Abstract
The persistence of effects of prenatal drug exposure (PDE) on brain functioning during adolescence is poorly understood. We explored neural activation to a visuospatial working memory (VSWM) versus a control task using functional magnetic resonance imaging (fMRI) in adolescents with PDE and a community comparison group (CC) of non-exposed adolescents. We applied graph theory metrics to resting state data using a network of nodes derived from the VSWM task activation map to further explore connectivity underlying WM functioning. Participants (ages 12–15 years) included 47 adolescents (27 PDE and 20 CC). All analyses controlled for potentially confounding differences in birth characteristics and postnatal environment. Significant group by task differences in brain activation emerged in the left middle frontal gyrus (BA 6) with the CC group, but not the PDE group, activating this region during VSWM. The PDE group deactivated the culmen, whereas the CC group activated it during the VSWM task. The CC group demonstrated a significant relation between reaction time and culmen activation, not present in the PDE group. The network analysis underlying VSWM performance showed that PDE group had lower global efficiency than the CC group and a trend level reduction in local efficiency. The network node corresponding to the BA 6 group by task interaction showed reduced nodal efficiency and fewer direct connections to other nodes in the network. These results suggest adolescence reveals altered neural functioning related to response planning that may reflect less efficient network functioning in youth with PDE.
Keywords: Prenatal drug exposure, fMRI, graph theory, visuospatial working memory, adolescence, pediatric
1. INTRODUCTION
The long-term impact of prenatal exposure to illicit drugs of abuse (PDE) on brain functioning remains poorly understood with relatively few published reports documenting significant effects (e.g., (Li, Milivojevic et al. 2006, Hurt, Giannetta et al. 2008, Li, Coles et al. 2009, Sheinkopf, Lester et al. 2009, Li, Santhanam et al. 2011, Roussotte, Rudie et al. 2012, Li, Santhanam et al. 2013, Li, Zhu et al. 2013). Evidence for altered brain functioning in children and adolescents with a history of PDE may be difficult to detect because the effects of postnatal environmental factors are often confounded with the effects of the prenatal exposure (Frank, Augustyn et al. 2001, Ackerman, Riggins et al. 2010, Buckingham-Howes, Berger et al. 2013). With many of the original cohorts entering adolescence, however, there is renewed interest in the population due to the recognition that cortical brain regions that may be affected by PDE undergo significant developmental changes during adolescence. Furthermore, evidence from nonhuman primate models of PDE suggests that disruption of performance on learning tasks may not emerge until adolescence (Lidow 2003). Previous studies exploring cognitive functions, such as working memory (WM) demonstrate subtle differences in the PDE population (Schroder, Snyder et al. 2004, Burden, Jacobson et al. 2005, Mayes, Snyder et al. 2007, Li, Coles et al. 2009, Ackerman, Riggins et al. 2010, Buckingham-Howes, Berger et al. 2013); but not universally (e.g., Betancourt, Yang, Brodsky, et al., 2011). These effects may persist after potentially confounding variables are controlled (Li, Coles et al. 2009) (e.g., birth head circumference, alcohol and tobacco prenatal exposure), however studies vary in attempts to control for such factors. Most functional magnetic resonance imaging (fMRI) studies do not consider confounding environmental variables (Ackerman, Riggins et al. 2010, Buckingham-Howes, Berger et al. 2013). Thus, the controversy regarding the contribution of the postnatal environment versus PDE continues for adolescent-aged individuals with PDE.
Our group (Riggins, Cacic et al. 2012) showed that children with PDE demonstrated worse memory performance in comparison to a community comparison (CC) group on standardized memory measures of list learning and story recall using the California Verbal Learning Test —Child Version (CVLT-C, (Delis, Kramer et al. 1994), and Children's Memory Scale (CMS, (Cohen 1997)). Our results suggested intact initial learning and recall of information on the simple recall task, but difficulty with increased task demands, such as under conditions involving interference in recall conditions. Furthermore, hippocampal volume, a structure known to support memory, was associated with memory performance. These group differences remained, even after controlling for early childhood environment. This current study further examines the relation between WM performance and neural functioning using functional magnetic resonance imaging (fMRI) and adjusting for early childhood environmental variables. WM, the ability to briefly maintain and manipulate information mentally (Baddeley 2010), continues to develop during adolescence and into young adulthood with maturation and better performance associated with brain activity in frontal, parietal and cerebellar brain regions (Kwon, Reiss et al. 2002, Crone, Wendelken et al. 2006, O'Hare, Lu et al. 2008). WM is a core cognitive function associated with academic performance, goal achievement and self-control (Hinson, Jameson et al. 2003, Barkley 2006, Shamosh, Deyoung et al. 2008, Gropper and Tannock 2009, Alloway, Gathercole et al. 2010). Dopamine is related to WM functioning (Backman and Nyberg 2013) and evidence from non-human primate models suggests that PDE can affect dopamine functioning in adult animals (Hamilton, Czoty et al. 2010).
PDE research is beginning to consider how exposure is associated with the integrity of brain functioning in large-scale networks via connectivity measures (Li, Milivojevic et al. 2006, Li, Santhanam et al. 2013, Li, Zhu et al. 2013). Li et al., (Li, Santhanam et al. 2011) investigated the relation between PDE and functional connectivity in the default mode network (DMN). A seed-based approach demonstrated that PDE was associated with stronger functional connectivity during resting state and less deactivation in the DMN during WM performance than in a comparison group. This same group (Li, Zhu, Guo et al., 2013) identified a set of cortical landmarks associated with adolescents who experienced PDE, and used the landmarks to discover functional connectomic signatures that differentiate the PDE brain from control subjects. They identified 10 structural landmarks that were altered in the adolescents with PDE that were associated with the functional connectomic signatures. The authors noted that the structural landmarks they identified as discrepant in PDE studies are brain networks associated with processes thought to be affected in PDE including working memory, language, executive function, motor, attention and vision processing. Graph theory analyses have the potential to further characterize network functioning by quantifying the topological organization of connectivity within the brain (He and Evans 2010); however, this method has yet to be applied to the PDE population. Networks based on functional and anatomical nodes involved in cognitive functioning are related to age (Dosenbach, Nardos et al. 2010, Fair, Nigg et al. 2012), IQ (Li, Liu et al. 2009, van den Heuvel, Stam et al. 2009) and clinical disorders (Fair, Nigg et al. 2012, Tye and Bolton 2013). Individual task performance is positively associated with topological efficiency of brain networks during both resting (Giessing, Thiel et al. 2013, Langer, von Bastian et al. 2013) and task performance (Bassett, Bullmore et al. 2009). Graph theory may enhance understanding of the neuronal integrity of brain regions associated with task performance as functional connectivity alterations may impact behavioral performance.
