Abstract
Metabolic adaptations permit tumor cells to metastasize to and thrive in the brain. Brain metastases continue to present clinical challenges due to rising incidence and resistance to current treatments. Therefore, elucidating altered metabolic pathways in brain metastases may provide new therapeutic targets for the treatment of aggressive disease. Due to the high demand for glucose in the brain, increased glycolytic activity is favored for energy production. Primary tumors that undergo Warburg-like metabolic reprogramming become suited to growth in the brain microenvironment. Indeed, elevated metabolism is a predictor of metastasis in many cancer subtypes. Specifically, metabolic alterations are seen in primary tumors that are associated with the formation of brain metastases, namely breast cancer, lung cancer, and melanoma. Because of this selective pressure, inhibitors of key metabolic factors may reduce tumor cell viability, thus exploiting metabolic pathways for cancer therapeutics. This review summarizes the metabolic advantages and vulnerabilities of brain metastases.
Keywords: Brain metastases, metabolic adaptation, cancer metabolism
Introduction to Brain Metastases
An estimated 90% of cancer deaths are caused by metastatic disease (1). The metastasis of primary cancers to the brain remains an urgent clinical issue due to the increasing frequency of cases. The current incidence of brain metastases (BMs) ranges from 9–17% of all cancer patients, and has increased due to enhanced imaging techniques for BM diagnosis and improved treatment of primary tumors, which increases time to progression and inflicts selective pressure towards a more aggressive, brain-penetrating phenotype (2). BMs cause severe side effects, such as impaired neurological function and coma, leading to a sharp decline in quality of life. BMs are also associated with poor prognosis, as the average survival of a patient with untreated BMs is less than two months (3). Typically, BMs come from specific primary cancers, including lung cancer (39–56%), breast cancer (13–30%), melanoma (6–11%), and colorectal cancer (3–8%) (2). BMs of different primary origins exhibit various median survival times after first treatment (in months): breast cancer (13.8), renal cell carcinoma (9.63), non-small cell lung carcinoma (7.0), melanoma (6.74), GI cancer (5.36), and small cell lung carcinoma (4.9), though additional prognostic factors can be used to further stratify patients (4). Substantial heterogeneity between cases complicates the study and treatment of BMs.
Metastasis is a complex process, requiring cells to enter and travel through the bloodstream, then exit the bloodstream and colonize foreign tissues. Therefore, metastatic cells often possess advantageous adaptations that promote survival. Ongoing research seeks to identify alterations in signaling pathways, gene and protein expression levels, and metabolic phenotypes that are characteristic of metastatic cells. Identifying these pro-metastatic factors may lead to new therapeutic options to improve the survival of patients with BMs.
Tumor Metabolism and Profiling
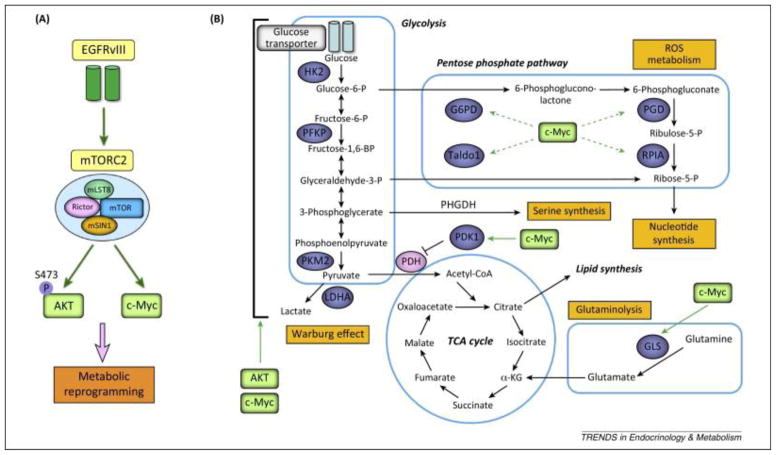
Both cell extrinsic and intrinsic factors affect cancer metabolism and metastasis. Extrinsically, interactions with the extracellular matrix, surrounding cells, and available nutrients affect cell metabolism. For example, ~20% of the body’s glucose-derived energy products are devoted to the brain (5). The brain’s elevated glucose supply and demand provides an ideal, nutrient-rich environment to fuel cancer cell growth. Due to rapid proliferation, cancer cells have high energetic and biosynthetic demands; therefore, tumors often undergo metabolic reprogramming to accommodate the need for nutrients and energy (6). The shift from oxidative phosphorylation to glycolysis in cancer cells is classically known as the Warburg effect (7). An example of factors involved in metabolic reprogramming is shown in Figure 1. Intrinsically, to support this metabolic shift, mutations that cause changes in gene expression can directly alter the levels or activity of metabolic enzymes present in a cancer cell. In addition, mutations that inactivate negative regulators of glycolysis, such as p53 or its target TIGAR, allow for constitutive glycolytic activity (8). Together, these metabolic adaptations promote tumor growth in the brain.
Figure 1. EGFR amplification promotes metabolic reprogramming.
A) Amplification of EGFR activates mTORC2, AKT, and c-Myc to promote metabolic reprogramming. EGFR variant III is shown as a representative mutation causing EGFR activation because it is commonly found in glioblastoma and breast cancer (77, 78). B) AKT and c-Myc (in green) activate enzymes (in blue) involved in glycolysis, the pentose phosphate pathway, and glutamine catabolism to supply energy and macromolecules to rapidly proliferating cancer cells.
Reprinted by permission from Elsevier: Cell Press, copyright 2014.
Masui K, Cavenee WK, Mischel PS. (2014) mTORC2 in the center of cancer metabolic reprogramming. Trends in endocrinology and metabolism: TEM. 25: 364–73. PMID: 24856037; PMCID: PMC4077930
Techniques used to establish metabolic profiles for tumors include FDG-PET imaging, metabolomics, and metabolic flux analysis. FDG-PET imaging uses the radiotracer 18F-FDG to visualize tumors throughout the body, as uptake correlates with the metabolic rates of different tissues. However, restricted uptake of FDG by gray matter in the brain limits the usefulness of this technique in cases of BMs (9). An alternative metabolomics approach employs spectroscopic methods to profile the metabolites present at a point in time in specific tissues. This discovery technique can reveal the accumulation or depletion of specific metabolite pools in response to drug treatment or tumor burden. In mouse models of BMs, biofluid metabolomics using NMR spectroscopy was used to distinguish tumor burden through differential metabolic profiles (10). This approach noninvasively detects micrometastases and aims to enhance early diagnosis of BMs to allow for earlier treatment.
