Abstract
Freezing of gait (FOG) is a common and debilitating symptom in Parkinson’s disease (PD). The current study investigated alterations of resting-state spontaneous brain activity in PD patients with FOG. A total of 29 patients with FOG, 28 patients without FOG and 31 controls were included. All subjects underwent resting-state functional MRI, and the amplitude of low-frequency fluctuation (ALFF) was calculated to measure the spontaneous brain activity. Between-group differences and correlations with FOG severity (both subjective and objective measures) were analyzed. Compared to those without FOG, patients with FOG showed increased ALFF in right anterior cingulate cortex (ACC) and left inferior parietal lobule (IPL), as well as decreased ALFF in right superior frontal gyrus (SFG), bilateral cerebellum and left thalamus. Correlation analyses demonstrated that ALFF within the right SFG, right ACC and bilateral pallidum were positively correlated with FOG; while ALFF within the thalamus, putamen, cerebellum and sensorimotor regions were negatively correlated. Our results indicate that FOG is associated with dysfunction within frontal-parietal regions, along with increased inhibitory outputs from basal ganglia. Additionally, altered activity of cerebellum implicates its role in the pathophysiology of FOG. These findings provide further insight into the underlying neural mechanisms of FOG in PD patients.
Introduction
Freezing of gait (FOG) is described as a ‘brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk’1, generally occurring during gait initiation and/or turning2. FOG is one of the most debilitating symptoms in Parkinson’s disease (PD), contributing to falls and reduced mobility and quality of life2,3. Common treatments such as anti-Parkinson medication do not consistently provide adequate benefit4. FOG is usually observed in the advanced stages of PD, but it can also present at early phase2. It has been suggested that FOG is not associated with the cardinal features of PD (tremor, bradykinesia or rigidity)4,5, but significantly associated with other factors, such as postural instability6 and impaired executive function7.
Although it is difficult to accompany gait into neuroimaging, much insight into FOG has been gained from neuroimaging techniques8. Using virtual reality and motor imagery paradigms, recent task-based functional MRI (fMRI) studies reported decreased neural activity within the bilateral sensorimotor regions and a concomitant increased response within fronto-parietal cortical regions in PD patients who have FOG compared to those without9,10. Decreased neural response had also been observed in a number of subcortical nuclei, including the bilateral caudate head, thalamus, subthalamic nucleus and globus pallidus internus during FOG episodes9. In addition, one previous multimodal study using resting-state fMRI and diffusion tensor imaging demonstrated altered functional and structural connectivity in the mesencephalic locomotor region and cerebellar locomotor region, involving mainly those connecting the peduncolopontine nucleus with the frontal cortices and cerebellum11,12.
While the above-mentioned studies provided essential information to unravel the neural correlates of FOG in PD, current knowledge of the underpinnings underlying FOG remains limited. It has been suggested that cerebral alternations observed in the resting-state, in the absence of experimental tasks, can take full advantage of the neural origin of spontaneous blood-oxygen-level-dependent (BOLD) signal fluctuations13. To date, several resting-state fMRI studies have investigated FOG related functional connectivity changes in PD patients, showing altered functional connectivities not only in the locomotor network11, but also in “cognitive” circuits such as the executive-attention related frontoparietal and visual occipito-temporal networks14. Although the result of abnormal functional connectivity between two remote areas is comprehensive and integrative, one could not draw any conclusion about which area is abnormal from such an examination15. Regarding the amplitude of low-frequency fluctuation (ALFF), Zang et al.15 proposed that ALFF measures the amplitude of low-frequency (0.01~0.08 Hz) BOLD signal and can be used as an index of local spontaneous neural activity in resting state. In addition, this technique provides insight into the neural substrates underlying PD at a local level15,16, which may have further implications for identifying treatment targets and thus guiding the development of new therapies for brain stimulation.
To our knowledge, no studies have investigated the spontaneous brain activity related to FOG in PD patients in the resting-state. ALFF has been used to study neurophysiological mechanisms in PD patients with depression17, mild cognitive impairment16 and rapid eye movement sleep behavior disorder18. Accordingly, we used ALFF to study the spontaneous brain activity in PD patients with FOG. Furthermore, we also explored the neural correlates with several gait deficits which are more impaired or specific in patients with FOG when comparing to patients without FOG and healthy controls in the gait analysis.
Results
Clinical and demographic characteristics
Participant demographics and clinical features are described in Table 1. Briefly, patients with FOG had longer disease duration, more severe parkinsonism as assessed by the Hoehn and Yahr (H-Y) stage and Movement Disorder Society Unified Parkinson’s Disease Rating Scale motor scores (MDS-UPDRS III), and higher levodopa equivalent daily dose (LEDD). Therefore, we controlled for these factors in the further analysis. There were no significant differences in onset age and onset side between patients with and without FOG. In the FOG group, the mean FOG duration was 1.95 ± 1.62 years, while the mean new freezing of gait questionnaire (NFOGQ) Part II and Part III scores were 15.13 ± 3.32 and 5.77 ± 2.32 respectively. Both patients with and without FOG had higher Hamilton Depression Rating Scale (HAMD) scores than healthy controls, whereas no difference was found between the two disease groups. Other clinical variables were similar among the three groups, including sex, age, Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA) total score and Executive Function sub-score.
Table 1.
Demographic and clinical features of all participants.
| Features | Patients with FOG (n = 31) | Patients without FOG (n = 31) | Healthy controls (n = 32) | F/T | p-value |
|---|---|---|---|---|---|
| Sex (m/f) | 14/17 | 20/11 | 17/15 | 2.365 | 0.307a |
| Age, years | 60.87 ± 8.14 | 58.03 ± 9.78 | 58.34 ± 7.25 | 1.057 | 0.352b |
| Disease Duration, years | 7.90 ± 5.06 | 5.23 ± 3.46 | — | 2.430 | 0.018c* |
| Onset Age, years | 52.68 ± 8.23 | 52.84 ± 10.56 | — | −0.067 | 0.947c |
| Onset Side (R/L/B) | 11/15/5 | 10/18/3 | — | 0.820 | 0.664a |
| H-Y stage (OFF) | 2.56 ± 0.73 | 1.92 ± 0.62 | — | 3.757 | 0.000c* |
| MDS-UPDRS III (OFF) | 41.48 ± 17.18 | 30.81 ± 14.41 | — | 2.651 | 0.010c* |
| FOG duration, years | 1.95 ± 1.62 | — | |||
| NFOGQ-II | 15.13 ± 3.32 | — | — | — | — |
| NFOGQ-III | 5.77 ± 2.32 | — | — | — | — |
| LEDD, mg/d | 704.67 ± 381.15 | 396.67 ± 296.06 | — | 3.495 | 0.000c* |
| MMSE | 28.48 ± 2.03 | 27.84 ± 1.73 | 28.59 ± 1.43 | 1.704 | 0.188b |
| MoCA | 25.58 ± 3.66 | 24.81 ± 2.99 | 26.16 ± 3.41 | 1.274 | 0.285b |
| Executive Function sub-score | 3.23 ± 0.99 | 3.03 ± 0.96 | 3.13 ± 0.98 | 0.296 | 0.744b |
| HAMD | 7.74 ± 4.70 | 6.32 ± 4.90 | 3.03 ± 2.92 | 10.180 | 0.000b* |
Means and SD are shown for continuous variables. FOG: freezing of gait; Onset Side (R/L/B): Right/Left/Bilateral onset; H-Y stage: Hoehn and Yahr stage; MDS-UPDRS III: Movement Disorder Society Unified Parkinson’s Disease Rating Scale motor score; NFOGQ: new freezing of gait questionnaire; LEDD: levodopa equivalent daily dose; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; HAMD: Hamilton Depression Scale. achi-square test; bvariance analysis; ctwo independent sample t-test.
