Abstract
Over the last decade, ankyrin-B has been identified as a prominent player in cardiac physiology. Ankyrin-B has a multitude of functions, with roles in expression, localization, and regulation of proteins critical for cardiac excitability, cytoskeletal integrity, and signaling. Further, human ANK2 variants that result in ankyrin-B loss-of-function are associated with ‘Ankyrin-B syndrome’, a complex cardiac phenotype that may include bradycardia and heart rate variability, conduction block, atrial fibrillation, QT interval prolongation, and potentially fatal catecholaminergic polymorphic ventricular tachycardia. However, our understanding of the molecular mechanisms underlying ankyrin-B function at baseline and in disease is still not fully resolved due to the complexity of ankyrin-B gene regulation, number of ankyrin-B-associated molecules, multiple roles of ankyrin-B in the heart and other organs that modulate cardiac function, and a host of unexpected clinical phenotypes. Here, we summarize known roles of ankyrin-B in the heart and the impact of ankyrin-B dysfunction in animal models and in human disease, as well as highlight important new findings illustrating the complexity of ankyrin-B signaling.
Keywords: ankyrin-B, ANK2, arrhythmias, ion channel, channelopathy
Introduction
Ankyrin-B is a member of the adapter protein family of ankyrins that includes ankyrin-R (ANK1), ankyrin-B (ANK2), and ankyrin-G (ANK3). These proteins display significant homology, yet maintain distinct functions and spatiotemporal dynamics. Within the heart, ankyrin-B (AnkB) and ankyrin-G (AnkG) are the major ankyrin gene products, although ankyrin-R (AnkR) isoforms have also been identified.1, 2 AnkG functions in the targeting of voltage-gated sodium channel Nav1.5 to the intercalated disc. Further, human variants in Nav1.5 that block interaction with AnkG are associated with Brugada syndrome, an arrhythmia syndrome associated with ST segment elevation in precordial leads V1–V3 and susceptibility to sudden cardiac death.3, 4 On the other hand, AnkB is primarily localized at the myocyte M-line and transverse-tubule (T-tubule) membranes where it associates with select membrane and signaling proteins to regulate excitation-contraction (EC) coupling. Human ANK2 loss-of-function variants are associated with a variety of arrhythmia phenotypes, including sinus node disease, atrial fibrillation, ventricular arrhythmia, and risk of sudden cardiac death.
To understand the phenotypic effects of loss-of-function ANK2 variants, it is first critical to understand excitation-contraction (EC) coupling. EC coupling is the process by which electrical stimuli ultimately lead to cardiac contraction. The initiation of cardiac contraction begins at pacemaker cells in the sinoatrial node with generation of an action potential (AP). The cardiac impulse spreads rapidly to the atrioventricular node, and then depolarization moves quickly though the ventricles, originating at the His Bundle before bifurcating to the left and right bundles. Purkinje fibers then rapidly propagate the AP into the ventricles. As the cardiac impulse spreads from myocyte to myocyte through gap junctions, action potentials are initiated by the activation of voltage-gated sodium channels (Nav). Subsequently, calcium channels (Cav) in the sarcolemma membrane at the T-tubules open. The influx of Ca2+ activates adjacent ryanodine receptors (RyR2) to release stored Ca2+ from the sarcoplasmic reticulum (SR). Free Ca2+ then binds cardiac Troponin-C, inducing a conformational change of Troponin that releases Tropomyosin from myosin. Actin then binds myosin to initiate contraction. Finally, the myosin head binds ATP to pull the actin filament towards the sarcomere. For relaxation to occur, Ca2+ is removed from the cytoplasm by the SR Ca2+ ATPase (SERCA2) and the Na+/Ca2+ exchanger (NCX1). Cytosolic Ca2+ moves into the extracellular space via NCX and is sequestered into the SR by SERCA2 until it is required for the next contraction. As Ca2+ is depleted from the cytosol, Tropomyosin and Troponin return to myosin to inhibit actin binding and relax the actin filament. This sequence repeats with the next AP.5–8 As described below, AnkB plays a critical role in the expression and localization of key proteins required throughout the cardiac excitation pathway. Thus, it is not surprising that deficiency or altered AnkB function has significant impact on cardiac function.
