Summary
Rucaparib, a polyADPribose polymerase (PARP) inhibitor, was approved recently for use in women with high grade serous ovarian cancer (HGSOC). It is now one of 3 approved PARPi for use in recurrent ovarian cancer, a family of agents that has changed the HGSOC treatment landscape and outcome.
In this issue of Clinical Cancer Research, Balasubramaniam and colleagues discuss the details that led to the US Food and Drug Administration approval of the PARP inhibitor (PARPi), rucaparib, for the treatment of patients with deleterious BRCA mutation-associated advanced high grade serous ovarian cancer (HGSOC) (1). Rucaparib, the second of 3 approved PARPi, and is licensed for use in patients with germline or somatic BRCA mutation-associated HGSOC (g or sBRCAm), for third or later line treatment.
Ovarian cancer incidence remains relatively stable in the US with 24,000+ new cases and over 14,000 women dying of disease each year; despite these concerning numbers, women are living longer and having improved quality of life (2). Continuing this advance requires new therapeutic opportunities. PARPi are the first new class of agents to be approved uniquely for ovarian cancer in over two decades. Their potential clinical utility was identified as the biochemical effects of deleterious germline mutations in BRCA1 and BRCA2 demonstrated their integral role in maintaining functional high fidelity DNA repair through homologous recombination (3). The single agent activity in several treatment aspects of HGSOC treatment suggests untapped potential for this new class. Concomitant with progress comes the conundrum of how, when, for whom, and in what combinations to use these agents in treatment of ovarian cancer.
Five PARPi are in clinical development, olaparib, rucaparib, niraparib, talazoparib, and veliparib. The first three listed are licensed for specific indications in HGSOC (Figure 1). PARPi are divided into two classes, weak-PARP-trappers (veliparib) and strong PARP-trappers (all others) (4). The ability to trap PARP enzyme on damaged DNA prevents DNA repair, stabilizes toxic PARP1/2-DNA complexes, and allows degeneration of stalled replication forks into double stranded DNA breaks. PARP enzyme inhibition in the absence of intact HR promotes poor fidelity repair through alternative end-joining. This results in increased cancer cell susceptibility to catastrophic repair events. Trapping comes at the cost of enhanced toxicity, most often myelosuppression and thrombocytopenia; equitoxic doses of trapping PARPi result in relatively similar clinical activity.
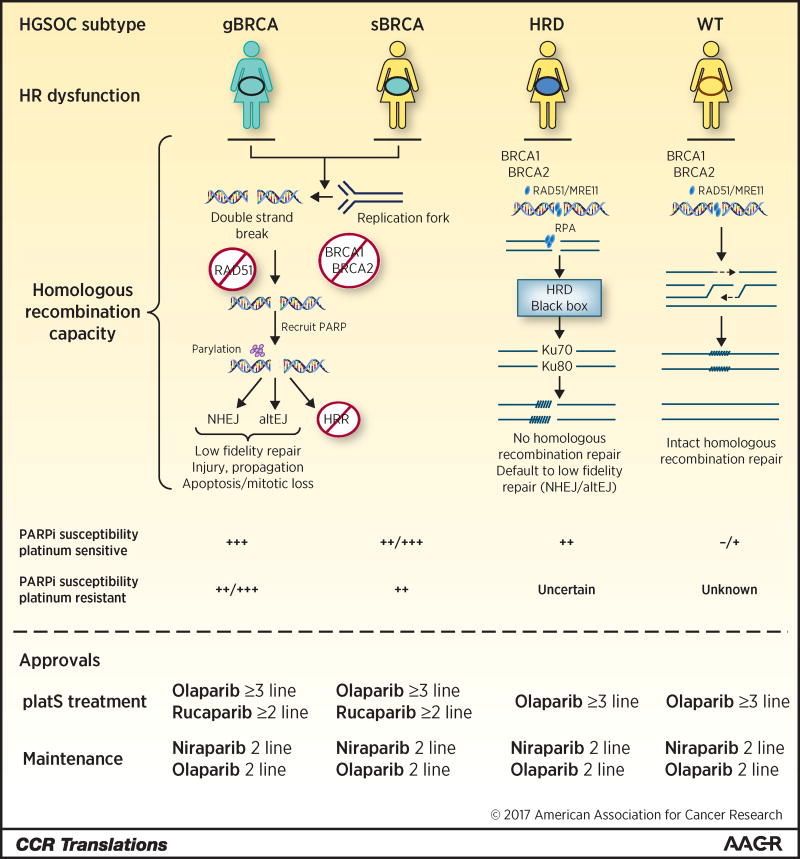
Figure 1. PARP inhibitor application and pathway events as a function of homologous recombination capacity.
