Abstract
Preterm infants are at elevated risk for a host of neurodevelopmental problems, including disorders that appear later in life. Gene–environment interactions and prematurity may combine to increase the risk for poor neurodevelopmental outcomes. Increasing evidence supports a genetic link to risk for atypical development; however, no genomic risk profiles are currently used for infants without apparent genetic disorders. The purpose of this review was to synthesize recent evidence of genetic associations with atypical neurodevelopmental outcomes that may affect preterm infants who do not have a rare genetic disease. Electronic and hand-search strategies were used to find relevant articles that were English-language, peer-reviewed primary research or meta-analysis reports published between July 2009 and July 2014, involving human participants. Articles included in the analysis (N = 29) used a wide range of study designs and methodologies, complicating the analysis. An integrative-review design was used to synthesize the data. Numerous genes (n = 43) and additional large deletion copy number variants were associated with neurodevelopmental outcomes, including cognition, attention, perception, psychiatric disease, autism spectrum disorder, cerebral palsy, infant behavior, and alterations in brain architecture. The creation of genetic risk profiles for complex disorders of neurodevelopment is presently hindered by inconsistent genetic-association evidence, methodological considerations, reporting problems, and lack of replication. However, several avenues of investigation offer promise, including large (>100 kb) copy number variants and the candidate genes MET, NRG3, and SLC6A4, each of which were reported to have associations with neurodevelopmental outcomes in multiple, high-quality studies.
Keywords: genetics, preterm infant, neurodevelopment, cognition, genomics, prematurity
Prematurity affects more than 12% of all infants born in the United States, accounting for a third of all infant deaths. Many preterm infants who survive the neonatal period are at elevated risk of cognitive impairment, behavioral problems, developmental delay, and disability (Centers for Disease Control and Prevention, 2013). For example, individuals born preterm had increased risk of depression, anxiety, and attention-deficit hyperactivity disorder (ADHD; Sullivan, Msall, & Miller, 2012) and significantly more autistic features than their classmates born at term (Johnson et al., 2010). In addition, those born preterm have heightened risks for psychotic disorders, organic/neuropsychiatric disorders, suicide attempts and completions, and addictive disorders with comorbid psychiatric illness (Lindström, Lindblad, & Hjern, 2009) as well as increased risk for depression and schizophrenia (SZ) later in life (Knud Larsen, Bendsen, Foldager, & Munk-Jørgensen, 2010).
Evidence suggests that both genetic and environmental factors contribute to neurodevelopmental disorders. For example, Wang et al. (2009) found that 10 single-nucleotide polymorphisms (SNPs) in cell adhesion genes clustered in a single region of one chromosome (5p14.1) were strongly associated with autism spectrum disorders (ASDs). SZ, long recognized as heritable, is associated with multiple genes (Braff, Freedman, Schork, & Gottesman, 2007). Likewise, 76–92% of the variance between ADHD phenotypes is explained by genetic factors (Rietveld, Hudziak, Bartels, van Beijsterveldt, & Boomsma, 2004).
Despite the major risk posed by preterm birth, not all individuals born preterm exhibit atypical neurodevelopment. Neurodevelopmental phenotypes of children born preterm are widely diverse. This heterogeneity may be explained by gene–environment interactions (G × E; Caspi & Moffitt, 2006), with preterm birth and genetic risk alleles forming multiple “hits” that affect outcomes in the neonatal period and throughout life. Examining genetic risk factors may provide a clearer picture of who is most at risk for adverse neurodevelopmental outcomes, which may lead to the development of risk-mitigating interventions. The purpose of this integrative review was to synthesize recent evidence of genes associated with atypical neurodevelopmental outcomes that may affect preterm infants who do not have a rare, genetic disease.
Method
We used an integrative review design, a type of systematic review, to synthesize the diverse literature (Whittemore & Knafl, 2005). We followed reporting guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for systematic reviews, with modification as appropriate for integrative review (Moher, Liberati, Tetzlaff, Altman, & the PRISMA Group, 2009). Genomics terms are defined in Table 1.
Table 1.
Glossary.
| Term | Definition |
|---|---|
| Candidate gene | A gene that is hypothesized to cause disease |
| Copy number variant (CNV) | Variations in the number of copies of a gene or gene segment described in terms of deletion (fewer copies than average) or duplication (more copies than average) |
| Genetics | The study of particular heritable traitsa |
| Genomics | The study of genes and gene products (e.g., proteins or messenger RNA)a |
| Genome-wide association study (GWAS) | A study that examines the many common DNA variants, typically in a large sample, to identify associations between specific variants and a particular traita |
| Haplotype | A sequence of consecutive alleles that are close together on a strand of DNA and are likely to be inherited together |
| Hardy–Weinberg equilibrium (HWE) | A mathematical principle stating that the frequency of a particular genetic variation will remain constant across a population from one generation to the next, assuming the population is intermingling at random, and as such is considered a mathematical “ideal” rather than a practical limitation (Wittke-Thompson, Pluzhnikov, & Cox, 2005) |
| Oncogene | A gene that is associated with the development of cancer |
| Phenotype | Observable characteristics of the interaction between the environment and the genotype |
| Proto-oncogene | A gene with the potential to become an oncogene with overexpression or mutation |
| Single nucleotide polymorphism (SNP) | A variation in a single DNA base pair occurring in at least 1% of the population |
aU.S. Department of Energy Human Genome Project (2012).