Ankyrin-B domains and binding partners
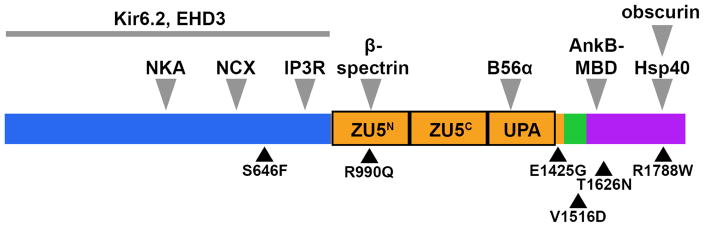
Canonical AnkB is 220 kDa and consists of four primary domains – a membrane- binding domain (MBD), a spectrin-binding domain (SBD), a death domain (DD), and a C-terminal domain (Figure 1). The MBD consists of 24 consecutive ANK repeats, and it is responsible for AnkB-dependent interactions with ion channels, transporters, and cell adhesion molecules. While ankyrin-binding partners are often organ- and cell-selective, the AnkB MBD in vertebrate cardiomyocytes directly associates with membrane Na/Ca exchanger (NCX1), Na/K ATPase (NKA),9, 10 the alpha subunit of the ATP-sensitive potassium channel (Kir6.2),11 and in atria, the L-type calcium channel Cav1.3.12 Of note, AnkB also regulates Cav2.1 expression and localization in the brain.13. While AnkB has been suggested to regulate Nav1.5 in heart, adult mice deficient in AnkB display no alteration in Nav1.5 expression, localization, or INa.14 Beyond the plasma membrane, the MBD associates with the inositol 1,4,5-trisphosphate receptor (IP3R) at the sarcoplasmic reticulum.11, 15, 16 The SBD interacts with β-spectrin at the N-terminal ZU5 domain to provide structural continuity between proteins associated with the MBD and the cytoskeleton of the cell.17 Through a direct interaction with the B56α subunit, the SBD is also responsible for localization of protein phosphatase 2A (PP2A), a regulator of NKA, NCX, RyR2, and IP3R that has a significant role in Ca2+ modulation via phosphorylation of these key players in the EC machinery.18 Finally, the regulatory domain (RD), so called because of its influence on MBD and SBD interactions, is comprised of the DD and the C-terminal domain. Interestingly, while highly unstructured, the C-terminal domain has been shown to bind the AnkB MBD to regulate localization of its binding partners.19 It is in this region where the highest number of pathogenic variants in ANK2 have been identified, yet this is also an area of innate amino acid variability between species and across human populations.20 This variability contributes to the difficulty of determining variant pathogenicity within the RD.
Figure 1.
Schematic of Ankyrin-B depicting protein domains and binding sites. Kir6.2 and EHD1–4 bind an undetermined location within the membrane-binding domain. Disease-causing variants in familial arrhythmogenic disease are shown. Blue, membrane-binding domain; orange, spectrin-binding domain; green, death domain; purple, C-terminal domain.
Role of ankyrin-B in cardiac excitability
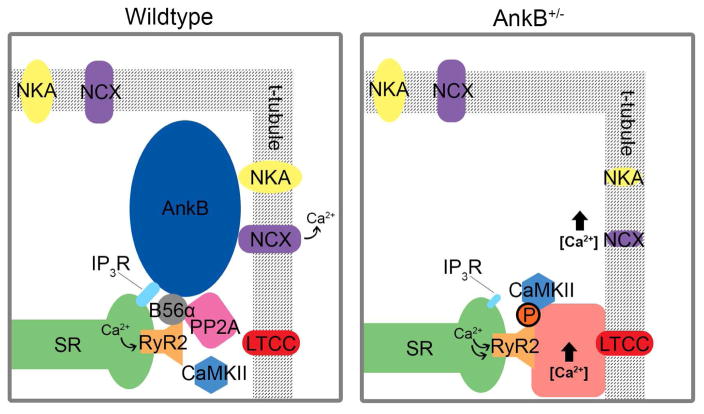
Mouse models of AnkB deficiency have been critical to dissect the role of AnkB in vertebrate physiology. Mice homozygous for an AnkB null mutation suffer neonatal lethality.21 However, mice heterozygous for an AnkB null mutation (AnkB+/−) have been indispensable in elucidating the mechanisms of AnkB in cardiomyocytes. AnkB+/− mice display bradycardia and heart rate variability, atrial arrhythmias, minor defects in QT interval, catecholamine-induced ventricular arrhythmias, and sudden death.22 At the cellular level, consistent with the roles of AnkB in binding NKA, NCX, and IP3R, loss of AnkB has been shown to decrease the expression and normal localization of NKA, NCX, and IP3R (Figure 2).23 Decreased expression of AnkB also causes an attenuated response to ouabain, a cardiac glycoside that inhibits NKA, resulting in altered calcium oscillations.23 AnkB+/− cardiomyocytes have unaltered diastolic [Ca2+], yet they display larger Ca2+ transients, SR Ca2+ content, and fractional SR Ca2+ release, causing an increased frequency in Ca2+ sparks. Furthermore, Cav1.2 expression and function is reduced in a mouse model of cardiac-specific deficiency of EHD3, an endosomal protein that binds and traffics AnkB. 24, 25 Alterations in calcium signaling due to dysregulation of the RyR2 are attributed to enhanced coupling of RyR2 openings in AnkB+/− cardiomyocytes.26 RyR2 pS1814 phosphorylation by calcium/calmodulin-dependent kinase (CaMKII) may also be amplified in AnkB+/− mice, potentially via disruption of the AnkB-dependent B56α targeting mechanism.27 Interestingly, hyperphosphorylation of RyR2 through CaMKII has also been identified in cardiomyocytes from patients with paroxysmal and chronic atrial fibrillation.28, 29 By an independent mechanism, activity of CaMKII may be activated due to increased levels of Ca2+ in the junctional cleft that are associated with decreased NCX activity in AnkB+/− mice.30 These Ca2+ sparks can lead to aberrant release of Ca2+ that compromises the specificity of EC signaling, ultimately increasing the propensity for arrhythmias and electrical dysfunction 31
Figure 2.