The present study explores whether adolescents with PDE versus a non-exposed, community comparison group (CC) group evidence differences in brain functioning associated with performance during a VSWM task. We derived a network of nodes supporting WM performance from the fMRI data and then applied a graph theory analysis to the resting data to test for group differences in coherence in regions associated with WM performance. We hypothesized group differences in task-related activation with reduced measures of topological efficiency in the underlying network supporting WM functioning, which would serve as evidence for the long-term consequences of PDE on neural functioning in an important cognitive domain.
2. METHODS
2.1 Participants
We recruited 12 to 15 year old participants with intrauterine exposure to cocaine and/or heroin from a larger longitudinal study of drug-using women and their infants at a university hospital serving a predominately inner-city, African American population (Nair, Black et al. 2008). Women were eligible in the original study if they or their infants had a urine toxicology screen at birth positive for cocaine and/or heroin or a self-reported history of cocaine and/or heroin use during pregnancy (≥ 2×/week), and their infants were ≥ 32 weeks gestational age and 1750 gms birth weight. The initial enrollment included 265 women who met eligibility. Non-drug-exposed CC participants were recruited at 5 years of age (n=70) or 14 years of age (n=24). The CC participants were born in the same hospital as the PDE group, with negative toxicology screens, no history of drug use in the mother’s medical records and denial of drug use by the mother. At the early adolescent follow up, 76 PDE and 62 CC were in the main study and approached for the imaging study if they met the additional criteria of no neurological or medical illness (e.g., diabetes, HIV, endocrinopathies, epilepsy, anemia or hypertension) that might confound data interpretation, no regular use of medications that might affect the imaging results (e.g., albuterol inhaler within 24 hours of scan session), no pregnancy or current illegal drug use (both verified by urine screens). At the time of the imaging study none of the participants had been diagnosed with a psychiatric disorder or were receiving psychotropic medications. Left-handed participants were included, as subtle differences in PDE may affect handedness preference (Olsen 1995). Of the original sample, 45% were ineligible and 68% of eligible participants were scanned.
The final cohort of 47 youth (27 PDE and 20 CC, after excluding one PDE for excessive movement) did not differ by exposure in age, gestational age, sex, IQ, birth head circumference and handedness, but differed on percent in continuous maternal care from birth to 6 years old, prenatal exposure to alcohol and cigarettes and birth weight and length (see Table 1). The resting network analysis included one fewer PDE and CC participant due to a participant request to stop the scan session early and a technical problem respectively. The groups remained similar in age, sex, IQ, birth head circumference and handedness without these two participants. WM behavioral data were missing for one CC subject due to a computer malfunction, however data from the behavioral practice suggested adequate performance, thus the imaging data were retained for all 47 participants. In this sample 12/27 were exposed to both heroin and cocaine, 13/27 were exposed to cocaine but not heroin and 2/27 were exposed to heroin but not cocaine. Our cohort is consistent with the majority of studies in PDE in that there was poly-substance abuse (e.g., (Rao, Wang et al. 2007, Li, Santhanam et al. 2011),(see;(Buckingham-Howes, Berger et al. 2013) for a review) in the mothers during pregnancy including exposure to non-illicit substances (e.g., nicotine. As Lester (1998) and Bauer (Bauer, Shankaran et al. 2002) demonstrated polydrug experience is much more common than use of a single drug during pregnancy when the mother is using illicit drugs and therefore, illicit drug use is typically now considered polydrug exposure. The Bauer analysis of data from 11,811 mothers in the Maternal Lifestyle Study (Bauer, Shankaran et al. 2002) found that 93% of women using cocaine or opiates admitted to using other substances, such as alcohol or tobacco, that are known to produce negative outcomes on a fetus.
Table 1.
Demographics and participant characteristics
| VSWM PDE Group | VSWM CC Group | Group Difference Statistics | |
|---|---|---|---|
| (N=27) | N=20) | Bold indicates significant difference | |
| Current Characteristics: | |||
| Age at scan (years (SD)) | 14.7, (1.1) | 14.2, (1.3) | F(1,45) = 2.46, p = .12 |
| Sex | 13 male, 14 female | 7 male, 13 female | Chi square(1) = 0.81, p = .37 |
| Participant's IQ (WASI (SD)) Vocabulary & Matrix Reasoning | 88.00, (12.37) | 91.80, (13.25) | F(1,45) = 1.02, p = .32 |
| Discontinuous maternal care birth to 6 y.o. | 56% | 0% | Chi square(1) = 16.32, p = .000 |
| Current caregiver IQ (WASI (SD)) Vocabulary & Matrix Reasoning | 83.26, (13.09) | 89.84, (11.78)* | F(1,44) = 3.06, p = .09 |
| Handedness | 24 right, 3 left | 18 right, 2 left | Chi square(1) = 0.02, p = .903 |
| Head Circumference at Birth (cm, (SD)) | 33.47, (3.55) | 35.25, (3.64)* | F(1,44) = 2.73, p = .105 |
| Birth Weight (gm, (SD)) | 2807, (480) | 3449, (712)* | F(1,44) = 13.3, p = .001 |
| Birth Length (cm, (SD)) | 47.3, (4.1) | 50.8, (2.8)* | F(1,44) = 10.49, p = .002 |
| Gestational Age (weeks, Dubowitz, (SD)) | 38.2, (2.5) | 38.7, (1.4)* | F(1,44) = .642, p = .427 |
| Prenatal exposure to alcohol | 56% | 15% | Chi square(1) = 8.00, p = .005 |
| Intensity of exposure (PDE only) | >2×/wk in 2nd and 3rd trimesters = 11% 1st trimester only = 44% no exposure = 44% | ||
| Prenatal exposure to cigarettes | 78% | 15% | Chi square(1) = 18.12, p<0.000 |
| Intensity of exposure (PDE only) | >2×/wk in 2nd and 3rd trimesters = 74% 1st trimester only = 4% no exposure = 22% |
n=19
Participants and caregivers received gift certificates to compensate their time. Parents/guardians gave written informed consent; and participants gave written assent, as approved by the University of Maryland, School of Medicine, IRB and NIDA Intramural Research Program IRB.
2.2 Procedures
All participants completed a series of measures and psychological tasks to gather demographic and clinical characteristics relevant to the current study (see Table 1) and broader project. The measures relevant to this study include age, handedness and presence of psychiatric diagnosis. This also included the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI), which were administered by an examiner blind to the group membership of the adolescent. The Vocabulary subtest assessed general word knowledge, verbal concept formation and fund of knowledge. The Matrix Reasoning subtest assessed visual intelligence, spatial ability and perceptual organization.
