Abstract
Background
Heath-related quality of life (HRQoL) among survivors with unresectable locally-advanced non-small cell lung cancer (LA-NSCLC) treated with radiotherapy and chemotherapy still is not clear. The current study were performed to determine HRQoL for long-term survivors with unresectable LA-NSCLC and to identify risk factors for poor HRQoL.
Methods
Among patients with LA-NSCLC receiving radiotherapy and chemotherapy between January 2006 and December 2010, 82 long-term survivors beyond 5 years were identified in this cross-sectional study. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30 and the lung cancer-specific questionnaire QLQ-LC13 were employed to gather information on HRQoL. HRQoL scores were compared between different subgroups to analyze factors related to HRQoL.
Results
Fifty-five out of 82 (67%) long-term survivors completed the HRQoL survey. They reported a mild reduction in global health status and physical and emotional functioning. Fatigue, dyspnea, coughing, and financial difficulties ranked the highest scores in the symptom scales. Analysis of risk factors for HRQoL showed age, exercise, smoking status, and treatment regimen were associated with global health status and functional scores, while age, gender, radiation pneumonitis, weight loss, and exercise were associated with symptom scores.
Conclusions
This study provides the first description of the HRQoL of long-term LA-NSCLC survivors receiving radiotherapy and chemotherapy who may experience a relatively high HRQoL. Factors related to poorer HRQoL are potential targets for intervention.
Keywords: Health-related quality of life, Non-small cell lung cancer, Survivor, Radiotherapy, Chemotherapy
Background
For decades, health practitioners have improved the outcome of locally advanced NSCLC (LA-NSCLC) by using concurrent chemoradiotherapy. Thus, a substantial goal of LA-NSCLC management is to maintain or improve the survivors’ health-related quality of life (HRQoL). Information about the HRQoL of long-term LA-NSCLC survivors is essential to anticipate consequences and problems. Moreover, it is useful to analyze factors that affect HRQoL and find optimal intervention to improve HRQoL.
Long-term lung cancer survivors experienced quality of life (QoL) impairment in 35% of cases and reported declined QoL and a worsened symptom load [1]. A cross-sectional study of QoL showed that a distressed mood was the most important predictor of the QoL of long-term NSCLC survivors [2]. Most patients in both studies were stageIand treated with surgery. However, little is known about the HRQoL status of long-term LA-NSCLC survivors who have survived beyond 5 years receiving radiotherapy and chemotherapy. This study aimed to determine the HRQoL of long-term LA-NSCLC survivors receiving radiotherapy and chemotherapy and to explore the relationships between demographic, clinical characteristics, and HRQoL.
Methods
Patients
Patients diagnosed with histologically/cytologically confirmed LA-NSCLC (stages IIIA or IIIB) and treated with radiotherapy in our department between January 2006 and December 2010 were reviewed in the current study. We defined patients who are still living at least five years after diagnosis with LA-NSCLC as long-term survivors. Patients’ demographics and clinical characteristics were uniformly collected from their medical records. Tumor stages of all patients were reclassified using the 7th edition of the American Joint Committee on Cancer Staging. Eligibility included histologically or cytologically confirmed NSCLC, older than age 18 at the time of diagnosis, and medical records indicating at least 60 months of follow-up. No specific post-treatment rehabilitation program was provided for all eligible patients, but the smokers were encouraged to stop smoking. The protocol of this study was approved by the ethics committees of the institution.
Assessment of HRQoL
Health-related QoL was assessed using a validated, cancer-specific core questionnaire, the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 (version 3.0) and a lung-specific module, the EORTC QLQ LC-13 [3]. Questions were scaled and scored using the recommended EORTC quality of life group procedures [4]. All scores on the EORTC QLQ-C30 and the QLQ-LC 13 were transformed to a 0 to 100 scale according to the guidelines of EORTC. Higher scores for the global QL and functional domains indicate better global health and function, while higher scores for symptoms indicate greater symptom severity. Given that a 10-point change in each of the EORTC multi-item scales is generally considered to be a moderate or meaningful change [5, 6], for the single-item scales, four levels of responses were defined: not at all, a little, quite a bit, and very much. Demographic characteristics were collected at the instructions of the survey. Smoking status was classified as current, former, and never smoker. Pack years of smoking were calculated as the number of packs of cigarettes smoked per day during the years of smoking. Exercise was defined as at least 30 min of moderate aerobic exercise a day, at least five days a week.
