Abstract
As the population ages, more elderly patients require radiotherapy-based treatment for their pelvic malignancies, including muscle-invasive bladder cancer, as they are unfit for major surgery. Therefore, there is an urgent need to find radiosensitising agents minimally toxic to normal tissues, including bowel and bladder, for such patients. We developed methods to determine normal tissue toxicity severity in intestine and bladder in vivo, using novel radiotherapy techniques on a small animal radiation research platform (SARRP). The effects of panobinostat (PAN) on in vivo tumour growth delay were evaluated using subcutaneous xenografts in athymic nude mice. PAN concentration levels in xenografts, plasma and normal tissues were measured in CD1-nude mice. CD1-nude mice were treated with drug/irradiation combinations to assess acute normal tissue effects in small intestine using the intestinal crypt assay, and later effects in small and large intestine at 11 weeks by stool assessment and at 12 weeks by histological examination. In vitro effects of PAN were assessed by qPCR and of PAN, TMP195 and mocetinostat by clonogenic assay, and western blot. PAN resulted in growth delay in RT112 bladder cancer xenografts but did not significantly increase acute (3.75 days) or 12 weeks’ normal tissue radiation toxicity. Radiosensitisation by PAN was effective in hypoxic bladder cancer cells and associated with class I HDAC inhibition, and protein downregulation of HDAC2 and MRE11. Pan-HDAC inhibition is a promising strategy for radiosensitisation, but more selective agents may be more useful radiosensitisers clinically, resulting in fewer systemic side effects.
Keywords: bladder cancer, normal tissue effects, panobinostat, radiosensitisation, small animal radiation research platform (SARRP)
Introduction
Muscle-invasive bladder cancer (MIBC) is a disease of an increasingly elderly population (1); Gray et al (2) highlighted the underuse of aggressive therapy in over 80 year-olds in the United States, and in the UK Other Cause Mortality accounts for only 34% of deaths in 80+ year-old MIBC patients (3). MIBC can be treated with radiotherapy (RT) or cystectomy with similar outcomes (1,4,5). While RT is well tolerated by elderly patients, concurrent chemoradiation (CRT) is more effective (6–8). However, CRT causes systemic toxicities and more severe bowel and bladder toxicity than RT alone; these side effects may be difficult for elderly patients to tolerate. As the population ages, new radiosensitisers which are less toxic to normal tissues, and hence more suitable for CRT in elderly patients, are urgently needed.
Over the last decade, histone deacetylases (HDAC) have emerged as important cancer therapeutic targets. HDAC inhibitors are promising radiosensitisers in preclinical studies, and although in clinical studies combined treatment appears well-tolerated acutely (9), data are lacking (especially for HDAC inhibitors in combination with radiotherapy) for longer term, particularly bowel, toxicities (10). HDAC expression is aberrant in multiple cancer types including bladder cancer (11,12). HDACs are classified as class I (HDAC1, 2, 3, 8), IIa (HDAC4, 5, 7 and 9), IIb (HDAC6 and 10), III (sirtuins) and IV (HDAC11). Conventional HDAC inhibitors target only the Zn2+-dependent class I, II and IV enzymes.
The pan-HDAC inhibitor panobinostat is one of the most potent HDAC inhibitors (13) and was recently reported in phase I trials in combination with RT in glioma and lung cancer. We previously found panobinostat to be an efficient radiosensitiser in the nanomolar range in vitro associated with downregulation of the DNA damage signalling proteins MRE11 and NBS1 and the homologous recombination (HR) protein RAD51 (14).
Here, we tested the hypothesis that panobinostat can spare normal tissues whilst being an effective tumour radiosensitiser in vivo. Panobinostat proved to be an efficient radiosensitiser in a bladder cancer xenograft model. However, in contrast to the known radiation modifier gemcitabine, clinically relevant doses of panobinostat did not increase the severity of acute normal tissue toxicity bowel surrounding mouse bladder irradiated using a small animal radiation research platform (SARRP). We also developed a model which can be used to investigate later normal tissue effects in large intestine and bladder which could also be used to investigate other pelvic malignancies pre-clinically. Furthermore, we demonstrated that radiosensitisation and effects on the MRE11-RAD50-NBS1 (MRN) complex were primarily class I-mediated, using class-selective HDAC inhibitors and transient HDAC1 or 2 knockdown.
Materials and Methods
All animal work was done in accordance with UK Home Office Guidelines and institutional guidelines, approved by the University of Oxford Animal Welfare and Ethical Review Body (AWERB), under University of Oxford project licences PPL 30/2922 and 30/3172. Group sizes were chosen to detect large effect sizes.
Cell lines
RT112 cells were a gift from Margaret Knowles (University of Leeds, obtained 2009) and were authenticated by extensive genomic analysis (15) and used within 10 passages. CAL29 and T24 cell lines were purchased from DSMZ (in 2011) and used within 15 passages. A stably transfected Ku80 knockdown cell line (KuKD), was created from RT112 (in 2010) as previously described, and used up to passage 10 (16). RT112, T24 and KuKD, but not Cal29, cell lines were validated by short tandem repeat analysis in 2015 (DNA Diagnostics Centre). No traces of mycoplasma/bacteria were found in any of the cell lines by DAPI staining in 2016. All four cell lines were tested for mycoplasma in June 2017 by TOKU-E PCR Mycoplasma Detection Kit and found to be negative. Culture conditions are described in Supplementary Materials.
Drugs and drug treatments
Panobinostat (for in vitro studies) and mocetinostat (17) were purchased from Selleck Chemicals. Gemcitabine was purchased from Accord Healthcare Ltd. TMP195 (18) was a generous gift from GlaxoSmithKline and, for in vivo experiments, panobinostat was a generous gift from Novartis.
Xenograft model for growth delay study
Cells were prepared in phenol red-free Matrigel (BD Biosciences) and RPMI medium 1:1, and 100 μL (2 x 106 cells) injected into the flank of 6-week old female athymic nude mice (Harlan Laboratories). Tumours were measured every second day with callipers. Xenograft volumes were calculated using [a x b x c x π/6] and xenografts allowed to reach 100 mm3 before randomising mice into four groups and treating with: mock treatment (4% DMSO, 4% β-cyclodextrin in distilled water intraperitoneally (IP); n=5 RT112, n=4 Ku knockdown (KuKD)), panobinostat (PAN, Novartis Pharmaceuticals, diluted in 4% DMSO, 4% β-cyclodextrin in water; n=7 RT112, n=6 KuKD) to a dose of 10 mg/kg, days 1, 3 and 5 IP, daily irradiation (IR, 20 Gy in 5 fractions, 300kV, using a Gulmay-320 cabinet irradiator (15); n=6) or PAN+IR (n=6).
Panobinostat concentration levels in plasma, xenografts, bladder and intestines
Panobinostat concentrations were measured in CD1-nude mice bearing 100 mm3 RT112 xenografts as described in Supplementary Methods.
Normal tissue response models
Drug treatment alone
Six to eight-week old CD1-nude mice (Charles River) were treated with mock treatment or panobinostat (10 mg/kg, IP) for 2, 6, or 24 hours (n=1 per group). A further four mice were treated similarly with mock treatment or gemcitabine (100 mg/kg, diluted in sterile water IP). Bromodeoxyuridine (BrdU, 60 mg/kg, diluted in phosphate buffered saline) was injected IP 30 minutes prior to sacrifice. The effects of drug on apoptosis and replication were assessed by Swiss roll as outlined in Supplementary Methods.