Experimental Task
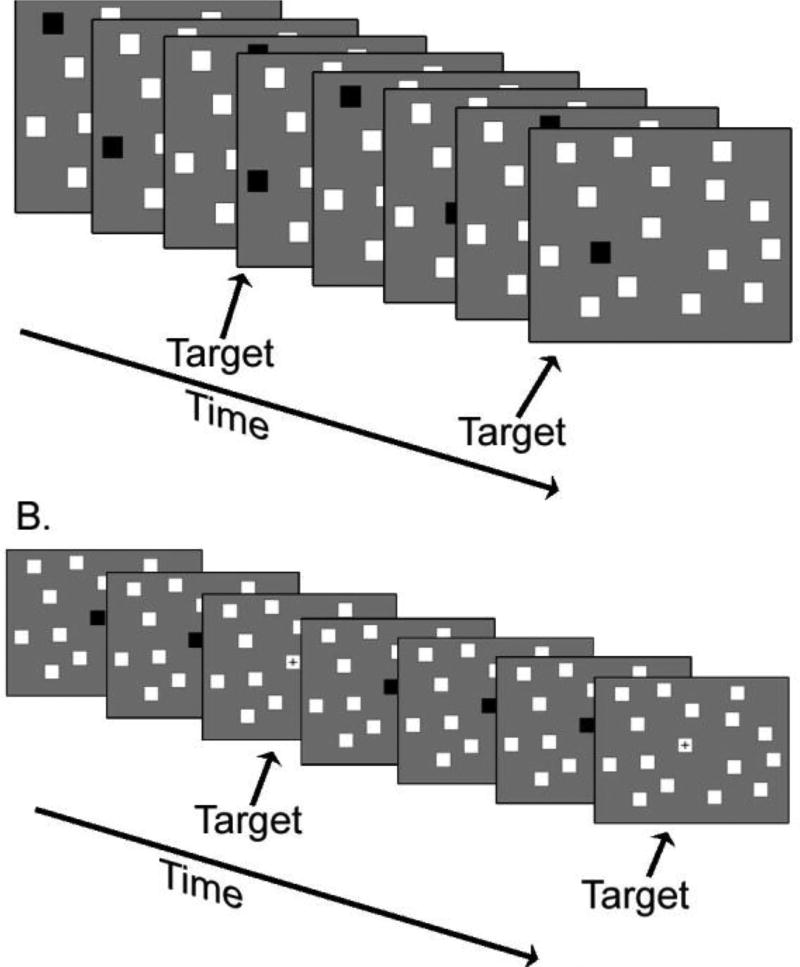
Participants performed a 2-back VSWM paradigm of visually-presented, spatial information and a control task requiring observation of matching visual stimuli, sustained attention and the same motor response. In the spatial VSWM task (Carlson, Martinkauppi et al. 1998, Owen, McMillan et al. 2005), individual darkened squares were presented sequentially in 1 of 16 different spatial locations (Figure 1A). Participants pressed a button whenever the darkened square returned to the same location as that presented two back (arrow). During the control task, an individual darkened square alternated with an occasional plus sign in the center spatial location (Figure 1B). Participants pressed a button when the plus sign appeared. Individual stimulus duration for each condition was 1 second, with four targets in each 24-second block. Responses within 200 msec of stimulus presentation were assigned to the previous stimulus, resulting in a response window of 200–1200 msec. Participants practiced the paradigm at a desktop computer and in a mock scanner. The program collected data on percent correct responses and reaction time (RT) for the target and control conditions. Validation of the task as a measure of memory and visual spatial performance was conducted in two separate samples using the identical VSWM 2-back task in relation to standardized measures of performance on the Wechsler Scales of Intelligence in typically developing young adults (17–23 years of age) and children and young adults (12 to 21 years of age) with attention-deficit/hyperactivity disorder (ADHD). Performance on the VSWM was positively correlated with percent correct target identification on a standardized test of recall and working memory, the Digit Span test on the Wechsler Adult Intelligence Scale (WAIS; n = 22; Pearson r = .45; p = 0.037) (Schweitzer, unpublished data). In a sample of adolescent and young adults with ADHD performance for percent correct target identification on the VSWM task was correlated with performance on a test of visual-spatial ability, the Block Design subtest from the Wechsler Scales of Intelligence (n=42; r = .35; p = 0.024; (Schweitzer, unpublished data)). N-back tasks, including the spatial identity matching n-back task tested in this study, are most frequently associated with activity in the premotor region (e.g., BA 6), dorsal lateral prefrontal cortex (BA 46, 9), dorsal cingulate and medial premotor (SMA, 32, 6) medial posterior parietal (BA 7), inferior parietal lobule (BA 40) and medial cerebellum (Owen, McMillan et al. 2005).
Figure 1.
A. 2-back visuo-spatial working memory task. B. Control task with the same number of targets per block.
2.3 FMRI data acquisition
Participants completed one 7-minute run containing 6 repeats of the following: 12 sec of rest (fixation on a smiley face), 4 sec instruction screen, 24 sec of the control task, 4 sec instruction screen, 24 sec of the VSWM task in a block design. All participants completed the tasks in the same order. An extra rest block ended the task. A 3-T Siemens Allegra scanner acquired whole-brain BOLD EPI; 39 oblique axial (30° axial to coronal), 4mm slices; TR = 2 sec; TE = 27 ms; Flip Angle = 80°; FOV = 22 × 22cm2. A whole-brain oblique axial T1-weighted structural image (MPRAGE) was acquired for anatomical reference (1-mm3 isotropic voxels: TR = 2.5 sec; TE = 4.38 ms; FA = 8°). Five minutes of eyes open resting data were gathered using the same scan parameters after completion of the VSWM task.
2.4 Analysis
Demographic comparisons
A one-way ANOVA (for continuous variables) and Chi-square (for categorical variables) tested for differences between groups in age, sex, IQ, caregiver IQ, birth head circumference, continuity of maternal care to age 6, and prenatal exposure to alcohol and tobacco.
2.4.1. Behavioral data analysis
A one-way ANOVA compared percent correct and RT data between groups. Intra-individual variability (IIV) in reaction time (IIV-RT) was calculated using an ex-Gaussian model (see (Heathcote, Brown et al. 2004, Fassbender, Zhang et al. 2009) and compared between groups using ANOVA. Relationships between behavioral variables were examined with bivariate correlations within each group with follow up moderated regression models looking for a significant group by measure interaction effect on the measure of interest to determine if there were significant differences in correlations.
2.4.2. fMRI processing and analysis
2.4.2.1. fMRI data preprocessing
fMRI data were preprocessed and analyzed using AFNI (Cox 1996). Both resting and task functional data were motion corrected, aligned with anatomical images, and normalized into Talairach space (3 mm3 voxels) and spatial smoothed to an 8mm full width at half maximum (Friedman, Glover et al. 2006). The resting fMRI images were further processed including band-pass filtering (0.01–0.1 Hz) and removal of nuisance signals including motion-correction parameters and the first three principal components from the time courses of white matter and cerebrospinal fluid voxels. Furthermore, to reduce potential motion-related confounds in resting fMRI data (Power, Barnes et al. 2012, Satterthwaite, Wolf et al. 2012, Van Dijk, Sabuncu et al. 2012), we excluded volumes with large volume-to-volume displacement greater than 0.35 mm/°. Participants with greater than 30% of volumes (one male PDE participant) excluded were excluded from further analysis.