In contrast, metabolic flux analysis monitors whole pathway activity by examining the formation and consumption rates of many metabolites. This targeted method requires stable-isotopes for mass spectrometry and provides a dynamic view of metabolism by quantifying the amount of specific metabolites over time (11). A metabolite with a stable isotope tracer, such as 13C, is introduced into a system and this tracer is transferred as the metabolite is processed. Metabolic flux accounts for not only metabolite concentrations, but also uses stoichiometric models to calculate enzyme activity. In orthotopic mouse models of glioblastoma, tracking various 13C-labeled nutrients revealed tumor accumulation of glutamine and increased mitochondrial glucose oxidation in tumor tissue compared to surrounding brain tissue (12). This analysis broadens the strict view of dependence on aerobic glycolysis into a more complex model involving glucose utilization by the citric acid cycle. Due to its targeted nature, metabolic flux is a powerful tool for scientific analysis and may uncover distinguishing metabolic characteristics that are useful for diagnostic and/or therapeutic purposes.
Signaling Pathways and Metabolism
Few cancer cells survive the journey from the primary tumor to the brain, where they can establish a metastatic lesion. The metastatic cells that inhabit the brain have a genetic predisposition for adaptability or the ability to crosstalk with host cells (13). Some common patterns of gene expression, protein levels, and signaling pathway activation have been identified in cells that colonize the brain. For example, factors involved in Notch signaling (notch1 and jagged-2) are expressed in melanoma cells with a pro-brain metastasizing phenotype (14). Note that MDA-MB-435 cells were used in this study and erroneously classified as breast cancer cells (15). Additionally, Il-1β is expressed in BMs from MDA-MB-231 breast cancer cells; this factor activates astrocytes to produce jagged-1, which activates Notch signaling in the tumor cells (16). Indeed, inhibition of Notch signaling in these cells reduces BMs in mice, suggesting that this may have a potential therapeutic benefit in humans (17).
In addition to its roles in cell development and communication, Notch has recently been recognized as a regulator of metabolism. Specifically, Notch regulates hepatocyte gluconeogenesis through FOXO1, hepatocyte lipogenesis through mTORC1 stabilization, and adipocyte thermogenesis through HES1 activation (18). Though tissue-specific differences in metabolism must be accounted for, it would be worth investigating whether Notch plays similar metabolic roles in brain tissue. Furthermore, the influence of signal pathway crosstalk on metabolism offers potential biological insights. Studies have shown that cell cycle control genes and Wnt signaling are upregulated in BMs (19). In endothelial cells from rat brains, the Wnt/β-catenin pathway interacts with the Notch pathway to increase the amount of monocarboxylic acid transporter 1 (MCT1) protein (20). MCT1 promotes pyruvate export and cell proliferation and is upregulated in glycolytic cancer cells, therefore MCT1 inhibitors block proliferation and may be useful cancer therapeutics (21).
The propensity of a tumor cell to metastasize to the brain depends on its cancer subtype. This phenomenon is the outcome of varied molecular signatures of gene/protein/receptor expression associated with different subtypes. For example, normal brain cells express heregulin, which increases the migration of cells expressing human epidermal growth factor receptor 2 (HER2) and HER3, suggesting that overexpression of these receptors provides a brain-metastatic advantage (22). Additional factors that favor metastatic colonization of the brain include amplification of epidermal growth factor receptor (EGFR), specifically in triple negative breast cancer (TNBC), HER2+ breast cancer, and EGFR-mutant lung cancer patients (23, 24). Amplification of EGFR is often associated with loss of phosphatase and tensin homolog (PTEN), a negative regulator of downstream EGFR effectors. Specifically, BMs are associated with downregulation of PTEN mRNA, allelic imbalance (differential expression of two alleles) at PTEN loci, and PTEN mutations (25). EGFR signaling activates the phosphoinositide 3-kinase (PI3K)/Akt pathway (Figure 1A), which promotes cell survival and proliferation and regulates c-Myc to facilitate metabolic reprogramming (26). Specifically, c-Myc activates enzymes involved in the pentose phosphate pathway, glutamine metabolism, and glycolysis, while reducing pyruvate flux into the citric acid cycle (Figure 1B). Together, these changes promote the Warburg effect, shifting cancer cells towards a more glycolytic state. Because these pathways support metabolic reprogramming and tumor growth, many involved factors are valuable therapeutic targets. Because signaling pathways are interconnected, simultaneous targeting of multiple pathways may be required for efficacious antitumor activity.
Adapting to the Brain Microenvironment
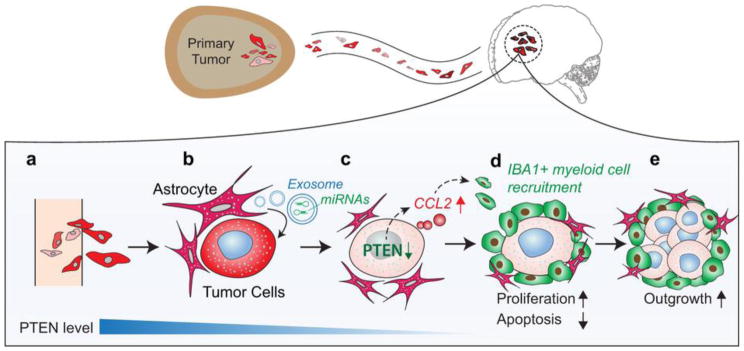
Crosstalk also occurs between cancer cells and other cells in the brain. This crosstalk can be indirect, through signaling pathways, or direct, through physical contact between cells. Both astrocytes and microglia inadvertently promote cancer growth through such interactions. For example, melanoma cells use gap junctions to hijack the neuro-protective function of reactive astrocytes to avoid chemotherapy-induced apoptosis (27). In addition, breast cancer cells secrete Il-1β, which activates nearby astrocytes (16). Once activated, astrocytes secrete factors that promote cancer cell proliferation, migration, and survival; such oncogenic signals include TGFβ, interleukins, cytokines, chemokines, MMP2, MMP9, and Wnt (28–31). Astrocytes also induce the loss of PTEN through the release of exosomes containing miRNAs that inhibit PTEN expression (32). Loss of PTEN increases the level of chemokine C-C motif ligand 2 (CCL2), which recruits myeloid cells that promote tumor outgrowth in the brain (Figure 2). Therefore, interactions between malignant and host cells in the brain perpetuate cancer growth. Blocking these interactions may inhibit brain metastasis.