Drug/irradiation combinations
To assess the acute effects on surrounding normal tissues of adding a radiosensitising drug to irradiation in vivo, 5-7-week old CD1-nude mice were treated with mock treatment, panobinostat (10 mg/kg IP) or gemcitabine (100 mg/kg IP). Approximately 6 hours later, mice were treated supine with 10, 12 or 14 Gy to the lower abdomen, including lower small intestine, in a SARRP (Xstrahl) with 220 kV X-rays, 13 mA, half-value layer 0.794 mm Cu, using a 178-degree arc treatment with a 14 mm circular collimator. Mice were sacrificed at 3.75 days and tissue processed as described in Supplementary Methods.
For assessment of late normal tissue effects, mice were treated vertically, head down in the SARRP with 5 Gy daily over 5 days, using a 356-degree arc treatment and 10 mm collimator, with the isocentre positioned at the posterior caudal bladder wall, to avoid the small intestine, with some mice receiving panobinostat 10 mg/kg day 1, 3 and 5. Eleven weeks later mice were isolated for 24 hours and faeces collected, weighed, counted and measured. Following sacrifice at 12 weeks, the intestines were formalin-fixed and paraffin-embedded, and examined histologically by a veterinary pathologist. Lesions were scored semi-quantitatively on a scale of 0 to 5 (0: no lesion present; 5: entire tissue affected (19)).
Crypt assay
H&E-stained Swiss roll slides were analysed in a crypt assay modified for use in mice receiving partial abdominal irradiation, using Swiss rolls, as outlined in Supplementary Methods.
The percentage of surviving crypts was calculated as:
Fluorescence slides of BrdU and cleaved caspase 3 were scanned using an Aperio FL digital pathology scanner (Leica). Twenty-five crypts from 4 samples under different time points were analysed to determine how many cells in the crypt were BrdU or cleaved caspase 3 positive.
Clonogenic assays
Clonogenic assays were performed as previously described (15). Clonogenic survival assays under hypoxic conditions were performed as described in Pires et al using an H34 Hypoxystation (Don Whitley Scientific) (20). Briefly, cells were seeded in 5 cm dishes, treated with DMSO or 25 nM panobinostat 2 hours later, and incubated at 2% O2 before IR. Cells were irradiated 24 hours later and the medium replaced 15 minutes post-IR. Dishes were incubated for a further 12 days before staining and counting.
Real-time quantitative polymerase chain reaction (qPCR)
Real-time qPCR was performed by ∆∆Ct method (see Supplementary Materials for further details).
Western blots
Western blot samples were prepared as previously described (15,16) with antibodies detailed in Supplementary Table 1 and details in Supplementary Materials.
siRNA transfection
Cells (4 x 105) were plated 24 hours prior to treatment with 120 nM siRNA (Invitrogen) or non-silencing control (NSC). siRNA was combined with Oligofectamine (Invitrogen) and added to serum-free medium for 4 hours before adding medium containing 10% v/v FBS. Twenty-four hours later, cells were harvested for western blot or trypsinised and replated for clonogenic assay.
Homologous recombination (HR) assays
The I-Sce1 assay was performed as in (21) and a PCR-based method as per manufacturer’s instructions (see Supplementary Materials for further details).
Statistical analysis
GraphPad Prism 6.0 was used for all statistical analyses, with results represented as mean and standard error of the mean (SEM). A two-way ANOVA with Bonferroni’s multiple comparison was used to analyse clonogenic survival curves. A one-way ANOVA with Bonferroni correction or Tukey’s multiple comparison was used to compare more than two samples; a two-tailed unpaired Student’s t-test was used to compare two samples. The Kaplan-Meier method was used to present time to tumour trebling with log-rank (Mantel-Cox) used to test significance.
Results
Panobinostat increases growth delay in irradiated bladder cancer cell xenografts
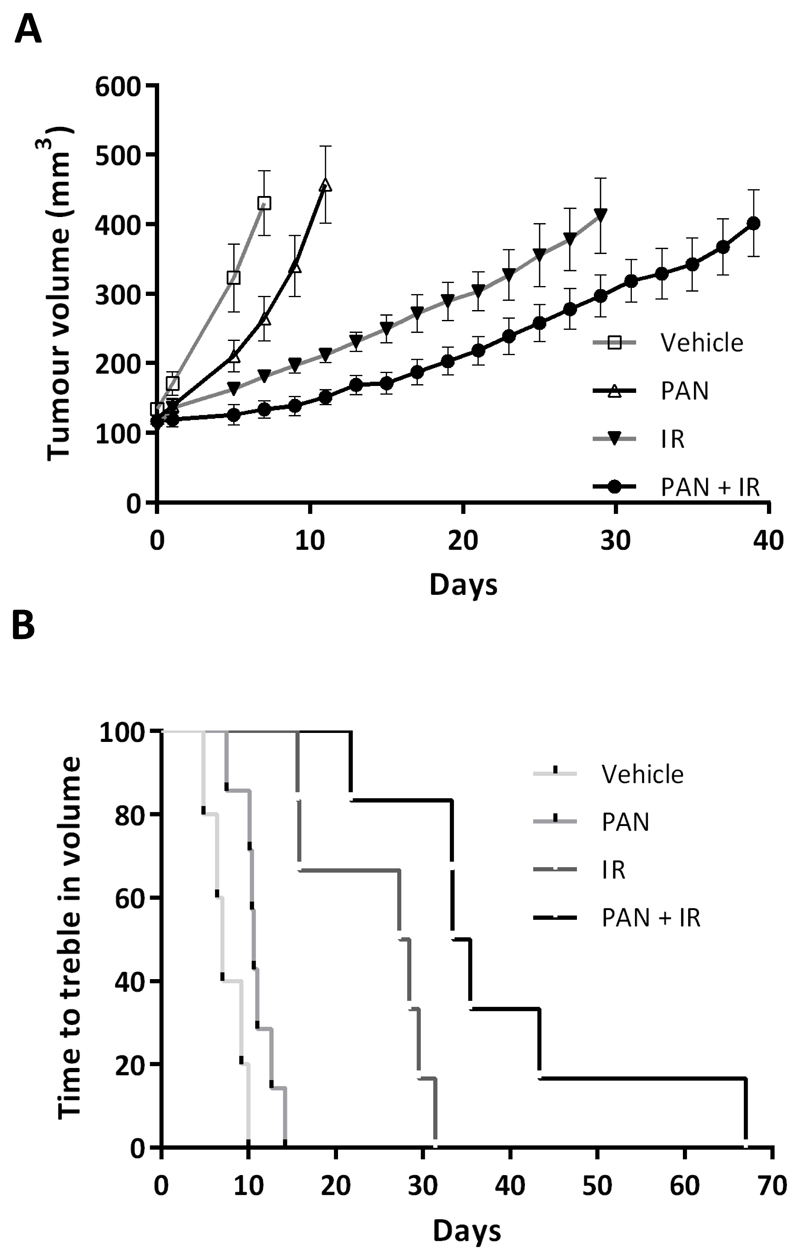
To test panobinostat (PAN) as a radiosensitiser in vivo, RT112 cells were injected into the flank of athymic nude mice, and mice were treated once xenografts reached 100 mm3 (Figure 1A). The Kaplan-Meier curve for time to treble tumour volume was significantly prolonged in the PAN+IR group compared with IR (p=0.007, Figure 1B and Supplementary Figure 1A). The loss in body weight from baseline for mice treated with PAN+IR was significantly greater than that for IR alone (p=0.03) but this was short-lived (Supplementary Figure 1B).