2.4.2.2. fMRI data analysis
Ideal waveforms were created by convolving a square-wave function with a hemodynamic response function. Multiple regression analyses generated percent signal change for the control and VSWM relative to the rest blocks for each participant. Motion parameters were modeled as variables of no interest. Any activation outside of a mask of brain plus 5 mm was set to zero to avoid the possibility of incidentally removing true brain activation.
A second-level group analysis was performed using the program 3dLME within AFNI. The model was a 2 (Group: PDE or CC) × 2 (Condition: VSWM or Control) design. LME analyses included potentially confounding variables that were significantly different between the groups, which, included controlling for prenatal exposure to alcohol and cigarettes and continuity of maternal care from birth to 6 years old. A corrected p < 0.05 level of significance (defined as a minimum cluster size of 23 voxels (621µl) at a voxel-wise threshold p = 0.001 as determined using the program AlphaSim) was considered significant. Post-hoc analyses investigating significant Group × Condition interactions were conducted at the whole brain level (pcorrected < 0.05) for each group separately and significant clusters were examined for spatial overlap with the original clusters showing significant Group × Condition interactions. We examined the relation between activations emerging from significant Group × Condition interaction regions of interest (ROIs) and behavioral measures (i.e., VSWM RT, percentage accuracy and IIV-RT using bivariate correlations in each group separately, since these regions were identified as being used differently by the two groups during this task. Significant correlations in either group were followed up with linear models in the whole group using the behavioral measure as the dependent variable and ROI activation (VSWM-Control), group as independent variables with an interaction term included to determine if the groups used the region in question in a significantly different way.
2.4.2.3. Network Analysis of Resting State fMRI Data
Nodes for the network analysis were derived by taking the local maxima from the VSWM task map (WM minus control) for the whole cohort (PDE and CC combined; See Supplemental Figure 1). Local maxima were determined by the 3dmaxima command in AFNI using a minimum threshold of F > 18.16 and minimum distance between peaks of 6, 3-mm isomorphic voxels, resulting in 78 nodes (see Supplemental Table 1). Mean resting fMRI time courses were extracted from 6 mm spheres centered at each of the 78 peak locations from the resting scan. The interregional correlation matrix of each subject was then obtained by calculating the partial correlation coefficients between the mean time courses of every pair of nodes. We used a minimum threshold approach (resulting in networks with a fixed minimum connectivity between nodes, but allowing different numbers of connections between participants) across a range of thresholds as opposed to a sparcity approach in order to avoid including node pairs with no or minimal connectivity. The minimum partial correlation coefficient for inclusion in the network ranged between 0.2 and 0.35 in increments of 0.01. The lower threshold limit was set to remove weak correlations so that only the correlations whose corresponding p values passed a statistical threshold (p < 0.01) were retained. The higher threshold limit is well accepted in the literature and ensures the resultant networks are fully connected and not arbitrarily subdivided (Lynall, Bassett et al. 2010, Power, Fair et al. 2010)(Hayasaka and Laurienti 2010). Topological metrics of local efficiency and global efficiency were determined for each network, using standard calculations (Watts and Strogatz 1998, Latora and Marchiori 2001). Local efficiency and global efficiency measure the ability of information transfer of a network at the local and global level, respectively, and provide a more clearly physical meaning for topological characterization of the networks. They are derived from each node’s clustering coefficient (a measure of how well connected its nearest neighbors are) and path length (the average shortest path from a node to any other node in the network). To provide threshold-independent results, each network metric was integrated across thresholds [i.e., the areas under the curves of network parameters across thresholds]. We then compared the network metrics between groups using moderated regression models, with age and sex as nuisance variables including interactions of age and sex with group in the model. Age and sex were included although they did not differ between the groups because they have been shown to alter network functioning (Tian, Wang et al. 2011, Yan, Gong et al. 2011, Fair, Bathula et al. 2012, Fair, Nigg et al. 2012, Wu, Taki et al. 2013). Interactions between group and age and group and sex were included because PDE may alter maturation and have differential effects by gender (Delaney-Black, Covington et al. 2004, Bennett, Bendersky et al. 2007).
Because the main imaging analysis of the task (see below) yielded a group by condition interaction in an area that was a node in the VSWM network, we compared the integrated nodal degree (number of connections of this node) and integrated nodal efficiency (inversely related to the path length of connections to the rest of the network) between groups using a moderated regression model, controlling for age and sex effects as above.
3. RESULTS
3.1. Behavioral Results
Accuracy, RT and IIV-RT did not differ between groups (see Table 2). In CC participants, percent correct on the VSWM condition was negatively correlated with RT (r(19) = −0.813, p <.001) and IIV-RT correlated negatively with accuracy (r (19) = −.673, p=.002). PDE participants, on the other hand, did not have a significant correlation between accuracy on the VSWM task and RT (r(27)=−.100, p=.621). The PDE group, however, demonstrated a similar negative correlation of IIV-RT to accuracy (r(27)=−.408, p=.034) as the CC group. A follow up moderated regression model looking for a group X RT interaction effect on accuracy, demonstrated this relation differed significantly between the groups (F(1,42)=11.39, p=.002). In regard to the relationship between accuracy and IIV-RT, the groups did not differ from one another (group X IIV-RT F(1,42)=2.828, p=.100). Thus, slower responders in the CC group tended to be less accurate and more variable, while PDE demonstrated no relation between RT and other behavioral measures, but did show a similar relationship between accuracy and variability (IIV-RT) as CC with better accuracy associated with less variability in response time for both groups.
Table 2.