Survey procedures
Eligible patients were asked to sign the consent form if they would be interested in the survey and complete Chinese versions of the questionnaires, if feasible, during their latest visit to the hospital. Others were mailed a consent form, questionnaires, and a stamped envelope. After the letter, a trained interviewer telephoned the patient to ask if the survivor was interested in the survey. Those who agreed to take part in the survey either completed a letter in reply or responded through a trained interviewer via telephone for survivors who could not complete the questionnaires by themselves.
Statistical analysis
The chi-squared test was applied for categorical variables to evaluate whether survey respondents differed significantly from nonrespondents of all patients. Cumulative survival was estimated according to the Kaplan-Meier method. Descriptive statistics, as appropriate, were used to provide a profile of HRQoL. HRQoL data were analyzed using nonparametric methods because the data were not normally distributed. Univariate data were compared by applying the Mann-Whitney U test or the KrusKal-Wallis test. Statistical analyses were performed using statistical software SPSS 19.0. The level of statistical significance was set to 0.05.
Results
Patient characteristics
The records of 505 patients with LA-NSCLC who underwent radiotherapy and chemotherapy were retrospectively reviewed. Of the 82 patients who were still living at 5 years or more after diagnosis, 55 patients completed the Chinese versions of the EORTC QLQ-C30 questionnaires and the QLQ-LC13 module. Patients’ characteristics are summarized in Table 1. Those who completed the survey (Survey Completed Group, SCG) included more patients treated with concurrent chemoradiotherapy (CCRT) and higher objective response rates than those who did not complete the survey (non-Survey Completed Group, nSCG) (p < 0.05), but they did not differ with regard to other factors.
Table 1.
Comparison of characteristics of patients between nSCG and SCG
| Characteristics | nSCG (450) n (%) | SCG (55) n (%) | P value | |
|---|---|---|---|---|
| Age | Mean | 61 | 60 | 0.563 |
| SD | 11 | 10 | ||
| Gender | Male | 372 (82.7%) | 45 (81.8%) | 0.876 |
| Female | 78 (17.3%) | 10 (18.2%) | ||
| KPS Scorea | 90–100 | 116 (25.8%) | 19 (34.5%) | 0.165 |
| 60–80 | 334 (74.2%) | 36 (65.5%) | ||
| Histology | Squamous | 266 (59.1%) | 28 (50.9%) | 0.244 |
| Non-squamous | 184 (40.9%) | 27 (49.1%) | ||
| Stages (AJCC 2002) | IIIA | 156 (34.7%) | 25 (45.5%) | 0.115 |
| IIIB | 294 (65.3%) | 30 (54.5%) | ||
| Smoking status | Never | 89 (19.8%) | 13 (23.6%) | 0.501 |
| Former/current | 361 (80.2%) | 42 (76.4%) | ||
| Weight lossb | ≥5 % | 121 (26.9%) | 15 (27.3%) | 0.959 |
| <5 % | 328 (73.1%) | 40 (72.7%) | ||
| Regimens | RT | 122 (27.1%) | 5 (9.1%) | 0.006 |
| CRT | 147 (32.7%) | 18 (32.7%) | ||
| CCRTc | 181 (40.2%) | 32 (58.2%) | ||
| RT dose | <60Gy | 138 (30.7%) | 11 (20%) | 0.102 |
| ≥60Gy | 312 (69.3%) | 44 (80%) | ||
| RT-technique | 2D–RT | 41 (9.1%) | 2 (3.6%) | 0.343 |
| 3D–CRT | 22 (4.9%) | 2 (3.6%) | ||
| IMRT | 387 (86%) | 51 (92.7%) | ||
| Response rated | CR + PR | 285 (64.2%) | 43 (78.2%) | 0.039 |
| SD + PD | 159 (35.8%) | 12 (21.8%) | ||
| Radiation esophagitise | <grade 2 | 269 (64.4%) | 37 (67.3%) | 0.670 |
| ≥grade 2 | 149 (35.6%) | 18 (32.7%) | ||
| Radiation pneumonitisf | <grade 2 | 330 (85.3%) | 46 (83.6%) | 0.750 |
| ≥grade 2 | 57 (14.7%) | 9 (16.4%) | ||
aone patient <80 in SCG, bone patient without data of weight loss, cCCRT including:induction chemotherapy + CCRT,CCRT + consolidation chemotherapy,induction + CCRT + consolidation chemotherapy and CCRT, dsix patients without data of response rate, e32 patients without data of radiation esophagitis, f63 patients without data of radiation pneumonitis. CCRT Concurrent chemo-radiotherapy, nSCG non-Survey completed group, RT Radiotherapy, SCG Survey completed group
The demographic characteristics at the time of the interview of the 55 patients in SCG group are listed in Table 2. Thirty-one patients reported no comorbidities. Three patients had second primary cancer (lung cancer, rectal cancer, and bladder cancer, respectively), two patients had metastasis (brain and liver, respectively), and five patients received target therapy (3 with erlotinib and 2 with gefitinib). The majority (54/55, 98%) could perform their daily life activities by themselves (KPS ≥ 80). They also reported their educational level, employment status, income per month, and exercise status. Main types of aerobic exercise reported were brisk walking, jogging, Tai chi, and Qigong. All 55 patients had health insurance.