Figure 2. Interactions between astrocytes and tumor cells support tumor growth.
A) Circulating tumor cells extravasate in the brain. B) Astrocytes (in pink) release exosomes containing miRNAs that reduce PTEN expression in nearby tumor cells. C) Loss of PTEN results in release of the chemoattractant CCL2. D) CCL2 recruits IBA1+ myeloid cells (in green), which promote tumor cell proliferation and reduce apoptosis. E) The myeloid cells support tumor outgrowth in the brain.
Reprinted by permission from Macmillan Publishers Ltd: Nature, copyright 2015.
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, Cheerathodi M, McCarty JH, Palmieri D, Saunus J, Lakhani S, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D. (2015) Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 527: 100–4. PMID: 26479035; PMCID: PMC4819404
In addition to signaling pathway similarities, metastatic cancer cells share certain metabolic characteristics with neuronal cells. In the brain, cancer cells adapt to utilize endogenous substrates for metabolism. For example, neurons typically catabolize gamma-aminobutyric acid (GABA) to create NADH to support biosynthetic processes. Previous studies on breast-to-brain metastases have shown that breast cancer cells with a GABAergic phenotype possess a strong growth advantage in the brain by converting GABA to succinate to augment the citric acid cycle (33). Thus, metastatic cells with neuron-like properties thrive in the brain microenvironment. Additionally, acetate is commonly metabolized by both primary brain cancers and BMs from melanoma, breast, and lung cancer (34). The ability to use acetate in addition to glucose as a carbon and energy source provides greater metabolic flexibility to these cells. Despite their origin or subtype, these common metabolic adaptations provide an advantage for rapid cancer growth in the brain.
Many metabolites and metabolic enzymes correlate with tumor invasiveness and may serve as indicators of disease progression. For example, glutamine metabolism is altered in lung cancer and in melanoma (35, 36). In addition, glutamine and lactate are associated with breast cancer proliferation and invasiveness, and enzymes involved in lipolysis support cancer growth and metastasis (37). Lipolysis creates lipid signaling messengers that affect a variety of cellular processes. In particular, oncogenic lipid signaling supports the metastasis of breast cancer cells to the brain by promoting cell survival, migration, and invasion (37). Key enzymes in lipolysis include monoacylglycerol lipase (MAGL) and alkylglyceronephosphate synthase (AGPS). Inhibition of MAGL or AGPS decreases the metastatic potential of breast cancer cells and reduces tumor growth and invasiveness (37, 38). Therefore, enzymes involved in lipid metabolism may be targeted to prevent the formation of BMs.
Breast-to-Brain Metastases
Improved treatment of primary tumors has increased the observed frequency of breast cancer metastases to secondary sites, including the brain. For example, HER2+ breast cancer patients treated with trastuzumab show a higher incidence of breast-to-brain metastasis (39). This issue is twofold: 1) primary disease is better controlled so patients are living longer, allowing time for metastasis to occur; and 2) the blood-brain barrier (BBB) protects tumor cells from most targeted therapies. Breast cancer commonly metastasizes to the bone, lung, and brain (40). Preference for metastatic sites is determined by many factors, including proximity to the primary tumor site, immune protection from the BBB, and breast cancer subtype. For example, luminal (especially ER+) and HER2+ breast cancers primarily metastasize to the bone, though 15.4% of luminal and 28.7% of HER2+ breast cancers metastasize to the brain (41). While basal subtypes prefer the lung, 10.9% of basal-like and 7.2% of TN non-basal-like breast cancers metastasize to the brain (19, 41). Thus, the subtype of the primary breast cancer affects future disease progression. This suggests an important link between gene expression patterns and metastasis of breast cancer. Further detailed examination of pro-metastatic genes in these subtypes may reveal additional metabolic mechanisms supporting breast-to-brain metastasis.
Metabolic phenotypes vary between different breast cancer subtypes. For example, luminal subtypes exhibit reverse-Warburg/null phenotypes that are metabolically inactive, while TNBC/basal-like subtypes exhibit Warburg/mixed phenotypes that are metabolically active (42). Because metabolically active tumors are typically more aggressive, metabolic status may predict disease progression and the likelihood of metastasis. Due to an elevated metabolism, TNBC and TNBC-derived BMs may benefit the most from metabolic intervention therapies.