Performance during VSWM** and Control Tasks
| Prenatal Drug Exposure Group |
Comparison Group |
Statistic | |
|---|---|---|---|
|
| |||
| Task | n=27 | n=19* | |
| Control Task | |||
|
|
|||
| Mean % Correct (SD) | 90.28 (11.67) | 90.56 (7.59) | F (1,44) = 0.009, p= 0.927 |
| Mean RT in ms (SD) | 490.21 (44.42) | 511.94 (72.41) | F (1,44) = 1.591, p= 0.214 |
| Mean IIV*** (SD) | 98.88 (31.58) | 108.64 (29.32) | F(1.44) =.910, p=.345 |
| VSWM Task** | |||
|
|
|||
| Mean % Correct (SD) | 85.49 (10.03) | 85.31 (16.57) | F(1,44) = 0.002, p= .962 |
| Mean RT in ms (SD) | 536.59 (59.13) | 539.82 (80.46) | F (1,44) = 0.25, p = 0.876 |
| Mean IIV*** (SD) | 160.85 (40.57) | 169.43 (50.59) | F(1,44)=.388, p=.537 |
Task data missing for one subject
VSWM = Visual spatial working memory task
IIV=Intra-individual variability
3.2. VSWM Task Activation Results
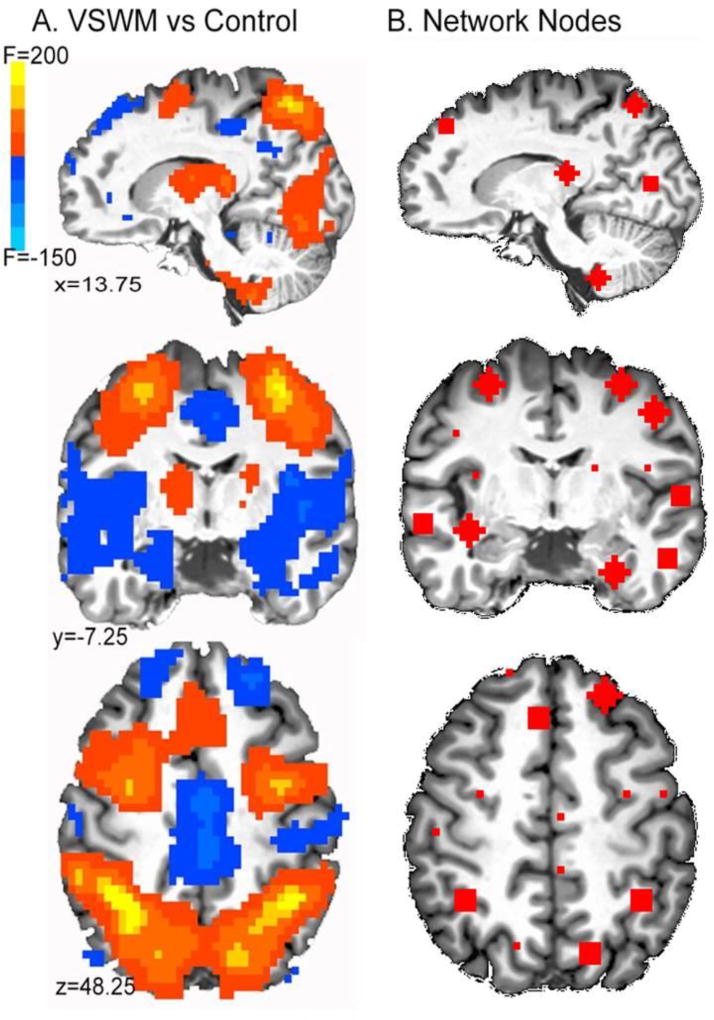
Significant activations from the whole-brain analysis showed main effects of Condition within the entire group of subjects in the frontal-parietal attention network (see Figure 2 below and Supplemental Figures 1 & 2) including the bilateral medial and middle frontal gyri (BA 6, 8, and 9), bilateral inferior (BA 40) and superior parietal (BA 7) lobules. The task also activated the precuneus (BA 7), bilateral lingual gyrus (BA 17 and 18), bilateral fusiform (BA 37), temporal (BA 37), occipital (BA 19) gyri and bilateral cerebellum (declive, uvula, pyramis, and culmen). Significant deactivations were observed in the parahippocampal cortices and regions of the “default network,” including bilateral superior frontal gyri (BA 9), medial frontal gyri (BA 10), anterior cingulate (BA 24, 30, 31) gyrus, medial frontal gyrus (BA 6), and posterior cingulate (BA 29 and 30).
Figure 2.
A. Contrast of working memory and control task across all subjects. (F-statistic is colored by direction of task effect). B. Nodes for network analysis derived from local maxima of task map in A.
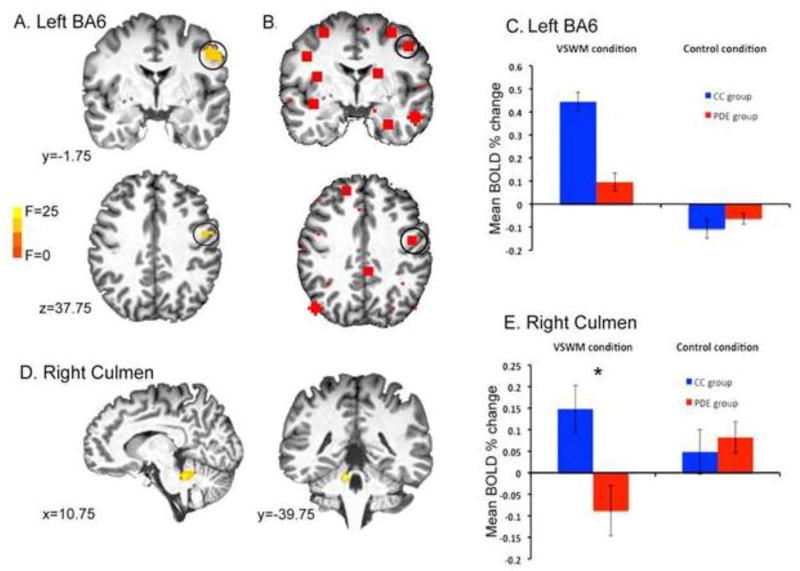
Whole-brain analyses showed no main effect of group (see Supplemental Figures 2 & 3 for individual group VSWM vs Control task montages). There was a significant Group × Condition interaction in left BA 6, with only the CC group activating this region during VSWM (see Figure 3A,C). Further, there was a significant interaction in the right culmen, which was not significantly activated in either group’s task map, but showed a trend towards differential activation in the PDE group who deactivated this region during VSWM but activated it during the control condition. In addition, the CC group appeared to activate the region more than the PDE group during the VSWM task (see Figure 3D,E).
Figure 3.
Group by task condition interactions: A. Left BA6. B. Corresponding node in network analysis. C. BOLD percent change versus rest by group and condition. D. Right culmen (no corresponding node in the network analysis). E. BOLD percent change versus rest by group and condition (error bars=SEM)* p < 0.001 CC versus PDE.
Activation in left BA 6 (VSWM-control task) did not significantly correlate with RT, task accuracy or IIV-RT in either group. CC did demonstrate a significant correlation between right culmen activation (VSWM-control task) and RT (r(19)=−.471, p=.042) that was not present in the PDE group (r(27)=.003, p=.989). A moderated regression revealed a trend level interaction effect on RT for group X right culmen activation (F(1,42)=3.802, p=.058). Neither group demonstrated a relation between culmen activation, accuracy or IIV-RT.
3.3. Network Results on Resting State Data
Integrated global efficiency of the VSWM network was significantly less in PDE than in CC controlling for age and sex (F(1,39)=6.206, p=0.017). Integrated local efficiency showed a trend level reduction in PDE (F(1,39)=3.711, p=0.061). Integrated nodal efficiency and degree for the node corresponding to the BA6 group × condition interaction were significantly reduced in PDE (Integrated node efficiency: F(1,39)=4.139, p=0.049; Integrated node degree: F(1,39)=4.568, p=0.039).