Table 2.
Demographic characteristics of 55 long-term survivors
| Characteristic | n (%) |
|---|---|
| Comorbidities | |
| Emphysema/COPD | 4 (7.3%) |
| Heart disease | 7 (12.7%) |
| Hypertension | 11 (20%) |
| Diabetes | 4 (7.3%) |
| Cancer | |
| Second primary cancer | 3 (5.5%) |
| Metastasis | 2 (3.6%) |
| Other comorbidities | 5 (9.1%) |
| Target therapy | 5 (9.1%) |
| Smoking status | |
| Never | 13 (23.6%) |
| Former | 38 (69.1%) |
| Current | 4 (7.3%) |
| < 20 pack-y | 21 (38.2%) |
| ≥ 20 pack-y | 34 (61.8%) |
| Education | |
| Less than high school | 21 (38.2%) |
| High school graduate or more | 34 (61.8%) |
| Employment status | |
| Employed | 10 (18.2%) |
| Retired and unemployed | 45 (81.8%) |
| Income per month | |
| < 3000 | 16 (29.1%) |
| ≥ 3000 | 39 (70.9%) |
| Medical insurance | |
| URBMI | 51 (92.7%) |
| NRCMS | 4 (7.3%) |
| Taking exercise | |
| yes | 45 (81.8%) |
| no | 10 (18.2%) |
URBMI Urban resident basic medical insurance, NRCMS New rural cooperative medical scheme
Survival and compliance
Of 505 patients, the median survival time was 21 months (95% CI, 19.14 to 22.86 months), and the overall 1, 2, and 5-year survival rates were 73.2%, 44.4%, and 17.1%, respectively. Because of death or no follow-up, only 55 of the 82 (67%) long-term survivors completed the survey. Fourteen patients died, including 2 patients who died from pancreatitis and a heart attack, respectively in the 5th year. Besides those patients, there were 9 patients lost to follow-up and 4 patients who did not want to complete the questionnaires.
Health-related quality of life
Self-reported HRQoL scores for the QLQ-C30 and the QLQ-LC13 are displayed in Table 3. The greatest impairment was seen in global health status (76.67 ± 15.91), followed by physical impairment (86.06 ± 14.53), and emotional (89.58 ± 14.65) functioning. There were relatively high levels of role, social, and cognitive functioning with scores above 90. Most symptom scores were very low, except for scores of fatigue (27.02 ± 15.72), dyspnea (24.85 ± 19.48 in QLQ-C30; 18.08 ± 13.74 in QLQ-LC13), coughing (23.03 ± 19.11), and financial difficulties (14.55 ± 21.05). The patients of four levels of responses of single-item symptom scales are shown in Fig. 1. The most prevalent symptoms were fatigue (49/55, 89%), dyspnea (QLQ-LC13, 48/55, 87.3%; QLQ-C30, 37/55, 67.3%), coughing (35/55, 63.6%), and financial difficulties (20/55, 36.4%).
Table 3.