Fig 1. Panobinostat causes increased growth delay in irradiated bladder cancer cell xenografts.
A) Treatment of RT112 xenografts with vehicle, PAN, IR (5 x 4 Gy) or PAN+IR (n=5 vehicle, n=7 PAN, n=6 IR, n=6 PAN+IR). B) Kaplan-Meier survival curve of mice with RT112 xenografts showing plots of time to treble tumour volume.
There was no significant difference in the observed transient body weight reduction between IR and PAN+IR in xenografts derived from 30% KuKD bladder cancer cells (Supplementary Figures 1C and D). These grew significantly more slowly than the parental RT112 xenografts (p<0.001) but displayed a striking sensitivity to radiation, and with PAN+IR there was only a 1.3-fold increase in growth in 55 days (Supplementary figure 1E).
Panobinostat preferentially accumulates in xenografts relative to plasma
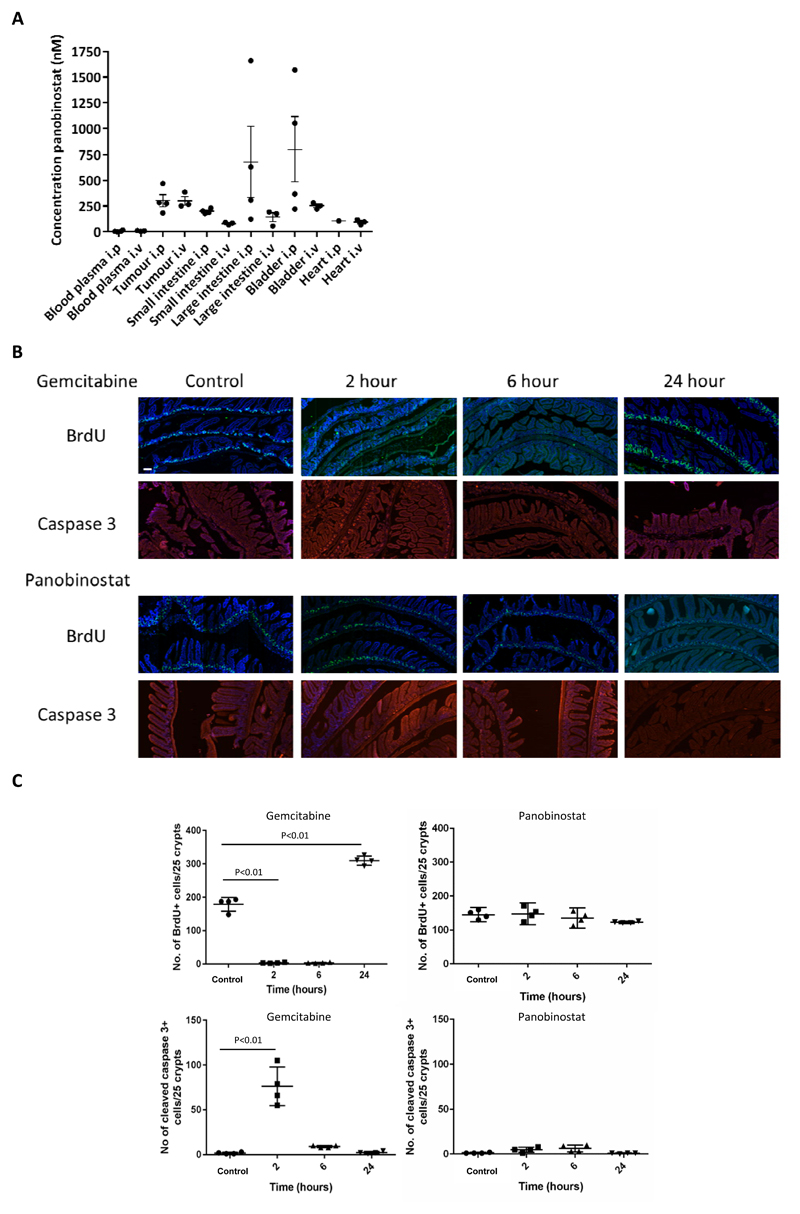
Mice bearing RT112 xenografts of approximately 100 mm3 were treated with IP (n=4) or intravenous (IV, n=3) PAN (with vehicle alone control, n=2) and tissue and blood collected 6 hours later. IP concentrations were similar to IV in plasma, tumour and heart (Figure 2A and Supplementary Table 2). However, small intestinal panobinostat concentration was significantly lower in the IV arm (p=0.001). The IV concentrations in large intestine and bladder were not significantly different from the IP concentrations due to a large range of values in the IP group (p=0.24 and p=0.20). Therefore, panobinostat preferentially accumulates in xenografts relative to plasma but IP administration resulted in high local concentrations of drug (above median tumour concentrations) in bladder and large intestine in most samples.
Fig 2. Panobinostat accumulation in normal tissues and methodology to assess acute toxicity.
A) Xenograft-bearing mice were treated with PAN 10 mg/kg IP (n=4) or IV (n=3), or mock treated (n=2). Xenograft, bladder, 4-5 cm of small and large intestine, heart and blood were collected 6 h later. B) Mice were treated with PAN or GEM and 30 min prior to sacrifice at 2, 4 or 24 h injected with BrdU (n=1 per group). The upper panel represents areas of fluorescent BrdU expression (green); the lower panel images show cleaved caspase 3 (red). The nucleus was stained by DAPI in the BrdU images. The 24 hour mouse of PAN treatment was a repeat as the first mouse had to be sacrificed within 22 h after drug administration on welfare grounds. Examination of limited tissues post mortem did not determine whether this was due to panobinostat. C) Quantification of number of BrdU positive cells per 25 crypts and number of cleaved caspase 3 positive cells per 25 crypts following treatments in (B). Four areas were scored per condition (n=1 for each time point).
Panobinostat has no effect on apoptosis or cell proliferation within 24 hours of treatment
Small intestine from mice treated with gemcitabine or panobinostat and culled at 2, 6 and 24 hours showed strikingly different effects following gemcitabine compared to panobinostat in terms of apoptosis and cellular proliferation (Figure 2B and C). While gemcitabine treatment reduced cellular proliferation as measured by BrdU expression, panobinostat treatment had no effect on proliferation, and while gemcitabine resulted in an increase in cellular apoptosis at 2 hours as shown by cleaved caspase 3 staining, panobinostat did not.