Gait performances
Descriptive statistics of the gait performances during the Instrumented Stand and Walk test are shown in Table 2. Briefly, both patients with and without FOG had significantly reduced first step range of motion, stride length, stride velocity and turn peak velocity compared to healthy control subjects. Furthermore, post-hoc t-tests demonstrated that all of these measures were more significantly impaired in patients with FOG than in those without FOG. Post-hoc t-tests also revealed that patients with FOG had significantly longer total duration and turn duration, more turn steps, as well as more reduced arm swing amplitude compared to both healthy controls and patients without FOG. We report no differences in the other parameters among the three groups.
Table 2.
Descriptive statistics of gait performance in all participates.
| Measurements | Patients with FOG | Patients without FOG | Healthy controls | F | p | p1 | p2 | p3 |
|---|---|---|---|---|---|---|---|---|
| Total Duration | 59.01 ± 25.87 | 46.99 ± 6.33 | 42.07 ± 1.80 | 10.130 | 0.000* | 0.003# | 0.000# | 0.206 |
| Step Initiation | ||||||||
| APA duration (s) | 0.51 ± 0.24 | 0.58 ± 0.32 | 0.56 ± 0.31 | 0.598 | 0.552 | 0.292 | 0.445 | 0.764 |
| APA latency (s) | 0.83 ± 1.27 | 0.68 ± 0.95 | 0.36 ± 0.16 | 2.148 | 0.123 | 0.515 | 0.045 | 0.174 |
| First step range of motion (degrees) | 22.80 ± 9.27 | 32.94 ± 9.63 | 40.61 ± 7.42 | 32.289 | 0.000* | 0.000# | 0.000# | 0.001# |
| First step latency (s) | 0.83 ± 1.27 | 0.68 ± 0.95 | 0.36 ± 0.16 | 2.148 | 0.123 | 0.515 | 0.045 | 0.174 |
| Straight Walking | ||||||||
| Stride length (m) | 0.98 ± 0.29 | 1.26 ± 0.18 | 1.40 ± 0.09 | 34.927 | 0.000* | 0.000# | 0.000# | 0.006# |
| Stride velocity (m/s) | 0.94 ± 0.27 | 1.16 ± 0.19 | 1.34 ± 0.09 | 33.357 | 0.000* | 0.000# | 0.000# | 0.000# |
| Cadence (steps/min) | 118.92 ± 22.00 | 110.53 ± 8.71 | 115.00 ± 8.14 | 2.640 | 0.077 | 0.024# | 0.282 | 0.221 |
| Double support time (% gait cycle) | 23.65 ± 8.02 | 21.71 ± 4.66 | 20.91 ± 4.08 | 1.831 | 0.166 | 0.192 | 0.066 | 0.590 |
| Swing time (% gait cycle) | 38.17 ± 4.01 | 39.15 ± 2.33 | 39.54 ± 2.04 | 1.831 | 0.166 | 0.192 | 0.066 | 0.590 |
| Stance time (% gait cycle) | 61.83 ± 4.01 | 60.85 ± 2.33 | 60.46 ± 2.04 | 1.831 | 0.166 | 0.192 | 0.066 | 0.590 |
| Arm swing amplitude (degrees) | 9.73 ± 6.65 | 14.34 ± 9.40 | 16.79 ± 10.56 | 4.907 | 0.010* | 0.049# | 0.003# | 0.290 |
| Turning | ||||||||
| Turn duration (s) | 5.34 ± 3.21 | 3.03 ± 0.71 | 2.28 ± 0.50 | 21.710 | 0.000* | 0.000# | 0.000# | 0.123 |
| Turn steps | 9.89 ± 4.09 | 6.00 ± 1.28 | 4.88 ± 0.93 | 34.060 | 0.000* | 0.000# | 0.000# | 0.080 |
| Turn peak velocity (degrees/sec) | 100.46 ± 39.39 | 129.07 ± 24.57 | 164.79 ± 36.51 | 28.133 | 0.000* | 0.001* | 0.000* | 0.000* |
FOG: freezing of gait; APA: anticipatory postural adjustments. p1: patients with FOG vs patients without FOG; p2: patients with FOG vs healthy controls; p3: patients without FOG vs healthy controls. *p < 0.001 in the ANOVA analyses; #p < 0.05 in the post-hoc analyses.
Comparison of ALFF values among groups
As mentioned in the Method, we excluded 6 subjects (2 patients with FOG, 3 patients without FOG and 1 healthy control) due to the remarkable head motion. Thus, our final ALFF analysis included 29 patients with FOG, 28 patients without FOG, and 31 healthy controls.
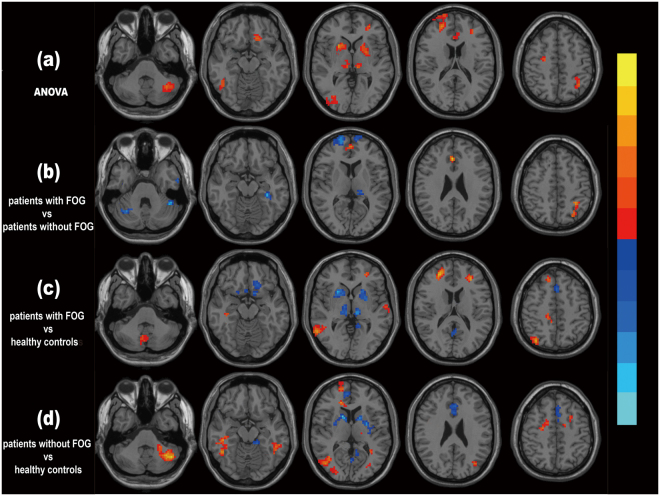
Figure 1a shows the brain regions presenting with significant differences in the ANOVA analysis. The three groups had significantly different ALFF values in the following regions: bilateral putamen and thalamus, left anterior cingulate cortex, Crus I of the left cerebellum, right superior frontal gyrus (SFG), left SFG (orbital part), left middle frontal gyrus, right inferior temporal gyrus, left inferior parietal lobule (IPL), right precentral gyrus and right middle occipital gyrus.
Figure 1.