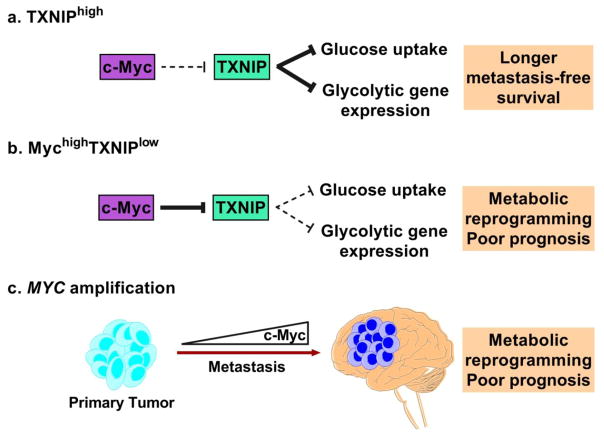
The metabolic state of a cell is regulated by a variety of molecular mechanisms. In TNBC, metabolic dysregulation is driven by factors such as glutathione S-transferase Pi 1 (GSTP1), forkhead box O 3a (FOXO3a), and EGFR-induced c-Myc (43–45). Specifically, c-Myc represses thioredoxin-interacting protein (TXNIP), an inhibitor of glycolytic gene expression and glucose uptake (46). Recent work has identified TXNIP as a suppressor of breast cancer metastasis, which strengthens the mechanistic link between metabolism and metastasis (47). MYC amplification is acquired during the metastatic process (48). Therefore, c-Myc-induced TXNIP inhibition drives glycolytic metabolism and provides a metastatic advantage to breast cancer cells (Figure 3). As certain types of TNBC are also sensitive to kinase inhibitors, a combination of kinase inhibitors with drugs targeting these metabolic differences may promote synthetic lethality (49). Due to certain molecular similarities between BMs and their tissue of origin, these therapies may also benefit TNBC-derived BMs. Cancer subtypes must be accounted for during drug development because they affect drug response and overall survival in patients.
Figure 3. Potential role of c-Myc in metabolic dysregulation.
A) Thioredoxin-interacting protein (TXNIP) typically inhibits glucose uptake and glycolytic gene expression. High TXNIP expression is associated with longer metastasis-free survival (46). B) MychighTXNIPlow signature is associated with metabolic reprogramming and poor prognosis in TNBC patients through reduced glucose uptake and glycolytic gene expression. C) MYC amplification is acquired during the metastatic process, which supports a general mechanism of metabolic dysregulation in BMs. This results in an aggressive, glycolytic tumor with a poor prognosis.
Lung-to-Brain Metastases
Lung cancer is divided into two major groups: non-small cell lung carcinoma (NSCLC) and small cell lung carcinoma (SCLC). Around 20–40% of patients with NSCLC develop BMs, and the adenocarcinoma subtype is generally more invasive than the squamous cell carcinoma subtype (50). Despite its less metastatic nature, squamous cell carcinoma exhibits an elevated glucose metabolism due to impaired blood vessel growth and tumor hypoxia (51). Therefore, it is important to evaluate metabolic state concurrently with other phenotypic and prognostic markers when assessing metastatic potential. NSCLC often harbors mutant LKB1, a kinase that activates the energy sensor AMPK, thus rendering tumors sensitive to metabolic stress (52). Mutations in LKB1 and KRAS, which are commonly associated, correlate with the formation of BMs in NSCLC patients (53). Therefore, patients with LKB1-mutant BMs may respond well to metabolic-targeted therapeutics.
SCLC is an aggressive disease that presents at advanced stages and leads to brain metastases in 80% of cases (54). In SCLC, a highly active metabolic phenotype, as assessed by volumetric metabolic parameters in FDG-PET imaging, correlates with poor prognosis (55, 56). Parameters assessed include metabolic tumor volume, total lesion glycolysis, and average standardized FDG uptake. Together, these parameters provide critical metrics for clinicians to interpret the metabolic phenotypes of tumors for the diagnosis of clinical stage and expected survival. Molecular characteristics observed in SCLC include c-Kit overexpression, EGFR mutation, VEGF overexpression, constitutively active PI3K, PTEN mutation, and Myc overexpression (57). These molecular abnormalities represent potentially actionable targets for drug development to treat aggressive SCLC.
Melanoma-to-Brain Metastases
Although melanoma accounts for a small percentage of skin cancers, it has the worst prognosis. Almost 45% of stage IV melanomas metastasize to the brain, and only 10% of these patients respond to systemic chemotherapy (58). Melanoma is often associated with BRAF mutations, which drive malignancy and have been targeted by successful therapeutics such as Vemurafenib and Dabrafenib (59). Melanoma cells that exhibit neuronal-like phenotypes such as glutamate and calcium signaling during early metastatic growth possess a brain-colonizing advantage (60). In addition, loss of claudin 1, a factor in tight junctions that interacts with endothelial cells in the brain, promotes melanoma-to-brain metastasis (61). Together, this suggests that both direct and indirect interactions with the microenvironment foster colonization of metastatic melanoma in the brain. Exposure of melanoma cells to the micro-environmental factor S100A4 causes a Warburg-like shift in metabolism, which promotes an invasive, malignant phenotype (62). In addition, metastatic melanoma cells exhibit increased oxidative phosphorylation, glutaminolysis, and β-oxidation compared to non-metastatic cells (63). Upregulation of these pathways supports rapid proliferation and invasiveness, demonstrating an additional link between metabolic reprogramming and metastasis.
Implications for Therapeutics
Currently, patients with BMs are treated by surgical resection or radiation. Radiation is targeted to the entire brain (whole brain radiation therapy, WBRT) or to a specific area of the brain (stereotactic radiotherapy). Though radiation may cause potential side effects such as cognitive impairment and radionecrosis of exposed brain tissue, resection followed by WBRT offers acceptable control of local disease (64). Treatment planning depends on the size, location, and number of metastases. Systemic therapy is still beneficial to many patients because it controls primary disease. Clinical trials have shown that some agents, such as anti-HER2 therapy in breast-BMs and anti-EGFR therapy in lung-BMs, provide benefits to a small subset of patients (65–67). However, the lack of response in the majority of patients may be attributed to poor drug distribution in the brain or acquired drug resistance of BMs (68, 69). Therefore, these therapies are not sufficient to treat the majority of patients with BMs.
Metabolic alterations observed in BMs offer potential targets for new therapeutics. However, because normal brain cells use the same metabolic pathways as BMs it is important to account for the potential off-target toxicities of metabolic-targeted therapeutics. Some metabolic interventions already show minimal toxicity profiles. For example, the treatment of cancer cells with dichloroacetate, an inhibitor of mitochondrial pyruvate dehydrogenase kinase, “normalizes” glucose oxidation, making the cancer cells susceptible to apoptosis while normal cells are unaffected (70). Dichloroacetate is also well tolerated in mice and in humans (71, 72). For more hazardous compounds, toxicity may be reduced by identifying mutations or unique isoforms of receptors, enzymes, or signaling proteins that are specific to tumor cells. Such targeted therapeutics should exhibit limited off-target toxicity. In general, side effects must be minimized for treatments to be worthwhile.