Panobinostat does not increase acute intestinal toxicity following ionising radiation
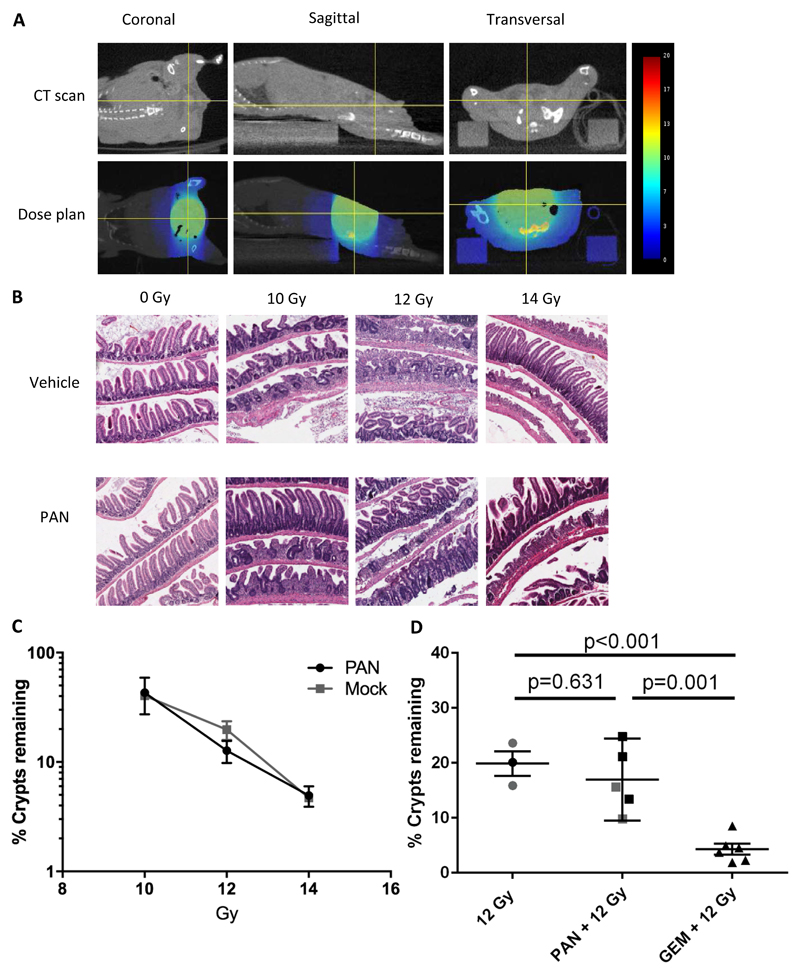
The effects of adding IP panobinostat and gemcitabine (GEM) on acute radiation intestinal toxicity were tested in CD-1 nude mice using our modified crypt assay, with irradiation to the lower abdomen only (Figure 3A)(22). No loss of small intestinal crypts was observed in mice treated with vehicle or drug alone (number of crypts per mm for mock=18.6 (n=2), PAN =19.6 (n=1), GEM =18.0 (n=1), Figure 3B). There was no significant difference in crypt loss between PAN+IR and IR alone at 10 Gy (p=0.87), 12 Gy (p=0.28), or 14 Gy (p=0.84, n=2 per group, Figure 3B and C). Further analysis was made with mice treated with 12 Gy alone, PAN +12 Gy or GEM +12 Gy (Figure 3D, n=3 for 12 Gy, n=5 for PAN +12 Gy, n=6 for 12 GEM +12 Gy). There was no significant difference in crypt loss between 12 Gy and PAN +12 Gy (p=0.63). However, GEM +12 Gy showed a significant crypt loss (4.3% crypts remained in GEM +12 Gy; 12 Gy vs GEM +12 Gy, p<0.001, PAN +12 Gy vs GEM +12 Gy, p=0.001) similar to that seen for 14 Gy alone (4.7%). In large bowel Swiss rolls there was no difference in IR alone or PAN+IR in terms of crypt regeneration and inflammation, as assessed by a veterinary pathologist (n=2 per group, Supplementary Table 3A).
Fig 3. Methodology to assess acute toxicity using the small animal radiation research platform.
A) Supine SARRP setup showing dosimetry for 14 mm arc treatment. B) H&E-stained sections of small intestinal Swiss rolls demonstrating lost and regenerating crypts after 10, 12 and 14 Gy radiation. C) Small intestinal crypt assay survival for panobinostat and IR, n=2 per group. Data were normalised to mean crypts per mm of two mock and one PAN only for each of three small intestine segments. D) Effect on small intestinal crypt survival of 12 Gy IR (n=3), PAN+12 Gy (n=5) and GEM+12 Gy (n=6) (including data from C) marked in grey) and from 4 independent studies. Three mice from 12 Gy IR and one mouse from PAN+12 Gy were excluded because the slide did not meet the criteria: either no damage or crypt loss < 3 mm. New data were normalised to mean crypts per mm of two mock, one PAN only and one gemcitabine only for each of three small intestine segments.
Panobinostat does not increase radiation intestinal and bladder toxicity at 12 weeks
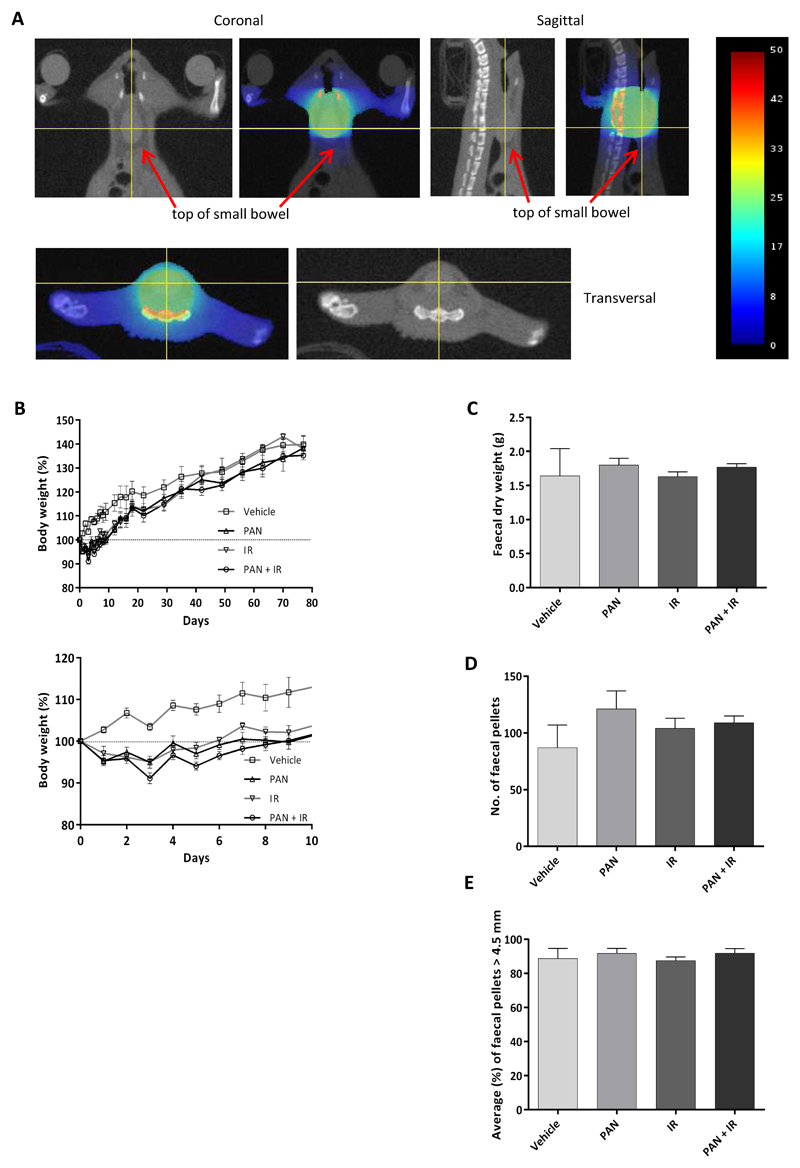
Mouse small intestine is exquisitely sensitive to radiation at the doses proposed; irradiation of even small segments of bowel can be fatal. We therefore developed a novel method to assess potential late effects of PAN+IR compared to IR alone. Mice were treated vertically, head-down in the SARRP, with gravity assisting the removal of small intestine from the treatment volume. Preliminary experiments using freshly euthanased mice, irradiated then immediately subjected to autopsy, reassured us that the entire small intestine would be at a safe distance from the field edge (Supplementary Figure 2). The 10 mm field was centred on the caudal posterior bladder wall, to include lower large bowel in the field, avoiding the small intestine (Figure 4A). Mice were treated with five fractions of 5 Gy (total 25 Gy) over five days. The loss of body weight from baseline of mice treated with PAN+IR was significantly greater compared to IR alone (p=0.03) and mock-treated mice (p<0.001), but weights had returned to baseline within 9 days (PAN+IR) and 6 days (IR) post treatment (Figure 4B).