Schematic of cardiomyocyte signaling changes with loss of AnkB. Deficiency of AnkB has been shown to decrease the expression and normal localization of Na/K ATPase (NKA) and Na/Ca exchanger (NCX), ultimately resulting in altered SR calcium load. Secondarily, altered ankyrin-B dependent-targeting of PP2A subunit B56α alters the PP2A/CaMKII balance favoring RyR2 hyperphosphorylation. Together, at the tissue level, these cellular events support arrhythmia, particularly in response to catecholamines.
Beyond the ventricle, AnkB directly binds a subgroup of voltage-gated Ca2+ channels (Cav1.3) that is predominately expressed in the atria and is responsible for voltage-activated L-type Ca2+ current. Heterozygosity of AnkB in mouse sinoatrial node and atrial myocytes is associated with reduced expression of 12, 32 Additionally, Cav1.3.cardiac-specific deletion of EHD3 results in dysregulated expression and localization of Cav3.1 and Cav3.2 and reduced T-type mediated Ca2+ current.33 AnkB+/− mice display sinus node and atrial electrophysiological dysfunction, abnormal SAN electrical activity (including altered diastolic depolarization), shortened atrial action potentials, atrial fibrosis, and increased susceptibility to atrial fibrillation.12, 32, 34 Additionally, Glukhov et al found that isoproterenol-treated AnkB+/− mice exhibited competing multiple pacemakers and beat-to-beat variability within the leading pacemaker.35 As with ventricular myocytes, AnkB+/− atrial and sinus node myocytes also display loss of NCX and NKA.12
Human ANK2 variants
Loss-of-function variants in ANK2 have been associated with a variety of cardiovascular phenotypes, and most notably present as Ankyrin-B Syndrome (Table 1). Formerly known as Type 4 Long QT Syndrome, Ankyrin-B Syndrome presents with an autosomal-dominant pattern of inheritance and displays a wide spectrum of phenotypes, including sinus node bradycardia, conduction block, prolonged rate corrected QT interval, and catecholaminergic polymorphic ventricular tachycardia.14, 36 Mice heterozygous for a null mutation in Ank2 (AnkB+/− mice) display similar arrhythmogenic disease to humans, exhibiting altered Ca2+ signaling and decreased expression and localization of the AnkB binding proteins that are necessary to maintain normal EC coupling.22 AnkB is required for T-tubule/SR localization of NCX, NKA and IP3R, and this interaction is lost in both AnkB+/− mice and in the human arrhythmia causing p.E1425G variant. Normal localization of NCX and AnkB was also found to be disrupted in the MBD variant p.S646F, indicating that NCX localization is regulated by two AnkB domains – MBD and RD.38 ANK2 variants have also been found to cause sinus node disease (SND), and AnkB heterozygous (AnkB+/−) mice phenocopy human SND patients, presumably due to loss of AnkB-mediated organization and signaling in sinoatrial node (SAN) cells.34 Another variant in ANK2 that disrupts the AnkB/βII-spectrin interaction (AnkB p.R990Q) causes severe arrhythmia phenotypes. By studying this variant, Smith et al discovered that βII-spectrin regulates the localization of NCX, AnkB, and RyR2. Furthermore, βII-spectrin-deficient mice display lethal arrhythmias and an accelerated heart failure phenotype.39
Table 1.