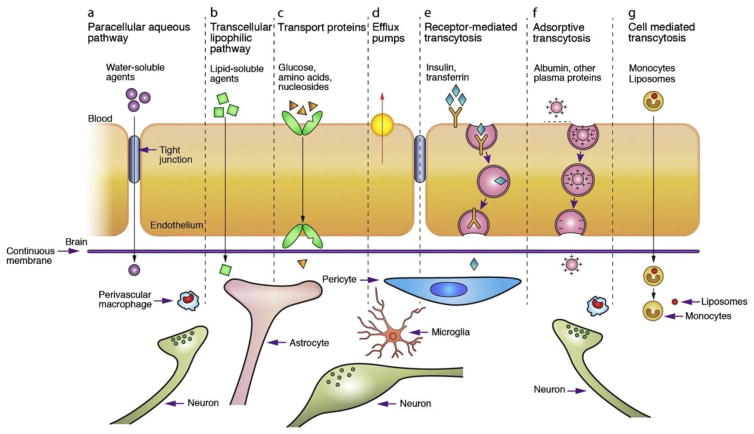
Experimental drug delivery methods combined with metabolic targets may provide needed advances in the treatment of BMs. Many delivery methods take advantage of endogenous transport pathways across the BBB (Figure 4). Methods in development include drug-loaded nanoparticles and liposomes, which accumulate in areas of leaky vasculature via the enhanced permeability and retention (EPR) effect (73). In addition, upregulation of the receptors for transferrin, insulin, and folate allow for receptor-mediated transcytosis of drugs conjugated to receptor ligands (74, 75). For example, transferrin-conjugated liposomes have been use to enhance the in vivo uptake and efficacy of 5-fluorouracil in brain tissue (76). Instead of traditional chemotherapeutic agents, these methods could be used to deliver drugs that target specific metabolic enzymes. However, many of these new delivery methods remain in preclinical testing and will require extensive evaluation before treatment of patients with BMs in the clinic.
Figure 4. Methods of transport across the BBB and potential drug delivery routes.
A–D) Common transport routes for solute molecules that are needed for normal brain metabolism. E–G) Transport routes that can be hijacked to deliver drugs to the brain. Drugs can be conjugated to insulin, transferrin, or albumin or loaded into liposomes, nanoparticles, or immune cells to utilize transcytosis pathways.
Reprinted by permission from Elsevier: Advanced Drug Delivery Reviews, copyright 2011.
Chen Y, Liu L. (2012) Modern methods for delivery of drugs across the blood-brain barrier. Advanced drug delivery reviews. 64: 640–65. PMID: 22154620
Conclusion
In conclusion, brain metastasis remains a growing health concern as treatment of primary tumors improves. The seclusion of the brain behind the BBB protects cancer cells from many chemotherapeutic agents and complicates treatment options. Further analysis of the differences between primary cancer subtypes and their matched BMs is necessary to develop molecular signatures and diagnosis-specific therapeutics. Cancer cells that metastasize are highly adaptable, and those able to mimic neuronal patterns of gene and protein expression, signaling, and metabolism can survive in the brain microenvironment. New therapies are needed to target factors unique to brain metastatic cells in order to enhance the survival of cancer patients with advanced disease.
Abbreviations
- AGPS
Alkylglyceronephosphate synthase
- BMs
Brain metastases
- BBB
Blood-brain barrier
- CCL2
Chemokine C-C motif ligand 2
- EGFR
Epidermal growth factor receptor
- EPR
Enhanced permeability and retention
- ER
Estrogen receptor
- FDG
Fluorodeoxyglucose
- FOXO3a
Forkhead box O 3a
- GABA
Gamma-aminobutyric acid
- GSTP1
Glutathione S-transferase Pi 1
- HER2
Human epidermal growth factor receptor 2
- MAGL
Monoacylglycerol lipase
- MCT1
Monocarboxylic acid transporter 1
- NMR
Nuclear magnetic resonance
- NSCLC
Non-small cell lung carcinoma
- PET
Positron emission tomography
- PI3K
Phosphoinositide 3-kinase
- PTEN
Phosphatase and tensin homolog
- SCLC
Small cell lung carcinoma
- TXNIP
Thioredoxin-interacting protein
- TNBC
Triple negative breast cancer
- WBRT
Whole brain radiation therapy
- VEGF
Vascular endothelial growth factor
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nature reviews. Cancer. 2006;6:449–58. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 2.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Current oncology reports. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 3.Markesbery WR, Brooks WH, Gupta GD, Young AB. Treatment for patients with cerebral metastases. Archives of neurology. 1978;35:754–6. doi: 10.1001/archneur.1978.00500350058012. [DOI] [PubMed] [Google Scholar]
- 4.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:419–25. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends in neurosciences. 2013;36:587–97. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nature cell biology. 2015;17:351–9. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY) 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–20. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 9.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nature reviews. Cancer. 2002;2:683–93. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 10.Larkin JR, Dickens AM, Claridge TD, Bristow C, Andreou K, Anthony DC, Sibson NR. Early Diagnosis of Brain Metastases Using a Biofluids-Metabolomics Approach in Mice. Theranostics. 2016;6:2161–69. doi: 10.7150/thno.16538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamboni N, Saghatelian A, Patti GJ. Defining the metabolome: size, flux, and regulation. Molecular cell. 2015;58:699–706. doi: 10.1016/j.molcel.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z, Cai L, Good L, Tu BP, Hatanpaa KJ, Mickey BE, Mates JM, Pascual JM, Maher EA, Malloy CR, Deberardinis RJ, Bachoo RM. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell metabolism. 2012;15:827–37. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witzel I, Oliveira-Ferrer L, Pantel K, Muller V, Wikman H. Breast cancer brain metastases: biology and new clinical perspectives. Breast cancer research: BCR. 2016;18:8. doi: 10.1186/s13058-015-0665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam DH, Jeon HM, Kim S, Kim MH, Lee YJ, Lee MS, Kim H, Joo KM, Lee DS, Price JE, Bang SI, Park WY. Activation of notch signaling in a xenograft model of brain metastasis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:4059–66. doi: 10.1158/1078-0432.CCR-07-4039. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix M. Persistent use of “false” cell lines. International journal of cancer. 2008;122:1–4. doi: 10.1002/ijc.23233. [DOI] [PubMed] [Google Scholar]