Fig 4. Panobinostat causes no increased radiation normal tissue toxicity at 11-12 weeks.
A) Dosimetry for 10 mm field centred on the caudal posterior bladder wall, including lower large bowel in the field and avoiding the small intestine. B) Body weight percentage changes from baseline for vehicle (n=4), PAN (n=5), IR (n=3) or PAN+IR (n=4). The lower panel focusses on the first 10 days. C) Mice were isolated for 24 h and their faeces collected and weighed. D) The number of faecal pellets was counted and E) the length of faecal pellets measured. In two IR cages the water bottle leaked, so the faeces became waterlogged and could not be processed.
There was no significant difference in mean weight of dried faeces or mean number of faecal pellets (23) across the groups at 11 weeks (Figure 4C-E). In all groups over 85% of pellets were >4.5 mm in length. On histological examination of the large intestines at 12 weeks, cage 4 mouse 4, treated with PAN+IR, had histological appearances consistent with a moderate diffuse ulcerative colitis. Although helicobacter was a possible cause we were unable to rule out the possible involvement of panobinostat or radiation in its development. Otherwise, only one mouse per treatment group had mild (grade 2 on a 0-5 semi-quantitative scale) changes, including inflammation and crypt hyperplasia (Supplementary Table 3B).
Panobinostat radiosensitises hypoxic bladder cancer cell lines
Our use of subcutaneous human bladder cancer cell xenografts meant that we could not directly investigate the effects of panobinostat on the tumour microenvironment in vivo. However, bladder tumours are known to have hypoxic regions which respond less well to radiotherapy than well oxygenated areas. Moreover, as hypoxic conditions which mimic the tumour microenvironment have significant effects on the chromatin (24), it is important to test HDAC inhibition in this context. On clonogenic assay, surviving fractions at 6 Gy (SF6) for normoxic and hypoxic (2% O2) RT112 cells were 0.27 and 0.35, respectively (p=0.37), with 25 nM panobinostat radiosensitising normoxic RT112 cells (SF6=0.10) (14) and significantly radiosensitising hypoxic RT112 cells (SF6=0.18, p=0.04,). The oxygen enhancement ratio for PAN+IR at 10% survival was 1.22, and for IR alone was 1.12 (Supplementary Figure 3). These data imply that panobinostat could overcome resistance to radiotherapy in the hypoxic regions of bladder cancer xenografts.
Radiosensitisation and expression of proteins are mediated via class I HDAC effects
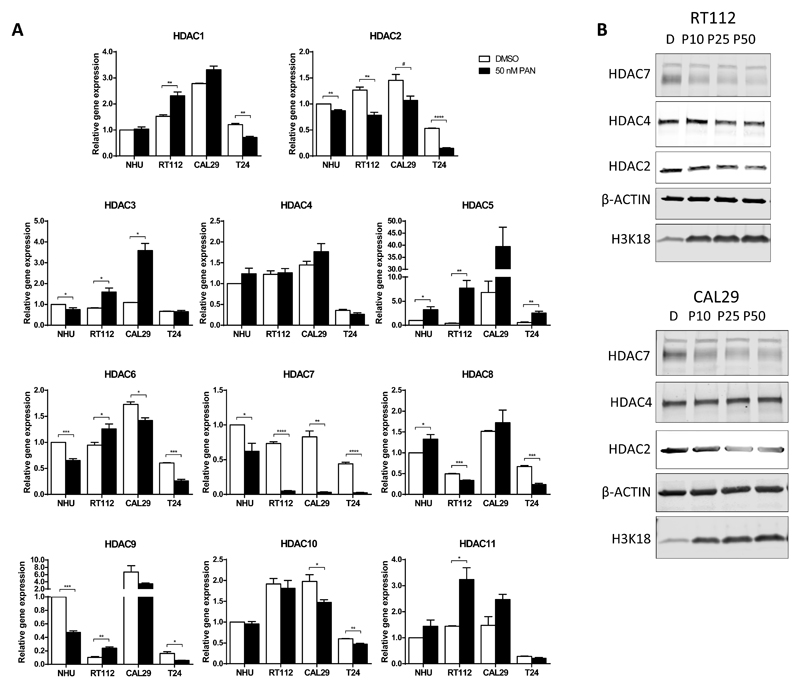
As panobinostat is a non-selective HDAC inhibitor, we hypothesised that its radiosensitising action could be mediated via an individual HDAC or HDAC class rather than its pan-HDAC effects. We established the baseline mRNA expression of 11 individual HDAC genes, normalised to GAPDH expression, in a normal human urothelial (NHU) cell line and RT112, CAL29 and T24 bladder cancer cell lines (Supplementary Figure 4A). mRNA levels of HDAC1 and HDAC2 were the highest across the cell lines, with approximately 5-fold higher expression than other HDACs. mRNA expression levels of untreated (DMSO) and panobinostat-treated samples were compared using a clinically achievable dose of 50 nM (25) (Figure 5A). HDAC2 (Class I HDAC) was downregulated by panobinostat in all treated cells. HDAC7 (Class IIa) was markedly downregulated in tumour cells up to 22-fold compared to untreated cells; in marked contrast, in NHU cells, downregulation was only 1.5-fold. At the protein level, panobinostat caused downregulation of HDAC2 and HDAC7 protein in RT112 and CAL29 cells (Figure 5B and Supplementary Figure 4B).
Fig 5. HDAC expression in response to panobinostat in a panel of bladder cell lines.
