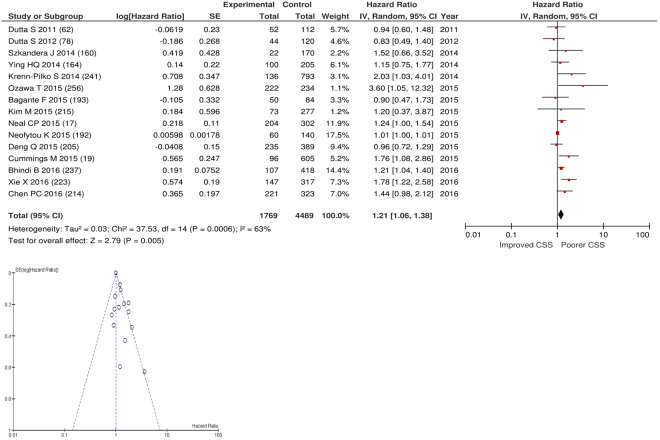
After exclusions fifteen studies examined the relationship with cancer specific survival including 4,489 patients (1,769 deaths), as the primary outcome measure. On meta-analysis there was a significant association between an elevated PLR and cancer specific survival (HR 1.21 95% CI 1.06–1.38, p = 0.005) with a substantial degree of heterogeneity (I2 = 63%, Fig. 25). The most common PLR threshold examined was ≥300 (n = 4). Other thresholds used were >150 (n = 1), ≥25.4 (n = 1), >103 (n = 1), ≥132 (n = 1), ≥176 (n = 1), >190 (n = 1), ≥200 (n = 1), ≥240 (n = 1), ≥292 (n = 1), PLR as continuous variable (n = 1) and PLR per 100 units (n = 1). These included studies on oesophageal (n = 3), colorectal (n = 3), gastric (n = 2), colorectal liver metastases (n = 1), adrenal (n = 1), renal (n = 1), endometrial (n = 1), bladder (n = 1), soft tissue sarcoma (n = 1) and breast cancers (n = 1). Geographically studies were located in the UK (n = 5), China (n = 4), Austria (n = 2), Japan (n = 1), US (n = 1), South Korea (n = 1) and Canada (n = 1). The proportion of patients who had an elevated PLR was 12% in the UK, 55% in China, 23% in Japan, 38% in US and 3% in South Korea. No specific PLR thresholds had more than four studies and therefore no further meta-analysis was carried out.
Figure 25.
Forrest and Funnel Plot of Studies investigating the prognostic value of PLR in terms of CSS in an unselected cohort of patients with operable cancer.
Studies of the prognostic value of lymphocyte monocyte ratio (LMR) in patients with primary operable cancer
Twenty one articles with both OS and/or CSS as their primary outcome measures were identified (Supplementary Table). This comprised data on 15,386 patients (4,298 deaths) reporting the significant prognostic value of LMR in cohorts of patients with primary operable cancer (Supplementary Table). All 21 studies were retrospective. Nineteen studies used multivariate and two used univariate survival analysis (Supplementary Table).
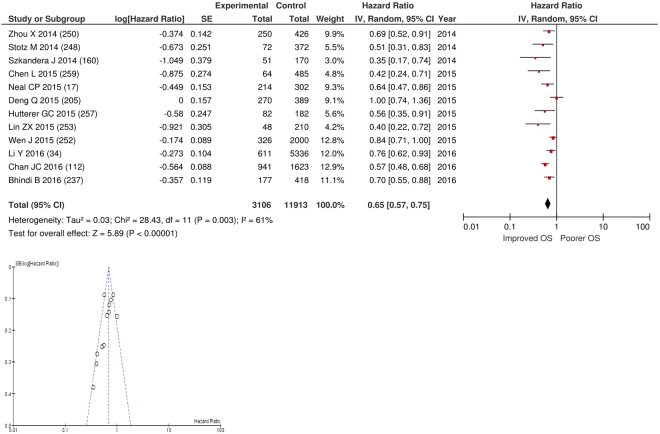
After exclusion twelve studies examined the relationship with overall survival including 11,913 patients (3,106 deaths), as the primary outcome measure. On meta-analysis there was a significant association between a elevated LMR and overall survival (HR 0.69 95% CI 0.63–0.74, p < 0.00001) with a substantial degree of heterogeneity (I2 = 61%, Fig. 26). There was a variety of LMR cut-offs used in each study including ≥2, (n = 1), ≥2.14 (n = 1), >2.35 (n = 1), >2.38 (n = 1), >2.83 (n = 1), ≥2.85 (n = 1), >2.87 (n = 1), >3.23 (n = 1), ≥3.80 (n = 1), ≥4 (n = 1), ≥4.32 (n = 1) and ≥4.95 (n = 1). These included studies on colorectal (n = 3), bladder (n = 2), liver only colorectal metastases (n = 1), gastric (n = 1), renal (n = 1), liver (n = 1), breast (n = 1), soft tissue sarcoma (n = 1) and cervical cancers (n = 1). Geographically the studies were carried out in China (n = 6), Austria (n = 3), the UK (n = 1), Canada (n = 1) and Australia (n = 1). The proportion of patients who had high LMRs was 71% in China, 68% in Japan, 64% in the UK, 49% in Australia and 48% in Austria. No specific LMR thresholds had more than four studies and therefore no further meta-analysis was carried out.
Figure 26.
Forrest and Funnel Plot of Studies investigating the prognostic value of LMR in terms of OS in an unselected cohort of patients with operable cancer.
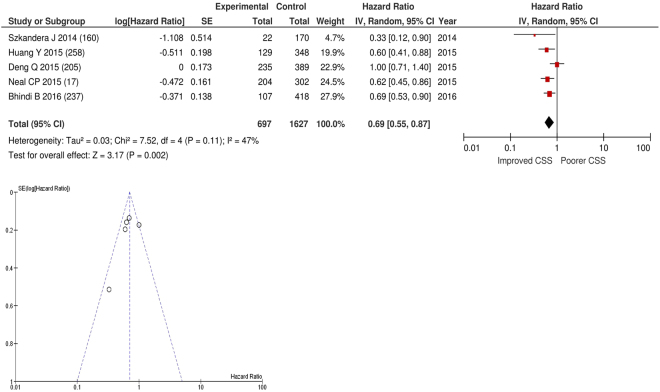
After exclusion five studies examined the relationship with cancer specific survival including 1,627 patients (697 deaths), as the primary outcome measure. On meta-analysis there was a significant association between a elevated LMR and cancer specific survival (HR 0.70 95% CI 0.60–0.82, p < 0.00001) with a moderate degree of heterogeneity (I2 = 47%, Fig. 27). There was a variety of LMR cut-offs used in each study including >2.35 (n = 1), ≥2.85 (n = 1), >2.93 (n = 1) and ≥4.95 (n = 1). One study expressed LMR in terms of log. These included studies on liver only colorectal metastases (n = 1), gastric cancer (n = 1), oesophageal cancer (n = 1), bladder cancer (n = 1) and soft tissue sarcoma (n = 1). Geographically the studies were carried out in the China (n = 2), UK (n = 1), Austria (n = 1), and Canada (n = 1). The proportion of patients who had high LMRs was 68% in Japan, 64% in the UK, 50% in Austria and 40% in China. No specific LMR thresholds had more than four studies and therefore no further meta-analysis was carried out.
Figure 27.
Forrest and Funnel Plot of Studies investigating the prognostic value of LMR in terms of CSS in an unselected cohort of patients with operable cancer.
Studies of the prognostic value of other scores of the systemic inflammatory response in patients with primary operable cancer
Thirty five articles reported a variety of other scores reported in less than 10 studies each. These included the PNI (Prognostic Nutritional Index), COP-NLR (combined platelet count and NLR), NLR/PLR combination, CAR (CRP/albumin ratio), SI (systemic inflammatory score), SII (systemic inflammatory index), NLR/CRP combination, (HALP) haemoglobin, albumin, lymphocyte and platelet, NLR/ESR (erythrocyte sedimentation rate) combination, (WLR) white cell count to lymphocyte count ratio, (APRI) AST-platelet ratio index, PI/CRP/WCC combination, Canton score, (AGR) albumin/globulin ratio, CRP/Neutrophil combination, (PIS) Prognostic Inflammation Score, and the CONUT score.
Eight articles with both overall survival (OS) and/or cancer specific survival (CSS) as their primary outcome measures were identified (Supplementary Table). This comprised data on 2,666 patients (1,387 deaths) reporting the significant prognostic value of PNI in cohorts of patients with primary operable cancer. All eight studies were carried out in a retrospective manner (Supplementary Table). Six studies used multivariate and two used univariate survival analysis (Supplementary Table).
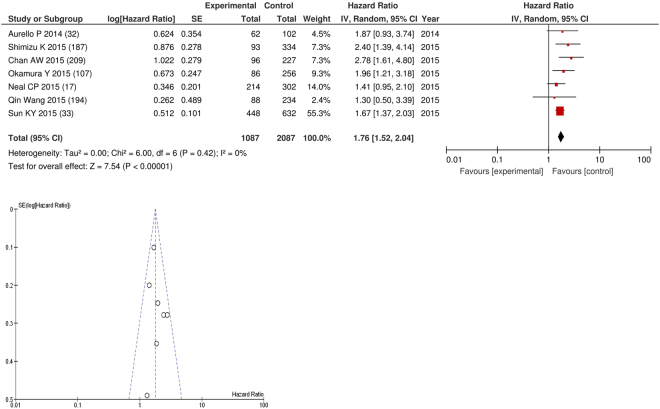
After exclusion seven studies examined the relationship with overall survival including 2,087 patients (1,087 deaths), as the primary outcome measure. On meta-analysis there was a significant association between PNI and overall survival (HR 1.76 95% CI 1.52–2.04, p < 0.00001) with minimal heterogeneity (I2 = 0%, Fig. 28). The most common PNI threshold examined was ≤45 (n = 3), ≤50 (n = 1), ≤50.5 (n = 1), 48.5 (n = 1), 48.2 (n = 1). These included hepatocellular (n = 3), gastric (n = 2), lung (n = 1) and colorectal liver metastases (n = 1). In these eight studies, there was a variation in their geographical locations including Japan (n = 2), UK (n = 1), Hong Kong (n = 1), China (n = 1), US (n = 1) and Italy (n = 1).The proportion of patients who with an elevated PNI was 74% in Hong Kong, 59% in Japan, 59% in Italy, 52% in China and 17% in the UK. No tumour site had more than four studies and therefore no further meta-analysis was carried out. Two studies examined the relationship with cancer specific survival including 579 patients (300 deaths), as the primary outcome measure. Both of these studies used a PNI threshold of ≤45. No threshold was used in ≥4 studies and thus, meta-analysis was not carried out.