HRQoL Scores of Long-term LA-NSCLC Survivors
| QoL Scale | Mean (SD) | Median (IQR) | Range |
|---|---|---|---|
| QLQ-C30 | |||
| Global health status/QoL | 76.67 (15.91) | 83.33 (66.67–91.67) | (25–100) |
| Physical functioning | 86.06 (14.53) | 93.33 (80.0–93.3) | (20–100) |
| Role functioning | 91.21(17.53) | 100 (83.3–100) | (0–100) |
| Emotional functioning | 89.58 (14.65) | 100 (75.0–100) | (50–100) |
| Cognitive functioning | 94.24 (12.51) | 100 (100–100) | (33.3–100) |
| Social functioning | 93.94 (13.75) | 100 (100–100) | (33.3–100) |
| Fatigue | 27.02 (15.77) | 33.33 (22.22–33.33) | (0–100) |
| Nausea and vomiting | 0.40 (2.99) | 0 (0–0) | (0–22.22) |
| Pain | 5.76 (12.92) | 0 (0–0) | (0–66.67) |
| Dyspnea | 24.85 (19.48) | 33.33 (0–33.33) | (0–66.67) |
| Insomnia | 6.67 (16.23) | 0 (0–0) | (0–66.67) |
| Appetite loss | 2.42 (8.74) | 0 (0–0) | (0–33.33) |
| Constipation | 2.42 (8.74) | 0 (0–0) | (0–33.33) |
| Diarrhea | 1.82 (7.64) | 0 (0–0) | (0–33.33) |
| Financial difficulties | 14.55 (21.05) | 0 (0–33.33) | (0–66.67) |
| QLQ LC-13 | |||
| Dyspnea | 18.08 (13.74) | 11.11 (11.11–22.22) | (0–66.67) |
| Coughing | 23.03 (19.11) | 33.33 (0–33.33) | (0–66.67) |
| Hemoptysis | 4.24 (12.92) | 0 (0–0) | (0–66.67) |
| Sore mouth | 0 (0) | 0 (0–0) | (0–0) |
| Dysphagia | 1.82 (9.98) | 0 (0–0) | (0–66.67) |
| Peripheral neuropathy | 2.42 (8.74) | 0 (0–0) | (0–33.33) |
| Alopecia | 5.45 (14.00) | 0 (0–0) | (0–66.67) |
| Pain in chest | 2.42 (8.74) | 0 (0–0) | (0–33.33) |
| Pain in arm/shoulder | 3.64 (10.49) | 0 (0–0) | (0–33.33) |
| Pain in other parts | 1.21 (6.30) | 0 (0–0) | (0–33.33) |
IQR Interquartile range, SD Standard deviation
Fig. 1.
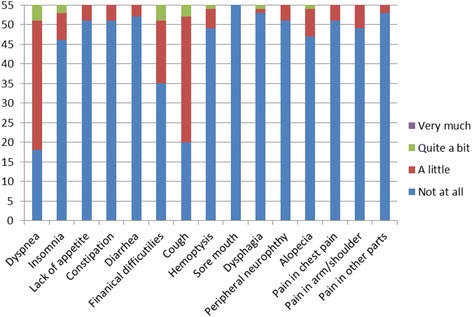
Number of patients in four levels of responses of single-item symptom scales
Risk factors for poor HRQoL
In global health status and functional scales, survivors of a younger age (≤60 years old), exercise, without comorbidity, and never smoker were significantly associated with more favorable scores for global health status. Likely, those with age (≤60 years old), exercise and without comorbidity had better physical, role and social functioning (p < 0.05). In contrast, CCRT was negatively associated with emotional functioning (p < 0.05) (Table 4). On the examination of the association between symptom load and the characteristics of survivors, fatigue was significantly negatively affected by weight loss <5% and lack of exercise (p < 0.05), while dyspnea was significantly negatively affected by gender: male, with at least one comorbidity, radiation pneumonitis (RP, ≥ grade 2), and age (>60 years old) (p < 0.05) (Table 5). HRQoL was not significantly affected by other factors.
Table 4.