A) Samples were treated with DMSO alone or with 50 nM panobinostat for 24 h and mRNA extracted. Expression was normalised to GAPDH from untreated NHU cells. B) Protein expression of HDAC2, HDAC4 and HDAC7 in RT112 and CAL29 cells, after 24-hour 10, 25 and 50 nM panobinostat incubation. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
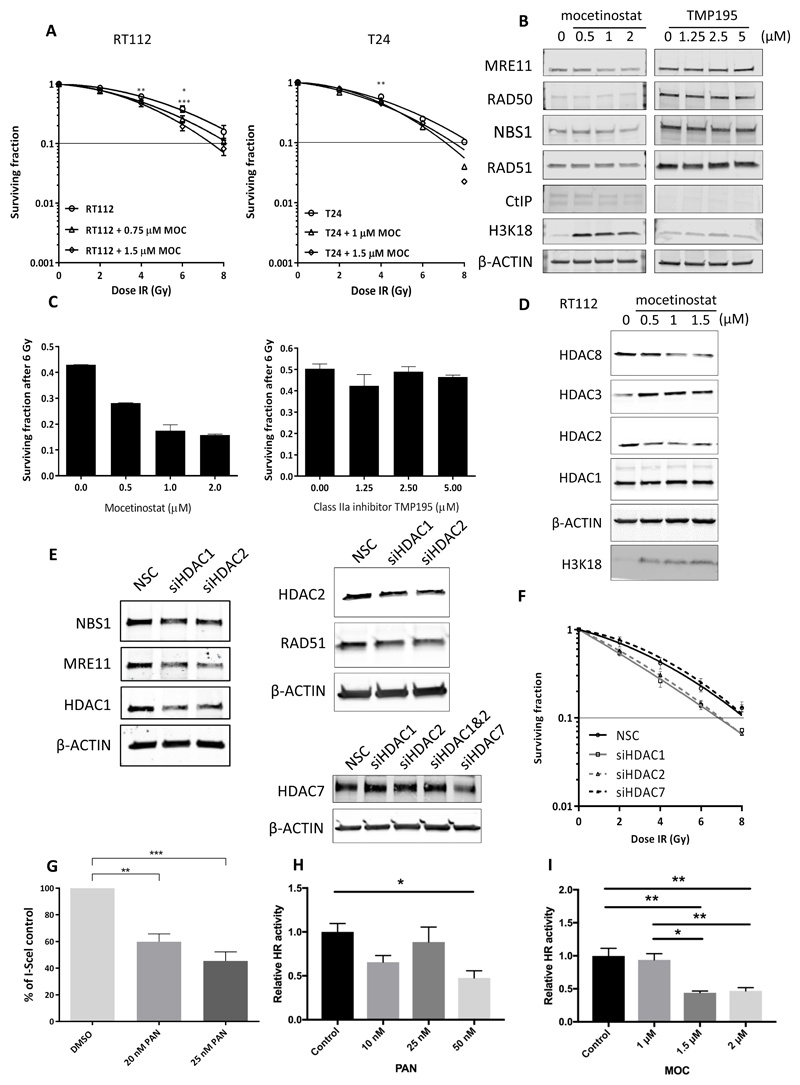
The class I selective agent mocetinostat (MOC) and class IIa selective agent TMP195 were then studied in RT112 and T24 bladder cancer cell lines by clonogenic assay. After 24 h treatment, mocetinostat was effective in the micromolar range in bladder cancer cells (Supplementary Figure 4C) and radiosensitised both cell lines (Figure 6A), although to a lesser degree than panobinostat, with sensitiser enhancement ratios all less than 1.25. Similarly to panobinostat, mocetinostat downregulated MRE11 (and to a lesser extent NBS1 and RAD51) protein levels in a concentration-dependent manner (Figure 6B). In contrast to mocetinostat, the class IIa selective inhibitor TMP195 was non-toxic to RT112 cells, did not downregulate the MRN proteins or RAD51 (Supplementary Figure 4D and Figure 6B), and did not radiosensitise cells in clonogenic assay (Figure 6C). Furthermore, TMP195 did not increase acetylation of histone H3 at residue 18 (Figure 6B), in contrast to panobinostat and mocetinostat (Supplementary Figure 4E and Figure 6B). Mocetinostat downregulated HDAC2 and HDAC8 (class I) protein expression (Figure 6D).
Fig 6. Effects of class I HDAC inhibitors on bladder cancer cells.
A) Clonogenic assay of RT112 and T24 cells incubated with mocetinostat (MOC) for 24 h (n=3). 10% cell survival represented by horizontal lines. B) Effects of MOC or TMP195 on MRN complex and selected HR proteins in RT112 cells (n=2). C) Radiosensitising effects of MOC and TMP195 at 6 Gy in RT112 cells (n=3). D) Effects of MOC on protein expression of class I HDACs in RT112 cells (n=2). E) Effects of transient knock-down of HDAC1 and HDAC2 genes on MRE11, NBS1 and RAD51 protein expression (n=2) and effects on protein levels of HDAC2 and HDAC7 following knockdown by siRNA, in RT112 cells. F) Clonogenic assay after transient knockdown of HDACs 1, 2 and 7 in RT112 cells. G) I-Sce1 assay for PAN (n=3). H, I) HR assays for PAN (n=4) and MOC (n=3). * p<0.05, ** p<0.01,*** p<0.001.
Transient knockdown of HDAC1 or HDAC2 protein levels in RT112 resulted in downregulation of levels of MRE11 and NBS1 (n=2, Figure 6E and Supplementary Figure 4F). Furthermore, transient knockdown of HDAC1 or 2, but not HDAC7, resulted in radiosensitisation (Figure 6F). Overall, these results imply that the radiosensitising effects of panobinostat are primarily mediated via its class I selective effects, in particular through its effects on HDAC1 and 2.
Using both I-SceI based and PCR-based HR assays, we demonstrated that panobinostat treatment resulted in reduced HR activity, and this was also seen for Class I inhibitor mocetinostat (Figure 6G-I) but not the Class IIa inhibitor TMP195 (Supplementary Figure 4G).
Discussion
There is an urgent need to find non-toxic radiosensitising agents to treat elderly bladder cancer patients. In most patients, radiotherapy treatment to the bladder causes tiredness and increased urinary and bowel frequency, and long-term some experience bladder shrinkage, increased bowel frequency and rectal bleeding. Currently used radiosensitising agents tend to exacerbate these side effects (6–8). HDAC inhibitors are promising as radiosensitisers as they have minimal effects on normal cells in vitro (26,27) whilst targeting tumour cells (28–32), with even the suggestion of normal cell sparing with H6CAHA in vitro (26).
To our knowledge this study is the first to address the mechanism and interrelation of the effect of HDAC inhibitors in both tumours and early normal tissue damage, although recently Kalanxhi et al (33) found that mice treated with suberoylanilide hydroxamic acid (SAHA) without radiation showed a significant decrease in body weigh but no apoptosis 3 hours after the last of five daily doses of drug. We developed a modified crypt assay which could be used to study acute effects of local abdominal treatments rather than whole abdominal irradiation. We showed that panobinostat does not add to the acute intestinal toxicity caused by 10-14 Gy IR. On the other hand, another common radiation modifier, gemcitabine, demonstrated crypt damage as early as 2 hours after administration based on our BrdU and caspase 3 staining. Furthermore, gemcitabine significantly radiosensitised the small intestine, such that GEM+12 Gy gave the equivalent crypt loss to 14 Gy alone. Our modified assay method could be useful to others investigating pelvic malignancies which require assessment of bowel toxicity where only part of the abdomen is irradiated. However, a limitation of our current method is that in two mice, the small intestine appeared to receive no irradiation and in another two mice less than 3 mm had been irradiated, perhaps due to a large caecum and the bladder receiving most of the dose. Since these experiments were undertaken, we have modified the set up so that the same sized field is moved slightly superior to encompass more bowel while still treating at least the top of the bladder. The transient weight loss seen after treatment was less marked in non-tumour-bearing animals, suggesting that in xenograft studies weight loss may be due to systemic effects and/or effects associated with tumour kill, possibly cytokine-mediated, rather than reflecting acute intestinal toxicity.
We also developed a novel SARRP method to look at late effects in bowel and bladder. The SARRP permits focussed delivery of ionising radiation, to a relatively homogeneous dose, simulating treatments delivered to human patients. Mice were treated vertically which allowed gravity to remove the small intestine from the treatment field (34), and our pre-treatment cone-beam CT scans gave us confidence that the intestine was outside the field for each treatment. This method will also allow us to irradiate orthotopic bladder tumours, produced by the method of Jager et al (35). A recent report described a method of treating C3H mice using a SARRP to model chronic radiation cystitis, but this involved radiation to the bladder alone without surrounding bowel (36). Our animal licence prevented study of animals beyond 12 weeks, but true late effects would require more than 90 days to elapse, so we cannot exclude the possibility that such effects could have emerged at a later time point. Twenty-five Gy in 5 fractions was chosen as this dose schedule is at the bottom of the sigmoid dose response curve for late effects (23), and so if panobinostat were to increase toxicity, this should be observable at a 30 week time point. One mouse developed ulcerative colitis of unknown aetiology, although panobinostat and/or IR could not be ruled out as a possible contributing factor. Therefore more mice would need to be treated out to 30 weeks to establish any lack of long term toxicity from panobinostat as a radiosensitiser. Of note, one mouse out of 26 mice receiving panobinostat had to be sacrificed within 22 hours after drug administration showing clinical signs of shaking and reluctance to move. The only significant change in the limited tissues (liver, kidney, heart, thymus and intestine) examined from this animal were minimal lymphoid atrophy, depleted hepatocyte glycogen and diffuse bilateral renal tubular dilatation. It is unclear whether these findings are related to panobinostat administration.