- 16.Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Wilber A, Mo YY, Moore BE, Liu W, Fukuda K, Iiizumi M, Sharma S, Liu Y, Wu K, Peralta E, Watabe K. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO molecular medicine. 2013;5:384–96. doi: 10.1002/emmm.201201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGowan PM, Simedrea C, Ribot EJ, Foster PJ, Palmieri D, Steeg PS, Allan AL, Chambers AF. Notch1 inhibition alters the CD44hi/CD24lo population and reduces the formation of brain metastases from breast cancer. Molecular cancer research: MCR. 2011;9:834–44. doi: 10.1158/1541-7786.MCR-10-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi P, Kuang S. Notch signaling as a novel regulator of metabolism. Trends in endocrinology and metabolism: TEM. 2015;26:248–55. doi: 10.1016/j.tem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer research. 2008;68:3108–14. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Sneve M, Haroldson TA, Smith JP, Drewes LR. Regulation of Monocarboxylic Acid Transporter 1 Trafficking by the Canonical Wnt/beta-Catenin Pathway in Rat Brain Endothelial Cells Requires Cross-talk with Notch Signaling. The Journal of biological chemistry. 2016;291:8059–69. doi: 10.1074/jbc.M115.710277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong CS, Graham NA, Gu W, Espindola Camacho C, Mah V, Maresh EL, Alavi M, Bagryanova L, Krotee PA, Gardner BK, Behbahan IS, Horvath S, Chia D, Mellinghoff IK, Hurvitz SA, Dubinett SM, Critchlow SE, Kurdistani SK, Goodglick L, Braas D, Graeber TG, Christofk HR. MCT1 Modulates Cancer Cell Pyruvate Export and Growth of Tumors that Co-express MCT1 and MCT4. Cell reports. 2016;14:1590–601. doi: 10.1016/j.celrep.2016.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Momeny M, Saunus JM, Marturana F, McCart Reed AE, Black D, Sala G, Iacobelli S, Holland JD, Yu D, Da Silva L, Simpson PT, Khanna KK, Chenevix-Trench G, Lakhani SR. Heregulin-HER3-HER2 signaling promotes matrix metalloproteinase-dependent blood-brain-barrier transendothelial migration of human breast cancer cell lines. Oncotarget. 2015;6:3932–46. doi: 10.18632/oncotarget.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hohensee I, Lamszus K, Riethdorf S, Meyer-Staeckling S, Glatzel M, Matschke J, Witzel I, Westphal M, Brandt B, Muller V, Pantel K, Wikman H. Frequent genetic alterations in EGFR- and HER2-driven pathways in breast cancer brain metastases. The American journal of pathology. 2013;183:83–95. doi: 10.1016/j.ajpath.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Yatabe Y, Takahashi T, Mitsudomi T. Epidermal growth factor receptor gene amplification is acquired in association with tumor progression of EGFR-mutated lung cancer. Cancer research. 2008;68:2106–11. doi: 10.1158/0008-5472.CAN-07-5211. [DOI] [PubMed] [Google Scholar]
- 25.Wikman H, Lamszus K, Detels N, Uslar L, Wrage M, Benner C, Hohensee I, Ylstra B, Eylmann K, Zapatka M, Sauter G, Kemming D, Glatzel M, Muller V, Westphal M, Pantel K. Relevance of PTEN loss in brain metastasis formation in breast cancer patients. Breast cancer research: BCR. 2012;14:R49. doi: 10.1186/bcr3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masui K, Cavenee WK, Mischel PS. mTORC2 in the center of cancer metabolic reprogramming. Trends in endocrinology and metabolism: TEM. 2014;25:364–73. doi: 10.1016/j.tem.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Q, Balasubramanian K, Fan D, Kim SJ, Guo L, Wang H, Bar-Eli M, Aldape KD, Fidler IJ. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia (New York, NY) 2010;12:748–54. doi: 10.1593/neo.10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, Maya M, He J, Kim SW, Weihua Z, Balasubramanian K, Fan D, Mills GB, Hung MC, Fidler IJ. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia (New York, NY) 2011;13:286–98. doi: 10.1593/neo.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pukrop T, Dehghani F, Chuang HN, Lohaus R, Bayanga K, Heermann S, Regen T, Van Rossum D, Klemm F, Schulz M, Siam L, Hoffmann A, Trumper L, Stadelmann C, Bechmann I, Hanisch UK, Binder C. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia. 2010;58:1477–89. doi: 10.1002/glia.21022. [DOI] [PubMed] [Google Scholar]
- 30.Sierra A, Price JE, Garcia-Ramirez M, Mendez O, Lopez L, Fabra A. Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Laboratory investigation; a journal of technical methods and pathology. 1997;77:357–68. [PubMed] [Google Scholar]
- 31.Termini J, Neman J, Jandial R. Role of the neural niche in brain metastatic cancer. Cancer research. 2014;74:4011–5. doi: 10.1158/0008-5472.CAN-14-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, Cheerathodi M, McCarty JH, Palmieri D, Saunus J, Lakhani S, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–4. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neman J, Termini J, Wilczynski S, Vaidehi N, Choy C, Kowolik CM, Li H, Hambrecht AC, Roberts E, Jandial R. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:984–9. doi: 10.1073/pnas.1322098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, Huang Z, Barnett S, Mickey BE, DeBerardinis RJ, Tu BP, Maher EA, Bachoo RM. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–14. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohamed A, Deng X, Khuri FR, Owonikoko TK. Altered glutamine metabolism and therapeutic opportunities for lung cancer. Clinical lung cancer. 2014;15:7–15. doi: 10.1016/j.cllc.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filipp FV, Ratnikov B, De Ingeniis J, Smith JW, Osterman AL, Scott DA. Glutamine-fueled mitochondrial metabolism is decoupled from glycolysis in melanoma. Pigment cell & melanoma research. 2012;25:732–9. doi: 10.1111/pcmr.