Differences in ALFF values among groups. (a) ANOVA results of the ALFF values among the three groups. (b) Post-hoc analysis showing differences in ALFF values between patients with FOG and without FOG, with MDS-UPDRS III score, H-Y stage, disease duration and LEDD as covariates. (c) Post-hoc analysis showing differences in ALFF values between patients with FOG and healthy controls. (d) Post-hoc analysis showing differences in ALFF values between patients without FOG and healthy controls. Brain regions presenting with significantly increased ALFF values are shown in hot colors, whereas decreased ALFF values are shown in cold colors. P value thresholds were set at a corrected P < 0.05 with a voxel-level p < 0.001, determined by AlphaSim correction.
Figure 1b–d shows the brain regions presenting with significant differences in the post-hoc analysis. Compared to those without FOG, patients with FOG had decreased ALFF values in the bilateral Crus I of cerebellum, bilateral SFG and left thalamus; along with increased ALFF values in the right anterior cingulate cortex and left IPL. Comparison between patients with FOG and healthy controls revealed that, ALFF values in the bilateral putamen and thalamus, left inferior frontal gyrus (orbital part), left supplementary motor area and left precuneus were decreased; while ALFF values in the lobule VIII of vermis, left middle frontal gyrus, right SFG, right superior parietal lobule and right middle temporal gyrus were increased. Post-hoc t test also demonstrated that relative to healthy controls, patients without FOG had decreased ALFF values in the bilateral putamen and anterior cingulate cortex, left supplementary motor area and right precuneus; and increased ALFF values in the left Crus I of cerebellum, right SFG, right middle occipital gyrus and bilateral inferior temporal gyrus (Table 3).
Table 3.
Differences of ALFF values among the groups.
| Brain regions (AAL atlas) | MNI coordinates | T value | Cluster size (mm3) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| ANOVA result | |||||
| Thalamus_R | 13 | −22 | 1 | 4.401 | 137 |
| Occipital_Mid_R | 30 | −84 | 2 | 4.718 | 65 |
| Cingulum_Ant_L | −3 | 21 | 30 | 5.163 | 107 |
| Temporal_Inf_R | 45 | −55 | −14 | 5.677 | 78 |
| Precentral_R | 30 | −9 | 54 | 6.274 | 118 |
| Parietal_Inf_L | −36 | −54 | 51 | 6.947 | 45 |
| Putamen_L | −20 | 3 | 10 | 7.079 | 260 |
| Frontal_Mid_L | −30 | 48 | 3 | 7.321 | 65 |
| Cerebellum_Crus1_L | −36 | −75 | −30 | 7.437 | 210 |
| Thalamus_L | −18 | −21 | 15 | 8.368 | 53 |
| Frontal_Sup_R | 24 | 48 | 18 | 8.939 | 149 |
| Frontal_Sup_Orb_L | −15 | 27 | −18 | 9.575 | 55 |
| Putamen_R | 24 | 15 | −3 | 13.457 | 494 |
| Patients with FOG vs patients without FOG | |||||
| Thalamus_L | −9 | −18 | 15 | −2.930 | 39 |
| Cerebellum_Crus1_L | −44 | −47 | −30 | −3.293 | 284 |
| Frontal_Sup_R | 30 | 63 | 15 | −3.604 | 113 |
| Cerebellum_Crus1_R | 36 | −63 | −27 | −4.140 | 53 |
| Parietal_Inf_L | −33 | −48 | 51 | 3.257 | 55 |
| Cingulum_Ant_R | 6 | 42 | 3 | 3.584 | 165 |
| Patients with FOG vs healthy controls | |||||
| Precuneus_L | 0 | −54 | 21 | −2.893 | 66 |
| Supp_Motor_Area_L | 0 | 21 | 48 | −3.228 | 38 |
| Putamen_L | −23 | 6 | −6 | −3.316 | 239 |
| Thalamus_R | 12 | −14 | 8 | −3.432 | 126 |
| Frontal_Inf_Orb_L | −18 | 27 | −18 | −3.840 | 122 |
| Thalamus_L | −10 | −20 | 8 | −3.936 | 167 |
| Putamen_R | 24 | 15 | −3 | −4.626 | 370 |
| Vermis_8 | 5 | −64 | −36 | 2.612 | 48 |
| Temporal_Mid_R | 60 | −54 | 0 | 3.575 | 108 |
| Parietal_Sup_R | 36 | −72 | 51 | 3.814 | 49 |
| Frontal_Mid_L | −30 | 39 | 15 | 3.968 | 137 |
| Frontal_Sup_R | 24 | 48 | 18 | 4.064 | 90 |
| Patients without FOG vs healthy controls | |||||
| Putamen_R | 23 | 8 | −1 | −2.710 | 66 |
| Cingulum_Ant_R | 4 | 21 | 28 | −2.799 | 96 |
| Cingulum_Ant_L | −3 | 20 | 26 | −2.824 | 125 |
| Putamen_L | −21 | 8 | 0 | −2.952 | 106 |
| Supp_Motor_Area_L | −3 | −3 | 63 | −3.359 | 57 |
| Precuneus_R | 0 | −48 | 72 | −4.135 | 160 |
| Temporal_Inf_L | −48 | −43 | −15 | 2.952 | 138 |
| Temporal_Inf_R | 49 | −51 | −15 | 3.120 | 124 |
| Occipital_Mid_R | 34 | −84 | 3 | 3.895 | 563 |
| Frontal_Sup_R | 24 | 48 | 18 | 3.961 | 801 |
| Cerebellum_Crus1_L | −33 | −75 | −30 | 4.147 | 424 |
MNI: Montreal Neurological Institute; AAL: Automated Anatomical Labeling.
Relationships between ALFF values and FOG severity in patients with FOG
Figure 2a–d shows brain regions that were proven to be significantly correlated with four measures for FOG severity in the FOG group, including one subjective measure (NFOGQ-II) and three relative objective measures/gait parameters (first step range of motion, stride length and turn steps).
Figure 2.
Correlation between ALFF values and FOG severity as well as Executive Function sub-score in PD patients with FOG. (a) Pearson’s correlation analysis between ALFF values and NFOGQ-II score. (b) Pearson’s correlation analysis between ALFF values and first step range of motion; (c) Pearson’s correlation analysis between ALFF values and stride length; (d) Pearson’s correlation analysis between ALFF values and turn steps; (e) Pearson’s correlation analysis between ALFF values and Executive Function sub-score. Brain regions showing a significantly increased positive correlation are shown in hot colors, whereas those showing a negative correlation are shown in cold colors. P value thresholds were set at a corrected P < 0.05 with a voxel-level p < 0.001, determined by AlphaSim correction.