HGSOC subtypes are designated as germline deleterious (gBRCA) or somatic (sBRCA) mutation of BRCA1/2, or HRD, homologous recombination deficient, as determined by HRD testing, or wild type. DNA DSBs occur directly or as a result of stalled replication fork degeneration. Inability to repair due to loss of HR capacity in g/sBRCA and HRD play out using different mechanisms. s/gBRCA have HR dysfunction caused by the inability to recruit and load RAD51 at DNA DSBs as occurs in wild type HR. Absent HR, PARP is recruited and PARylation of DNA ends occurs followed by activation of nonhomologous end-joining (NHEJ) or alternative end-joining (altEJ), both low fidelity repair mechanisms ultimately leading to cell injury, apoptosis, or mitotic arrest or catastrophe. HRD may also occur in the absence of defined mutations; in such cases, the causative event may not be known, hence the “black box”. In HRD without known mutation, recruitment of BRCA1/2 and downstream partners may occur but normal HR repair does not; similar to g/sBRCA, low fidelity repair occurs and PARP susceptibility is observed. PARP inhibitor susceptibility is a function of HR capacity and of platinum-sensitivity as shown. Current FDA approvals are outlined.
Three PARPi are now approved: rucaparib, olaparib, and niraparib (Figure 1). The phase II ARIEL2 study, for which the approval was given, examined rucaparib 600 mg twice daily in patients with HGSOC or high grade endometrioid (HGEOC) ovarian cancer after ≥1 prior platinum-based chemotherapy regimen and whose last treatment was platinum-based (5). It evaluated rucaparib activity as a function of potential predictive biomarkers: g/sBRCAm, a signature of HR dysfunction (HRD) by a loss of heterozygosity assay (LOH high; Foundation Medicine, Cambridge, MA) and no mutation, and neither mutation nor HRD [LOH low]. RECIST response rate and PFS were monitored, with greatest activity in g/sBRCAm patients (80%, 12.8 months), reduced response rate in LOH high (29%, 5.7 months), and least activity in LOH low (10%, 5.2 months). Liver enzyme elevation, anemia, and fatigue were the most common grade 3 events occurring in ≥ 9% of patients. The phase III ARIEL3 study, just reported at ESMO 2017 examined maintenance therapy with rucaparib 600 mg twice daily in patients with platinum-sensitive (platS) HGSOC after 1 or 2 prior platinum-based chemotherapy regimens. PFS was the primary endpoint. Results showed an improvement from 5.4 months to 16.6 mo in g/sBRCAm patients (HR=0.23), 13.6 mo in LOH high (HR=0.32), and 10.8 mo in the full intent-to-treat group (HR=0.36).
Olaparib, the first approved PARPi, now is approved for treatment of women with HGSOC, independent of gBRCA status, with ≥3 recurrences, and for maintenance of first recurrence treatment response for platS HGSOC (6). SOLO1, maturing, evaluates olaparib in maintenance of primary treatment response (NCT01844986). Retrospective studies have shown that prior exposure to olaparib does not abrogate platinum-sensitivity in subsequent lines of therapy (7). Niraparib is approved for maintenance of first recurrence treatment response in unselected recurrent platS HGSOC. Niraparib 300 mg BID improved PFS for all tested patient subsets, with best response in gBRCA HGSOC patients (21 vs 5.5 months; HR 0.27, p<0.0001). The exploratory population of HRD-negative BRCA wild type (BRCAwt) patients derived benefit with niraparib (6.9 v 3.8 mo; HR 0.58, p=0.02). The impact of niraparib (PARPi) exposure on response and PFS to subsequent therapy, whether PARPi induce cross-resistance to the subsequent chemotherapy such as platinums, and the long-term risks and benefits remain unknown.