Figure 28.
Forrest and Funnel Plot of Studies investigating the prognostic value of PNI in terms of OS in an unselected cohort of patients with operable cancer.
Four studies reported the COP-NLR score. The first such study was by Ishizuka and coworkers14 from Japan. In this multivariate survival analysis on patients with colorectal cancer, low COP-NLR was shown to be related to a statistically better cancer specific survival (OR: 0.464 95% CI 0.267–0.807 p = 0.007). The second such study was also by Ishizuka and coworkers15 from Japan. In this multivariate survival analysis on patients with gastric cancer, elevated COP-NLR was shown to be related to a statistically significant worse overall survival (HR: 1.781 95% CI 1.094–2.899 p = 0.020). The third such study was by Zhang and coworkers16 from China. In this multivariate survival analysis on patients with lung cancer, elevated COP-NLR was shown to be related to a statistically significant worse overall survival (HR: 1.810 95% CI 1.587–2.056 p < 0.001). The fourth such study was by Neal and coworkers17 from the UK. In this univariate survival analysis on patients with colorectal liver metastases, elevated COP-NLR was shown to be related to a statistically significant worse overall survival (HR: 1.230 95% CI 1.005–1.505 p = 0.045) and worse cancer specific survival (HR: 1.243 95% CI 1.003–1.541 p = 0.047).
Three studies reported the combination of the NLR and PLR. The first such study was by Feng and coworkers18 from China. The combination of NLR and PLR is collectively named the CNP. The CNP was calculated based on data obtained on the day of admission, where patients with both elevated NLR (>3.45) and PLR (>166.5) were allocated a score of 2, and patients showing one or neither were allocated a score of 1 or 0, respectively. In this multivariate survival analysis on patients with oesophageal cancer, CNP 1 or 2 was shown to be related to a statistically worse overall survival (HR: 1.964 95% CI 1.371–2.814 p < 0.001). The second such study was by Cummings and coworkers19 from the UK. In this multivariate survival analysis on patients with endometrial cancer, both high NLR and PLR was shown to be related to a statistically significant worse overall survival (HR: 2.54 95% CI 1.61–4.01 p < 0.001) and worse cancer specific survival (HR: 2.26 95% CI 1.24–4.13 p = 0.008). The third such study was by Chuan Li and coworkers20 from China. In this multivariate survival analysis on patients with liver cancer, elevated postoperative NLR-PLR was shown to be related to a statistically significant worse overall survival (HR: 2.894 95% CI 1.992–4.2 p < 0.001).
Two studies reported the CAR. The first such study was by Ishizuka and coworkers21 from Japan. In this multivariate survival analysis on patients with colorectal cancer, CAR >0.038 was shown to be related to a statistically worse overall survival (HR: 2.613 95% CI 1.621–4.212 p < 0.001). The second such study was by Xu and coworkers22,23 from China. In this multivariate survival analysis on patients with oesophageal cancer, CRP/Albumin ratio >0.50 was shown to be related to a statistically significant worse overall survival (HR: 2.44 95% CI 1.82–3.26 p < 0.0001).
One study reported the SI a score involving leucocyte count, serum albumin and haemoglobin level. High leucocyte count (>9,500 µl), low serum albumin level (3.5 g/dl) and low haemoglobin level (<12.5 mg/dl) was each allocated a score of 1.The study was conducted by Miyata and coworkers24 from Japan. In this multivariate survival analysis on patients with oesophageal cancer, SI score of 2/3 was shown to be related to a statistically significant worse overall survival (HR: 3.17 95% CI 1.74–5.78 p = 0.0002).
One study reported on the SII which was determined as neutrophil × platelet/lymphocyte. The study was conducted by Ha and coworkers25,26 from South Korea. In this multivariate survival analysis on patients with ampulla of vater cancer, SII ≤780 was shown to predict better overall survival (HR: 0.924 95% CI 0.44–1.93 p = 0.833).
One study reported on the combination of the NLR and CRP. The study was conducted by Tomita and coworkers27 from Japan. In this multivariate survival analysis on patients with lung cancer, low NLR and low CRP (compared to both high) was shown to predict better overall survival (RR: 0.403 95% CI 0.240–0.689 p = 0.0012).
One study reported on preoperative HALP. The study was conducted by Chen and coworkers28 from China. In this multivariate survival analysis on patients with gastric cancer, HALP ≥56.8 was shown to predict better overall survival (HR: 0.700 95% CI 0.496–0.987 p = 0.042).
One study reported on the combination of the NLR and ESR. The study was conducted by Hyun and coworkers29 from Korea. Patients were divided into three groups: those with ESR and NLR in the normal range (group 0), those with either elevated ESR or elevated NLR (group I), and those with both elevated ESR and elevated NLR (group II). In this multivariate survival analysis on patients with renal cancer, both elevated ESR and NLR was shown to predict worse overall survival (HR: 3.521 95% CI 1.888–6.567 p < 0.001) and worse cancer specific survival (HR: 4.367 95% CI 1.987–9.597 p < 0.001).
One study reported on the WLR. The study was conducted by East and coworkers30 from the UK. In this multivariate survival analysis on patients with colon cancer, WLR ≥3.4 was shown to predict worse overall survival (HR: 4.10 95% CI 3.13–7.42 p = 0.03).
One study reported on the APRI. The study was conducted by Shen and coworkers31 from China. In this multivariate survival analysis on patients with liver cancer, APRI ≥0.62 was shown to predict worse overall survival (HR: 1.508 95% CI 1.127–2.016 p = 0.006).
One study reported on the combination of the PI, CRP and white cell count (0 if both low, 1 if either high, 2 if both high). The study was conducted by Aurello and coworkers32 from Italy. In this multivariate survival analysis on patients with gastric cancer, PI 2 was shown to predict worse overall survival (HR: 0.37 95% CI 0.16–0.82 p = 0.01).
One study reported on the Canton score involving PNI, NLR and platelet. The study was conducted by Sun and coworkers33 from China. In this multivariate survival analysis on patients with gastric cancer, elevated Canton score was shown to predict worse overall survival (HR: 1.643 95% CI 1.142–2.364 p = 0.007).
One study reported on the AGR. The study was conducted by Li and coworkers34 from China. In this multivariate survival analysis on patients with colorectal cancer, AGR ≥1.50 was shown to predict better overall survival (HR: 0.646 95% CI 0.543–0.767 p < 0.001).
One study reported on the combination of CRP and neutrophils. The study was conducted by Christina and coworkers35 from Austria. In this multivariate survival analysis on patients with oral cancer, high CRP/ neutrophil was shown to predict worse overall survival (HR: 2.7 95% CI 0.68–10.75 p = 0.16).
One study reported on the PIS involving a combination of NLR and serum albumin. PIS was defined as follows: patients with increased NLR and decreased serum albumin were assigned score 0; patients with either increased NLR or decreased serum albumin were assigned score 1; patients with decreased NLR and increased serum albumin were assigned score 2. The study was conducted by Wang and coworkers36 from China. In this multivariate survival analysis on patients with ovarian cancer, PIS 2 was shown to predict better overall survival (HR: 0.18 95% CI 0.09–0.38 p < 0.001).
Finally, the last study reported on the CONUT score involving serum albumin concentration, total lymphocyte count and total cholesterol concentration. The study was conducted by Toyokawa and coworkers 37 from Japan. In this multivariate survival analysis on patients with oesophageal cancer, high CONUT score was shown to predict worse overall survival (HR: 2.303 95% CI 1.191–4.455 p = 0.013).
Assessment of bias using funnel plot analysis of studies carried out in patients with primary operable cancer
Funnel plot analysis containing ten or more studies revealed bias towards studies reporting a relationship between an increased SIR as evidenced by the GPS/GPS (multiple tumour types Figs 2 and 8; colorectal cancer Figs 3 and 9), NLR (multiple tumour types Figs 10 and 18; NLR >5 Fig. 11), PLR (multiple tumour types Figs 22 and 25; PLR >300 Fig. 23), LMR (multiple tumour types Fig. 26) and poorer survival. The funnel plots also showed that a clear majority of studies had high patient numbers. This is particularly true for studies focusing on GPS/mGPS (Figs 2 and 8), NLR (Figs 10 and 18), PLR (Fig. 22) and LMR (Fig. 26).
Discussion
In the present review 244 reports of the prognostic value of systemic inflammation based prognostic scores were identified. This is in contrast to the initial review by Roxburgh and McMillan (2010) where 18 such studies were identified. In particular, those scores based on the ratio of components of a white cell count have been the subject of intense interest with, over the intervening 7 years, 158 studies reporting the value of the NLR, 68 reporting PLR and 21 reporting LMR. Also, the cumulative GPS/mGPS has been the subject of 80 reports. The majority of these studies have been carried out in lung and gastrointestinal cancer. For example, the GPS/mGPS had prognostic value in lung (5 studies), gastric cancer (7 studies), pancreatic (5 studies), and colon cancer (3 studies). A feature of this up to date review of systemic inflammation based prognostic scores is the identification of the proliferation of new scores derived from routinely available markers of the SIR. Most notable among these that have been validated in several studies are PINI (7 studies), COP-NLR (4 studies) and CNP (3 studies). It remains to be established whether any of the scores will have prognostic value in addition to the GPS/mGPS and NLR. Irrespective, there is increasing recognition and acceptance of the clinical utility of systemic inflammation based prognostic scores prior to surgery for cancer.