Panobinostat at 10 mg/kg days 1, 3 and 5 was sufficient to increase growth delay with 20 Gy in 5 fractions of IR in xenografts. The striking effect of panobinostat in KuKD xenografts supports our hypothesis that, due to synthetic lethality, panobinostat may be useful in non-homologous end-joining (NHEJ)-defective bladder cancer (37). Panobinostat accumulated preferentially in xenografts compared to plasma as previously seen by Atadja et al (13). Our plasma concentrations are clinically achievable (25). We found that IP administration resulted in higher concentrations of panobinostat in small and large intestine and bladder compared to IV administration. However, this local ‘bathing’ of the bowel and bladder with drug following IP injection neither increased its toxic effects in this area nor exacerbated radiation side effects. Furthermore, whilst gemcitabine reduced BrdU and increased cleaved caspase 3 levels, panobinostat did not.
Our subcutaneous xenograft human bladder cancer cell model is limited compared to orthotopic syngeneic models as it cannot assess tumour invasiveness or the immune response, nor is it an ideal model with which to examine the tumour microenvironment. Bladder cancers are known to have a hypoxic fraction of around 10%, with high levels of hypoxia associated with lower survival, and a survival advantage was found in the BCON Phase III trial for carbogen breathing and nicotinamide with radiotherapy versus radiotherapy alone (7). Panobinostat reduces resistance to cisplatin in hypoxic cells (38) and SAHA radiosensitises hypoxic cells (39). Our similar findings for panobinostat imply it may be useful in hypoxic tumours.
We initially hypothesised that the striking differential effect on HDAC7 expression seen in NHUs and tumour cells could explain the differential effects of panobinostat in combination with IR on tumour and normal tissue seen in vivo. However, HDAC1 and 2 were much more highly expressed than the remaining HDACs and we found radiosensitisation and inhibition of MRE11 protein expression via class I (HDAC1 to some extent and 2 with a greater effect) but not IIa (HDAC7) inhibition. HDAC1 and HDAC2 have been shown to form a heterodimer with different localisation and functions (40) when the dimer is disrupted, which account for the dual effect of knockdown of either protein. Furthermore, radiosensitisation was seen following HDAC1 and HDAC2 but not HDAC7 siRNA knockdown. Our HR assay results also confirmed this result as MOC decreased the HR activity while TMP195 failed to show any effect. Therefore, class I-selective inhibitors might be as effective as panobinostat with fewer systemic side effects from off target effects on other HDACs. Although mocetinostat is only effective in the micromolar range, the HDAC1 and 2-selective inhibitor romidepsin (41) showed promise at nanomolar concentrations with radiotherapy for cutaneous T-cell lymphoma (42).
Whilst a phase I clinical trial of panobinostat as a radiosensitiser could rapidly be commenced in bladder cancer, as panobinostat is already in clinical use, potent class I-selective HDAC inhibitors should be studied pre-clinically, as they may have fewer systemic side effects, and such studies might lead to clinical trials.
Supplementary Material
Acknowledgments
We thank Novartis and GlaxoSmithKline for supplying panobinostat and TMP195, respectively, as generous gifts, Prof Freddie Hamdy for funding HS, Karla Watson and Dr Sally Hill for help with animal work, Prof Ruth Muschel for use of her PPL, Alexa Walker for helpful technical advice, and Prof Eric O’Neill and Dr Anderson Ryan for critical reading of the manuscript.
Financial support: This work was supported by Cancer Research UK (grant C5255/A15935), Nuffield Department of Surgical Sciences, University of Oxford, Rosetrees Trust (grant M331), OCRC Development Grants 0213 and 0713 and The Slovene Human Resources Development and Scholarship Fund.
References
- 1.Kotwal S, Choudhury A, Johnston C, Paul AB, Whelan P, Kiltie AE. Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a United Kingdom specialist treatment center. Int J Radiat Oncol Biol Phys. 2008;70:456–63. doi: 10.1016/j.ijrobp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Gray PJ, Fedewa SA, Shipley WU, Efstathiou JA, Lin CC, Zietman AL, et al. Use of Potentially Curative Therapies for Muscle-invasive Bladder Cancer in the United States: Results from the National Cancer Data Base. Eur Urol. 2013;63:823–9. doi: 10.1016/j.eururo.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Noon AP, Albertsen PC, Thomas F, Rosario DJ, Catto JWF. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer. 2013;108:1534–40. doi: 10.1038/bjc.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rödel C, Weiss C. Organ-Sparing Multimodality Treatment for Muscle-Invasive Bladder Cancer: Can We Continue to Ignore the Evidence? J Clin Oncol. 2014;32:3787–8. doi: 10.1200/JCO.2014.58.5521. [DOI] [PubMed] [Google Scholar]
- 5.Efstathiou JA, Spiegel DY, Shipley WU, Heney NM, Kaufman DS, Niemierko A, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol. 2012;61:705–11. doi: 10.1016/j.eururo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Choudhury A, Swindell R, Logue JP, Elliott PA, Livsey JE, Wise M, et al. Phase II study of conformal hypofractionated radiotherapy with concurrent gemcitabine in muscle-invasive bladder cancer. J Clin Oncol. 2011;29:733–8. doi: 10.1200/JCO.2010.31.5721. [DOI] [PubMed] [Google Scholar]
- 7.Hoskin PJ, Rojas AM, Bentzen SM, Saunders MI. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol. 2010;28:4912–8. doi: 10.1200/JCO.2010.28.4950. [DOI] [PubMed] [Google Scholar]
- 8.James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–88. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 9.Ree AH, Dueland S, Folkvord S, Hole KH, Seierstad T, Johansen M, et al. Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: the Pelvic Radiation and Vorinostat (PRAVO) phase 1 study. Lancet Oncol. 2010;11:459–64. doi: 10.1016/S1470-2045(10)70058-9. [DOI] [PubMed] [Google Scholar]
- 10.Groselj B, Sharma NL, Hamdy FC, Kerr M, Kiltie AE. Histone deacetylase inhibitors as radiosensitisers: effects on DNA damage signalling and repair. Br J Cancer. 2013;108:748–54. doi: 10.1038/bjc.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.New M, Olzscha H, La Thangue NB. HDAC inhibitor-based therapies: Can we interpret the code? Mol Oncol. 2012;6:637–56. doi: 10.1016/j.molonc.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozawa A, Tanji N, Kikugawa T, Sasaki T, Yanagihara Y, Miura N, et al. Inhibition of bladder tumour growth by histone deacetylase inhibitor. BJU Int. 2010;105:1181–6. doi: 10.1111/j.1464-410X.2009.08795.x. [DOI] [PubMed] [Google Scholar]