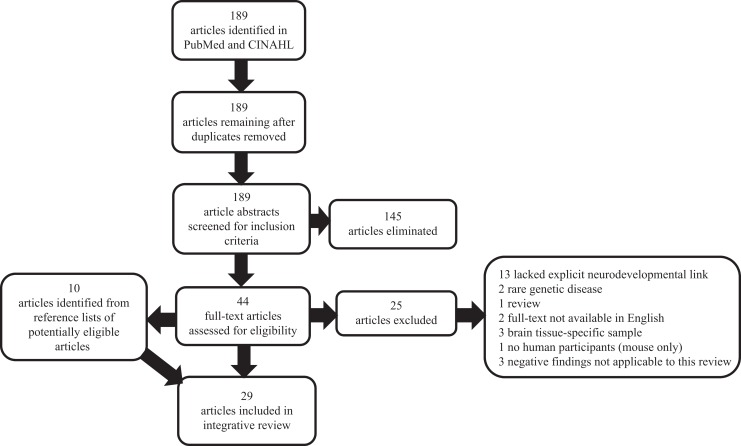
We performed electronic database searches from June through July 2014 in PubMed and the Cumulative Index to Nursing and Allied Health Literature with combinations of the following search terms: (1) neurodevelopment or neurologic and (2) genetic or gene. Searches were restricted to English-language articles with human participants published between June 2009 and June 2014, resulting in 189 articles. For the purposes of this review, we define atypical neurodevelopment as any functional deficit in higher order neurodevelopmental process (e.g., emotional regulation, cognition, or perception). As many neurodevelopmental problems do not become apparent during the neonatal or early childhood periods, we did not limit search criteria to infants. Articles were eligible for inclusion if they met the following criteria, assessed using the article abstract: meta-analysis or original research report, peer-reviewed, and human subjects. Because the purpose of this review was to ascertain risk factors in living individuals free of rare genetic disorders, articles that focused exclusively on brain tissue–specific sampling or rare genetic disease were eliminated, resulting in 44 remaining articles. We added 10 articles after reviewing the reference lists for those 44 articles. We included articles in which there was no evidence of correlation between neurodevelopment and genes studied only if another included article reported a correlation between those genes and a neurodevelopmental outcome. Specific reasons for elimination and final sample details are displayed in Figure 1.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses diagram showing the flow of information for this review.
During the full-text review phase, we recorded study design, target genes, methodologic approach, DNA sources, sample size, a summary of findings, and sample characteristics on data forms, along with odds ratios and corrected p values, where available. We collated data forms into a spreadsheet and reviewed articles with any missing data again prior to analysis. In addition, we assigned each included article a quality score (no = 0, yes = 1) for each of the following criteria: (1) presence of a comparison group; (2) appropriate statistical control for multiple tests; (3) adequate sample, defined as attrition < 20%, response rate > 70%, and appropriately selected controls; (4) potential confounding characteristics reported and statistically adjusted; and (5) findings of association that correspond to gene targets in another included article. We assigned quality scores following the selection of the sample in order to facilitate evaluation and report them here to clarify potential limitations of the articles included.
Once we analyzed the included articles, several trends became apparent that informed the design of this review. First, authors used multiple approaches to examine the genetic variation, making the studies not directly comparable. Second, researchers used a wide variety of study designs and neurodevelopmental outcome measures, which also contributes complexity to the process of synthesizing the available literature.
In addition, specific neurodevelopmental disorders such as SZ, bipolar disorder (BPD), ADHD, and ASD share a much larger volume of genetic literature than general neurodevelopmental (e.g., poor cognition) or infant-specific outcomes. However, since SZ, BPD, ADHD, and ASD are commonly understood to be due in part to impaired neurodevelopment (Harrison, 2007), many of the findings specific to these disorders may be relevant to impaired neurodevelopment from a broader perspective. As neurodegenerative and environmental elements may also play key roles in the etiology of disorders or phenotypes appearing later in life, a clear theoretical or hypothetical statement linking genomic findings to neurodevelopment was required for a disorder-specific study to be included.
Results
Among the 29 included articles, authors used four methodologies for genetic approaches: (1) polymorphism association studies, which involved examining individual SNP or haplotypes of 2–5 consecutive nucleotides (SNP studies); (2) genome-wide association studies (GWAS); (3) copy number variation (CNV) evaluations; and (4) phenotype mining (PM). A single study employed a combination of GWAS and CNV approaches. Similarly, authors measured a wide range of neurodevelopmental outcomes. To facilitate analysis, we divided articles into five categories by primary outcome: (1) infant behavioral and developmental outcomes; (2) childhood-onset disorders; (3) adolescent- and adult-onset disorders; (4) general measures of cognition, attention, and perception; and (5) alterations to brain structure or function. The design, study characteristics, approach, outcomes category, and findings are presented in Table 2.
Table 2.
Article Characteristics by Outcome Category and Quality Score.
| Study | Quality | Approach | N | Sample Characteristics | Target Gene(s) | Outcomes |
|---|---|---|---|---|---|---|
| Infant behavioral and developmental outcomes | ||||||
| Brummelte, Galea, Devlin, and Oberlander (2013) | 43a | CNV | 86 | Pregnant Canadians with or without exposure to SSRI | SLC6A4 | Infants with two short variants had increased reelin levels (p = .041, η2 = .074), increased vigor and irritability (p < .008) and decreased alertness and orientation (p = .03, η2 = .12) compared to those with any long variants |
| Hill et al. (2013) | 45 | CNV | 209 | U.K. mothers and their infants | MAOA | G × E study: homogeneity of low-activity allele + negative prenatal life events was associated with negative infant emotionality at 5 weeks (p = .017, CI [1.18, 1.53]) |
| Clark et al. (2010) | 32,5 | SNP | 272 | U.S. 2-year-olds surviving preterm birth following assignment in an RCT of antenatal corticosteroid exposure | IL1B-511, IL4R-148, IL6-174, IL6-176 | All four SNP variants were associated with decreased scores on the Bayley’s at 2 years of age (ps < .05). Increased odds of developmental delay at age 2 among carriers of IL1B-511 (OR = 3.1, CI [1.2, 8.2]) and IL6-176 (OR = 2.2, CI [1.2, 3.9]) |
| Childhood-onset disorders | ||||||
| Loo et al. (2012) | 45 | GWAS | 656 | U.S. family trios with an autistic child | KIF16B, PAX5, ELSPBP1, AOX1, PISD, RXRA, CSMD2, LUZP2, CNTN4, COL1AZ, UCN3, CYCSP14 | GWAS used sliding haplotype windows to analyze additive effect of multiple SNPs across genes on intelligence (within ASD case–control design). Larger number of minor alleles for target genes were associated with higher IQ (p = .0000005) |
| Toma et al. (2013) | 45 | SNP | 676 | White Spanish ASD patients and healthy controls | DDC | Haplotype C-T-A-T overrepresented in ASD cases (OR = 2.44, p = .000099, CI [1.55, 3.83]) |