It is perhaps surprising that, given apparent the superior prognostic value of the GPS/ mGPS3 the relatively larger numbers of reports of the prognostic value of ratios based on components of the white cell count. However, the pre-operative differential white cell count is part of the standard pre-operative workup for the majority of cancer resections as it is used to help identify patients who may have an infection prior to surgery. Also, the white cell count is used to identify any pre-existing conditions that may affect the surgical procedure such as the hypercoagulability of thrombocytosis. Thus, these results are routinely available for retrospective studies. This might also explain the variety of prognostic thresholds reported for NLR, PLR and LMR. In contrast, reports on the prognostic value of the GPS/mGPS, not routinely assessed as part of the standard pre-operative workup, were more likely to be examined in prospective studies. This might explain the consistent adherence to the original thresholds reported for GPS/ mGPS. From the above there is a strong case for the GPS/mGPS to be incorporated into pre-operative workup of patients undergoing surgery for cancer.
It is of interest that while there is general uniformity of thresholds used in the GPS/mGPS studies, with most adhering to the original abnormal thresholds (CRP >10 mg/l and albumin <35 g/l), studies in East Asia particularly Japan have used thresholds of 7.5 mg/l38, 5 mg/l39,40 and 3 mg/l41–43. Such lower CRP thresholds are above the normal reference ranges in Japan/ East Asia cohorts and results in fewer patients breaching the CRP >10 mg/l threshold. This observation of a greater proportion of patients with elevated systemic inflammation markers in Western countries compared with Eastern Asian countries is also apparent in white cell derived ratios. Given the objective and reproducible nature of systemic inflammation based prognostic scores it is likely that such observations are real. Indeed, there are recognized ethnic differences in the normal range of neutrophils and lymphocytes44–46. For example, Azab and co-workers recently reported that, in more than 9,000 patients in the United States, there were ethnic differences in the NLR46. Specifically, in the cohort as a whole the mean NLR was 2.15. In contrast, black Americans had a mean NLR of 1.76, Hispanic Americans had a mean NLR of 2.08 and white Americans had a mean NLR of 2.2446. Also, within ethnicities, patients who had diabetes, cardiovascular disease, a high BMI and were smokers had a significantly higher NLR46. Although, similar data for the GPS/mGPS has not yet appeared in the literature it is likely that there would be a similar effect on the GPS/mGPS. Therefore, given that the most common abnormal thresholds used for NLR are >5 and >3 it is likely that a combination of tumour and host genetic and environmental factors are responsible for such consistent East/West differences. These and the present results emphasise the importance of not only staging the tumour but also the host systemic inflammatory response in patients with operable disease7.
Recently, studies have directly compared the prognostic value of the two most common combined markers of the systemic inflammatory response, the NLR and the GPS/mGPS. Guthrie and coworkers47 reported a comparison in both the preoperative and follow-up settings in patients with resectable colorectal cancer. In this study of 206 patients undergoing a surgical resection at a single institution it was reported that both preoperative mGPS (HR: 1.97, CI 1.16–3.34, p < 0.005) and NLR (HR: 3.07, CI 1.23–7.63, p < 0.05) were independently associated with cancer specific survival47. However in the postoperative follow-up only mGPS (HR: 4.81, CI 2.13–10.83, p < 0.001) maintained its significance in terms of cancer specific survival47. In contrast, Wang and coworkers (2012) reported that, in 177 patients with pancreatic cancer treated with surgery and palliative chemotherapy, although NLR and mGPS predicted overall survival only NLR was independently associated with overall survival (HR: 2.54 CI 1.31–4.90, p = 0.006)48. Finally, Okuno and coworkers (2016) reported that, in 534 patients with perihilar cholangiocarcinoma, both the NLR and mGPS had prognostic value49. However, on multivariate analysis, only the mGPS was independently associated with overall survival (HR: 1.58 CI 1.21–2.06, p = 0.001)49.
The present review and meta-analysis has a number of limitations50–261. For example funnel plot analysis, even after fixed effect analysis, showed that there was for all systemic inflammation based prognostic scores some asymmetry. This would suggest that there may be some reporting bias. The basis of this bias is not clear. Other than statistically significant results being more likely to be published other possible contributors may be that the studies included in the analysis were English language only publication, had small study size, included multiple tumour types and included multiple thresholds. Nevertheless the consistency of prognostic value over a variety of systemic inflammation based prognostic scores and across larger studies, single tumour types and single thresholds would indicate that although there was evidence of bias in the meta-analysis, such scores do indeed have prognostic value. Similarly when only univariate analysis was available it was entered into the analysis. The majority of studies had HR derived from multivariate analysis (181 studies) and therefore harmonisation of HR results was not attempted. In the present meta-analysis there was considerable heterogeneity in the HR of some of the markers of the SIR. However, this was less when a consistent threshold for the marker was used. There are other potential contributors to such heterogeneity including geographical location. Such sub-analysis was limited by the number of studies available for meta-analysis. The strength of this present review is its comprehensive nature.
In summary, the results of this review consolidate the prognostic value of combined markers of the systemic inflammatory response including GPS/mGPS NLR, PLR and LMR in patients with resectable cancers. This is particularly true for the GPS/mGPS and NLR and in lung and GI cancers. These should form part of the routine preoperative workup and follow-up for all such patients undergoing resection for cancer.
Electronic supplementary material
Author Contributions
Mr. Dolan and Mr. Lim both contributed equally to the compiling of papers and their statistical analysis as part of this systematic review and meta-analysis. They also both contributed equally to the writing of the final paper. As such they should be classed as joint first authors. Mr. McSorley provided day to day support and feedback for statistical analysis and the final paper. Professors McMillan and Horgan also provided day to day support and made significant input to the final paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16955-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Organization, W. H. World Health Organization Cancer Fact Sheethttp://www.who.int/mediacentre/factsheets/fs297/en/ (2017).
- 2.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future oncology (London, England) 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 3.Proctor MJ, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. European journal of cancer (Oxford, England: 1990) 2011;47:2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Bosanquet N, Sikora K. The economics of cancer care in the UK. The Lancet. Oncology. 2004;5:568–574. doi: 10.1016/S1470-2045(04)01569-4. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet (London, England) 2008;371:771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. Colorectal Cancer, Systemic Inflammation, and Outcome: Staging the Tumor and Staging the Host. Annals of surgery. 2016;263:326–336. doi: 10.1097/SLA.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 8.McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
- 9.Guthrie GJ, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Critical reviews in oncology/hematology. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Templeton AJ, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Journal of the National Cancer Institute. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 11.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer treatment reviews. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Dolan, R. D., McSorley, S. T., Horgan, P. G., Laird, B. & McMillan, D. C. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Critical Reviews in Oncology/Hematology, 10.1016/j.critrevonc.2017.06.002. [DOI] [PubMed]
- 13.Higgins, J. G. S. Vol. 1 (ed. Green Higgins, J. S.) 675 (John Wiley & Sons Ltd, London 2011).
- 14.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. British journal of cancer. 2013;109:401–407. doi: 10.1038/bjc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishizuka M, Oyama Y, Abe A, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients undergoing surgery for gastric cancer. Journal of surgical oncology. 2014;110:935–941. doi: 10.1002/jso.23753. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, et al. Prognostic Significance of Combination of Preoperative Platelet Count and Neutrophil-Lymphocyte Ratio (COP-NLR) in Patients with Non-Small Cell Lung Cancer: Based on a Large Cohort Study. PloS one. 2015;10:e0126496. doi: 10.1371/journal.pone.0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neal CP, et al. Prognostic performance of inflammation-based prognostic indices in patients with resectable colorectal liver metastases. Med Oncol. 2015;32:144. doi: 10.1007/s12032-015-0590-2. [DOI] [PubMed] [Google Scholar]
- 18.Feng JF, Huang Y, Liu JS. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2013;6:1605–1612. doi: 10.2147/OTT.S52501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings M, et al. Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br J Cancer. 2015;113:311–320. doi: 10.1038/bjc.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, et al. Postoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes of hepatocellular carcinoma. J Surg Res. 2015;198:73–79. doi: 10.1016/j.jss.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Ishizuka M, et al. Clinical Significance of the C-Reactive Protein to Albumin Ratio for Survival After Surgery for Colorectal Cancer. Annals of surgical oncology. 2016;23:900–907. doi: 10.1245/s10434-015-4948-7. [DOI] [PubMed] [Google Scholar]
- 22.Xu XL, Yu HQ, Hu W, Song Q, Mao WM. A Novel Inflammation-Based Prognostic Score, the C-Reactive Protein/Albumin Ratio Predicts the Prognosis of Patients with Operable Esophageal Squamous Cell Carcinoma. PloS one. 2015;10:e0138657. doi: 10.1371/journal.pone.0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ubukata H, et al. Evaluations of interferon-gamma/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010;102:742–747. doi: 10.1002/jso.21725. [DOI] [PubMed] [Google Scholar]