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra P, Ambs S. Metabolic Signatures of Human Breast Cancer. Molecular & cellular oncology. 2015;2 doi: 10.4161/23723556.2014.992217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjamin DI, Cozzo A, Ji X, Roberts LS, Louie SM, Mulvihill MM, Luo K, Nomura DK. Ether lipid generating enzyme AGPS alters the balance of structural and signaling lipids to fuel cancer pathogenicity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14912–7. doi: 10.1073/pnas.1310894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson EM, Abdel-Rasoul M, Maly J, Wu CS, Lin NU, Shapiro CL. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2013;24:1526–33. doi: 10.1093/annonc/mdt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sihto H, Lundin J, Lundin M, Lehtimaki T, Ristimaki A, Holli K, Sailas L, Kataja V, Turpeenniemi-Hujanen T, Isola J, Heikkila P, Joensuu H. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast cancer research: BCR. 2011;13:R87. doi: 10.1186/bcr2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:3271–7. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 42.Choi J, Kim DH, Jung WH, Koo JS. Metabolic interaction between cancer cells and stromal cells according to breast cancer molecular subtype. Breast cancer research: BCR. 2013;15:R78. doi: 10.1186/bcr3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor S, Lam M, Pararasa C, Brown JE, Carmichael AR, Griffiths HR. Evaluating the evidence for targeting FOXO3a in breast cancer: a systematic review. Cancer cell international. 2015;15:1. doi: 10.1186/s12935-015-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louie SM, Grossman EA, Crawford LA, Ding L, Camarda R, Huffman TR, Miyamoto DK, Goga A, Weerapana E, Nomura DK. GSTP1 Is a Driver of Triple-Negative Breast Cancer Cell Metabolism and Pathogenicity. Cell chemical biology. 2016;23:567–78. doi: 10.1016/j.chembiol.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim SO, Li CW, Xia W, Lee HH, Chang SS, Shen J, Hsu JL, Raftery D, Djukovic D, Gu H, Chang WC, Wang HL, Chen ML, Huo L, Chen CH, Wu Y, Sahin A, Hanash SM, Hortobagyi GN, Hung MC. EGFR Signaling Enhances Aerobic Glycolysis in Triple-Negative Breast Cancer Cells to Promote Tumor Growth and Immune Escape. Cancer research. 2016;76:1284–96. doi: 10.1158/0008-5472.CAN-15-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen L, O’Shea JM, Kaadige MR, Cunha S, Wilde BR, Cohen AL, Welm AL, Ayer DE. Metabolic reprogramming in triple-negative breast cancer through Myc suppression of TXNIP. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:5425–30. doi: 10.1073/pnas.1501555112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen D, Dang BL, Huang JZ, Chen M, Wu D, Xu ML, Li R, Yan GR. MiR-373 drives the epithelial-to-mesenchymal transition and metastasis via the miR-373-TXNIP-HIF1alpha-TWIST signaling axis in breast cancer. Oncotarget. 2015;6:32701–12. doi: 10.18632/oncotarget.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singhi AD, Cimino-Mathews A, Jenkins RB, Lan F, Fink SR, Nassar H, Vang R, Fetting JH, Hicks J, Sukumar S, De Marzo AM, Argani P. MYC gene amplification is often acquired in lethal distant breast cancer metastases of unamplified primary tumors. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:378–87. doi: 10.1038/modpathol.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fink LS, Beatty A, Devarajan K, Peri S, Peterson JR. Pharmacological profiling of kinase dependency in cell lines across triple-negative breast cancer subtypes. Molecular cancer therapeutics. 2015;14:298–306. doi: 10.1158/1535-7163.MCT-14-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Current oncology (Toronto, Ont) 2013;20:e300–6. doi: 10.3747/co.20.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuurbiers OC, Meijer TW, Kaanders JH, Looijen-Salamon MG, de Geus-Oei LF, van der Drift MA, van der Heijden EH, Oyen WJ, Visser EP, Span PN, Bussink J. Glucose metabolism in NSCLC is histology-specific and diverges the prognostic potential of 18FDG-PET for adenocarcinoma and squamous cell carcinoma. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9:1485–93. doi: 10.1097/JTO.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 52.Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS, Shaw RJ. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer cell. 2013;23:143–58. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao N, Wilkerson MD, Shah U, Yin X, Wang A, Hayward MC, Roberts P, Lee CB, Parsons AM, Thorne LB, Haithcock BE, Grilley-Olson JE, Stinchcombe TE, Funkhouser WK, Wong KK, Sharpless NE, Hayes DN. Alterations of LKB1 and KRAS and risk of brain metastasis: comprehensive characterization by mutation analysis, copy number, and gene expression in non-small-cell lung carcinoma. Lung cancer (Amsterdam, Netherlands) 2014;86:255–61. doi: 10.1016/j.lungcan.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lekic M, Kovac V, Triller N, Knez L, Sadikov A, Cufer T. Outcome of small cell lung cancer (SCLC) patients with brain metastases in a routine clinical setting. Radiology and oncology. 2012;46:54–9. doi: 10.2478/v10019-012-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee YJ, Cho A, Cho BC, Yun M, Kim SK, Chang J, Moon JW, Park IK, Choi HJ, Kim JH. High tumor metabolic activity as measured by fluorodeoxyglucose positron emission tomography is associated with poor prognosis in limited and extensive stage small-cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:2426–32. doi: 10.1158/1078-0432.CCR-08-2258. [DOI] [PubMed] [Google Scholar]