| Bi et al. (2012) | 31,5 | GWAS | 67 | Autistic Caucasians (age unreported) | CNVs, ANK3 | Seven missense de novo mutations identified. ANK3 CNV was present in 4 of the 67 participants |
| Lien et al. (2013) | 33a,5 | SNP | 255 | Norwegian children with CP | ApoEε4 | SNP variant associated with comorbid epilepsy (OR = 2.19, p = .02, CI [1.13, 4.24]), presence of a gastrostomy (OR = 2.72, p =.027, CI [1.12, 6.59]), and (in spastic unilateral CP only) severity of fine motor impairment (OR = 2.6, p = .007, CI [1.31, 6.88]) |
| Costantine et al. (2012) | 22,4 | SNP | ǂ | U.S. toddlers following assignment in an RCT of antenatal exposure to magnesium sulfate for risk of preterm birth | VIP, GRIN3A, AGER, (−) BDNF, (−) RELN | Increased odds of CP for each copy of minor allele (A) of VIP (OR = 2.67, p = .03, CI [1.09, 6.55]) and for each minor allele (T) of GRIN3A (OR = 4.67, p = .01, CI [1.36, 16.01]). Association between mental delay and AGER was moderated by antenatal exposure to magnesium sulfate (p = .02) |
| Rosenfeld et al. (2010) | 21,4,5 | GWAS CNV | 1,461 | Laboratory database | Large CNVs | 180 Abnormal (113 potentially causative) CNVs identified in samples referred for evaluation for ASD |
| Sorte, Gjevik, Sponheim, Eiklid, and Rødningen (2013) | 23b,4,5 | CNV | 50 | Children with autism | de novo CNVs | Case reports of nine large deletion CNVs detected in eight cases |
| Adolescent- and adult-onset disorders | ||||||
| Burdick, DeRosse, Kane, Lencz, and Malhotra (2010) | 5 | SNP | 529 | U.S. Caucasian SZ patients, healthy controls | MET | Associations found between multiple haplotype variants and SZ (p = .00025, CI [0.24, 0.65], OR = 0.4), and cognition (ps < .001) |
| Meier et al. (2013) | 5 | SNP | 469 | SZ patients and BPD patients | NRG3 | 1 SNP variant of NRG3 was associated with SZ symptom profiles (p = .037) and multiple tests of cognitive processing speed (ps < .05) |
| Tost et al. (2014) | 5 | SNP | 611 | Caucasians of European descent with SZ, their siblings, unrelated controls | NRG3 | Single SNP test, with C-carrier associated with low IQ (p = .008) in cases and greater activation of the prefrontal cortex in controls with a genotype-group interaction effect (p = .016) |
| Kao et al. (2010) | 42 | SNP | 1,545 | U.S. Caucasian SZ probands, their parents, unrelated controls | NRG3 | Multiple NRG3 SNP variants were associated with SZ and symptom dimensions (ps < .05) |
| Kirov, Rujescu, Collier, O’Donovan, and Owen (2009) | 45 | CNV | 50,843 | Pooled sample from multiple SZ GWASs | NRXN1 | Deletion CNVs > 100 kb were overrepresented in cases (OR = 4.78, p = .000013, CI [2.44, 9.37]). Those deletions that disrupt exons, specifically, had OR = 7.44, p = .00037, CI [3.22, 17.18] |
| Shaikh et al. (2011) | 43a | SNP | 764 | Caucasian SZ patients, family, unrelated controls | (−)COMT, (−)BDNF, (−)NRG1 | P50 suppression tests show no significant results by genotype |
| Tosato et al. (2012) | 44 | SNP | 27 | European SZ patients | NRG1 | Homogenous minor allele (C/C) associated with superior temporal gyrus volumes (ps < .006) |
| Zakharyan et al. (2011) | 44 | SNP | 208 | Armenian SZ patients and healthy controls | BDNF | Homogenous minor allele (Met/Met) associated with SZ diagnosis (OR = 2.28, p = .006, CI [1.14, 1.98]) and lower age of onset (p = .024) |
| Alaerts et al. (2009) | 32,3b | SNP | 1,000 | Adult Swedish SZ patients and healthy controls | NRG1 | 5 SNP variants across the intron were significantly associated with SZ (p < .04). One previously identified candidate variant was nonsignificant |
| Chandler et al. (2010) | 34,5 | SNP | 508 | Australian SZ patients, healthy controls | NRN1 | Associations of IQ with haplotype variant in the full sample (p ≤ .01) and 1 SNP variant with lower IQ in cases (p = .0013) |
| Håvik et al. (2012) | 32,5 | SNP | 9,895 | Pooled sample of European GWASs in SZ/BPD/ADHD patients, healthy controls | DCLK1 | SNP variants were associated with SZ, BPD, and ADHD individually and combined (ps < .05), SZ + ADHD (OR = 1.32, p = .00004, CI [1.17, 1.49]), BPD + ADHD (p = .00004, OR = 1.32) |
| Kuswanto et al. (2012) | 33a,3b,5 | SNP | 200 | Chinese SZ patients, hospital staff and convenience controls | ZNF804A | SNP variant was associated with volumes in multiple brain areas for cases and right temporal lobe only for controls (ps < .009) |
| General measures of cognition, attention, and perception | ||||||
| Beevers, Wells, Ellis, and McGeary (2009) | 5 | CNV | 182 | U.S. healthy college students | SLC6A4 | Short allele of 5-HTTLPR was associated with difficulty disengaging from emotional stimuli (p < .05, η2 = .17). |
| Lin et al. (2012) | 5 | SNP | 182 | Healthy Chinese Han | MET, AKT | MET gene variant (rs2237717 [C/T]) was associated with improved facial recognition (p = .048) |
| Pietiläinen et al. (2011) | 22,4,5 | PM | 4,932 | Subset of Finnish birth cohort (1966) with seven phenotypes | Large deletion CNVs | CNVs (>500 kb) associated with IQ < 85 (OR = 3.79, p = .0024, CI [1.54, 8.14]) and repeating grade (OR = 2.02, p = .071, CI [1.47, 4.67]) |
| Alterations in brain architecture | ||||||
| Cattaneo et al. (2010) | 5 | SNP | 139 | Amniotic fluid from healthy, Caucasian women at 15–17 weeks | BDNF | Met variant carriers (Met/Met, Val/Met) had decreased BDNF in amniotic fluid compared to Val/Val (p = .002) |
| Dutt et al. (2011) | 44 | SNP | 62 | Adults with history of preterm (<33 weeks) birth, term controls | COMT | Homogenous Met/Met variant associated with greater corpus callosum volumes (ps < .03) |
| Jahanshad et al. (2012) | 31,5 | SNP | 615 | Healthy Australian adult twins and siblings | HFE | HFE variant associated with transferrin. Low transferrin associated with decreased white matter fiber integrity (ps < .05) |
| Kobiella et al. (2011) | 32,4 | CNV | 54 | Healthy adult smokers and nonsmokers | SLC6A4 | Short allele associated with brain volumes (ps < .017) and left amygdala activation (p = .015). G × E effect found with smoking (p =.037) |
Note. In the primary sources, p values and odds ratios (OR) or η2 are reported for positive findings related to genotype only. Confidence intervals (CIs) are reported at 95% threshold unless otherwise noted. (−) = nonsignificant findings; ADHD = attention-deficit hyperactivity disorder; ASD = autism spectrum disorder; BPD = bipolar disorder; CNV = copy number variant; CP = cerebral palsy; G × E = gene and environment interactions; GWAS = genome-wide association study; IQ = intelligence quotient; PM = phenotype mining; SNP = single-nucleotide polymorphism; SSRI = selective serotonin reuptake inhibitor; SZ = schizophrenia. ǂ = N is indeterminate based on primary source.