- 24.Miyata H, et al. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Exp Ther Med. 2011;2:879–885. doi: 10.3892/etm.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha HR, et al. Survival Outcomes According to Adjuvant Treatment and Prognostic Factors Including Host Immune Markers in Patients with Curatively Resected Ampulla of Vater Cancer. PloS one. 2016;11:e0151406. doi: 10.1371/journal.pone.0151406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilie M, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118:1726–1737. doi: 10.1002/cncr.26456. [DOI] [PubMed] [Google Scholar]
- 27.Tomita M, Shimizu T, Ayabe T, Nakamura K, Onitsuka T. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer research. 2012;32:3535–3538. [PubMed] [Google Scholar]
- 28.Chen XL, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. 2015;6:41370–41382. doi: 10.18632/oncotarget.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sung HH, et al. Clinical significance of prognosis using the neutrophil-lymphocyte ratio and erythrocyte sedimentation rate in patients undergoing radical nephroureterectomy for upper urinary tract urothelial carcinoma. BJU international. 2015;115:587–594. doi: 10.1111/bju.12846. [DOI] [PubMed] [Google Scholar]
- 30.East JM, et al. Ratios derived from an array of standard haematological indices predict the oncological outcome in colon cancer. Colorectal Dis. 2014;16:442–449. doi: 10.1111/codi.12561. [DOI] [PubMed] [Google Scholar]
- 31.Shen SL, et al. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. 2014;21:3802–3809. doi: 10.1245/s10434-014-3771-x. [DOI] [PubMed] [Google Scholar]
- 32.Aurello P, et al. Value of preoperative inflammation-based prognostic scores in predicting overall survival and disease-free survival in patients with gastric cancer. Annals of surgical oncology. 2014;21:1998–2004. doi: 10.1245/s10434-014-3533-9. [DOI] [PubMed] [Google Scholar]
- 33.Sun KY, et al. Novel immunological and nutritional-based prognostic index for gastric cancer. World journal of gastroenterology. 2015;21:5961–5971. doi: 10.3748/wjg.v21.i19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. International journal of cancer. 2016;139:220–231. doi: 10.1002/ijc.30071. [DOI] [PubMed] [Google Scholar]
- 35.Christina EC, Cornelia C, Edgar S. Neoadjuvant radiotherapy plus radical surgery for locally advanced stage III/IV oral cancer: Analysis of prognostic factors affecting overall survival. Oral oncology. 2016;60:1–7. doi: 10.1016/j.oraloncology.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Wang YQ, et al. A novel prognostic inflammation score predicts outcomes in patients with ovarian cancer. Clinica chimica acta; international journal of clinical chemistry. 2016;456:163–169. doi: 10.1016/j.cca.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Toyokawa T, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016;16:722. doi: 10.1186/s12885-016-2696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura M, et al. New prognostic score for the survival of patients with esophageal squamous cell carcinoma. Surg Today. 2014;44:875–883. doi: 10.1007/s00595-013-0628-z. [DOI] [PubMed] [Google Scholar]
- 39.Arigami T, et al. Analysis of the Fibrinogen and Neutrophil-Lymphocyte Ratio in Esophageal Squamous Cell Carcinoma: A Promising Blood Marker of Tumor Progression and Prognosis. Medicine (Baltimore) 2015;94:e1702. doi: 10.1097/MD.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirashima K, et al. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. Journal of gastroenterology. 2014;49:1040–1046. doi: 10.1007/s00535-013-0855-5. [DOI] [PubMed] [Google Scholar]
- 41.Kawashima M, et al. Significance of the Glasgow Prognostic Score as a prognostic indicator for lung cancer surgery. Interactive cardiovascular and thoracic surgery. 2015;21:637–643. doi: 10.1093/icvts/ivv223. [DOI] [PubMed] [Google Scholar]
- 42.Takeno S, et al. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor in patients with resectable gastric cancer. Oncology. 2014;87:205–214. doi: 10.1159/000362601. [DOI] [PubMed] [Google Scholar]
- 43.Ishizuka M, et al. Usefulness of a modified inflammation-based prognostic system for predicting postoperative mortality of patients undergoing surgery for primary hepatocellular carcinoma. J Surg Oncol. 2011;103:801–806. doi: 10.1002/jso.21857. [DOI] [PubMed] [Google Scholar]
- 44.Bain B, Seed M, Godsland I. Normal values for peripheral blood white cell counts in women of four different ethnic origins. Journal of clinical pathology. 1984;37:188–193. doi: 10.1136/jcp.37.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. Journal of clinical pathology. 1996;49:664–666. doi: 10.1136/jcp.49.8.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azab B, Camacho-Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PloS one. 2014;9:e112361. doi: 10.1371/journal.pone.0112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guthrie GJ, Roxburgh CS, Farhan-Alanie OM, Horgan PG, McMillan DC. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. British journal of cancer. 2013;109:24–28. doi: 10.1038/bjc.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang DS, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Medical oncology (Northwood, London, England) 2012;29:3092–3100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- 49.Okuno M, et al. Evaluation of inflammation-based prognostic scores in patients undergoing hepatobiliary resection for perihilar cholangiocarcinoma. Journal of gastroenterology. 2016;51:153–161. doi: 10.1007/s00535-015-1103-y. [DOI] [PubMed] [Google Scholar]
- 50.Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Annals of surgery. 2007;246:1047–1051. doi: 10.1097/SLA.0b013e3181454171. [DOI] [PubMed] [Google Scholar]
- 51.Leitch EF, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. British journal of cancer. 2007;97:1266–1270. doi: 10.1038/sj.bjc.6604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobayashi T, et al. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery. 2008;144:729–735. doi: 10.1016/j.surg.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Annals of surgery. 2009;249:788–793. doi: 10.1097/SLA.0b013e3181a3e738. [DOI] [PubMed] [Google Scholar]
- 54.Ishizuka M, et al. Systemic inflammatory response predicts postoperative outcome in patients with liver metastases from colorectal cancer. J Surg Oncol. 2009;100:38–42. doi: 10.1002/jso.21294. [DOI] [PubMed] [Google Scholar]
- 55.Crozier JE, et al. Relationship between emergency presentation, systemic inflammatory response, and cancer-specific survival in patients undergoing potentially curative surgery for colon cancer. American journal of surgery. 2009;197:544–549. doi: 10.1016/j.amjsurg.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 56.Roxburgh CS, Wallace AM, Guthrie GK, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative surgery for colon cancer. Colorectal Dis. 2010;12:987–994. doi: 10.1111/j.1463-1318.2009.01961.x. [DOI] [PubMed] [Google Scholar]
- 57.Richards CH, et al. The relationship between patient physiology, the systemic inflammatory response and survival in patients undergoing curative resection of colorectal cancer. British journal of cancer. 2010;103:1356–1361. doi: 10.1038/sj.bjc.6605919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hefler-Frischmuth K, et al. The inflammation-based modified Glasgow Prognostic Score in patients with vulvar cancer. Eur J Obstet Gynecol Reprod Biol. 2010;149:102–105. doi: 10.1016/j.ejogrb.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi T, et al. Inflammation-based prognostic score and number of lymph node metastases are independent prognostic factors in esophageal squamous cell carcinoma. Dig Surg. 2010;27:232–237. doi: 10.1159/000276910. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi T, et al. Elevated C-reactive protein and hypoalbuminemia measured before resection of colorectal liver metastases predict postoperative survival. Dig Surg. 2010;27:285–290. doi: 10.1159/000280021. [DOI] [PubMed] [Google Scholar]
- 61.Moug SJ, et al. Comparison of positive lymph node ratio with an inflammation-based prognostic score in colorectal cancer. Br J Surg. 2011;98:282–286. doi: 10.1002/bjs.7294. [DOI] [PubMed] [Google Scholar]
- 62.Dutta S, Crumley AB, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg. 2011;35:1861–1866. doi: 10.1007/s00268-011-1130-7. [DOI] [PubMed] [Google Scholar]
- 63.Dutta S, Al-Mrabt NM, Fullarton GM, Horgan PG, McMillan DC. A comparison of POSSUM and GPS models in the prediction of post-operative outcome in patients undergoing oesophago-gastric cancer resection. Annals of surgical oncology. 2011;18:2808–2817. doi: 10.1245/s10434-011-1676-5. [DOI] [PubMed] [Google Scholar]
- 64.Crumley AB, et al. Interrelationships between tumor proliferative activity, leucocyte and macrophage infiltration, systemic inflammatory response, and survival in patients selected for potentially curative resection for gastroesophageal cancer. Annals of surgical oncology. 2011;18:2604–2612. doi: 10.1245/s10434-011-1658-7. [DOI] [PubMed] [Google Scholar]
- 65.Jamieson NB, et al. A prospective comparison of the prognostic value of tumor- and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Annals of surgical oncology. 2011;18:2318–2328. doi: 10.1245/s10434-011-1560-3. [DOI] [PubMed] [Google Scholar]
- 66.Roxburgh CS, et al. Relationship between preoperative comorbidity, systemic inflammatory response, and survival in patients undergoing curative resection for colorectal cancer. Annals of surgical oncology. 2011;18:997–1005. doi: 10.1245/s10434-010-1410-8. [DOI] [PubMed] [Google Scholar]
- 67.Vashist YK, et al. Glasgow Prognostic Score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Annals of surgical oncology. 2011;18:1130–1138. doi: 10.1245/s10434-010-1383-7. [DOI] [PubMed] [Google Scholar]
- 68.Nozoe T, et al. Significance of modified Glasgow prognostic score as a useful indicator for prognosis of patients with gastric carcinoma. American journal of surgery. 2011;201:186–191. doi: 10.1016/j.amjsurg.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 69.Roxburgh C, et al. Adjuvant chemotherapy for resected colon cancer: comparison of the prognostic value of tumour and patient related factors. Int J Colorectal Dis. 2011;26:483–492. doi: 10.1007/s00384-010-1120-5. [DOI] [PubMed] [Google Scholar]
- 70.Dutta S, et al. The relationship between tumour necrosis, tumour proliferation, local and systemic inflammation, microvessel density and survival in patients undergoing potentially curative resection of oesophageal adenocarcinoma. British journal of cancer. 2012;106:702–710. doi: 10.1038/bjc.2011.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishizuka M, et al. Impact of an inflammation-based prognostic system on patients undergoing surgery for hepatocellular carcinoma: a retrospective study of 398 Japanese patients. American journal of surgery. 2012;203:101–106. doi: 10.1016/j.amjsurg.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 72.Richards CH, et al. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg. 2012;99:287–294. doi: 10.1002/bjs.7755. [DOI] [PubMed] [Google Scholar]