Relationship between selected demographic/clinical features and global health status, functional scale scores, mean (SD)
| Age | p | Exercise | p | Comorbidity | p | Smoking status | p | Regimens | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤60 (n = 25) | >60 (n = 30) | Yes (n = 45) | No (n = 10) | Yes (n = 24) | No (n = 31) | Never (n = 13) | Former/current (n = 42) | RT (n = 5) | RT + CT (n = 18) | CCRT (n = 32) | ||||||
| Global health status | 82.64 (12.98) | 71.39 (16.48) | 0.011 | 79.81 (14.81) | 62.5 (13.18) | 0.001 | 69.79 (17.34) | 81.99 (12.56) | 0.007 | 85.71(12.42) | 73.58 (15.91) | 0.01 | 73.33 (21.57) | 79.63 (11.15) | 75.52 (17.45) | 0.775 |
| Physical functioning | 93.06 (7.22) | 80.0 (16.33) | 0.000 | 88.59 (13.9) | 74.67 (12.09) | 0.001 | 80.28 (17.42) | 90.54 (10.00) | 0.007 | 90.95 (8.52) | 84.39 (15.82) | 0.162 | 78.67 (17.26) | 85.56 (11.72) | 87.5 (15.59) | 0.293 |
| Role functioning | 98.61 (4.71) | 85.0 (21.60) | 0.001 | 93.7 (17.51) | 80.0 (13.15) | 0.000 | 85.42 (22.69) | 95.70 (10.00) | 0.019 | 96.43 (9.65) | 89.43 (19.28) | 0.157 | 86.67 (18.26) | 90.74 (13.06) | 92.19 (19.85) | 0.414 |
| Emotional functioning | 90.97 (13.66) | 88.11 (15.63) | 0.492 | 90.59 (14.53) | 85.0 (15.11) | 0.110 | 87.22 (16.90) | 91.40 (12.64) | 0.284 | 91.07 (14.05) | 89.07 (14.99) | 0.619 | 95.0 (11.18) | 95.37 (9.58) | 85.47 (16.31) | 0.035 |
| Social functioning | 100 (0) | 88.89 (17.14) | 0.001 | 95.93 (12.39) | 85.0 (16.57) | 0.002 | 88.20 (18.04) | 98.39 (6.60) | 0.002 | 98.81 (4.45) | 92.28 (15.41) | 0.114 | 86.67 (21.73) | 94.44 (9.90) | 94.79 (14.32) | 0.395 |
CCRT Concurrent chemoradiotherapy, CT Chemotherapy, RT Radiotherapy, SD Standard deviation
Table 5.
Relationship between selected demographic/clinical features and symptom scale scores, mean (SD)
| Age | p | Gender | p | Comorbidity | p | Exercise | p | Weight loss (≥5%) | p | RP | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤60 (n = 25) | >60 (n = 30) | Male (n = 45) | Female (n = 10) | Yes (n = 24) | No (n = 31) | Yes (n = 45) | No (n = 10) | Yes (n = 15) | No (n = 40) | <grade 2 (n = 46) | ≥grade 2 (n = 9) | |||||||
| Fatigue | 23.15 (13.48) | 30.28 (17.18) | 0.110 | 26.85 (16.6) | 27.78 (12.0) | 0.491 | 29.98 (19.71) | 24.73 (11.73) | 0.190 | 25.68 (16.72) | 33.06 (8.73) | 0.026 | 17.04 (13.19) | 30.76 (15.14) | 0.001 | 25.3 (12.45) | 35.8 (26.51) | 0.255 |
| LCDY | 10.88 (9.62) | 24.07 (14.02) | 0.000 | 19.88 (13.74) | 10.0 (11.05) | 0.019 | 25.00 (15.46) | 12.72 (9.43) | 0.001 | 16.42 (12.64) | 25.56 (16.6) | 0.064 | 20.74 (9.26) | 17.08 (15.06) | 0.118 | 15.34 (9.74) | 32.1 (21.83) | 0.018 |
LCDY Dyspnea in QLQ LC13, RP Radiation pneumonitis, SD Standard deviation
Discussion
Obtaining a satisfactory level of HRQoL is a fundamental goal of the therapy for LA-NSCLC patients. To better understand HRQoL in long-term LA-NSCLC survivors, based on QLQ-C30 and QLQ-LC13 using the most frequently used instruments in patients with lung cancer [7], we report the patient-reported HRQoL outcomes from the cross-sectional study for long-term LA-NSCLC survivors treated with radiotherapy and chemotherapy. Our findings suggest that long-term LA-NSCLC survivors have a relatively high HRQoL, while the long-term NSCLC survivors reported a slight reduction in global health status, physical and emotional functioning, and the predominant symptom load includes fatigue, dyspnea, coughing, and financial difficulties.