4. DISCUSSION
The behavioral data demonstrated the expected coupling between RT, IIV-RT and accuracy measures in the CC group for the VSWM task that was not seen in the PDE group, even though their overall performance was equivalent. The behavioral findings may reflect subtle indications of altered attentional and response preparatory skills in the PDE group. We found a reduced extent of activation associated with WM performance in left BA 6 in the PDE adolescents compared to a well-matched CC group. The culmen also appears affected by PDE as we found significant group by task differences in culmen activity. The CC activated this region while the PDE group showed deactivation during the VSWM task and activation during the control condition. Additional analyses showed that in a network derived from this population’s map of task-related activity the PDE group demonstrated significantly less global efficiency in comparison to the CC group and a trend toward reduced local efficiency. Further, the node corresponding to the group by task interaction in the left BA6, showed significantly reduced nodal degree and efficiency at rest.
WM-related activity in BA 6 is consistent with other pediatric studies (Kwon, Reiss et al. 2002, Ciesielski, Lesnik et al. 2006, Fassbender, Schweitzer et al. 2011) and may reflect the continuous updating that is required in performing n-back tasks (Wager and Smith 2003). Decreased frontal activity during a VSWM may not be specific to prenatal cocaine or heroin exposure, as other groups have found decreased frontal activity during a VSWM task for children prenatally exposed to methamphetamine (Roussotte, Bramen et al. 2011). The culmen, a region in which we found more activation for the VSWM task in the CC group, compared to the PDE group, is associated with response preparation and selection in Go/No-Go tasks (Simmonds, Fotedar et al. 2007). A previous study found that better attention, as evidenced through lower RT variability was associated with greater activity in the culmen (Simmonds, Fotedar et al. 2007). The CC group appears to be able to modulate brain activity in the culmen in response to task demands as activity in the culmen was associated with RT for the CC, but not the PDE group. Group differences in premotor activity found in BA 6 may be linked to group differences in activity in the culmen as they appear to be part of an interacting cerebro-cerebellar circuit (Kelly and Strick 2003). Our findings are also consistent with a recent study (Li, Zhu et al. 2013), that suggested that PDE is identified with functional connectomic signatures and processes involved in working memory, language, executive function, motor and attention.
The 2-back VSWM task used in this study was relatively easy with both groups achieving over 85% correct. Task activation differences in the face of comparable performance can be interpreted in different ways: reduced activation may be interpreted as a reflection of a less well-developed neural system (e.g., (Jolles 2011) but is often, especially in WM studies, taken to be indicative of increased neural efficiency (e.g., (Basten, Stelzel et al. 2012). Whereas the reduced extent of activation in the PDE group in left BA6 may represent improved efficiency, it may also represent failure to utilize the traditional network supporting WM and response preparation that might result in performance deficits in a more demanding task. Our graph theory based analysis of the network underlying the current VSWM task suggests multiple reduced efficiency measures reflecting altered functioning in VSWM-related regions (i.e., BA6) corresponding to our group by task interaction. The behavioral data, with the absence of the traditional relationship between RT and accuracy in the PDE group, provide further support that the PDE group is not using regions associated with response preparation and attention, optimally. Future studies employing event-related designs with tasks resulting in group differences in behavioral performance may further reveal cognitive vulnerabilities associated with PDE.
Findings from this project add to growing literature suggesting that there are long-term neural effects of PDE on memory functioning. Notably, our group (Riggins, Cacic et al. 2012) demonstrated in a larger sample from which this study was drawn, that PDE participants who perform worse on behavioral memory tasks have larger hippocampal volumes in comparison to the CC group. The hippocampal volumes are negatively correlated with memory performance, with lower memory scores associated with larger hippocampal volumes.
Both the frontal region (i.e., BA 6) and the culmen are likely to continue maturing into young adulthood (Kwon, Reiss et al. 2002, Tiemeier, Lenroot et al. 2010). It is possible that these regions are typically involved in a cerebro-cerebellar circuit that is used to subserve the WM processes in typically-developing individuals (Kirschen, Chen et al. 2010, Bostan, Dum et al. 2013), that is atypical or underdeveloped in the PDE group. The decreased nodal efficiency and degree evidenced in the BA6 node by the PDE group in our study and impaired DMN brain activity (i.e., (Li, Santhanam et al. 2011)) suggest that PDE brain functioning is more consistent with a younger maturational state in development. These findings support theories suggesting that some of the effects of PDE may not be detectable until middle adolescence or young adulthood is reached, a period associated with the development of sophisticated cognitive and emotional regulation. Furthermore, the use of connectivity measures, which assess how well brain regions work together, may be superior at detecting subtle differences in brain functioning related to development over region-by-region comparisons. Measures of functional connectivity may someday be used to predict vulnerability for mental illness and/or cognitive functioning.
5. STRENGTHS AND LIMITATIONS
Strengths of this study on PDE include the use of multiple image analysis methods and inclusion of multiple covariates (e.g., age, sex, IQ, prenatal exposure to alcohol and tobacco, and caregiver changes). The overall findings suggest that PDE produces subtle, but lasting impact on brain functioning. Similar to the majority of longitudinal studies of PDE, our sample had subjects with poly-substance exposure and frequent caregiver changes. Thus, our findings have high ecological validity and are relevant and generalizable to the majority of PDE children as poly-substance abuse is the norm (e.g., (Bauer, Shankaran et al. 2002);(Lester, LaGasse et al. 1998)) and children of women engaging in polysubstance abuse experience common environmental risks, regardless of the specific substance abused by the mother. We acknowledge, though that a limitation of this sample, is that we cannot specifically identify which substance of abuse (i.e., heroin or cocaine) may be responsible for the alterations present in the PDE group, although we did control for cigarette and alcohol exposure. While we were able to statistically control for caregiver changes we cannot totally rule out the effects of environment on our participants’ functioning or link the findings to a specific drug. We recognize that stimulants, such as cocaine and opiates likely have different effects on the developing brain (Lu, Liu et al. 2012). Animal model studies suggest that prenatal exposure to both cocaine and heroin can lead to impairments in spatial memory, but that the mechanisms underlying those impairments may be different (Lu, Liu et al. 2012). As noted in Riggins et al., (Riggins, Cacic et al. 2012) it is also likely that there are other nondirect PDE factors that impact brain functioning in our PDE participants that were not controlled for, such as the mental health of the parent or other aspects of the caregiving environment. For example, in Riggins et al., (Riggins, Cacic et al. 2012) we found that maternal depression was a significant predictor of recall ability.
Another potential concern is that we did not control for brain activations associated with saccades given that the target in the control task was fixed, rather than moving, as it was in our VSWM task. We chose to present the control target in a fixed spot due to concerns that the participants would inadvertently practice where the target was if it was moving and thus, perform the control task as a WM task. We recommend that future studies collect saccadic information during the tasks to identify brain activation related to the saccadic movement rather than WM. The regions in which we found of activation (e.g., BA 6) are historically associated with WM performance (Kwon, Reiss et al. 2002, Ciesielski, Lesnik et al. 2006, Fassbender, Schweitzer et al. 2011) and thus, we think it is unlikely that the activation is due solely to eye movements.