- 73.Qayyum T, et al. Prospective study of the role of inflammation in renal cancer. Urol Int. 2012;88:277–281. doi: 10.1159/000334971. [DOI] [PubMed] [Google Scholar]
- 74.Sugimoto K, et al. Glasgow prognostic score as a prognostic factor in patients undergoing curative surgery for colorectal cancer. Dig Surg. 2012;29:503–509. doi: 10.1159/000346002. [DOI] [PubMed] [Google Scholar]
- 75.Kubota T, et al. Significance of the inflammation-based Glasgow prognostic score for short- and long-term outcomes after curative resection of gastric cancer. J Gastrointest Surg. 2012;16:2037–2044. doi: 10.1007/s11605-012-2036-x. [DOI] [PubMed] [Google Scholar]
- 76.Powell AG, et al. The relationship between tumour site, clinicopathological characteristics and cancer-specific survival in patients undergoing surgery for colorectal cancer. Colorectal Dis. 2012;14:1493–1499. doi: 10.1111/j.1463-1318.2012.03048.x. [DOI] [PubMed] [Google Scholar]
- 77.La Torre M, et al. The glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Annals of surgical oncology. 2012;19:2917–2923. doi: 10.1245/s10434-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 78.Dutta S, Crumley AB, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour and patient related factors in patients undergoing potentially curative resection of gastric cancer. American journal of surgery. 2012;204:294–299. doi: 10.1016/j.amjsurg.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 79.Wang DS, et al. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2012;33:749–756. doi: 10.1007/s13277-011-0285-z. [DOI] [PubMed] [Google Scholar]
- 80.Lamb GW, Aitchison M, Ramsey S, Housley SL, McMillan DC. Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. British journal of cancer. 2012;106:279–283. doi: 10.1038/bjc.2011.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Inflammation-based prognostic system predicts postoperative survival of colorectal cancer patients with a normal preoperative serum level of carcinoembryonic antigen. Annals of surgical oncology. 2012;19:3422–3431. doi: 10.1245/s10434-012-2384-5. [DOI] [PubMed] [Google Scholar]
- 82.Jiang X, et al. Prognostic importance of the inflammation-based Glasgow prognostic score in patients with gastric cancer. British journal of cancer. 2012;107:275–279. doi: 10.1038/bjc.2012.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jamieson NB, et al. The relationship between tumor inflammatory cell infiltrate and outcome in patients with pancreatic ductal adenocarcinoma. Annals of surgical oncology. 2012;19:3581–3590. doi: 10.1245/s10434-012-2370-y. [DOI] [PubMed] [Google Scholar]
- 84.Stotz M, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. British journal of cancer. 2013;109:416–421. doi: 10.1038/bjc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shiba H, et al. Glasgow prognostic score predicts therapeutic outcome after pancreaticoduodenectomy for carcinoma of the ampulla of vater. Anticancer research. 2013;33:2715–2721. [PubMed] [Google Scholar]
- 86.Oshiro Y, et al. Inflammation-based prognostic score is a useful predictor of postoperative outcome in patients with extrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2013;20:389–395. doi: 10.1007/s00534-012-0550-6. [DOI] [PubMed] [Google Scholar]
- 87.Horino K, et al. Glasgow Prognostic Score as a useful prognostic factor after hepatectomy for hepatocellular carcinoma. Int J Clin Oncol. 2013;18:829–838. doi: 10.1007/s10147-012-0451-3. [DOI] [PubMed] [Google Scholar]
- 88.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Inflammation-based prognostic system predicts survival after surgery for stage IV colorectal cancer. American journal of surgery. 2013;205:22–28. doi: 10.1016/j.amjsurg.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 89.Son HJ, et al. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Annals of surgical oncology. 2013;20:2908–2913. doi: 10.1245/s10434-013-2968-8. [DOI] [PubMed] [Google Scholar]
- 90.Nozoe T, Matono R, Ijichi H, Ohga T, Ezaki T. Glasgow Prognostic Score (GPS) can be a useful indicator to determine prognosis of patients with colorectal carcinoma. Int Surg. 2014;99:512–517. doi: 10.9738/INTSURG-D-13-00118.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pinato DJ, et al. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. British journal of cancer. 2014;110:1930–1935. doi: 10.1038/bjc.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang J, et al. The inflammation-based scores to predict prognosis of patients with hepatocellular carcinoma after hepatectomy. Medical oncology (Northwood, London, England) 2014;31:883. doi: 10.1007/s12032-014-0883-x. [DOI] [PubMed] [Google Scholar]
- 93.Feng JF, Zhao Q, Chen QX. Prognostic significance of Glasgow prognostic score in patients undergoing esophagectomy for esophageal squamous cell carcinoma. Saudi. J Gastroenterol. 2014;20:48–53. doi: 10.4103/1319-3767.126319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Forrest R, et al. Comparison of visual and automated assessment of tumour inflammatory infiltrates in patients with colorectal cancer. European journal of cancer (Oxford, England: 1990) 2014;50:544–552. doi: 10.1016/j.ejca.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 95.Wu XS, et al. Evaluation of two inflammation-based prognostic scores in patients with resectable gallbladder carcinoma. Annals of surgical oncology. 2014;21:449–457. doi: 10.1245/s10434-013-3292-z. [DOI] [PubMed] [Google Scholar]
- 96.Sun ZQ, et al. Prognostic significance of preoperative fibrinogen in patients with colon cancer. World J Gastroenterol. 2014;20:8583–8591. doi: 10.3748/wjg.v20.i26.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakagawa K, et al. The modified Glasgow prognostic score as a predictor of survival after hepatectomy for colorectal liver metastases. Annals of surgical oncology. 2014;21:1711–1718. doi: 10.1245/s10434-013-3342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miyazaki T, et al. Inflammation-based scoring is a useful prognostic predictor of pulmonary resection for elderly patients with clinical stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. 2015;47:e140–145. doi: 10.1093/ejcts/ezu514. [DOI] [PubMed] [Google Scholar]
- 99.Matsuda S, et al. Cumulative prognostic scores based on plasma fibrinogen and serum albumin levels in esophageal cancer patients treated with transthoracic esophagectomy: comparison with the Glasgow prognostic score. Annals of surgical oncology. 2015;22:302–310. doi: 10.1245/s10434-014-3857-5. [DOI] [PubMed] [Google Scholar]
- 100.Farhan-Alanie OM, McMahon J, McMillan DC. Systemic inflammatory response and survival in patients undergoing curative resection of oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2015;53:126–131. doi: 10.1016/j.bjoms.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 101.Ferro M, et al. Modified Glasgow Prognostic Score is Associated With Risk of Recurrence in Bladder Cancer Patients After Radical Cystectomy: A Multicenter Experience. Medicine (Baltimore) 2015;94:e1861. doi: 10.1097/MD.0000000000001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ni XC, et al. Prognostic Value of the Modified Glasgow Prognostic Score in Patients Undergoing Radical Surgery for Hepatocellular Carcinoma. Medicine (Baltimore) 2015;94:e1486. doi: 10.1097/MD.0000000000001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hirahara N, et al. Impact of inflammation-based prognostic score on survival after curative thoracoscopic esophagectomy for esophageal cancer. Eur J Surg Oncol. 2015;41:1308–1315. doi: 10.1016/j.ejso.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 104.Shibutani M, et al. The prognostic significance of a postoperative systemic inflammatory response in patients with colorectal cancer. World J Surg Oncol. 2015;13:194. doi: 10.1186/s12957-015-0609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shiba H, et al. Glasgow prognostic score predicts outcome after surgical resection of gallbladder cancer. World J Surg. 2015;39:753–758. doi: 10.1007/s00268-014-2844-0. [DOI] [PubMed] [Google Scholar]
- 106.Watt DG, Martin JC, Park JH, Horgan PG, McMillan DC. Neutrophil count is the most important prognostic component of the differential white cell count in patients undergoing elective surgery for colorectal cancer. American journal of surgery. 2015;210:24–30. doi: 10.1016/j.amjsurg.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 107.Okamura Y, et al. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg. 2015;39:1501–1509. doi: 10.1007/s00268-015-2982-z. [DOI] [PubMed] [Google Scholar]
- 108.Abe T, et al. Prediction of long-term survival by using the Glasgow Prognostic Score in patients with hepatocellular carcinoma after liver transplantation. Hepatol Res. 2016;46:622–633. doi: 10.1111/hepr.12597. [DOI] [PubMed] [Google Scholar]
- 109.Park JH, et al. Mismatch repair status in patients with primary operable colorectal cancer: associations with the local and systemic tumour environment. British journal of cancer. 2016;114:562–570. doi: 10.1038/bjc.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fu YP, et al. A Novel and Validated Inflammation-Based Score (IBS) Predicts Survival in Patients With Hepatocellular Carcinoma Following Curative Surgical Resection: A STROBE-Compliant Article. Medicine (Baltimore) 2016;95:e2784. doi: 10.1097/MD.0000000000002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan H, et al. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol. 2016;142:1285–1297. doi: 10.1007/s00432-015-2113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chan, J. C. et al. The Lymphocyte-to-Monocyte Ratio is a Superior Predictor of Overall Survival in Comparison to Established Biomarkers of Resectable Colorectal Cancer. Annals of surgery, 10.1097/sla.0000000000001743 (2016). [DOI] [PMC free article] [PubMed]
- 113.Walsh SM, Casey S, Kennedy R, Ravi N, Reynolds JV. Does the modified Glasgow Prognostic Score (mGPS) have a prognostic role in esophageal cancer? J Surg Oncol. 2016;113:732–737. doi: 10.1002/jso.24225. [DOI] [PubMed] [Google Scholar]
- 114.Otowa Y, et al. Changes in modified Glasgow prognostic score after neoadjuvant chemotherapy is a prognostic factor in clinical stage II/III esophageal cancer. Dis Esophagus. 2016;29:146–151. doi: 10.1111/dote.12316. [DOI] [PubMed] [Google Scholar]
- 115.Melling N, et al. Glasgow Prognostic Score may be a prognostic index for overall and perioperative survival in gastric cancer without perioperative treatment. Surgery. 2016;159:1548–1556. doi: 10.1016/j.surg.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 116.Halazun KJ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 117.Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008;97:513–518. doi: 10.1002/jso.21001. [DOI] [PubMed] [Google Scholar]