The most compelling questions in PARPi incorporation into the treatment lifecycle of HGS/EOC remain--for whom are these agents most active, and when should they be used. Complicating whom to treat, beyond g/sBRCA and HRD+ patients, is the issue of variants of undetermined significance (VUS). Validation of the different HRD assays as predictive for benefit to PARPi awaits. Germline VUS raise a different flag. VUS determinations are now being made based upon in silico determination of deleterious function of the putative mutated protein; this need may be abrogated by a reliable, accurate HRD assay. Lastly, reclassification of patients previously described as having a VUS upon receipt of new information; however, this requires due diligence on the part of providers and genetic testing entities, with timely follow up of VUS patients.
Benefits of PARPi appear strongest in women with gBRCAm with platS disease. An incremental reduction in responsiveness occurs with progression to sBRCA, HRD, and then BRCAwt genomics. Similarly, there is incremental loss of responsiveness with platR disease. This would suggest better outcome with earlier use. However, we have no guidance as to when along the treatment timeline to best use PARPi or if combinations will change such recommendations. There is risk that these studies may be biased by access to PARPi in post-progression therapy, complicating OS endpoints. The question remains open as to the best timing to introduce these agents.
The future of PARPi use in HGS/EOC and in other cancers is wide open. The renaissance of the cellular DNA damage response as a therapeutic target has advanced our understanding of complex DNA repair and resistance mechanisms in general, and those that arise with PARP inhibition in particular (8). PARPi are intriguing combination therapy partners, to combine with other DNA repair, angiogenesis, cell cycle, and signaling inhibitors. These combinations provide the opportunity to leverage clinical synthetic lethality where the combined effects of the agents may be far more active than either single agent (9). Initial proof of concept comes from the activity of olaparib in combination with the VEGFR1–3 inhibitor, cediranib. This combination, now in phase III, demonstrated unexpected and striking activity in women with BRCAwt HGSOC, with >2-fold improvement in PFS (10). Other combination partners include small molecule and antibody inhibitors of angiogenesis, cyclin dependent kinases, phosphatidylinositol-3’kinase inhibitors, DNA repair/cell cycle regulation enzymes (ATM, ATR, CHK 1/2), and perhaps immune and cell cycle checkpoint modulation. Studies of most of these combinations are underway. We have entered an era of HGSOC treatment change yielding improvements in PFS and OS, and broadening applicability of PARPi to more women with ovarian cancer.
Acknowledgments
Funding: This work was supported by the Cancer Therapy Evaluation Program (ECK and SPI) and Center for Cancer Research (JmL) of the National Cancer Institute.
Footnotes
Conflict of Interest: The authors have no conflicts of interest.
References
- 1.Balasubramaniam S, Beaver JA, Horton S, Fernandes LL, Tang S, Horne HN, et al. FDA approval sumaray: Rucaparib for the treatment of patients with deleterious BRCA-mutation-associated advanced ovarian cancer. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-17-1337. in press. [DOI] [PubMed] [Google Scholar]
- 2.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–88. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor MJ. Targeting the DNA Damage Response in Cancer. Mol Cell. 2015;60:547–60. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 4.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72:5588–99. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swisher EM, Lin KK, Oza A, Scott C, Giordana H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. The Lancet Oncology. 2017;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 6.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–84. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 7.Ang JE, Gourley C, Powell CB, High H, Shapira-Frommer R, Castonguay V, et al. Efficacy of chemotherapy in BRCA1/2 mutation carrier ovarian cancer in the setting of PARP inhibitor resistance: a multi-institutional study. Clin Cancer Res. 2013;19:5485–93. doi: 10.1158/1078-0432.CCR-13-1262. [DOI] [PubMed] [Google Scholar]
- 8.Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355:1152–58. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivy SP, de Bono JS, Kohn EC. The ‘Pushmi-Pullyu’ of DNA REPAIR: Clinical Synthetic Lethality. Trends in Cancer. 2016;2:646–56. doi: 10.1016/j.trecan.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–14. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]