Correlation analysis between ALFF values and NFOGQ-II showed that ALFF values in the bilateral putamen, thalamus and precentral gyrus, right supplementary motor area, left precuneus, right inferior temporal gyrus and left IPL were negatively correlated with NFOGQ-II score; whereas ALFF values in the right Crus II of cerebellum, left anterior cingulate cortex and right SFG were positively correlated with NFOGQ-II score. Correlation analysis between ALFF values and first step range of motion demonstrated a negative correlation in bilateral globus pallidus, left SFG and left precentral gyrus. Correlation analysis between ALFF values and stride length showed a negative correlation in bilateral SFG and globus pallius, left anterior cingulate cortex, right insula and left middle temporal gyrus; and a positive correlation in bilateral postcentral gyrus, left superior parietal cortex and left superior occipital gyrus. Correlation between ALFF values and turn steps revealed a negative correlation in bilateral postcentral gyrus, left inferior parietal cortex and left precuneus, as well as a positive correlation in bilateral globus pallidus and right SFG (Table 4).
Table 4.
Correlation between ALFF values and FOG severity in PD patients with FOG.
| Brain regions (AAL atlas) | MNI coordinates | T value | Cluster size (mm3) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Correlation between ALFF values and NFOGQ-II score | |||||
| Parietal_Inf_L | −45 | −39 | 51 | −0.523 | 83 |
| Supp_Motor_Area_R | 7 | −19 | 67 | −0.530 | 115 |
| Precuneus_L | −9 | −46 | 60 | −0.548 | 130 |
| Putamen_L | −30 | −6 | 6 | −0.566 | 36 |
| Thalamus_R | 9 | −21 | 6 | −0.587 | 46 |
| Thalamus_L | −15 | −18 | 12 | −0.589 | 44 |
| Temporal_Inf_R | 39 | −60 | −6 | −0.624 | 168 |
| Precentral_R | 48 | −9 | 48 | −0.641 | 424 |
| Putamen_R | 33 | −3 | 9 | −0.723 | 75 |
| Precentral_L | −45 | −6 | 30 | −0.723 | 303 |
| Cingulum_Ant_L | −9 | 39 | 0 | 0.502 | 36 |
| Cerebellum_Crus2_R | 24 | −90 | −27 | 0.581 | 50 |
| Frontal_Sup_R | 30 | 33 | 54 | 0.616 | 86 |
| Correlation between ALFF values and first step range of motion | |||||
| Pallidum_L | −19 | 7 | 6 | −0.481 | 61 |
| Precentral_L | −30 | −3 | 46 | −0.496 | 50 |
| Precentral_L | −30 | −3 | 46 | −0.496 | 50 |
| Frontal_Sup_L | −15 | 24 | 51 | −0.583 | 42 |
| Temporal_Mid_L | −54 | −30 | 3 | −0.641 | 59 |
| Pallidum_R | 18 | 12 | 9 | −0.650 | 40 |
| Cerebellum_Crus1_R | 27 | −88 | −27 | −0.696 | 97 |
| Correlation between ALFF values and stride length | |||||
| Pallidum_L | −19 | −1 | 5 | −0.454 | 58 |
| Pallidum_R | 16 | 5 | 5 | −0.473 | 65 |
| Insula_R | 43 | 0 | 5 | −0.517 | 62 |
| Frontal_Sup_R | 10 | 62 | 8 | −0.543 | 168 |
| Cingulum_Ant_L | −4 | 42 | 21 | −0.554 | 248 |
| Temporal_Mid_L | −54 | −33 | 6 | −0.665 | 78 |
| Frontal_Sup_L | −27 | 57 | 6 | −0.672 | 331 |
| Parietal_Sup_L | −28 | −45 | 61 | 0.515 | 128 |
| Occipital_Sup_L | −7 | −99 | 21 | 0.581 | 195 |
| Postcentral_L | −57 | −9 | 39 | 0.604 | 135 |
| Postcentral_R | 42 | −9 | 48 | 0.621 | 234 |
| Correlation between ALFF values and turn steps | |||||
| Parietal_Inf_L | −27 | −45 | 54 | −0.509 | 52 |
| Postcentral_L | −48 | −9 | 40 | −0.537 | 154 |
| Precuneus_L | −6 | −57 | 66 | −0.599 | 54 |
| Postcentral_R | 48 | −6 | 28 | −0.666 | 172 |
| Pallidum_L | −17 | 5 | 2 | 0.520 | 92 |
| Frontal_Sup_R | 21 | 21 | 63 | 0.587 | 48 |
| Pallidum_R | 13 | 4 | 2 | 0.692 | 108 |
MNI: Montreal Neurological Institute; AAL: Automated Anatomical Labeling.
Relationships between ALFF values and Executive Function sub-score in patients with FOG
Figure 2e exhibits the brain regions that were significantly correlated with the Executive Function sub-score. Correlation analysis between ALFF values and Executive Function sub-score showed a negative correlation in the right Crus I of cerebellum, left SFG, left precuneus and left middle occipital gyrus, as well as a positive correlation in the left putamen and right thalamus (Table 5).
Table 5.
Correlation between ALFF values and Executive Function sub−score in PD patients with FOG.
| Brain regions (AAL atlas) | MNI coordinates | T value | Cluster size (mm3) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Precuneus_L | −5 | −58 | 26 | −0.416 | 111 |
| Cerebellum_Crus1_R | 36 | −55 | −38 | −0.430 | 173 |
| Occipital_Mid_L | −36 | −88 | 26 | −0.482 | 257 |
| Frontal_Sup_L | −13 | 61 | 26 | −0.485 | 96 |
| Thalamus_R | 11 | −18 | 0 | 0.482 | 73 |
| Putamen_L | −24 | 9 | 0 | 0.599 | 50 |
MNI: Montreal Neurological Institute; AAL: Automated Anatomical Labeling.
Discussion
The current study is the first to investigate the pattern of resting-state spontaneous brain activity in PD patients with FOG. In the present study, we used inertial sensors to acquire gait parameters during gait which is powerful and also permits more precise and specific correlations between clinical data and functional MRI results. Results presented here demonstrate that FOG is associated with decreased ALFF value in the prefrontal cortex, as well as increased ALFF values in the parietal cortex and anterior cingulate cortex (ACC). In addition, we also found that FOG is positively correlated with ALFF values in the bilateral globus pallidus and negatively correlated with ALFF values in the bilateral sensorimotor regions and thalamus. These findings indicate that FOG in PD is likely to be due to the impaired processing in the frontal and parietal regions8, and may also be associated with increased basal ganglia inhibitory output, which lead to decreased information processing in the thalamus and brainstem9,19. Interestingly, we also found that altered activity of Crus I of cerebellum plays a critical role in the pathophysiology of FOG.