Reasons for loss of quality points: 1no comparison group. 2No mention of statistical controls for multiple tests and large battery of tests. 3aSample concerns: attrition > 20%, response rate < 70%, population not generalizable. 3bControl population dissimilar to cases in multiple ways. 4Confounders not reported and/or not controlled. 5No other study in this review reported associations with target genes.
Of the 43 potential genetic markers of neurodevelopmental risk identified in the reviewed studies, only 6 were examined more than once. Of these, three (BDNF, COMT, and RELN) were inconsistently associated with neurodevelopmental outcomes of interest. The remaining three were consistently associated with neurodevelopmental outcomes: (1) MET, a proto-oncogene associated with SZ and facial recognition that is also strongly linked to cancers for which SZ patients and their first-degree relatives have decreased incidence (Burdick, DeRosse, Kane, Lencz, & Malhotra, 2010; Lin et al., 2012); (2) NRG3, a neuregulin gene associated with SZ, BPD, and intelligence quotient (IQ) <75 that affects the proliferation, migration, differentiation, and apoptosis of some neurological cells (Kao et al., 2010; Meier et al., 2013; Tost et al., 2014); and (3) SLC6A4 (also known as 5-HTT), a serotonin transporter gene associated with attention and alterations in infant behavior and levels of reelin, a protein important for neuronal migration in infancy and adult neuronal plasticity (Beevers, Wells, Ellis, & McGeary, 2009; Brummelte, Galea, Devlin, & Oberlander, 2013; Kobiella et al., 2011). All but one of the articles that examined MET, NRG3, and SCL6A4 received quality scores of 4–5 per criteria established in this review.
Infant Behavioral and Developmental Outcomes
The three articles that reported data about infant behavior and developmental outcomes used the Bayley’s Scales of Infant Development, the Neurobehavioral Assessment of the Preterm Infant, or the Neonatal Behavior Assessment as their primary outcome measures. Researchers used buccal cells, umbilical cord blood, umbilical cord blood serum, and placental tissue as sources of genetic samples. Clark et al. (2010) reported degradation in the umbilical samples and discordant findings between serum samples and placental samples for multiple infants who had both DNA sources available. In addition to this sample-quality limitation, Clark et al. drew their sample of preterm infants from a randomized controlled trial (RCT) examining antenatal exposure to single- or multiple-dose corticosteroids. Similarly, Brummelte, Galea, Devlin, and Oberlander (2013) examined infants exposed prenatally to serotonin reuptake inhibitors (SRIs). Both studies may thus have limited generalizability because their samples were drawn from infants who experienced and survived exogenous exposures that may have altered outcomes compared to unexposed infants. Finally, Hill et al. (2013) demonstrated a G × E effect between maternal stressful experiences during pregnancy and high- versus low-activity variants of the gene MAOA, which encodes the structure of monoamine oxidase A, an enzyme responsible for the breakdown of serotonin and other neurotransmitters. In high-stress prenatal environments, low-activity MAOA infants demonstrated more negative emotionality, while in low-stress prenatal environments, high-expression MAOA infants were more emotionally negative.
Childhood-Onset Disorders
We reviewed seven articles reporting on genetic links to childhood-onset neurodevelopmental disorders. The most frequently examined subgroup of disorders (n = 4) were ASDs. These articles were primarily reports of GWAS and CNV studies examining a relatively large number of target genes. Rosenfeld et al. (2010) reported findings of 180 CNVs in 1,461 cases referred to one lab for genetic analysis related to ASD diagnoses. The investigators used no controls or comparison groups, and the case population was poorly defined. Similarly, Sorte, Gjevik, Sponheim, Eiklid, and Rødningen (2013) examined 50 children with ASDs and found that those with large deletion CNVs were at increased risk of intellectual disability and severe behavioral phenotypes. CNVs were also implicated in ASDs among participants in a GWAS study, with 4 of the 67 autistic Caucasian participants exhibiting CNV of the gene ANK3 and 7 showing de novo missense mutations in the same gene. However, the study included no comparison group to determine whether these variations were associated with diagnosis (Bi et al., 2012).
In one GWAS family study, investigators used four-base pair sliding haplotype windows over 1,000,000 SNPs to examine the cumulative effects of multiple polymorphisms on intelligence in children with ADHD. They found that minor alleles of eight genes were associated with higher intelligence, and the effects were additive (Loo et al., 2012). A second study that examined four-base haplotypes found that a specific combination of SNPs in the gene DDC was associated with ASD diagnosis (Toma et al., 2013). Additionally, two articles were reports of neurodevelopmental outcomes related to cerebral palsy (CP). Lien et al. (2013) reported strong associations between apolipoprotein E (ApoE) genotype and symptom severity in CP, with increased odds of worse fine motor control, concurrent epilepsy, and presence of gastrostomy in children with variant ApoEε4, which has also been implicated in risk for late-onset Alzheimer’s disease (AD; APOE, 2014). Costantine et al. (2012) reported increased odds of CP for each minor allele (A) of VIP, a gene that encodes proteins active in glucose metabolism and lowering blood pressure, and for each minor allele (T) of GRIN3A, part of a genetic family involved in brain plasticity and excitatory synaptic signaling. However, two factors limit generalizability of Costantine et al.’s findings: (1) the sample included infants who were antenatal participants in an RCT of magnesium sulfate exposure and (2) the researchers used no statistical control for multiple testing.