- 118.Sarraf KM, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 119.Kishi Y, et al. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Annals of surgical oncology. 2009;16:614–622. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 120.Cho H, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith RA, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. American journal of surgery. 2009;197:466–472. doi: 10.1016/j.amjsurg.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 122.Halazun KJ, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Annals of surgery. 2009;250:141–151. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 123.Jagdev SP, et al. Improving the accuracy of pre-operative survival prediction in renal cell carcinoma with C-reactive protein. British journal of cancer. 2010;103:1649–1656. doi: 10.1038/sj.bjc.6605973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shimada H, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–176. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 125.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. American journal of surgery. 2010;200:197–203. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 126.Mohri Y, et al. Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J Surg. 2010;34:285–290. doi: 10.1007/s00268-009-0302-1. [DOI] [PubMed] [Google Scholar]
- 127.Liu H, et al. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in rectal carcinoma. J Gastrointest Cancer. 2010;41:116–120. doi: 10.1007/s12029-009-9125-4. [DOI] [PubMed] [Google Scholar]
- 128.Kao SC, et al. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol. 2011;6:1923–1929. doi: 10.1097/JTO.0b013e31822a3740. [DOI] [PubMed] [Google Scholar]
- 129.Jung MR, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104:504–510. doi: 10.1002/jso.21986. [DOI] [PubMed] [Google Scholar]
- 130.Sharaiha RZ, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Annals of surgical oncology. 2011;18:3362–3369. doi: 10.1245/s10434-011-1754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer research. 2011;31:2995–2998. [PubMed] [Google Scholar]
- 132.Hung HY, et al. Effect of preoperative neutrophil-lymphocyte ratio on the surgical outcomes of stage II colon cancer patients who do not receive adjuvant chemotherapy. Int J Colorectal Dis. 2011;26:1059–1065. doi: 10.1007/s00384-011-1192-x. [DOI] [PubMed] [Google Scholar]
- 133.Neal CP, et al. Preoperative systemic inflammation and infectious complications after resection of colorectal liver metastases. Arch Surg. 2011;146:471–478. doi: 10.1001/archsurg.2011.50. [DOI] [PubMed] [Google Scholar]
- 134.Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011;13:499–503. doi: 10.1007/s12094-011-0687-9. [DOI] [PubMed] [Google Scholar]
- 135.Wang GY, et al. A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PloS one. 2011;6:e25295. doi: 10.1371/journal.pone.0025295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136.Bertuzzo VR, et al. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation. 2011;91:1279–1285. doi: 10.1097/TP.0b013e3182187cf0. [DOI] [PubMed] [Google Scholar]
- 137.Idowu OK, Ding Q, Taktak AF, Chandrasekar CR, Yin Q. Clinical implication of pretreatment neutrophil to lymphocyte ratio in soft tissue sarcoma. Biomarkers. 2012;17:539–544. doi: 10.3109/1354750X.2012.699554. [DOI] [PubMed] [Google Scholar]
- 138.Ishizuka M, et al. Clinical significance of tumor pathology for postoperative survival of patients undergoing surgery for stage IV colorectal cancer. Anticancer research. 2012;32:3291–3297. [PubMed] [Google Scholar]
- 139.Gondo T, et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79:1085–1091. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 140.Kwon HC, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17:216–222. doi: 10.3109/1354750X.2012.656705. [DOI] [PubMed] [Google Scholar]
- 141.Carruthers R, et al. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Dis. 2012;14:e701–707. doi: 10.1111/j.1463-1318.2012.03147.x. [DOI] [PubMed] [Google Scholar]
- 142.Wang L, et al. Esophageal carcinosarcoma: a unique entity with better prognosis. Annals of surgical oncology. 2013;20:997–1004. doi: 10.1245/s10434-012-2658-y. [DOI] [PubMed] [Google Scholar]
- 143.Choi ES, Kim HS, Han I. Elevated preoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Annals of surgical oncology. 2014;21:778–785. doi: 10.1245/s10434-013-3418-3. [DOI] [PubMed] [Google Scholar]
- 144.Szkandera J, et al. Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients. British journal of cancer. 2013;108:1677–1683. doi: 10.1038/bjc.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Krane LS, et al. Preoperative neutrophil/lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. Journal of endourology. 2013;27:1046–1050. doi: 10.1089/end.2012.0606. [DOI] [PubMed] [Google Scholar]
- 146.Pichler M, et al. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. British journal of cancer. 2013;108:901–907. doi: 10.1038/bjc.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jankova L, Dent OF, Chan C, Chapuis P, Clarke SJ. Preoperative neutrophil/lymphocyte ratio predicts overall survival but does not predict recurrence or cancer-specific survival after curative resection of node-positive colorectal cancer. BMC Cancer. 2013;13:442. doi: 10.1186/1471-2407-13-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fu SJ, et al. Prognostic value of preoperative peripheral neutrophil-to-lymphocyte ratio in patients with HBV-associated hepatocellular carcinoma after radical hepatectomy. Medical oncology (Northwood, London, England) 2013;30:721. doi: 10.1007/s12032-013-0721-6. [DOI] [PubMed] [Google Scholar]
- 149.Shibutani M, et al. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer research. 2013;33:3291–3294. [PubMed] [Google Scholar]
- 150.Forget P, et al. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Annals of surgical oncology. 2013;20(Suppl 3):S650–660. doi: 10.1245/s10434-013-3136-x. [DOI] [PubMed] [Google Scholar]
- 151.Absenger G, et al. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. British journal of cancer. 2013;109:395–400. doi: 10.1038/bjc.2013.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mano Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Annals of surgery. 2013;258:301–305. doi: 10.1097/SLA.0b013e318297ad6b. [DOI] [PubMed] [Google Scholar]
- 153.Azuma T, et al. Preoperative neutrophil-lymphocyte ratio as an independent prognostic marker for patients with upper urinary tract urothelial carcinoma. Clinical genitourinary cancer. 2013;11:337–341. doi: 10.1016/j.clgc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 154.Dumitrascu T, Chirita D, Ionescu M, Popescu I. Resection for hilar cholangiocarcinoma: analysis of prognostic factors and the impact of systemic inflammation on long-term outcome. J Gastrointest Surg. 2013;17:913–924. doi: 10.1007/s11605-013-2144-2. [DOI] [PubMed] [Google Scholar]
- 155.Perisanidis C, et al. High neutrophil-to-lymphocyte ratio is an independent marker of poor disease-specific survival in patients with oral cancer. Medical oncology (Northwood, London, England) 2013;30:334. doi: 10.1007/s12032-012-0334-5. [DOI] [PubMed] [Google Scholar]
- 156.Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 2013;16:55–59. doi: 10.4048/jbc.2013.16.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liao Y, Ni Y, He R, Liu W, Du J. Clinical implications of fibroblast activation protein-alpha in non-small cell lung cancer after curative resection: a new predictor for prognosis. J Cancer Res Clin Oncol. 2013;139:1523–1528. doi: 10.1007/s00432-013-1471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bambury RM, et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013;114:149–154. doi: 10.1007/s11060-013-1164-9. [DOI] [PubMed] [Google Scholar]
- 159.Toiyama Y, et al. C-reactive protein as predictor of recurrence in patients with rectal cancer undergoing chemoradiotherapy followed by surgery. Anticancer research. 2013;33:5065–5074. [PubMed] [Google Scholar]
- 160.Szkandera J, et al. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int J Cancer. 2014;135:362–370. doi: 10.1002/ijc.28677. [DOI] [PubMed] [Google Scholar]
- 161.Dalpiaz O, et al. Validation of pretreatment neutrophil-lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. BJU international. 2014;114:334–339. doi: 10.1111/bju.12441. [DOI] [PubMed] [Google Scholar]
- 162.Luo HL, et al. Subclassification of upper urinary tract urothelial carcinoma by the neutrophil-to-lymphocyte ratio (NLR) improves prediction of oncological outcome. BJU international. 2014;113:E144–149. doi: 10.1111/bju.12582. [DOI] [PubMed] [Google Scholar]
- 163.Zhang T, et al. Evaluation of preoperative hematologic markers as prognostic factors and establishment of novel risk stratification in resected pN0 non-small-cell lung cancer. PloS one. 2014;9:e111494. doi: 10.1371/journal.pone.0111494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ying HQ, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Medical oncology (Northwood, London, England) 2014;31:305. doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 165.Linton A, et al. Factors associated with survival in a large series of patients with malignant pleural mesothelioma in New South Wales. British journal of cancer. 2014;111:1860–1869. doi: 10.1038/bjc.2014.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kubo T, et al. Impact of the perioperative neutrophil-to-lymphocyte ratio on the long-term survival following an elective resection of colorectal carcinoma. International journal of colorectal disease. 2014;29:1091–1099. doi: 10.1007/s00384-014-1964-1. [DOI] [PubMed] [Google Scholar]
- 167.Viers BR, et al. Preoperative neutrophil-lymphocyte ratio predicts death among patients with localized clear cell renal carcinoma undergoing nephrectomy. Urologic oncology. 2014;32:1277–1284. doi: 10.1016/j.urolonc.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 168.Koh YW, Lee HJ, Ahn JH, Lee JW, Gong G. Prognostic significance of the ratio of absolute neutrophil to lymphocyte counts for breast cancer patients with ER/PR-positivity and HER2-negativity in neoadjuvant setting. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:9823–9830. doi: 10.1007/s13277-014-2282-5. [DOI] [PubMed] [Google Scholar]
- 169.Hermanns T, et al. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. British journal of cancer. 2014;111:444–451. doi: 10.1038/bjc.2014.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tanaka N, et al. A multi-institutional validation of the prognostic value of the neutrophil-to-lymphocyte ratio for upper tract urothelial carcinoma treated with radical nephroureterectomy. Annals of surgical oncology. 2014;21:4041–4048. doi: 10.1245/s10434-014-3830-3. [DOI] [PubMed] [Google Scholar]
- 171.Jiang N, et al. The role of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after radical resection for gastric cancer. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals. 2014;19:444–451. doi: 10.3109/1354750X.2014.926567. [DOI] [PubMed] [Google Scholar]
- 172.Yuan D, et al. The preoperative neutrophil-lymphocyte ratio predicts recurrence and survival among patients undergoing R0 resections of adenocarcinomas of the esophagogastric junction. Journal of surgical oncology. 2014;110:333–340. doi: 10.1002/jso.23651. [DOI] [PubMed] [Google Scholar]
- 173.Ozdemir Y, Akin ML, Sucullu I, Balta AZ, Yucel E. Pretreatment neutrophil/lymphocyte ratio as a prognostic aid in colorectal cancer. Asian Pacific journal of cancer prevention: APJCP. 2014;15:2647–2650. doi: 10.7314/APJCP.2014.15.6.2647. [DOI] [PubMed] [Google Scholar]