The key spatiotemporal findings observed during straight walking in our study are consistent with those from most previous studies, demonstrating that patients with FOG walk more slowly with a shorter stride length than those without FOG20,21. No significant difference in cadence was found among the groups, indicating that PD patients are capable of modulating cadence, thereby at least partly compensating for their smaller step length22,23. Reduced arm swing amplitude is reflective of bradykinesia in the upper body, and has been reported to be associated with an increased risk of falls for patients with PD24. Patients with FOG have significantly reduced arm swing amplitude, which may be associated with the more advanced disease stage25. It has been suggested that patients with FOG spend more time in the double-support phase of gait26. Although not significant, a tendency of longer double support time was documented in our study, which could be due to the fact that patients were only moderately affected by FOG. With respect to gait initiation, patients with FOG show a much lower first step range of motion compared to patients without FOG. Unlike the spatial control of APAs, relative timing (APAs duration and latency, first step latency) is unaffected in PD patients27. As expected, turning in patients with FOG is characterized by slow velocity, additional steps and time needed to complete the turn28. Our results provide further evidence for the pronounced impairments during gait initiation and turning in patients with FOG2.
In patients with FOG, there was a decreased ALLF value in the right SFG and a concomitant increased ALLF value in the left IPL compared to patients without FOG. Our findings are consistent with a number of previous neuroimaging experiments that have implicated frontoparietal dysfunction in the pathophysiology of freezing8,9,14. The prefrontal and posterior parietal cortices have previously been suggested to co-activate as a functional network, described as the cognitive control network29. One recent task-based fMRI study demonstrated that regions within this network are significantly activated during freezing episodes, which underlies a compensatory recruitment of regions that are attempting to overcome a freezing episode9. Although both of the two PD groups had higher activity in the right SFG than healthy controls, the less increased ALFF value in patients with FOG might represent an unsatisfied or failing compensatory mechanism. Moreover, there was a positive correlation between the ALFF value in the right SFG and the severity of the FOG. These findings indicate that the spontaneous neural activity in the right SFG is decreased in patients with FOG compared to those without, and this decreased activity becomes more significant as FOG progresses. On the other hand, theoretically, the increased ALFF in the left IPL in patients with FOG might reflect a compensatory or pathological effect when comparing to patients without FOG. However, pathological impairments should be more severe as FOG progresses, which is inconsistent with our finding of the negative correlations with the NFOGQ-II score and turn steps. Therefore, we suggested that the increased ALFF value in the left IPL is more likely a reflection of the compensatory effect; however, this effect becomes less significant as the symptom progresses. Of note, a previous resting-state fMRI study14 revealed that patients with FOG exhibit significantly reduced functional connectivity in the frontal and parietal regions. These findings indicate that not only the functional connectivity but also the spontaneous neural activity within the frontal and parietal regions is impaired in patients with FOG.
Our results also show that the ALFF value in the left thalamus was significantly decreased in patients with FOG when comparing to patients without FOG. In addition, the ALFF values in the bilateral thalamus and putamen were negatively correlated with FOG severity. The thalamus and brainstem locomotor region, particularly the pedunculopontine nucleus, are the major terminals of the corticostriatal projections, receiving inhibitory outputs arising from the basal ganglia. Lewis and Barker proposed an “interference model” and explained the occurrence of FOG as a momentary breakdown of concurrent information processing of those competing yet complementary tasks, such as cognitive and limbic load during motor tasks. Decreased neural reserve in the basal ganglia leads to a “cross-talk” within these competing inputs; consequently, a paroxysmal excessive inhibition of the thalamus and pedunculopontine nucleus are induced, thus triggering a freezing episode19. The negative correlation between ALFF value in the left thalamus and the NFOGQ-II score indicates that this dysfunction becomes more pronounced as the FOG progresses.
The cerebellum is one of the major subcortical structures that influence multiple aspects of motor, cognitive and affective behavior30. Growing evidence suggests that the cerebellum plays a major role in the pathophysiology of PD, including both pathological and compensatory effects31. However, the implication of cerebellar dysfunction in FOG is rare. One previous diffusion tensor imaging study demonstrated that FOG is associated with poor white matter connectivity between the pedunculopontine nucleus and the cerebellum12. A more recent study utilizing the lesion network mapping technique identified that lesions causing FOG are located within a common functional network characterized by connectivity to the cerebellar locomotor region32. Among the cerebellum’s complicated lobular division, Crus I and II of cerebellum sends and receives projections from prefrontal cortex area33, forming a closed-loop circuit and linking to association networks involved with executive control34. We found that patients with FOG, in the present study, had significantly lower ALFF value in the bilateral Crus I of cerebellum than patients without FOG, whereas had no significant difference with healthy controls. Previous studies showed that during the cognitive performance, metabolism in the cerebellum is increased in PD patients35, which might play a compensatory effort to maintain the cognitive function12. Due to the cognitive feature of FOG36–38, we therefore proposed that, presumably, the failure to increase activity for the Crus I of cerebellum might reflect its impaired compensatory effort in patients with FOG. Our findings provide evidence that cerebellar dysfunction might also play a role in the pathophysiology of FOG, however, further investigations are needed to clarify the role of cerebellum plays in FOG.
Functions of the ACC are central to intelligent behaviors, including emotional self-control, focused problem solving, adaptive response to changing conditions, and switching action plans39–41. It has been suggested that dysfunctions within this area might predispose individuals to FOG42. Our finding of increased ALFF value in the right ACC in patients with FOG compared to those without FOG is consistent with the results from a previous task-based fMRI study9. This increased ALFF might be a compensatory effect to improve the limited ability to switch between motor programs in patients with FOG. The positive correlation of ALFF with the NFOGQ-II score suggests that this compensatory effect may become stronger as the FOG progresses.
In addition, we found that the impaired performances in FOG (first step range of motion, stride length, and turn steps) were all correlated with the ALFF value changes in the bilateral sensorimotor regions and globus pallidus. According to the “interference model” mentioned above, abnormally increased inhibitory outputs originate from the internal pallidum lead to an excessive inhibition of the thalamus and brainstem; consequently, a freezing episode might be triggered19. According to the classic basal ganglia model43, basal ganglia dysfunction leads to the excessive inhibition in the sensorimotor areas, which in turn results in some motor deficits in PD (e.g., akinesia).
We also found that the first step range of motion was negatively correlated with ALFF value in the left middle temporal gyrus. Functions of the middle temporal gyrus might be involved in conflict resolution of multiple inputs from vestibular and other sensory afferents during gait initiation44. Our findings indicate that abnormal activity in the temporal cortex is associated with the impaired gait initiation in PD patients with FOG. The stride length during straight walking was associated with abnormal activity within the temporal and occipital cortices. These areas contribute to the integration of visual sensory information, which is damaged in FOG45. Using FDG-PET, Lyoo et al. reported that metabolism within these regions was decreased in PD patients with FOG, and such hypometabolism limited the efficiency of STN-DBS on FOG46. Additionally, we found that turn steps were associated with activity in the left precuneus. One recent resting-state fMRI study also demonstrated that hyper-connectivity involving the precuneus was correlated with a higher step number during dual-task turning47. Both of these studies suggested that abnormal activity in the precuneus might play a role in turning in patients with FOG.