Adolescent- and Adult-Onset Disorders
Studies looking at SZ as the primary neurodevelopmental outcome comprised the largest group of articles to meet our inclusion criteria (n = 12). Burdick, DeRosse, Kane, Lencz, and Malhotra (2010) examined SZ patients and healthy controls for MET haplotype variants and found an association with SZ and neurocognition. Kao et al. (2010) tested 1,545 SZ patients, unaffected family members, and unrelated controls for NRG3 SNP variants and found associations between SNPs and SZ and symptom dimensions. Similarly, Meier et al. (2013) examined an NRG3 SNP variant and symptom profiles among individuals with SZ and BPD and identified an association between the variant and the genetic susceptibility to cognitive deficits. Kirov, Rujescu, Collier, O’Donovan, and Owen (2009) found that large deletion CNVs (>100 kb) of NRXN1 were overrepresented in SZ cases. Alaerts et al. (2009) found associations between several SNPs across the intron of NRG1 and SZ diagnosis in Swedish adults compared to healthy controls. Tost et al. (2014) examined a single SNP variant of NRG3 in individuals with SZ compared to their unaffected siblings and unrelated controls and found that minor allele (C) carriers outperformed those with the risk major allele (T/T) on IQ tests. Zakharyan et al. (2011) found associations of the BDNF gene Val66Met variant with SZ diagnosis and earlier age of onset. Håvik et al. (2012) identified significant associations of DCLK1 variants with diagnoses of SZ alone and SZ combined with ADHD.
There were several methodological concerns with studies in this group. For example, Tosato et al. (2012) examined brain architecture among SZ patients with a single variant of NRG1, a protein-encoding gene that influences growth and development of multiple organ systems. However, only 19% of participants completed both DNA sampling and magnetic resonance imaging (MRI). Another group of authors reported finding no correlations between several genes and P50 suppression, a specific test of the brain’s ability to filter stimuli that are unimportant or repetitive, but relative and healthy controls differed significantly from SZ cases on a number of reported measures (Shaikh et al., 2011). Kuswanto et al. (2012), who identified an association between a variant of the gene ZNF804A and brain regional volumes in SZ cases, used hospital staff and community members as controls despite significant differences from cases. Chandler et al. (2010), who found associations between multiple variants of NRN1 and IQ among SZ patients and healthy controls, did not report sample demographic characteristics.
General Measures of Cognition, Attention, and Perception
A total of three articles reported studies examining general cognition, attention, or perception in individuals who were not diagnosed with specific disorders of neurodevelopment. Measures included facial recognition tests, IQ testing, standardized school performance measures, and disengagement of attention in relation to emotional or neutral (i.e., not considered emotionally provocative) stimuli. Study participants were primarily healthy adults. Pietiläinen et al. (2011) reported increased odds of low IQ and repeating school grades for individuals with large deletion CNVs compared to unaffected cohort members. Beevers, Wells, Ellis, and McGeary (2009) found an association of short CNVs of 5-HTT with difficulty disengaging from emotional stimuli. Finally, Lin et al. (2012) found associations in 182 healthy people between facial recognition and MET variants.
Alterations in Brain Structure and Function
Finally, four articles were reports of examinations of brain structure and function in healthy individuals using MRI, functional MRI, positron emission tomography, and BDNF in amniotic fluid. Dutt et al. (2011) found that a COMT variant was associated with reduced corpus callosum size in adults born at <33 weeks’ gestation. Kobiella et al. (2011) found that short variants of 5-HTT were associated with smaller amygdala volumes and increased amygdala activation in response to unpleasant stimuli in healthy adults.
The remaining two articles used indirect measures that have been linked to neurodevelopmental outcomes. Cattaneo et al. (2010) found that the BDNF Val66Met variant was associated with reduced BDNF availability in amniotic fluid at 15–17 weeks of pregnancy, potentially resulting in decreased fetal brain growth and higher risk for BPD and affective disorders. A second study reported that variation in HFE was associated with transferrin, a protein important in iron binding that has been associated with decreased white matter fiber integrity (Jahanshad et al., 2012).
Discussion
This review demonstrates that authors have used multiple genomic approaches including GWAS, CNV studies, SNP and haplotype studies, and PM to examine genetic factors that influence neurodevelopment and neurodevelopmental disorders. These approaches have resulted in identification of numerous factors, including 43 genes and additional large deletion CNVs, associated with neurodevelopmental outcomes including cognition, attention, perception, psychiatric disease, ASD, CP, infant behavior, and alterations in brain structure.
Although authors reported a wide range of genetic contributions to neurodevelopmental phenotypes, readers should consider several methodological and reporting concerns when interpreting this literature. First, although technological advances have allowed genomic research to flourish, methods to correct for errors in genotyping were highly inconsistent across these studies. For example, multiple articles reviewed used Hardy–Weinberg equilibrium (HWE; Table 1) as a control measure, but in only two articles did authors discuss the potential problem with requiring HWE in cases as well as controls (Wittke-Thompson, Pluzhnikov, & Cox, 2005). The appropriateness of using HWE as a genotyping error control, particularly among cases in a case–control design, is dependent on multiple factors, including sample size and disease prevalence. In addition, the articles reviewed reported vastly different standards for data elimination based on genotyping error. Perhaps even more concerning was the lack of statistical control for multiple tests. As the number of SNPs examined ranged from 1 to 1,000,000 in the articles reviewed, failure to control for multiple tests could have escalated the uncorrected Type I error.
Multiple articles included no report of basic design features including the number of participants, participant characteristics, and collection and processing of genomic material. Failure to clearly describe the sample and report participant characteristics such as age, gender, and socioeconomic status can mask confounders. Failure to report collection and processing of genomic material is also troublesome, as different sources of DNA are prone to differential genotyping error rates. Inappropriate reporting reduces replicability and generalizability of these studies and hinders interpretation. In addition, authors generally discussed total number of SNPs examined, but identification of nonassociation is important in this emerging field, particularly given the often disparate findings between populations. Most of the articles reported inclusion of purposely homogenous samples, generally of White European descent. Although homogenous samples reduce complexity in genomic analysis, this choice affects interpretation and generalizability.