An additional methodological limitation was our use of a block-design. With an event-related task we would be able to specifically relate brain functioning to performance on a trial by trial basis. An advantage of the block design is the statistical power and speed at which block design data can be acquired and both factors are important in collecting data from pediatric populations.
5. Conclusions and Future Directions
This study found that children with PDE experience subtle attentional and response preparation challenges that are related to reduced activity and network-related functioning in frontal (i.e., BA 6) and cerebellar regions. Future studies should continue to follow these adolescents into adulthood as additional exposure effects may not be detectable until the adolescent-young adult period begins. Subsequent research should attempt to increase our ability to understand how neural-behavioral findings may suggest specific prevention and intervention strategies for children who have experienced prenatal insults to reduce potential learning problems. This includes the need to develop targeted interventions that can address issues such as impaired neural connectivity and working memory that are informed by the neuroscience findings for this at-risk population.
Supplementary Material
Acknowledgments
Funding
This research was funded by National Institutes of Health grants R01 DA07432 (Nair) and R01 DA021059 (Black) and the Intramural Research Program of the NIH, NIDA. The funders had not involvement in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication.
We would like to thank Elliot Stein, Ph.D., Kim Slater, and the Neuroimaging Research Branch of NIDA-IRP for support with data collection and analysis; Stacy Buckingham-Howes, Ph.D. and the F.U.T.U.R.E.S. team for participant recruitment and testing, and the families for their participation.
Abbreviations
- PDE
prenatal drug exposure
- VSWM
visual spatial working memory
- WM
working memory
- fMRI
functional magnetic resonance imaging
- DMN
default mode network
- CC
community comparison group
- IQ
intelligence quotient
- IRB
institutional review board
- NIDA
National Institutes of Drug Abuse, National Institutes of Health
- BOLD
blood-oxygen-level-dependent
- AFNI
Analysis of Functional Neuroimages
- RT
response time
- BA
Brodmann area
- WASI
Wechsler Abbreviated Scale of Intelligence.
Footnotes
Disclosure
The authors have no competing financial interests in relation to the work described.
References
- Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125(3):554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway TP, Gathercole SE, Elliott J. Examining the link between working memory behaviour and academic attainment in children with ADHD. Dev Med Child Neurol. 2010;52(7):632–636. doi: 10.1111/j.1469-8749.2009.03603.x. [DOI] [PubMed] [Google Scholar]
- Backman L, Nyberg L. Dopamine and training-related working-memory improvement. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Curr Biol. 2010;20(4):R136–140. doi: 10.1016/j.cub.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Barkley R. Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York: Guilford Press; 2006. [Google Scholar]
- Bassett DS, Bullmore ET, Meyer-Lindenberg A, Apud JA, Weinberger DR, Coppola R. Cognitive fitness of cost-efficient brain functional networks. Proc Natl Acad Sci U S A. 2009;106(28):11747–11752. doi: 10.1073/pnas.0903641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten U, Stelzel C, Fiebach CJ. Trait anxiety and the neural efficiency of manipulation in working memory. Cogn Affect Behav Neurosci. 2012;12(3):571–588. doi: 10.3758/s13415-012-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CR, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, Smeriglio VL, Finnegan LP, Maza PL, Verter J. The Maternal Lifestyle Study: drug exposure during pregnancy and short-term maternal outcomes. Am J Obstet Gynecol. 2002;186(3):487–495. doi: 10.1067/mob.2002.121073. [DOI] [PubMed] [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. J Dev Behav Pediatr. 2007;28(6):467–472. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17(5):241–254. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham-Howes S, Berger SS, Scaletti LA, Black MM. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics. 2013;131(6):e1917–1936. doi: 10.1542/peds.2012-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005;29(3):443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- Carlson S, Martinkauppi S, Rama P, Salli E, Korvenoja A, Aronen HJ. Distribution of cortical activation during visuospatial n-back tasks as revealed by functional magnetic resonance imaging. Cereb Cortex. 1998;8(8):743–752. doi: 10.1093/cercor/8.8.743. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Lesnik PG, Savoy RL, Grant EP, Ahlfors SP. Developmental neural networks in children performing a Categorical N-Back Task. Neuroimage. 2006;33(3):980–990. doi: 10.1016/j.neuroimage.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Cohen M. Children's Memory Scale. Psych Corp; 1997. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci U S A. 2006;103(24):9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan JH, Chiodo L, Sokol RJ. Prenatal cocaine: quantity of exposure and gender moderation. J Dev Behav Pediatr. 2004;25(4):254–263. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test-Children's Version. San Antonio: The Psychological Corporation, Harcourt Brace & Company; 1994. [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Jr, Barch DM, Petersen SE, Schlaggar BL. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang YF, Mostofsky S, Castellanos FX, Milham MP. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Schweitzer JB, Cortes CR, Tagamets MA, Windsor TA, Reeves GM, Gullapalli R. Working memory in attention deficit/hyperactivity disorder is characterized by a lack of specialization of brain function. PLoS One. 2011;6(11):e27240. doi: 10.1371/journal.pone.0027240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA. 2001;285(12):1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Glover GH, Krenz D, Magnotta V. Reducing inter-scanner variability of activation in a multicenter fMRI study: role of smoothness equalization. Neuroimage. 2006;32(4):1656–1668. doi: 10.1016/j.neuroimage.2006.03.062. [DOI] [PubMed] [Google Scholar]
- Giessing C, Thiel CM, Alexander-Bloch AF, Patel AX, Bullmore ET. Human brain functional network changes associated with enhanced and impaired attentional task performance. J Neurosci. 2013;33(14):5903–5914. doi: 10.1523/JNEUROSCI.4854-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropper RJ, Tannock R. A pilot study of working memory and academic achievement in college students with ADHD. J Atten Disord. 2009;12(6):574–581. doi: 10.1177/1087054708320390. [DOI] [PubMed] [Google Scholar]
- Hamilton LR, Czoty PW, Gage HD, Nader MA. Characterization of the dopamine receptor system in adult rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology (Berl) 2010;210(4):481–488. doi: 10.1007/s00213-010-1847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Laurienti PJ. Comparison of characteristics between region-and voxel-based network analyses in resting-state fMRI data. Neuroimage. 2010;50(2):499–508. doi: 10.1016/j.neuroimage.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Evans A. Graph theoretical modeling of brain connectivity. Curr Opin Neurol. 2010;23(4):341–350. doi: 10.1097/WCO.0b013e32833aa567. [DOI] [PubMed] [Google Scholar]