It has been generally suggested that FOG is significantly correlated with cognitive decline, particularly executive dysfunction37. Unfortunately, we did not find any significant difference in the total score or the Executive Function sub-score of MoCA among the three groups. This discrepancy might be attributed to the fact that we only assessed MoCA to evaluate the cognitive function, rather than using other assessments which are specific to test the executive function. Despite this limitation, we found that the spontaneous activity in the right Crus I of cerebellum had negative correlation, while the spontaneous activity in the left SFG and left putamen had positive correlations with the Executive Function sub-score. Previous functional imaging studies showed that the underactivity and pathological disorganization within the frontostriatal networks are involved in the executive dysfunction in PD patients48,49. Lewis et al.48, using event-related fMRI, found that the executively impaired PD patients exhibit significantly less activation in the dorsolateral and ventrolateral prefrontal cortex as well as putamen compared to patients with no such deficit. Consistent with their study, our finding of positive correlation with ALFF in the SFG and putamen demonstrated that the spontaneous activity within these regions decreased as the executive dysfunction progresses. Additionally, the involvement of cerebellum in the executive functions has been well established50–52, and the interconnection between the cerebellum and the prefrontal cortex plays an important role in the pathogenesis of executive function impairment50,53. In the present study, the negative correlation between the ALFF in the Crus I of cerebellum and Executive Function sub-score might be a reflection of the cerebellar compensatory effect to maintain the executive function in patients with FOG. Further studies are needed to investigate the executive dysfunction in PD patients with FOG.
Our study has some limitations that should be considered. First, our patients with FOG had greater disease severity, longer duration, and greater LEDD compared to patients without FOG. As we asked the patients to withdraw the medications at least 12 h before fMRI scanning, the confounding from mismatched levodopa dosage could be reduced. Moreover, to minimize the above potential confounds, we included MDS-UPDRS III, H-Y stage, disease duration, and LEDD as covariates when comparing the ALFF values between patients with FOG and without FOG. Second, the gait analyses performed in the present study are an indirect reflection of FOG. We did not measure other objective measures except for the NFOGQ (for instance, the percentage of time spent freezing during the Stand and Walk trials). Third, we did not evaluate assessments that are specific to the executive function, as discussed above. Future studies with these measurements are warranted to improve our understanding on FOG.
In conclusion, the present study demonstrates that there are spontaneous brain activity changes in the resting state in PD patients with FOG. Our findings suggest that FOG in PD is associated with impairment within frontal and parietal regions, along with increased basal ganglia inhibitory output. Also, we proposed that cerebellar dysfunction might be involved in the pathophysiology of FOG in PD patients. Future investigation of longitudinal changes in brain activity during the progression of FOG may provide more information.
Methods
Participants
We studied 62 PD patients (31 with FOG and 31 without FOG) recruited from the Movement Disorders Clinic of the Xuanwu Hospital of Capital Medical University. Exclusion criteria were: history of deep brain stimulation surgery; marked rest tremor; presence of contraindications for MRI; MMSE score ≤24; comorbidities of neurological disease other than PD. A group of 32 healthy sex- and age-matched volunteers served as controls. The study was approved by the local Medical Ethics Committee and written informed consent was obtained from all participants prior to the experiment. The experiments were performed according to the Declaration of Helsinki and were approved by the Institutional Review Board of Xuanwu Hospital.
Patients were classified as “with FOG” and “without FOG” based on the positive response to Part I of the NFOGQ54, a dichotomous question that asks whether FOG episodes were experienced during the past month or not. Recognition of FOG episodes was illustrated by the presentation of a short video (70 s) demonstrating typical freezing phenotypes. NFOGQ has previously been shown to be a reliable tool to detect FOG55. In 27 of the 31 (87%) self-reported freezers, FOG was confirmed during clinical testing or spontaneous behavior. None of the patients classified as non-freezers demonstrated FOG episodes during the physical examination.
Clinical assessments
Clinical assessments of patients were conducted in their practical off state, that is, at least after a 12-hour withdrawal of anti-Parkinson medication. The MDS-UPDRS III and H-Y stage assessed motor disability and disease severity. MoCA assessed general cognitive function56, and HAMD measured affective symptoms. Moreover, the executive domain of MoCA (trail making, phonemic fluency, and verbal abstraction; 4 points) was evaluated to assess executive function56. Parts II and III of the NFOGQ were used to evaluate FOG severity and disability, respectively.
Quantitative gait analysis
All participants underwent quantitative assessments of gait using Opal inertial sensors, Mobility Lab, the clinical user interface and automated algorithms by Ambulatory Parkinson’s Disease Monitoring (APDM Inc. http://apdm.com). Subjects wore six Opal sensors composed of 3D accelerometers, 3D gyroscopes, and 3D magnetometers. The sensors were positioned with Velcro belts on the posterior trunk, on the anterior shank of each leg, on each wrist, and on the sternum. Data were acquired and automatically analyzed with MobilityLab57,58. All participants performed four trials of the Instrumented Stand and Walk test, designed to assess several domains of gait initiation, straight walking and turning59. The Instrumented Stand and Walk test consisted of standing quietly for 30 seconds, followed by initiating gait with the most involved leg or dominant leg, walk 7 meters, turn 180 degrees after crossing a line on the ground, and return to the initial position. During quiet standing, subjects were asked to keep their arms at their sides and look straight ahead. A template was used to achieve consistent foot placement with 10 cm between heels and a 30-degree outward rotation of the feet58. We measured gait initiation (anticipatory postural adjustments duration and latency, first step range of motion and first step latency), straight walking (stride length, stride velocity, cadence, double support time%, swing time %, stance time % and arm swing amplitude) and turning (turn duration, turn steps and peak velocity) in each subject.
Functional MRI acquisition
Imaging was carried out in a SIEMENS Trio 3 T scanner. Participants were instructed to keep their head still and eyes closed during scanning. Earplugs and a head coil with foam pads were used to minimize machine noise and head motion. fMRI scans were acquired following a 12-hour period of medication withdrawal in all patients. For each participant, we acquired high-resolution T1- and T2-weighted anatomical images, and a radiologist viewed the images to exclude participants with space-occupying lesions and cerebrovascular diseases. BOLD images were obtained using the following SE-EPI sequence: repetition time = 2000 ms, echo time = 30 ms, voxel size = 3.0 × 3.0 × 3.0 mm3, slice thickness/gap = 4.0/0 mm, axial slices = 33 layers, flip angle = 90°, FOV = 256 mm × 256 mm, matrix size = 64 × 64, and scanning time = 8 min.