In spite of these methodological concerns, several articles presented results of rigorous research and identified three genes with high potential for association with neurodevelopmental disorders: MET, NRG3, and SLC6A4. MET, located at ch7q31, may be relevant to neurodevelopment due to its effects on neuronal migration and angiogenesis following birth (Life Map Sciences, 2014a). While we identified no studies examining MET specifically in preterm infants, investigators have linked the gene to the development of autistic features, a problem far more prevalent among former preterm infants than the general population (Kuzniewicz et al., 2013). We also identified no studies in preterm infants that examined NRG3 located at ch10q22-23 (Life Map Sciences, 2014b). However, variants of the gene have been associated with AD diagnosis and age of onset (Wang et al., 2014), which may indicate a role for this gene in brain plasticity. The serotonin transporter gene SLC6A4, located at ch17q11.2, is thought to affect sudden infant death syndrome, aggression among AD patients, and susceptibility to mood disorders (Life Map Sciences, 2014c). Investigators have studied SLC6A4 in the fetal and neonatal periods primarily to assess the effects of prenatal exposure to SRIs and concurrent risk of autism (Harrington, Lee, Crum, Zimmerman, & Hertz-Picciotto, 2013). Finally, a subset of articles demonstrated that CNVs may be important to the development of severe phenotypes of atypical neurodevelopment, especially large deletions (>100 kb; Brummelte et al., 2013; Kobiella et al., 2011; Rosenfeld et al., 2010; Sorte, Gjevik, Sponheim, Eiklid, & Rødningen, 2013).
The diversity of chromosome locations and functions exhibited by these genes, as well as the range of potential neurodevelopmental outcomes associated with them, points to the multifactorial, complex nature of neurodevelopment. Unfortunately, genomic science does not yet provide an explanation about the pathways by which these genes may lead to adverse neurodevelopmental outcomes or how the presence of these genes may combine with other exposures encountered by the preterm infant. However, further research in this area may increase understanding of why some infants of the same birth gestation and medical course do more poorly than others. In particular, studies reporting interaction effects between genotype and environmental influences have the potential to explain inconsistencies that have plagued association studies in complex diseases or phenotypes. Additionally, haplotype studies that examine the additive role of multiple polymorphisms on phenotype, rather than examining each SNP individually, may allow further development of genetic risk profiles.
The ethics and specific uses of genetic risk profiles lie outside the scope of this article. However, we acknowledge that the ethical concerns surrounding such profiles is particularly underscored in complex disease, where genetic risk may be small compared to environmental risks. Researchers should address the ethical implications of their findings in order to encourage public and professional discussion. Research into genetic associations with neurodevelopment is relatively new and thus precludes identification of specific interventions. However, this area of research may help identify those infants at greatest risk and provide understanding about pathways by which genes influence outcomes. Thus, a range of interventions could become possible including focused assessments, specific parent education, and referral for early intervention. Interventions to manage the infants’ early environment, including the neonatal intensive care unit, may be particularly important for improving outcomes in infants at risk.
The purpose of this integrative review was to identify and synthesize recent evidence of genetic factors associated with atypical neurodevelopmental outcomes that may affect preterm infants. The development of genetic risk profiles for complex disorders of neurodevelopment is hindered by inconsistent genetic association evidence, methodological considerations, and reporting problems. However, several avenues of investigation are promising, including the candidate genes MET, NRG3, and SLC6A4. Replication of these studies in samples of preterm infants is needed to test the validity of these genes as risk factors.
Footnotes
Author Contribution: L. Blair contributed to conception and design, acquisition, analysis, and interpretation; drafted the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. R. Pickler contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. C. Anderson contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Authors’ Note: The contents are the responsibility of the authors and do not necessarily represent the official views of the NIH.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The first author was supported by a Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32NR014225; Arcoleo, PI) from the National Institute of Nursing Research, National Institutes of Health (NINR, NIH) in affiliation with The Ohio State University. The work was additionally supported by R01NR012307 (Pickler, PI) from the NINR, NIH.
References
- Alaerts M., Ceulemans S., Forero D., Moens L. N., De Zutter S., Heyrman L.…Del-Favero J. (2009). Support for NRG1 as a susceptibility factor for schizophrenia in a northern Swedish isolated population. Archives of General Psychiatry, 66, 828–837. Retrieved from 10.1001/archgenpsychiatry.2009.82 [DOI] [PubMed]
- APOE. (2014, August 25). In Genetics home reference. Retrieved August 27, 2014, from http://ghr.nlm.nih.gov/gene/APOE
- Beevers C. G., Wells T. T., Ellis A. J., McGeary J. E. (2009). Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. Journal of Abnormal Psychology, 118, 670–681. Retrieved from 10.1037/a0016198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi C., Wu J., Jiang T., Liu Q., Cai W., Yu P.…Sun Z. S. (2012). Mutations of ANK3 identified by exome sequencing are associated with autism susceptibility. Human Mutation, 33, 1635–1638. Retrieved from 10.1002/humu.22174 [DOI] [PubMed]