Our study shows that the 5-year overall survival rate of all patients was 17.1%; this figure is comparable with other studies [8, 9]. Survivors who completed the survey have a higher proportion of CCRT, and an objective response rate may result from a strong association between survival and treatment effect [10]. Several studies have documented the impact of cancer diagnosis and treatment on HRQoL in patients and short-term survivors with LA-NSCLC [11–13]. It is possible that the HRQoL of long-term LA-NSCLC survivors may differ from those experienced around diagnosis and treatment. Baseline global health status and functioning scores in two studies [11, 13] were lower than the current finding. Besides, this study found that long-term survivors of LA-NSCLC had a stable or slight decline in global health status and functioning scores; in contrast, for patients who just finished treatment or short-term survivors, global health status and all functioning scores except for emotional functioning declined significantly [11, 13]. The worsening of emotional functioning of long-term survivors is presumably an effect of a long-term burden on health, financial status, and their families. Furthermore, our study indicated that fatigue, dyspnea, coughing, and financial difficulties were the most frequent and high-score symptoms, while a high proportion of patients who had just finished treatment or short-term survivors experienced fatigue, loss of appetite, respiratory problems, cough, pain, and blood in sputum [14, 15]. These differences may relate to the symptoms associated with lung cancer, chemotherapy, and around the time of radiotherapy that were significant during treatment but later improved [11]. Financial problems are more common among the long-term survivors possibly due to long-term medical expenses though most of the patients have health insurance.
According to our assessment of the risk factors for a poor HRQoL in the present study, among long-term survivors, the HRQoL reported by younger survivors (≤60 years old) had significantly better scores in their global health status, physical, role and social functioning, and dyspnea than the older patients. The study [16] conducted by Lemonnier et al. also found that older age was associated with low HRQoL. In addition, our study indicated that exercise had a positive effect on global health status, physical, role and social functioning, and fatigue; the increasing number of evidence also suggests that exercise may be able to enhance cancer survivorship [17, 18]. Survivors treated with CCRT and with a smoking history had lower scores on emotional functioning and global health status, respectively, while patients with comorbidities had significantly more disruption in global health, functional scales, and dyspnea. These findings suggest that treatment regimen, tobacco use, and comorbidities have a long-term effect on HRQoL. As expected, survivors with RP (≥ grade 2) during or after radiotherapy had significantly higher dyspnea scores. Our results suggest that the risk factors of HRQoL include general (age), lifestyle (exercise), side effect (RP), and treatment (CCRT), which should be emphasized to further understand mechanisms and lead to tailored intervention strategies to improve HRQoL. In this study, smoking status was only associated with global health, but the symptom burden, particularly dyspnea and cough, continued partly due to a relatively small number of survivors who continued to smoke.
Several limitations should be considered when interpreting these results. First, our results have a potential bias in follow-up. Of the 82 patients, there were 4 patients (5%) who declined to answer the survey and 9 patients (11%) were lost to follow-up. It is generally accepted that those with advanced cancer do not complete surveys, leaving only those in better health to provide data. Hence, our results may overestimate HRQoL in these patients. Second, in this cross-sectional study, we did not observe longitudinal HRQoL, dynamic time-dependent changes. Thus, we could not define any change in HRQoL, and further studies are warranted. Finally, the number of patients was relatively small, thus limiting the statistical power of subgroup analyses and not correcting for multivariate comparisons.
Conclusions
Long-term LA-NSCLC survivors treated with radiotherapy and chemotherapy have a relatively high HRQoL, with slight limitations in global health status and physical and emotional functioning and a higher symptom load of fatigue, dyspnea, coughing, and financial difficulties. Information about HRQoL of long-term LA-NSCLC survivors can be contributed to rehabilitation programs and long-term surveillance. Future prospective studies need to consider the long-term change of HRQoL and explore potential interventions.
Acknowledgements
Not applicable.
Funding
This work was founded by the National Natural Science Foundation of China (No.81272616).
Availability of data and materials
The datasets generated or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CCRT
Concurrent chemoradiotherapy
- CT
Chemotherapy
- EORTC
European Organization for Research and Treatment of Cancer
- HRQoL
Heath-related quality of life
- IQR
Interquartile range
- LA-NSCLC
Locally-advanced non-small cell lung cancer
- LCDY
Dyspnea in QLQ LC13
- NRCMS
New rural cooperative medical scheme’
- nSCG
Non-Survey completed group
- NSCLC
Non-small cell lung cancer
- QLQ
Quality of life questionnaire
- QoL
Quality of life
- RP
Radiation pneumonitis
- RT
Radiotherapy
- SCG
Survey completed group
- SD
Standard deviation
- URBMI
Urban resident basic medical insurance
Authors’ contributions
LW was responsible for management of the project, quality assessment, review, and approval of the manuscript. JR and LW were responsible for conception, research goals and aims. JR, JW, NB and WJ contributed to design of methodology, writing of the protocol, data analysis and interpretation. All authors were involved in recruitment and treatment of patients. JR, JW, NB and LW contributed to write, review and edit the completed report. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the ethics committees of the institution and in accordance with the Declaration of Helsinki and current ethics guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juntao Ran, Email: ranjt2014@126.com.