Data preprocessing
Data were preprocessed and analyzed using DPABI version 2.2 (http://rfmri.org/dpabi) and SPM12 software package (http://www.fil.ion.ucl.ac.uk/spm). The first 10 time points were discarded to allow the magnetization to approach a dynamic equilibrium and to allow participants to get used to the scanning noise. The remaining images were corrected for slice timing with the middle slice as a reference, realigned to remove excessive head motion. EPI data were then normalized into a standard brain space template (the Montreal Neurological Institute template), and resampled to 3.0 × 3.0 × 3.0 mm isotropic voxels. Linear trends were removed and images were smoothed with a 6-mm Gaussian kernel to increase the signal-to-noise ratio. We further reduced potential confounds of head motion with Friston-24 correction using Friston24-parameter model (6 head motion parameters, 6 head motion parameters one time point before and the 12 corresponding squared items60). To reduce possible effects of physiological artifacts, the nuisance covariates of cerebrospinal fluid signal and white matter signal were finally regressed-out. For each participate, the instantaneous head motion was calculated along with frame-wise displacement (FD) as defined by Jenkinson et al.61, which was preferable for its consideration of voxel-wise differences in its derivation62. Subjects were excluded from analysis if their head motion (mean FD Jenkinson) was greater than mean + 2 * SD (threshold 0.25 mm)63. FD correction led to exclusion of 6 participants (patients with FOG: n = 2; patients without FOG: n = 3; healthy controls: n = 1).
ALFF calculation
ALFF calculation was performed using RS-fMRI Data Processing Toolkit REST version 1.8 (REST, http://rest.restfmri.net)64. Each time series was transformed to the frequency domain through fast Fourier transform. The square root of the power spectrum was computed and the averaged square root obtained across 0.01–0.08 Hz was taken as the ALFF measurement. The resultant ALFF of each voxel value was then further divided by the global mean value for standardization.
Statistical analysis
Clinical characteristics
Demographic data were presented as mean ± SD for continuous variables. Independent two samples t-test and one-way analysis of covariance (ANOVA) were performed for the comparison of continuous variables, and the chi-squared test was used to compare categorical variables. Post-hoc analyses were then used to assess group differences in performance on MMSE, MoCA, and HAMD, as well as all the metrics of gait derived from the Opal sensors. The threshold for the level of significance was set at α = 0.05.
Functional imaging analysis
One-way ANOVA was performed to identify differences of regional ALFF values among patients with FOG, patients without FOG and healthy controls. Brain areas showing significant differences (voxel-level p < 0.001, cluster size >945 mm3/35 voxels, corresponding to a corrected p < 0.05 as determined by AlphaSim correction) in the ANOVA analysis were then extracted as a mask. Post-hoc analyses within this mask were used to explore pair-wise differences among the three groups. To account for the differences in motor severity, disease duration, H-Y stage, MDS-UPDRS III score and LEDD, these features were applied as covariate variables in the comparison between patients with and without FOG. The same AlphaSim correction was also used in the post-hoc analyses.
To explore the relationship between brain activity and the severity of FOG, Pearson’s correlation was computed between the ALFF values and several FOG measures, including the subjective measure (NFOGQ-II) as well as the objective measures/gait parameters which were more pronounced impaired or specific in patients with FOG. As shown in the Results of Gait Performances/Table 2, the first step range of motion, stride length, stride velocity and turn peak velocity were more pronounced impaired in PD patients with FOG, while turn duration, turn steps and arm swing amplitude were relatively specific to FOG. We then chose three of the above parameters from the three different gait domains to be performed in the correlation analyses, that is, “first step range of motion” from step initiation, “stride length” from straight walking, and “turn steps” from turning. In addition, although no significant difference was found among the three groups, we also performed the correlation analysis between ALFF values and Executive Function sub-score due to the cognitive nature of FOG36–38.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Acknowledgements
This work was supported by the grants from the Beijing Municipal government (PXM2017_026283_000002), Beijing Municipal Science & Technology Commission (Z161100005116011; Z171100000117013), Beijing Municipal Administration of Hospitals’ Mission Plan (SML20150803), National Natural Science Foundation of China (61473196), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (ZYLX201609), and Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2012BAI10B04).
Author Contributions
Chan P. and Wu T. designed the experiments and revised the manuscript; Mi T., Mei S. and Gao L. carried out data collection; Mi T. carried out data analysis and drafted the manuscript; Liang P. and Li K. carried out functional fMRI scan.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-23233-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tao Wu, Email: wutao69@gmail.com.
Piu Chan, Email: pbchan@hotmail.com.
References
- 1.Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Movement disorders: official journal of the Movement Disorder Society. 2008;23(Suppl 2):S423–425. doi: 10.1002/mds.21927. [DOI] [PubMed] [Google Scholar]
- 2.Nutt JG, et al. Freezing of gait: moving forward on a mysterious clinical phenomenon. The Lancet Neurology. 2011;10:734–744. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Movement disorders: official journal of the Movement Disorder Society. 2007;22:2192–2195. doi: 10.1002/mds.21659. [DOI] [PubMed] [Google Scholar]
- 4.Schaafsma JD, et al. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2003;10:391–398. doi: 10.1046/j.1468-1331.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- 5.Giladi N. Gait disturbances in advanced stages of Parkinson’s disease. Adv Neurol. 2001;86:273–278. [PubMed] [Google Scholar]
- 6.Giladi N. Freezing of gait. Clinical overview. Adv Neurol. 2001;87:191–197. [PubMed] [Google Scholar]
- 7.Yogev-Seligmann, G., Hausdorff, J. M. & Giladi, N. The role of executive function and attention in gait. Movement disorders: official journal of the Movement Disorder Society23, 329–342; quiz 472, 10.1002/mds.21720 (2008). [DOI] [PMC free article] [PubMed]
- 8.Bartels AL, Leenders KL. Brain imaging in patients with freezing of gait. Movement disorders: official journal of the Movement Disorder Society. 2008;23(Suppl 2):S461–467. doi: 10.1002/mds.21912. [DOI] [PubMed] [Google Scholar]
- 9.Shine JM, et al. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease. Brain: a journal of neurology. 2013;136:1204–1215. doi: 10.1093/brain/awt049. [DOI] [PubMed] [Google Scholar]
- 10.Shine JM, et al. Differential neural activation patterns in patients with Parkinson’s disease and freezing of gait in response to concurrent cognitive and motor load. PloS one. 2013;8:e52602. doi: 10.1371/journal.pone.0052602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fling BW, et al. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PloS one. 2014;9:e100291. doi: 10.1371/journal.pone.0100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fling BW, et al. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain: a journal of neurology. 2013;136:2405–2418. doi: 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tessitore A, et al. Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait. Parkinsonism & related disorders. 2012;18:781–787. doi: 10.1016/j.parkreldis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Zang YF, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Gao, L., Wu, X., Zhang, J., Chan, P. & Wu, T. Brain activity in Parkinson’s disease patients with mild cognitive impairment. (2016).