- Heathcote A, Brown S, Cousineau D. QMPE: estimating Lognormal, Wald, and Weibull RT distributions with a parameter-dependent lower bound. Behav Res Methods Instrum Comput. 2004;36(2):277–290. doi: 10.3758/bf03195574. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn. 2003;29(2):298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Hurt H, Giannetta JM, Korczykowski M, Hoang A, Tang KZ, Betancourt L, Brodsky NL, Shera DM, Farah MJ, Detre JA. Functional magnetic resonance imaging and working memory in adolescents with gestational cocaine exposure. J Pediatr. 2008;152(3):371–377. doi: 10.1016/j.jpeds.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Jolles DD, Kleibeuker SW, Rombouts SARB, Crone EA. Developmental Differences in Prefrontal Activation During Working Memory Maintenance and Manipulation for Different Memory Loads. Developmental Science. 2011 doi: 10.1111/j.1467-7687.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23(23):8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Desmond JE. Modality specific cerebro-cerebellar activations in verbal working memory: an fMRI study. Behav Neurol. 2010;23(1–2):51–63. doi: 10.3233/BEN-2010-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc. Natl. Acad. Sci. U.S.A. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer N, von Bastian CC, Wirz H, Oberauer K, Jancke L. The effects of working memory training on functional brain network efficiency. Cortex. 2013 doi: 10.1016/j.cortex.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87(19):198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Lester BM. The Maternal Lifestyles Study. Ann N Y Acad Sci. 1998;846:296–305. [PubMed] [Google Scholar]
- Lester BM, LaGasse LL, Bigsby R. Prenatal cocaine exposure and child development: what do we know and what do we do? Semin Speech Lang. 1998;19(2):123–146. doi: 10.1055/s-2008-1064041. [DOI] [PubMed] [Google Scholar]
- Li, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85(3):205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Li, Santhanam P, Coles CD, Ellen Lynch M, Hamann S, Peltier S, Hu X. Prenatal cocaine exposure alters functional activation in the ventral prefrontal cortex and its structural connectivity with the amygdala. Psychiatry Res. 2013;213(1):47–55. doi: 10.1016/j.pscychresns.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Zhu D, Guo L, Li Z, Lynch ME, Coles C, Hu X, Liu T. Connectomics signatures of prenatal cocaine exposure affected adolescent brains. Hum Brain Mapp. 2013;34(10):2494–2510. doi: 10.1002/hbm.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Brain anatomical network and intelligence. PLoS Comput Biol. 2009;5(5):e1000395. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Coles CD, Lynch ME, Hamann S, Peltier S, LaConte S, Hu X. Prenatal cocaine exposure alters emotional arousal regulation and its effects on working memory. Neurotoxicol Teratol. 2009;31(6):342–348. doi: 10.1016/j.ntt.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Lynch ME, Hamann S, Peltier S, Hu X. Increased "default mode" activity in adolescents prenatally exposed to cocaine. Hum Brain Mapp. 2011;32(5):759–770. doi: 10.1002/hbm.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS. Consequences of prenatal cocaine exposure in nonhuman primates. Brain Res Dev Brain Res. 2003;147(1–2):23–36. doi: 10.1016/j.devbrainres.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lu R, Liu X, Long H, Ma L. Effects of prenatal cocaine and heroin exposure on neuronal dendrite morphogenesis and spatial recognition memory in mice. Neurosci Lett. 2012;522(2):128–133. doi: 10.1016/j.neulet.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30(28):9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes L, Snyder PJ, Langlois E, Hunter N. Visuospatial working memory in school-aged children exposed in utero to cocaine. Child Neuropsychol. 2007;13(3):205–218. doi: 10.1080/09297040600888753. [DOI] [PubMed] [Google Scholar]
- Nair P, Black MM, Ackerman JP, Schuler ME, Keane VA. Children's cognitive-behavioral functioning at age 6 and 7: prenatal drug exposure and caregiving environment. Ambul Pediatr. 2008;8(3):154–162. doi: 10.1016/j.ambp.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. Neuroimage. 2008;42(4):1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. Is left-handedness a sensitive marker of prenatal exposures or indicators of fetal growth? Scand J Soc Med. 1995;23(4):233–235. doi: 10.1177/140349489502300403. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67(5):735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Wang J, Giannetta J, Korczykowski M, Shera D, Avants BB, Gee J, Detre JA, Hurt H. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120(5):e1245–1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- Riggins T, Cacic K, Buckingham-Howes S, Scaletti LA, Salmeron BJ, Black MM. Memory ability and hippocampal volume in adolescents with prenatal drug exposure. Neurotoxicol Teratol. 2012;34(4):434–441. doi: 10.1016/j.ntt.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Bramen JE, Nunez SC, Quandt LC, Smith L, O'Connor MJ, Bookheimer SY, Sowell ER. Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: the effects of methamphetamine, alcohol, and polydrug exposure. Neuroimage. 2011;54(4):3067–3075. doi: 10.1016/j.neuroimage.2010.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Rudie JD, Smith L, O'Connor MJ, Bookheimer SY, Narr KL, Sowell ER. Frontostriatal connectivity in children during working memory and the effects of prenatal methamphetamine, alcohol, and polydrug exposure. Dev Neurosci. 2012;34(1):43–57. doi: 10.1159/000336242. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder MD, Snyder PJ, Sielski I, Mayes L. Impaired performance of children exposed in utero to cocaine on a novel test of visuospatial working memory. Brain Cogn. 2004;55(2):409–412. doi: 10.1016/j.bandc.2004.02.062. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB. 2015 Unpublished data. [Google Scholar]
- Shamosh NA, Deyoung CG, Green AE, Reis DL, Johnson MR, Conway AR, Engle RW, Braver TS, Gray JR. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, Lagasse LL, Durston S, Casey BJ. Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Dev Neurosci. 2009;31(1–2):159–166. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45(9):2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Tian L, Wang J, Yan C, He Y. Hemisphere- and gender-related differences in small-world brain networks: a resting-state functional MRI study. Neuroimage. 2011;54(1):191–202. doi: 10.1016/j.neuroimage.2010.07.066. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2010;49(1):63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye C, Bolton P. Neural connectivity abnormalities in autism: Insights from the tuberous sclerosis model. BMC Med. 2013;11(1):55. doi: 10.1186/1741-7015-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29(23):7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T, Smith E. Neuroimaging Studies of Working Memory: A Meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of 'small-world' networks. Nature. 1998;393(6684):440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Hashizume H, Sassa Y, Takeuchi H, Thyreau B, He Y, Evans AC, Li X, Kawashima R, Fukuda H. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PLoS One. 2013;8(2):e55347. doi: 10.1371/journal.pone.0055347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Gong G, Wang J, Wang D, Liu D, Zhu C, Chen ZJ, Evans A, Zang Y, He Y. Sex- and brain size-related small-world structural cortical networks in young adults: a DTI tractography study. Cereb Cortex. 2011;21(2):449–458. doi: 10.1093/cercor/bhq111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.