Jingbo Wang, Email: wangjingbo303@yahoo.com.
Nan Bi, binan_email@163.com.
Wei Jiang, Email: jwmars@126.com.
Zongmei Zhou, Email: zhouzongmei2013@163.com.
Zhouguang Hui, Email: drdhuizg@163.com.
Jun Liang, Email: liang23400@163.com.
Qinfu Feng, Email: 13811300221@163.com.
Luhua Wang, Phone: (86) 010 8778 8799, Email: wlhwq@yahoo.com.
References
- 1.Yang P, Cheville AL, Wampfler JA, et al. Quality of life and symptom burden among long-term lung cancer survivors. J Thorac Oncol. 2012;7:64–70. doi: 10.1097/JTO.0b013e3182397b3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarna L, Padilla G, Holmes C, et al. Quality of life of long-term survivors of non-small-cell lung cancer. J Clin Oncol. 2002;20:2920–2929. doi: 10.1200/JCO.2002.09.045. [DOI] [PubMed] [Google Scholar]
- 3.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 4.Fayers PM, Aaronson NK, Bjordal K, et al. EORTCS QLQ-C30 scoring manual. 3rd ed. Brussels: EORTC; 2001.
- 5.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 6.Osoba D, Bezjak A, Brundage M, et al. Analysis and interpretation of health-related quality-of-life data from clinical trials: basic approach of the National Cancer Institute of Canada clinical trials group. Eur J Cancer. 2005;41:280–287. doi: 10.1016/j.ejca.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Damm K, Roeske N, Jacob C. Health-related quality of life questionnaires in lung cancer trials: a systematic literature review. Health Econ Rev. 2013;3:15. doi: 10.1186/2191-1991-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 study. J Clin Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 10.Blumenthal GM, Karuri SW, Zhang H, et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol. 2015;33:1008–1014. doi: 10.1200/JCO.2014.59.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallqvist A, Bergman B, Nyman J. Health related quality of life in locally advanced NSCLC treated with high dose radiotherapy and concurrent chemotherapy or cetuximab--pooled results from two prospective clinical trials. Radiother Oncol. 2012;104:39–44. doi: 10.1016/j.radonc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Auchter RM, Scholtens D, Adak S, et al. Quality of life assessment in advanced non-small-cell lung cancer patients undergoing an accelerated radiotherapy regimen: report of ECOG study 4593. Eastern cooperative oncology group. Int J Radiat Oncol Biol Phys. 2001;50:1199–1206. doi: 10.1016/S0360-3016(01)01604-2. [DOI] [PubMed] [Google Scholar]
- 13.Langendijk JA, Aaronson NK, de Jong JM, et al. Prospective study on quality of life before and after radical radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2001;19:2123–2133. doi: 10.1200/JCO.2001.19.8.2123. [DOI] [PubMed] [Google Scholar]
- 14.Iyer S, Taylor-Stokes G, Roughley A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer. 2013;81:288–293. doi: 10.1016/j.lungcan.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Iyer S, Roughley A, Rider A, et al. The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer. 2014;22:181–187. doi: 10.1007/s00520-013-1959-4. [DOI] [PubMed] [Google Scholar]
- 16.Lemonnier I, Baumann C, Jolly D, et al. Solitary pulmonary nodules: consequences for patient quality of life. Qual Life Res. 2011;20:101–109. doi: 10.1007/s11136-010-9719-0. [DOI] [PubMed] [Google Scholar]
- 17.Leach HJ, Devonish JA, Bebb DG, et al. Exercise preferences, levels and quality of life in lung cancer survivors. Support Care Cancer. 2015;23:3239–3247. doi: 10.1007/s00520-015-2717-6. [DOI] [PubMed] [Google Scholar]
- 18.Ferrer RA, Huedo-Medina TB, Johnson BT, et al. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41:32–47. doi: 10.1007/s12160-010-9225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analysed during the current study are available from the corresponding author on reasonable request.