- 17.Hu X, et al. Altered Resting-State Brain Activity and Connectivity in Depressed Parkinson’s Disease. PloS one. 2015;10:e0131133. doi: 10.1371/journal.pone.0131133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, D. et al. Abnormal baseline brain activity in Parkinson’s disease with and without REM sleep behavior disorder: A resting-state functional MRI study. Journal of magnetic resonance imaging: JMRI, 10.1002/jmri.25571 (2016). [DOI] [PubMed]
- 19.Lewis SJ, Barker RA. A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism & related disorders. 2009;15:333–338. doi: 10.1016/j.parkreldis.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Knobl P, Kielstra L, Almeida Q. The relationship between motor planning and freezing of gait in Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 2012;83:98–101. doi: 10.1136/jnnp-2011-300869. [DOI] [PubMed] [Google Scholar]
- 21.Smulders, K., Dale, M. L., Carlson-Kuhta, P., Nutt, J. G. & Horak, F. B. Pharmacological treatment in Parkinson’s disease: Effects on gait. Parkinsonism & related disorders, 10.1016/j.parkreldis.2016.07.006 (2016). [DOI] [PMC free article] [PubMed]
- 22.Morris ME, Iansek R, Matyas TA, Summers JJ. Ability to modulate walking cadence remains intact in Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 1994;57:1532–1534. doi: 10.1136/jnnp.57.12.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowes SG, et al. Determinants of gait in the elderly parkinsonian on maintenance levodopa/carbidopa therapy. Br J Clin Pharmacol. 1990;30:13–24. doi: 10.1111/j.1365-2125.1990.tb03738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. Journal of neurology, neurosurgery, and psychiatry. 2002;72:721–725. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirelman A, et al. Arm swing as a potential new prodromal marker of Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2016;31:1527–1534. doi: 10.1002/mds.26720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hausdorff JM, et al. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Experimental brain research. 2003;149:187–194. doi: 10.1007/s00221-002-1354-8. [DOI] [PubMed] [Google Scholar]
- 27.Rosin R, Topka H, Dichgans J. Gait initiation in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 1997;12:682–690. doi: 10.1002/mds.870120509. [DOI] [PubMed] [Google Scholar]
- 28.Bengevoord A, et al. Center of mass trajectories during turning in patients with Parkinson’s disease with and without freezing of gait. Gait & Posture. 2016;43:54–59. doi: 10.1016/j.gaitpost.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 30.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 31.Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain: a journal of neurology. 2013;136:696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Annals of neurology. 2017;81:129–141. doi: 10.1002/ana.24845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.E KH, Chen SH, Ho MH, Desmond JE. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Human brain mapping. 2014;35:593–615. doi: 10.1002/hbm.22194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habas C, et al. Distinct cerebellar contributions to intrinsic connectivity networks. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C, et al. Metabolic brain networks associated with cognitive function in Parkinson’s disease. NeuroImage. 2007;34:714–723. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naismith SL, Shine JM, Lewis SJ. The specific contributions of set-shifting to freezing of gait in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2010;25:1000–1004. doi: 10.1002/mds.23005. [DOI] [PubMed] [Google Scholar]
- 37.Amboni M, Cozzolino A, Longo K, Picillo M, Barone P. Freezing of gait and executive functions in patients with Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2008;23:395–400. doi: 10.1002/mds.21850. [DOI] [PubMed] [Google Scholar]
- 38.Giladi N, Hausdorff JM. The role of mental function in the pathogenesis of freezing of gait in Parkinson’s disease. Journal of the neurological sciences. 2006;248:173–176. doi: 10.1016/j.jns.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex - The evolution of an interface between emotion and cognition. Ann Ny Acad Sci. 2001;935:107–117. doi: 10.1111/j.1749-6632.2001.tb03476.x. [DOI] [PubMed] [Google Scholar]
- 40.Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. Journal of neurophysiology. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- 41.Helmich RC, Aarts E, de Lange FP, Bloem BR, Toni I. Increased dependence of action selection on recent motor history in Parkinson’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:6105–6113. doi: 10.1523/JNEUROSCI.0704-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snijders AH, et al. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain: a journal of neurology. 2011;134:59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- 43.DeLong MR, Wichmann T. Basal Ganglia Circuits as Targets for Neuromodulation in Parkinson Disease. JAMA neurology. 2015;72:1354–1360. doi: 10.1001/jamaneurol.2015.2397. [DOI] [PubMed] [Google Scholar]
- 44.Wagner J, et al. Mind the bend: cerebral activations associated with mental imagery of walking along a curved path. Experimental brain research. 2008;191:247–255. doi: 10.1007/s00221-008-1520-8. [DOI] [PubMed] [Google Scholar]
- 45.Lee MS, Kim HS, Lyoo CH. “Off” gait freezing and temporal discrimination threshold in patients with Parkinson disease. Neurology. 2005;64:670–674. doi: 10.1212/01.WNL.0000151961.14861.BA. [DOI] [PubMed] [Google Scholar]
- 46.Lyoo CH, et al. Different cerebral cortical areas influence the effect of subthalamic nucleus stimulation on parkinsonian motor deficits and freezing of gait. Movement disorders: official journal of the Movement Disorder Society. 2007;22:2176–2182. doi: 10.1002/mds.21609. [DOI] [PubMed] [Google Scholar]
- 47.Vervoort G, et al. Dual-task-related neural connectivity changes in patients with Parkinson’ disease. Neuroscience. 2016;317:36–46. doi: 10.1016/j.neuroscience.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 48.Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lozza C, et al. Executive processes in Parkinson’s disease: FDG-PET and network analysis. Human brain mapping. 2004;22:236–245. doi: 10.1002/hbm.20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolcekova E, et al. Cognitive impairment in cerebellar lesions: a logit model based on neuropsychological testing. Cerebellum Ataxias. 2017;4:13. doi: 10.1186/s40673-017-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum (London, England) 2007;6:184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- 52.Karatekin C, Lazareff JA, Asarnow RF. Relevance of the cerebellar hemispheres for executive functions. Pediatric neurology. 2000;22:106–112. doi: 10.1016/S0887-8994(99)00128-9. [DOI] [PubMed] [Google Scholar]
- 53.Komaba Y, Osono E, Kitamura S, Katayama Y. Crossed cerebellocerebral diaschisis in patients with cerebellar stroke. Acta neurologica Scandinavica. 2000;101:8–12. doi: 10.1034/j.1600-0404.2000.00002.x. [DOI] [PubMed] [Google Scholar]
- 54.Nieuwboer A, Giladi N. The challenge of evaluating freezing of gait in patients with Parkinson’s disease. Br J Neurosurg. 2008;22(Suppl 1):S16–18. doi: 10.1080/02688690802448376. [DOI] [PubMed] [Google Scholar]
- 55.Nieuwboer A, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture. 2009;30:459–463. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 56.Chou KL, et al. A recommended scale for cognitive screening in clinical trials of Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2010;25:2501–2507. doi: 10.1002/mds.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehabil Med. 2010;46:239–248. [PMC free article] [PubMed] [Google Scholar]
- 58.Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB. Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease. Movement disorders: official journal of the Movement Disorder Society. 2015;30:1361–1370. doi: 10.1002/mds.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mancini, M. et al. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. J Bioeng Biomed SciSuppl 1, 007, 10.4172/2155-9538.S1-007 (2011). [DOI] [PMC free article] [PubMed]
- 60.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 61.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- 62.Yan CG, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan CG, Craddock RC, Zuo XN, Zang YF, Milham MP. Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. NeuroImage. 2013;80:246–262. doi: 10.1016/j.neuroimage.2013.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song XW, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS one. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.