- 174.Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World journal of surgical oncology. 2014;12:58. doi: 10.1186/1477-7819-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Viers BR, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. European urology. 2014;66:1157–1164. doi: 10.1016/j.eururo.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 176.McNamara MG, et al. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. European journal of cancer (Oxford, England: 1990) 2014;50:1581–1589. doi: 10.1016/j.ejca.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 177.Malietzis G, et al. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Annals of surgery. 2014;260:287–292. doi: 10.1097/SLA.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 178.Grivas N, et al. Clinico-pathological prognostic factors of renal cell carcinoma: A 15-year review from a single center in Greece. Urology annals. 2014;6:116–121. doi: 10.4103/0974-7796.130552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shen L, et al. Baseline neutrophil-lymphocyte ratio (>/= 2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiation oncology (London, England) 2014;9:295. doi: 10.1186/s13014-014-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Neofytou K, et al. Elevated platelet to lymphocyte ratio predicts poor prognosis after hepatectomy for liver-only colorectal metastases, and it is superior to neutrophil to lymphocyte ratio as an adverse prognostic factor. Medical oncology (Northwood, London, England) 2014;31:239. doi: 10.1007/s12032-014-0239-6. [DOI] [PubMed] [Google Scholar]
- 181.Song Y, et al. Preoperative neutrophil-to-lymphocyte ratio as prognostic predictor for hypopharyngeal squamous cell carcinoma after radical resections. J Craniofac Surg. 2015;26:e137–140. doi: 10.1097/SCS.0000000000001235. [DOI] [PubMed] [Google Scholar]
- 182.Takahashi Y, et al. Prognostic Significance of Preoperative Neutrophil-Lymphocyte Ratios in Patients with Stage I Non-small Cell Lung Cancer After Complete Resection. Annals of surgical oncology. 2015;22(Suppl 3):S1324–1331. doi: 10.1245/s10434-015-4735-5. [DOI] [PubMed] [Google Scholar]
- 183.Tu XP, et al. Preoperative neutrophil-to-lymphocyte ratio is an independent prognostic marker in patients with laryngeal squamous cell carcinoma. BMC cancer. 2015;15:743. doi: 10.1186/s12885-015-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shin JS, Suh KW, Oh SY. Preoperative neutrophil to lymphocyte ratio predicts survival in patients with T1-2N0 colorectal cancer. Journal of surgical oncology. 2015;112:654–657. doi: 10.1002/jso.24061. [DOI] [PubMed] [Google Scholar]
- 185.Que Y, et al. Preoperative platelet-lymphocyte ratio is superior to neutrophil-lymphocyte ratio as a prognostic factor for soft-tissue sarcoma. BMC cancer. 2015;15:648. doi: 10.1186/s12885-015-1654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hsu JT, et al. Prognostic Value of the Preoperative Neutrophil to Lymphocyte Ratio in Resectable Gastric Cancer. Medicine. 2015;94:e1589. doi: 10.1097/MD.0000000000001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shimizu K, et al. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World journal of surgical oncology. 2015;13:291. doi: 10.1186/s12957-015-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Han S, et al. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC cancer. 2015;15:617. doi: 10.1186/s12885-015-1629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liao R, et al. Preoperative neutrophil-to-lymphocyte ratio predicts recurrence of patients with single-nodule small hepatocellular carcinoma following curative resection: a retrospective report. World journal of surgical oncology. 2015;13:265. doi: 10.1186/s12957-015-0670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Aldemir MN, et al. Prognostic Value of Baseline Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratios in Local and Advanced Gastric Cancer Patients. Asian Pacific journal of cancer prevention: APJCP. 2015;16:5933–5937. doi: 10.7314/APJCP.2015.16.14.5933. [DOI] [PubMed] [Google Scholar]
- 191.Kadota K, et al. Prognostic Impact of Immune Microenvironment in Lung Squamous Cell Carcinoma: Tumor-Infiltrating CD10+ Neutrophil/CD20+ Lymphocyte Ratio as an Independent Prognostic Factor. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015;10:1301–1310. doi: 10.1097/JTO.0000000000000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Neofytou K, et al. The Preoperative Lymphocyte-to-Monocyte Ratio is Prognostic of Clinical Outcomes for Patients with Liver-Only Colorectal Metastases in the Neoadjuvant Setting. Annals of surgical oncology. 2015;22:4353–4362. doi: 10.1245/s10434-015-4481-8. [DOI] [PubMed] [Google Scholar]
- 193.Bagante F, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio as predictors of disease specific survival after resection of adrenocortical carcinoma. Journal of surgical oncology. 2015;112:164–172. doi: 10.1002/jso.23982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang Q, et al. The Severity of Liver Fibrosis Influences the Prognostic Value of Inflammation-Based Scores in Hepatitis B-Associated Hepatocellular Carcinoma. Annals of surgical oncology. 2015;22(Suppl 3):S1125–1132. doi: 10.1245/s10434-015-4598-9. [DOI] [PubMed] [Google Scholar]
- 195.Pine JK, et al. Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. British journal of cancer. 2015;113:204–211. doi: 10.1038/bjc.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li J, Lin J, Luo Y, Kuang M, Liu Y. Multivariate Analysis of Prognostic Biomarkers in Surgically Treated Endometrial Cancer. PloS one. 2015;10:e0130640. doi: 10.1371/journal.pone.0130640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang H, et al. Clinical significance of preoperative neutrophil-lymphocyte vs platelet-lymphocyte ratio in primary operable patients with non-small cell lung cancer. American journal of surgery. 2015;210:526–535. doi: 10.1016/j.amjsurg.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 198.Zhang Y, Jiang C, Li J, Sun J, Qu X. Prognostic significance of preoperative neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in patients with gallbladder carcinoma. Clinical & translational oncology: official publication of the Federation of Spanish. Oncology Societies and of the National Cancer Institute of Mexico. 2015;17:810–818. doi: 10.1007/s12094-015-1310-2. [DOI] [PubMed] [Google Scholar]
- 199.Qu JL, et al. Prognostic Model Based on Systemic Inflammatory Response and Clinicopathological Factors to Predict Outcome of Patients with Node-Negative Gastric Cancer. PloS one. 2015;10:e0128540. doi: 10.1371/journal.pone.0128540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang WW, Liu KJ, Hu GL, Liang WJ. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:8831–8837. doi: 10.1007/s13277-015-3533-9. [DOI] [PubMed] [Google Scholar]
- 201.Yu L, Lv CY, Yuan AH, Chen W, Wu AW. Significance of the preoperative neutrophil-to-lymphocyte ratio in the prognosis of patients with gastric cancer. World journal of gastroenterology. 2015;21:6280–6286. doi: 10.3748/wjg.v21.i20.6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Duan H, et al. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World journal of gastroenterology. 2015;21:5591–5597. doi: 10.3748/wjg.v21.i18.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wen RM, et al. Preoperative Neutrophil to Lymphocyte Ratio as a Prognostic Factor in Patients with Non-metastatic Renal Cell Carcinoma. Asian Pacific journal of cancer prevention: APJCP. 2015;16:3703–3708. doi: 10.7314/APJCP.2015.16.9.3703. [DOI] [PubMed] [Google Scholar]
- 204.Choi WJ, et al. Preoperative Neutrophil-to-Lymphocyte Ratio is a Better Prognostic Serum Biomarker than Platelet-to-Lymphocyte Ratio in Patients Undergoing Resection for Nonmetastatic Colorectal Cancer. Annals of surgical oncology. 2015;22(Suppl 3):S603–613. doi: 10.1245/s10434-015-4571-7. [DOI] [PubMed] [Google Scholar]
- 205.Deng Q, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. Journal of translational medicine. 2015;13:66. doi: 10.1186/s12967-015-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Spolverato G, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. Journal of surgical oncology. 2015;111:868–874. doi: 10.1002/jso.23900. [DOI] [PubMed] [Google Scholar]
- 207.Han LH, et al. Prognostic significance of preoperative lymphocyte-monocyte ratio in patients with resectable esophageal squamous cell carcinoma. Asian Pacific journal of cancer prevention: APJCP. 2015;16:2245–2250. doi: 10.7314/APJCP.2015.16.6.2245. [DOI] [PubMed] [Google Scholar]
- 208.Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The Platelet-to-Lymphocyte Ratio Versus Neutrophil-to-Lymphocyte Ratio: Which is Better as a Prognostic Factor in Gastric Cancer? Annals of surgical oncology. 2015;22:4363–4370. doi: 10.1245/s10434-015-4518-z. [DOI] [PubMed] [Google Scholar]
- 209.Chan AW, et al. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma After Surgical Resection. Annals of surgical oncology. 2015;22:4138–4148. doi: 10.1245/s10434-015-4516-1. [DOI] [PubMed] [Google Scholar]
- 210.Choi JE, et al. Perioperative neutrophil:lymphocyte ratio and postoperative NSAID use as predictors of survival after lung cancer surgery: a retrospective study. Cancer medicine. 2015;4:825–833. doi: 10.1002/cam4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lee SK, et al. Immediate postoperative inflammation is an important prognostic factor in breast cancer. Oncology. 2015;88:337–344. doi: 10.1159/000368985. [DOI] [PubMed] [Google Scholar]
- 212.Chen ZY, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. British journal of cancer. 2015;112:1088–1097. doi: 10.1038/bjc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wuxiao ZJ, et al. A prognostic model to predict survival in stage III colon cancer patients based on histological grade, preoperative carcinoembryonic antigen level and the neutrophil lymphocyte ratio. Asian Pacific journal of cancer prevention: APJCP. 2015;16:747–751. doi: 10.7314/APJCP.2015.16.2.747. [DOI] [PubMed] [Google Scholar]
- 214.Chen Q, et al. The elevated preoperative neutrophil-to-lymphocyte ratio predicts poor prognosis in intrahepatic cholangiocarcinoma patients undergoing hepatectomy. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:5283–5289. doi: 10.1007/s13277-015-3188-6. [DOI] [PubMed] [Google Scholar]
- 215.Kim M, et al. Prognostic value of systemic inflammatory responses in patients with upper urinary tract urothelial carcinoma. World journal of urology. 2015;33:1439–1457. doi: 10.1007/s00345-015-1484-9. [DOI] [PubMed] [Google Scholar]