- Braff D. L., Freedman R., Schork N. J., Gottesman I. I. (2007). Deconstructing schizophrenia: An overview of the use of endophenotypes in order to understand a complex disorder. Schizophrenia Bulletin, 33, 21–32. Retrieved from 10.1093/schbul/sbl049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S., Galea L. A. M., Devlin A. M., Oberlander T. F. (2013). Antidepressant use during pregnancy and serotonin transporter genotype (SLC6A4) affect newborn serum reelin levels. Developmental Psychobiology, 55, 518–529. Retrieved from 10.1002/dev.21056 [DOI] [PubMed] [Google Scholar]
- Burdick K. E., DeRosse P., Kane J. M., Lencz T., Malhotra A. K. (2010). Association of genetic variation in the MET proto-oncogene with schizophrenia and general cognitive ability. American Journal of Psychiatry, 167, 436–443. Retrieved from 10.1176/appi.ajp.2009.09050615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Moffitt T. E. (2006). Gene-environment interactions in psychiatry: Joining forces with neuroscience. Nature Reviews. Neuroscience, 7, 583–590. [DOI] [PubMed] [Google Scholar]
- Cattaneo A., Bocchio-Chiavetto L., Zanardini R., Marchina E., Bellotti D., Milanesi E.…Gennarelli M. (2010). BDNF Val66Met polymorphism and protein levels in amniotic fluid. BMC Neuroscience, 11, 16 Retrieved from 10.1186/1471-2202-11-16 [DOI] [PMC free article] [PubMed]
- Centers for Disease Control and Prevention. (2013). Preterm birth. Retrieved February 11, 2014, from http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm
- Chandler D., Dragović M., Cooper M., Badcock J. C., Mullin B. H., Faulkner D.…Jablensky A. (2010). Impact of neuritin 1 (NRN1) polymorphisms on fluid intelligence in schizophrenia. American Journal of Medical Genetics. Part B, 153B, 428–437. Retrieved from 10.1002/ajmg.b.30996 [DOI] [PubMed]
- Clark E. A. S., Mele L., Wapner R. J., Spong C. Y., Sorokin Y., Peaceman A.…Rouse D. J. (2010). Association of fetal inflammation and coagulation pathway gene polymorphisms with neurodevelopmental delay at age 2 years. American Journal of Obstetrics and Gynecology, 203, 83.e1–83.e10. Retrieved from 10.1016/j.ajog.2010.01.047 [DOI] [PMC free article] [PubMed]
- Costantine M. M., Clark E. A. S., Lai Y., Rouse D. J., Spong C. Y., Mercer B. M.…Caritis S. N. (2012). Association of polymorphisms in neuroprotection and oxidative stress genes and neurodevelopmental outcomes after preterm birth. Obstetrics and Gynecology, 120, 542–550. Retrieved from 10.1097/AOG.0b013e318265f232 [DOI] [PMC free article] [PubMed]
- Dutt A., Shaikh M., Ganguly T., Nosarti C., Walshe M., Arranz M.…Allin M. P. G. (2011). COMT gene polymorphism and corpus callosum morphometry in preterm born adults. NeuroImage, 54, 148–153. Retrieved from 10.1016/j.neuroimage.2010.07.048 [DOI] [PubMed]
- Harrington R. A., Lee L.-C., Crum R. M., Zimmerman A. W., Hertz-Picciotto I. (2013). Serotonin hypothesis of autism: Implications for selective serotonin reuptake inhibitor use during pregnancy. Autism Research, 6, 149–168. Retrieved from 10.1002/aur.1288 [DOI] [PubMed] [Google Scholar]
- Harrison P. J. (2007). Schizophrenia susceptibility genes and their neurodevelopmental implications: Focus on neuregulin 1. Novartis Foundation Symposium, 288, 246–255; discussion 255–259, 276–281. [PubMed] [Google Scholar]
- Håvik B., Degenhardt F. A., Johansson S., Fernandes C. P. D., Hinney A., Scherag A.…Le Hellard S. (2012). DCLK1 variants are associated across schizophrenia and attention deficit/hyperactivity disorder. PloS One, 7, e35424 Retrieved from 10.1371/journal.pone.0035424 [DOI] [PMC free article] [PubMed]
- Hill J., Breen G., Quinn J., Tibu F., Sharp H., Pickles A. (2013). Evidence for interplay between genes and maternal stress in utero: Monoamine oxidase a polymorphism moderates effects of life events during pregnancy on infant negative emotionality at 5 weeks. Genes, Brain, and Behavior, 12, 388–396. Retrieved from 10.1111/gbb.12033 [DOI] [PubMed] [Google Scholar]
- Jahanshad N., Kohannim O., Hibar D. P., Stein J. L., McMahon K. L., de Zubicaray G. I.…Thompson P. M. (2012). Brain structure in healthy adults is related to serum transferrin and the H63D polymorphism in the HFE gene. Proceedings of the National Academy of Sciences, 109, E851–E859. Retrieved from 10.1073/pnas.1105543109 [DOI] [PMC free article] [PubMed]
- Johnson S., Hollis C., Kochhar P., Hennessy E., Wolke D., Marlow N. (2010). Autism spectrum disorders in extremely preterm children. Journal of Pediatrics, 156, 525.e2–531.e2. Retrieved from 10.1016/j.jpeds.2009.10.041 [DOI] [PubMed] [Google Scholar]
- Kao W.-T., Wang Y., Kleinman J. E., Lipska B. K., Hyde T. M., Weinberger D. R., Law A. J. (2010). Common genetic variation in neuregulin 3 (NRG3) influences risk for schizophrenia and impacts NRG3 expression in human brain. Proceedings of the National Academy of Sciences, 107, 15619–15624. Retrieved from 10.1073/pnas.1005410107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G., Rujescu D., Collier D., O’Donovan M., Owen M. (2009). Neurexin 1 (NRXN1) deletions in schizophrenia. Schizophrenia Bulletin, 35, 851–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knud Larsen J., Bendsen B. B., Foldager L., Munk-Jørgensen P. (2010). Prematurity and low birth weight as risk factors for the development of affective disorder, especially depression and schizophrenia: A register study. Acta Neuropsychiatrica, 22, 284–291. Retrieved from 10.1111/j.1601-5215.2010.00498.x [DOI] [PubMed] [Google Scholar]
- Kobiella A., Reimold M., Ulshöfer D. E., Ikonomidou V. N., Vollmert C., Vollstädt-Klein S.…Smolka M. N. (2011). How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: The roles of in vivo serotonin transporter expression and amygdala structure. Translational Psychiatry, 1, e37 Retrieved from 10.1038/tp.2011.29 [DOI] [PMC free article] [PubMed]
- Kuswanto C. N., Woon P.-S., Zheng X. B., Qiu A., Sitoh Y.-Y., Chan Y. H.…Sim K. (2012). Genome-wide supported psychosis risk variant in ZNF804A gene and impact on cortico-limbic WM integrity in schizophrenia. American Journal of Medical Genetics. Part B, 159B, 255–262. Retrieved from 10.1002/ajmg.b.32032 [DOI] [PubMed]
- Kuzniewicz M. W., Wi S., Qian Y., Walsh E. M., Armstrong M. A., Croen L. A. (2013). Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. Journal of Pediatrics, 164, 20–25. Retrieved from 10.1016/j.jpeds.2013.09.021 [DOI] [PubMed] [Google Scholar]