- 56.Park SB, Choi JY, Moon SH, Yoo J, Kim H, Ahn YC, Ahn MJ, Park K, Kim BT. Prognostic value of volumetric metabolic parameters measured by [18F]fluorodeoxyglucose-positron emission tomography/computed tomography in patients with small cell lung cancer. Cancer imaging: the official publication of the International Cancer Imaging Society. 2014;14:2. doi: 10.1186/1470-7330-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischer B, Marinov M, Arcaro A. Targeting receptor tyrosine kinase signalling in small cell lung cancer (SCLC): what have we learned so far? Cancer treatment reviews. 2007;33:391–406. doi: 10.1016/j.ctrv.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, Hamid O, Infante JR, Millward M, Pavlick AC, O’Day SJ, Blackman SC, Curtis CM, Lebowitz P, Ma B, Ouellet D, Kefford RF. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet (London, England) 2012;379:1893–901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. The New England journal of medicine. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nygaard V, Prasmickaite L, Vasiliauskaite K, Clancy T, Hovig E. Melanoma brain colonization involves the emergence of a brain-adaptive phenotype. Oncoscience. 2014;1:82–94. doi: 10.18632/oncoscience.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Izraely S, Sagi-Assif O, Klein A, Meshel T, Ben-Menachem S, Zaritsky A, Ehrlich M, Prieto VG, Bar-Eli M, Pirker C, Berger W, Nahmias C, Couraud PO, Hoon DS, Witz IP. The metastatic microenvironment: Claudin-1 suppresses the malignant phenotype of melanoma brain metastasis. International journal of cancer. 2015;136:1296–307. doi: 10.1002/ijc.29090. [DOI] [PubMed] [Google Scholar]
- 62.Bettum IJ, Gorad SS, Barkovskaya A, Pettersen S, Moestue SA, Vasiliauskaite K, Tenstad E, Oyjord T, Risa O, Nygaard V, Maelandsmo GM, Prasmickaite L. Metabolic reprogramming supports the invasive phenotype in malignant melanoma. Cancer letters. 2015;366:71–83. doi: 10.1016/j.canlet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 63.Rodrigues MF, Obre E, de Melo FH, Santos GC, Jr, Galina A, Jasiulionis MG, Rossignol R, Rumjanek FD, Amoedo ND. Enhanced OXPHOS, glutaminolysis and beta-oxidation constitute the metastatic phenotype of melanoma cells. The Biochemical journal. 2016;473:703–15. doi: 10.1042/BJ20150645. [DOI] [PubMed] [Google Scholar]
- 64.Le Rhun E, Dhermain F, Vogin G, Reyns N, Metellus P. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert review of neurotherapeutics. 2016 doi: 10.1080/14737175.2016.1184572. [DOI] [PubMed] [Google Scholar]
- 65.Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M, Miles D, Samant M, Welslau M, Dieras V. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26:113–9. doi: 10.1093/annonc/mdu486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga JY, Goncalves A, Leheurteur M, Domont J, Gutierrez M, Cure H, Ferrero JM, Labbe-Devilliers C. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. The Lancet. Oncology. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 67.Zhao X, Zhu G, Chen H, Yang P, Li F, Du N. Efficacy of icotinib versus traditional chemotherapy as first-line treatment for preventing brain metastasis from advanced lung adenocarcinoma in patients with epidermal growth factor receptor-sensitive mutation. Journal of cancer research and therapeutics. 2014;10(Suppl):C155–9. doi: 10.4103/0973-1482.145851. [DOI] [PubMed] [Google Scholar]
- 68.Ohashi K, Maruvka YE, Michor F, Pao W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:1070–80. doi: 10.1200/JCO.2012.43.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stricker T, Arteaga CL. Drug-Resistant Brain Metastases: A Role for Pharmacology, Tumor Evolution, and Too-Late Therapy. Cancer discovery. 2015;5:1124–6. doi: 10.1158/2159-8290.CD-15-1166. [DOI] [PubMed] [Google Scholar]
- 70.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 71.Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, Felitsyn NM, Gilmore RL, Greer M, Henderson GN, Hutson AD, Neiberger RE, O’Brien RG, Perkins LA, Quisling RG, Shroads AL, Shuster JJ, Silverstein JH, Theriaque DW, Valenstein E. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117:1519–31. doi: 10.1542/peds.2005-1226. [DOI] [PubMed] [Google Scholar]
- 72.Kinnaird A, Dromparis P, Saleme B, Gurtu V, Watson K, Paulin R, Zervopoulos S, Stenson T, Sutendra G, Pink DB, Carmine-Simmen K, Moore R, Lewis JD, Michelakis ED. Metabolic Modulation of Clear-cell Renal Cell Carcinoma with Dichloroacetate, an Inhibitor of Pyruvate Dehydrogenase Kinase. European urology. 2016;69:734–44. doi: 10.1016/j.eururo.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 73.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2009;71:409–19. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 74.Doi A, Kawabata S, Iida K, Yokoyama K, Kajimoto Y, Kuroiwa T, Shirakawa T, Kirihata M, Kasaoka S, Maruyama K, Kumada H, Sakurai Y, Masunaga S, Ono K, Miyatake S. Tumor-specific targeting of sodium borocaptate (BSH) to malignant glioma by transferrin-PEG liposomes: a modality for boron neutron capture therapy. Journal of neuro-oncology. 2008;87:287–94. doi: 10.1007/s11060-008-9522-8. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Advanced drug delivery reviews. 2012;64:640–65. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Soni V, Kohli DV, Jain SK. Transferrin-conjugated liposomal system for improved delivery of 5-fluorouracil to brain. Journal of drug targeting. 2008;16:73–8. doi: 10.1080/10611860701725381. [DOI] [PubMed] [Google Scholar]
- 77.Gan HK, Kaye AH, Luwor RB. The EGFRvIII variant in glioblastoma multiforme. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2009;16:748–54. doi: 10.1016/j.jocn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 78.Ge H, Gong X, Tang CK. Evidence of high incidence of EGFRvIII expression and coexpression with EGFR in human invasive breast cancer by laser capture microdissection and immunohistochemical analysis. International journal of cancer. 2002;98:357–61. doi: 10.1002/ijc.10224. [DOI] [PubMed] [Google Scholar]