- 216.Szkandera J, et al. The derived neutrophil/lymphocyte ratio predicts poor clinical outcome in soft tissue sarcoma patients. American journal of surgery. 2015;210:111–116. doi: 10.1016/j.amjsurg.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 217.Ben Q, et al. Validation of the pretreatment neutrophil-lymphocyte ratio as a predictor of overall survival in a cohort of patients with pancreatic ductal adenocarcinoma. Pancreas. 2015;44:471–477. doi: 10.1097/MPA.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 218.Graziosi L, et al. Prognostic value of preoperative neutrophils to lymphocytes ratio in patients resected for gastric cancer. American journal of surgery. 2015;209:333–337. doi: 10.1016/j.amjsurg.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 219.Takahashi R, et al. Prognostic significance of systemic neutrophil and leukocyte alterations in surgically treated endometrial cancer patients: a monoinstitutional study. Gynecologic oncology. 2015;137:112–118. doi: 10.1016/j.ygyno.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 220.Shirai Y, et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery. 2015;158:360–365. doi: 10.1016/j.surg.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 221.Chen Q, et al. Negative impact of preoperative platelet-lymphocyte ratio on outcome after hepatic resection for intrahepatic cholangiocarcinoma. Medicine. 2015;94:e574. doi: 10.1097/MD.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lian L, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer biomarkers: section A of Disease markers. 2015;15:899–907. doi: 10.3233/CBM-150534. [DOI] [PubMed] [Google Scholar]
- 223.Xie X, Luo KJ, Hu Y, Wang JY, Chen J. Prognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Dis Esophagus. 2016;29:79–85. doi: 10.1111/dote.12296. [DOI] [PubMed] [Google Scholar]
- 224.Mohri Y, et al. Impact of Preoperative Neutrophil to Lymphocyte Ratio and Postoperative Infectious Complications on Survival After Curative Gastrectomy for Gastric Cancer: A Single Institutional Cohort Study. Medicine. 2016;95:e3125. doi: 10.1097/MD.0000000000003125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Takahashi Y, et al. Neutrophil-Lymphocyte Ratio as a Prognostic Marker for Lung Adenocarcinoma After Complete Resection. World journal of surgery. 2016;40:365–372. doi: 10.1007/s00268-015-3275-2. [DOI] [PubMed] [Google Scholar]
- 226.Cheng YC, et al. The Prognostic Significance of Inflammation-Associated Blood Cell Markers in Patients with Upper Tract Urothelial Carcinoma. Annals of surgical oncology. 2016;23:343–351. doi: 10.1245/s10434-015-4781-z. [DOI] [PubMed] [Google Scholar]
- 227.Turner N, et al. Analysis of local chronic inflammatory cell infiltrate combined with systemic inflammation improves prognostication in stage II colon cancer independent of standard clinicopathologic criteria. International journal of cancer. 2016;138:671–678. doi: 10.1002/ijc.29805. [DOI] [PubMed] [Google Scholar]
- 228.Fu Y, Liu W, OuYang D, Yang A, Zhang Q. Preoperative Neutrophil-to-lymphocyte Ratio Predicts Long-term Survival in Patients Undergoing Total Laryngectomy With Advanced Laryngeal Squamous Cell Carcinoma: A Single-center Retrospective Study. Medicine. 2016;95:e2689. doi: 10.1097/MD.0000000000002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lu SD, et al. Preoperative Ratio of Neutrophils to Lymphocytes Predicts Postresection Survival in Selected Patients With Early or Intermediate Stage Hepatocellular Carcinoma. Medicine. 2016;95:e2722. doi: 10.1097/MD.0000000000002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen PC, Feng JF. A Novel Inflammation-Based Stage (I Stage) in Patients with Resectable Esophageal Squamous Cell Carcinoma. Mediators of inflammation. 2016;2016:5396747. doi: 10.1155/2016/5396747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang SC, et al. Pretreatment Neutrophil to Lymphocyte Ratio Independently Predicts Disease-specific Survival in Resectable Gastroesophageal Junction and Gastric Adenocarcinoma. Annals of surgery. 2016;263:292–297. doi: 10.1097/SLA.0000000000001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hodek, M. et al. Neoadjuvant chemoradiotherapy of rectal carcinoma: Baseline hematologic parameters influencing outcomes. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft… [et al.] 192, 632–640, 10.1007/s00066-016-0988-6 (2016). [DOI] [PubMed]
- 233.Morizawa Y, et al. Neutrophil-to-lymphocyte ratio as a detection marker of tumor recurrence in patients with muscle-invasive bladder cancer after radical cystectomy. Urologic oncology. 2016;34(257):e211–257. doi: 10.1016/j.urolonc.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 234.Kosumi K, et al. Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patients. Surgery today. 2016;46:405–413. doi: 10.1007/s00595-015-1197-0. [DOI] [PubMed] [Google Scholar]
- 235.Kawahara T, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in bladder cancer patients after radical cystectomy. BMC cancer. 2016;16:185. doi: 10.1186/s12885-016-2219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kang M, Jeong CW, Kwak C, Kim HH, Ku JH. The Prognostic Significance of the Early Postoperative Neutrophil-to-Lymphocyte Ratio in Patients with Urothelial Carcinoma of the Bladder Undergoing Radical Cystectomy. Annals of surgical oncology. 2016;23:335–342. doi: 10.1245/s10434-015-4708-8. [DOI] [PubMed] [Google Scholar]
- 237.Bhindi B, et al. Identification of the best complete blood count-based predictors for bladder cancer outcomes in patients undergoing radical cystectomy. British journal of cancer. 2016;114:207–212. doi: 10.1038/bjc.2015.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. Journal of gynecologic oncology. 2012;23:265–273. doi: 10.3802/jgo.2012.23.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Feng JF, Huang Y, Zhao Q, Chen QX. Clinical significance of preoperative neutrophil lymphocyte ratio versus platelet lymphocyte ratio in patients with small cell carcinoma of the esophagus. ScientificWorldJournal. 2013;2013:504365. doi: 10.1155/2013/504365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Baranyai Z, et al. The comparison of thrombocytosis and platelet-lymphocyte ratio as potential prognostic markers in colorectal cancer. Thrombosis and haemostasis. 2014;111:483–490. doi: 10.1160/TH13-08-0632. [DOI] [PubMed] [Google Scholar]
- 241.Krenn-Pilko S, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. British journal of cancer. 2014;110:2524–2530. doi: 10.1038/bjc.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Szkandera J, et al. The elevated preoperative platelet to lymphocyte ratio predicts decreased time to recurrence in colon cancer patients. American journal of surgery. 2014;208:210–214. doi: 10.1016/j.amjsurg.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 243.Zhang GM, et al. Preoperative lymphocyte-monocyte and platelet-lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:8537–8543. doi: 10.1007/s13277-015-3613-x. [DOI] [PubMed] [Google Scholar]
- 244.Messager M, Neofytou K, Chaudry MA, Allum WH. Prognostic impact of preoperative platelets to lymphocytes ratio (PLR) on survival for oesophageal and junctional carcinoma treated with neoadjuvant chemotherapy: A retrospective monocentric study on 153 patients. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2015;41:1316–1323. doi: 10.1016/j.ejso.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 245.Pang Q, et al. Platelet to lymphocyte ratio as a novel prognostic tool for gallbladder carcinoma. World journal of gastroenterology. 2015;21:6675–6683. doi: 10.3748/wjg.v21.i21.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ozawa T, et al. The preoperative platelet to lymphocyte ratio is a prognostic marker in patients with stage II colorectal cancer. International journal of colorectal disease. 2015;30:1165–1171. doi: 10.1007/s00384-015-2276-9. [DOI] [PubMed] [Google Scholar]
- 247.Saito H, et al. A new prognostic scoring system using factors available preoperatively to predict survival after operative resection of perihilar cholangiocarcinoma. Surgery. 2016;159:842–851. doi: 10.1016/j.surg.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 248.Stotz M, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. British journal of cancer. 2014;110:435–440. doi: 10.1038/bjc.2013.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hu P, et al. Prognostic significance of systemic inflammation-based lymphocyte- monocyte ratio in patients with lung cancer: based on a large cohort study. PloS one. 2014;9:e108062. doi: 10.1371/journal.pone.0108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhou X, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcomes in patients with stage II/III gastric cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:11659–11666. doi: 10.1007/s13277-014-2504-x. [DOI] [PubMed] [Google Scholar]
- 251.Hutterer GC, et al. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urologic oncology. 2014;32:1041–1048. doi: 10.1016/j.urolonc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 252.Wen J, et al. Prognostic Significance of Preoperative Circulating Monocyte Count in Patients With Breast Cancer: Based on a Large Cohort Study. Medicine. 2015;94:e2266. doi: 10.1097/MD.0000000000002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lin ZX, et al. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World journal of gastroenterology. 2015;21:10898–10906. doi: 10.3748/wjg.v21.i38.10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yoshida T, et al. A novel risk stratification model, involving preoperative lymphocyte-monocyte ratio and standard pathological factors, for overall survival in patients with bladder cancer undergoing radical cystectomy. Japanese journal of clinical oncology. 2015;45:1162–1167. doi: 10.1093/jjco/hyv057. [DOI] [PubMed] [Google Scholar]
- 255.Yamagishi T, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with malignant pleural mesothelioma. Lung cancer (Amsterdam, Netherlands) 2015;90:111–117. doi: 10.1016/j.lungcan.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 256.Ozawa T, et al. Impact of a lymphocyte to monocyte ratio in stage IV colorectal cancer. The Journal of surgical research. 2015;199:386–392. doi: 10.1016/j.jss.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 257.Hutterer GC, et al. Pretreatment lymphocyte-monocyte ratio as a potential prognostic factor in a cohort of patients with upper tract urothelial carcinoma. Journal of clinical pathology. 2015;68:351–355. doi: 10.1136/jclinpath-2014-202658. [DOI] [PubMed] [Google Scholar]
- 258.Huang Y, Feng JF. Low preoperative lymphocyte to monocyte ratio predicts poor cancer-specific survival in patients with esophageal squamous cell carcinoma. OncoTargets and therapy. 2015;8:137–145. doi: 10.2147/OTT.S73794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chen L, Zhang F, Sheng XG, Zhang SQ. Decreased pretreatment lymphocyte/monocyte ratio is associated with poor prognosis in stage Ib1-IIa cervical cancer patients who undergo radical surgery. OncoTargets and therapy. 2015;8:1355–1362. doi: 10.2147/OTT.S82174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Peng W, et al. Prognostic value of the platelet to lymphocyte ratio change in liver cancer. The Journal of surgical research. 2015;194:464–470. doi: 10.1016/j.jss.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 261.Peng W, et al. Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. The Journal of surgical research. 2014;192:402–408. doi: 10.1016/j.jss.2014.05.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.