- Lien E., Andersen G. L., Bao Y., Gordish-Dressman H., Skranes J. S., Vik T., Blackman J. A. (2013). Apolipoprotein E polymorphisms and severity of cerebral palsy: A cross-sectional study in 255 children in Norway. Developmental Medicine and Child Neurology, 55, 372–377. Retrieved from 10.1111/dmcn.12086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Life Map Sciences. (2014a). MET gene. In GeneCards human gene database. Retrieved January 8, 2015, from http://www.genecards.org/cgi-bin/carddisp.pl?gene=MET
- Life Map Sciences. (2014b). NRG3 gene. In GeneCards human gene database. Retrieved January 8, 2015, from http://www.genecards.org/cgi-bin/carddisp.pl?gene=NRG3
- Life Map Sciences. (2014c). SLC6A4 gene. In GeneCards human gene database. Retrieved January 8, 2015, from http://www.genecards.org/cgi-bin/carddisp.pl?gene=SLC6A4
- Lin M. T., Huang K. H., Huang C. L., Huang Y. J., Tsai G. E., Lane H. Y. (2012). MET and AKT genetic influence on facial emotion perception. PloS One, 7, e36143 Retrieved from 10.1371/journal.pone.0036143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström K., Lindblad F., Hjern A. (2009). Psychiatric morbidity in adolescents and young adults born preterm: A Swedish National Cohort Study. Pediatrics, 123, e47–e53. Retrieved from 10.1542/peds.2008-1654 [DOI] [PubMed] [Google Scholar]
- Loo S. K., Shtir C., Doyle A. E., Mick E., McGough J. J., McCracken J.…Nelson S. F. (2012). Genome-wide association study of intelligence: Additive effects of novel brain expressed genes. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 432.e2–440.e2. Retrieved from 10.1016/j.jaac.2012.01.006 [DOI] [PubMed]
- Meier S., Strohmaier J., Breuer R., Mattheisen M., Degenhardt F., Mühleisen T. W.…Wüst S. (2013). Neuregulin 3 is associated with attention deficits in schizophrenia and bipolar disorder. International Journal of Neuropsychopharmacology, 16, 549–556. Retrieved from 10.1017/S1461145712000697 [DOI] [PubMed]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., & the PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med, 6, e1000097 Retrieved from 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietiläinen O. P. H., Rehnström K., Jakkula E., Service S. K., Congdon E., Tilgmann C.…Peltonen L. (2011). Phenotype mining in CNV carriers from a population cohort. Human Molecular Genetics, 20, 2686–2695. Retrieved from 10.1093/hmg/ddr162 [DOI] [PMC free article] [PubMed]
- Rietveld M. J. H., Hudziak J. J., Bartels M., van Beijsterveldt C. E. M., Boomsma D. I. (2004). Heritability of attention problems in children: Longitudinal results from a study of twins, age 3 to 12. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 45, 577–588. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J. A., Ballif B. C., Torchia B. S., Sahoo T., Ravnan J. B., Schultz R.…Shaffer L. G. (2010). Copy number variations associated with autism spectrum disorders contribute to a spectrum of neurodevelopmental disorders. Genetics in Medicine, 12, 694–702. Retrieved from 10.1097/GIM.0b013e3181f0c5f3 [DOI] [PubMed]
- Shaikh M., Hall M.-H., Schulze K., Dutt A., Walshe M., Williams I.…Bramon E. (2011). Do COMT, BDNF and NRG1 polymorphisms influence P50 sensory gating in psychosis? Psychological Medicine, 41, 263–276. Retrieved from 10.1017/S003329170999239X [DOI] [PubMed]
- Sorte H. S., Gjevik E., Sponheim E., Eiklid K. L., Rødningen O. K. (2013). Copy number variation findings among 50 children and adolescents with autism spectrum disorder. Psychiatric Genetics, 23, 61–69. Retrieved from 10.1097/YPG.0b013e32835d718b [DOI] [PubMed] [Google Scholar]
- Sullivan M. C., Msall M. E., Miller R. J. (2012). 17-year outcome of preterm infants with diverse neonatal morbidities: Part 1—Impact on physical, neurological, and psychological health status. Journal for Specialists in Pediatric Nursing, 17, 226–241. Retrieved from 10.1111/j.1744-6155.2012.00337.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma C., Hervás A., Balmaña N., Salgado M., Maristany M., Vilella E.…Cormand B. (2013). Neurotransmitter systems and neurotrophic factors in autism: Association study of 37 genes suggests involvement of DDC. World Journal of Biological Psychiatry, 14, 516–527. Retrieved from 10.3109/15622975.2011.602719 [DOI] [PubMed]
- Tosato S., Bellani M., Bonetto C., Ruggeri M., Perlini C., Lasalvia A.…Brambilla P. (2012). Is neuregulin 1 involved in determining cerebral volumes in schizophrenia? Preliminary results showing a decrease in superior temporal gyrus volume. Neuropsychobiology, 65, 119–125. Retrieved from 10.1159/000330584 [DOI] [PubMed]
- Tost H., Callicott J. H., Rasetti R., Vakkalanka R., Mattay V. S., Weinberger D. R., Law A. J. (2014). Effects of neuregulin 3 genotype on human prefrontal cortex physiology. Journal of Neuroscience, 34, 1051–1056. Retrieved from 10.1523/JNEUROSCI.3496-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Energy Human Genome Project. (2012). Human Genome Project information archive 1990–2003. Retrieved from http://web.ornl.gov/sci/techresources/Human_Genome/glossary.shtml#G
- Wang K., Zhang H., Ma D., Bucan M., Glessner J. T., Abrahams B. S.…Hakonarson H. (2009). Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature, 459, 528–533. Retrieved from 10.1038/nature07999 [DOI] [PMC free article] [PubMed]
- Wang K.-S., Xu N., Wang L., Aragon L., Ciubuc R., Arana T. B.…Xu C. (2014). NRG3 gene is associated with the risk and age at onset of Alzheimer disease. Journal of Neural Transmission, 121 Retrieved from 10.1007/s00702-013-1091-0 [DOI] [PubMed]
- Whittemore R., Knafl K. (2005). The integrative review: Updated methodology. Journal of Advanced Nursing, 52, 546–553. Retrieved from 10.1111/j.1365-2648.2005.03621.x [DOI] [PubMed] [Google Scholar]
- Wittke-Thompson J. K., Pluzhnikov A., Cox N. J. (2005). Rational inferences about departures from Hardy-Weinberg equilibrium. American Journal of Human Genetics, 76, 967–986. Retrieved from 10.1086/430507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharyan R., Boyajyan A., Arakelyan A., Gevorgyan A., Mrazek F., Petrek M. (2011). Functional variants of the genes involved in neurodevelopment and susceptibility to schizophrenia in an Armenian population. Human Immunology, 72, 746–748. Retrieved from 10.1016/j.humimm.2011.05.018 [DOI] [PubMed] [Google Scholar]