- 13.Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280:233–41. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Groselj B, Kerr M, Kiltie AE. Radiosensitisation of bladder cancer cells by panobinostat is modulated by Ku80 expression. Radiother Oncol. 2013;108:429–33. doi: 10.1016/j.radonc.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr M, Scott HE, Groselj B, Stratford MRL, Karaszi K, Sharma NL, et al. Deoxycytidine Kinase Expression Underpins Response to Gemcitabine in Bladder Cancer. Clin Cancer Res. 2014;20:5435–45. doi: 10.1158/1078-0432.CCR-14-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao B, Kerr M, Groselj B, Teo MTW, Knowles MA, Bristow RG, et al. Imatinib radiosensitizes bladder cancer by targeting homologous recombination. Cancer Res. 2013;73:1611–20. doi: 10.1158/0008-5472.CAN-12-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fournel M, Bonfils C, Hou Y, Yan PT, Trachy-Bourget M-C, Kalita A, et al. MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther. 2008;7:759–68. doi: 10.1158/1535-7163.MCT-07-2026. [DOI] [PubMed] [Google Scholar]
- 18.Lobera M, Madauss KP, Pohlhaus DT, Wright QG, Trocha M, Schmidt DR, et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat Chem Biol. 2013;9:319–25. doi: 10.1038/nchembio.1223. [DOI] [PubMed] [Google Scholar]
- 19.Shackelford C, Long G, Wolf J, Okerberg C, Herbert R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol. 2002;30:93–6. doi: 10.1080/01926230252824761. [DOI] [PubMed] [Google Scholar]
- 20.Pires IM, Olcina MM, Anbalagan S, Pollard JR, Reaper PM, Charlton PA, et al. Targeting radiation-resistant hypoxic tumour cells through ATR inhibition. Br J Cancer. 2012;107:291–9. doi: 10.1038/bjc.2012.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao Z, Bozzella M, Seluanov A, Gorbunova V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair (Amst) 2008;7:1765–71. doi: 10.1016/j.dnarep.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moolenbeek C, Ruitenberg E. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15:57–9. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- 23.Terry N, Denekamp J. RBE values and repair characteristics for colo-rectal injury after caesium 137 gamma-ray and neutron irradiation. II. Fractionation up to ten doses. Br J Radiol. 1984;57:617–29. doi: 10.1259/0007-1285-57-679-617. [DOI] [PubMed] [Google Scholar]
- 24.Olcina MM, Foskolou IP, Anbalagan S, Senra JM, Pires IM, Jiang Y, et al. Replication Stress and Chromatin Context Link ATM Activation to a Role in DNA Replication. Mol Cell. 2013;52:758–66. doi: 10.1016/j.molcel.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F, et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12:4628–35. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- 26.Konsoula Z, Cao H, Velena A, Jung M. Adamantanyl-histone deacetylase inhibitor H6CAHA exhibits favorable pharmacokinetics and augments prostate cancer radiation sensitivity. Int J Radiat Oncol Biol Phys. 2011;79:1541–8. doi: 10.1016/j.ijrobp.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 27.Lee J-H, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci U S A. 2010;107:14639–44. doi: 10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crisanti MC, Wallace AF, Kapoor V, Vandermeers F, Dowling ML, Pereira LP, et al. The HDAC inhibitor panobinostat (LBH589) inhibits mesothelioma and lung cancer cells in vitro and in vivo with particular efficacy for small cell lung cancer. Mol Cancer Ther. 2009;8:2221–31. doi: 10.1158/1535-7163.MCT-09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng L, Cuneo KC, Fu A, Tu T, Atadja PW, Hallahan DE. Histone deacetylase (HDAC) inhibitor LBH589 increases duration of gamma-H2AX foci and confines HDAC4 to the cytoplasm in irradiated non-small cell lung cancer. Cancer Res. 2006;66:11298–304. doi: 10.1158/0008-5472.CAN-06-0049. [DOI] [PubMed] [Google Scholar]
- 30.Storch K, Eke I, Borgmann K, Krause M, Richter C, Becker K, et al. Three-dimensional cell growth confers radioresistance by chromatin density modification. Cancer Res. 2010;70:3925–34. doi: 10.1158/0008-5472.CAN-09-3848. [DOI] [PubMed] [Google Scholar]
- 31.Cha T-L, Chuang M-J, Wu S-T, Sun G-H, Chang S-Y, Yu D-S et al. Dual degradation of aurora A and B kinases by the histone deacetylase inhibitor LBH589 induces G2-M arrest and apoptosis of renal cancer cells. Clin Cancer Res. 2009;15:840–50. doi: 10.1158/1078-0432.CCR-08-1918. [DOI] [PubMed] [Google Scholar]
- 32.Berghauser Pont LME, Naipal K, Kloezeman JJ, Venkatesan S, van den Bent M, van Gent DC, et al. DNA damage response and anti-apoptotic proteins predict radiosensitization efficacy of HDAC inhibitors SAHA and LBH589 in patient-derived glioblastoma cells. Cancer Lett. 2015;356:525–35. doi: 10.1016/j.canlet.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 33.Kalanxhi E, Risberg K, Barua IS, Dueland S, Andersen SN, Pettersen SJ, et al. Induction of Apoptosis in Intestinal Toxicity to a Histone Deacetylase Inhibitor in a Phase I Study with Pelvic Radiotherapy. Cancer Res Treat. 2017;49:374–86. doi: 10.4143/crt.2016.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart F, Michael B, Denekamp J. Late radiation damage in the mouse bladder as measured by increased urination frequency. Radiat Res. 1978;75:649–59. [PubMed] [Google Scholar]
- 35.Jager W, Moskalev I, Janssen C, Hayashi T, Awrey S, Lange D, et al. Ultrasound-Guided Intramural Inoculation of Orthotopic Bladder Cancer Xenografts: A Novel High-Precision Approach. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0059536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zwaans BMM, Krueger S, Bartolone SN, Chancellor MB, Marples B, Lamb LE. Modeling of chronic radiation-induced cystitis in mice. Adv Radiat Oncol. 2016;1:373–43. doi: 10.1016/j.adro.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bentley J, Diggle CP, Harnden P, Knowles MA, Kiltie AE. DNA double strand break repair in human bladder cancer is error prone and involves microhomology-associated end-joining. Nucleic Acids Res. 2004;32:5249–59. doi: 10.1093/nar/gkh842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hrzenjak A, Moinfar F, Kremser M-L, Strohmeier B, Petru E, Zatloukal K, et al. Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo. Mol Cancer. 2010;9:49. doi: 10.1186/1476-4598-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saelen MG, Ree AH, Kristian A, Fleten KG, Furre T, Hektoen HH, et al. Radiosensitization by the histone deacetylase inhibitor vorinostat under hypoxia and with capecitabine in experimental colorectal carcinoma. Radiat Oncol. 2012;7:165. doi: 10.1186/1748-717X-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan DH, He S, Yu J, Winter S, Cao W, Seiser C, et al. Protein Kinase CK2 Regulates the Dimerization of Histone Deacetylase 1 (HDAC1) and HDAC2 during Mitosis. J Biol Chem. 2013;288:16518–28. doi: 10.1074/jbc.M112.440446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Adachi M, Zhao X, Kawamura R, Imai K. Histone deacetylase inhibitors FK228, N-(2-aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)amino-methyl]benzamide and m-carboxycinnamic acid bis-hydroxamide augment radiation-induced cell death in gastrointestinal adenocarcinoma cells. Int J Cancer. 2004;110:301–8. doi: 10.1002/ijc.20117. [DOI] [PubMed] [Google Scholar]
- 42.Akilov O, Grant C, Frye R, Bates S, Piekarz R, Geskin L. Low-dose electron beam radiation and romidepsin therapy for symptomatic cutaneous T-cell lymphoma lesions. Br J Dermatol. 2012;167:194–7. doi: 10.1111/j.1365-2133.2012.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.