Human ANK2 variants linked to in vitro loss-of-function
| Variant | Domain | Phenotype | Reference |
|---|---|---|---|
| S646F | MBD | Long QT, syncope, seizures, dilated cardiomyopathy with SCD, congenital heart defect, Wolff-Parkinson-White syndrome | 38 |
| R990Q | SBD | Long QT, syncope, ventricular fibrillation, sudden cardiac arrest | 39 |
| E1425G | SBD | Long QT, sinus node bradycardia, CPVT, atrial fibrillation, polyphasic t-waves, SCD | 22 |
| V1516D | DD | Atrial fibrillation, exercise-induced VT, drug-induced long QT, syncope, Brugada syndrome, bradycardia, CPVT | 40 |
| T1626N | CTD | Long QT, sinus arrhythmia, syncope, SCD | 14 |
| R1788W | CTD | Long QT, bradycardia, syncope, supraventricular and ventricular arrhythmias | 14 |
MBD, membrane-binding domain; SBD, spectrin-binding domain; DD, death domain; CTD, C-terminal domain; SCD, sudden cardiac death; CPVT, catecholaminergic polymorphic ventricular tachycardia
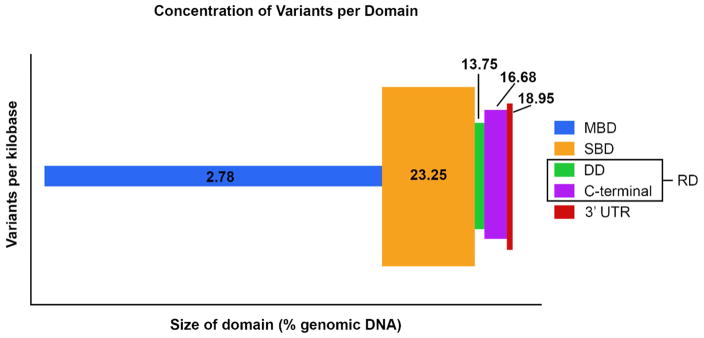
Although the function of the four protein domains of AnkB are well described, the heterogeneous nature of ANK2 variants remains unclear. To gain insight into the various clinical phenotypes observed with ANK2 variants, Mohler et al. characterized nine loss-of-function variants in vitro by monitoring contraction rates, calcium release, and channel/transporter expression and localization. They found that the phenotypes presented by these variants fell into three categories – negligible, minor, and severe loss-of-function, and that they were localized to one of two domains, the SBD or the RD.40 To date, over 2,500 ANK2 variants are reported in the Exome Aggregation Consortium (ExAC) at the Broad Institute and over 350 ANK2 variants have been logged in ClinVar at the National Institutes of Health. Despite the abundance of identified variants, few patterns have emerged to identify probable deleterious variants. Although the membrane-binding domain (MBD) comprises the majority of the genomic DNA and is directly responsible for interactions with membrane-bound ion channels and transporters, it is the least likely domain to harbor a variant (Figure 3). Only recently was the first account of a familial disease-causing, loss-of-function, ANK2 variant in the MBD reported. Segregation of the AnkB p.S646F variant was found in affected individuals from two multi-generational families within the Gitxsan First Nation with Long QT syndrome. The loss-of-function variant was found to prohibit normal membrane targeting of NCX, presumably due to the altered localization of AnkB p.S646F.38 Ichikawa et al also recently found possibly damaging variants in the MBD in isolated cases of arrhythmia. All other reported disease causing loss-of-function variants occur in the SBD, DD, or C-terminal domain.14, 22, 34, 42 Many loss-of-function variants in the SBD do not disrupt the interaction between AnkB and spectrin. Interestingly, Wang et al discovered that loss-of-function ANK2 variants within the SBD that did not affect spectrin-binding were located within a structural supramodule at the ZU5N-ZU5C-UPA domain, providing evidence of an additional function for the SBD.43–45
Figure 3.
Over 2,500 ANK2 variants listed in the Exome Aggregation Consortium at the Broad Institute were mapped to a protein domain. The length of the domain along the x-axis is based on percentage of genomic DNA that makes up that domain. Variants per kilobase on the y-axis depicts the average number of variants/Kb/domain. MBD, membrane-binding domain; SBD, spectrin-binding domain; DD, death domain; RD, regulatory domain; UTR, untranslated region.
Common variants in ANK2 may also be relevant, as they have been shown to influence the QT interval length in the general population. In large general population surveys, Cooperative Health Research in the Region of Augsburg (KORA S3 and KORA S4), Sedlacek et al. found that common genetic variants in the distant 5′ region of ANK2 significantly influenced rate-corrected QT intervals. Additionally, ANK2 p.L1622I is associated with mild loss-of-function and is disproportionately identified in individuals of African descent, a population that also experiences increased incidence of ventricular arrhythmias and sudden cardiac death.47 Similar common ANK2 variants may introduce compound phenotypes with other unrelated cardiac diseases. Recently, evidence has emerged of a non-arrhythmogenic role for AnkB in heart disease, as ANK2 variants are associated with greater maximum left ventricular wall thickness in patients with hypertrophic cardiomyopathy.48 AnkB dysregulation is also associated with ischemic heart disease. Five days after canine myocardial infarction, AnkB protein expression is downregulated in the border zone, which is accompanied by dysregulation of AnkB-associated ion channels and transporters. However, upregulation of Ank2 gene expression at this time results in recovery of AnkB levels at 14 days post-myocardial infarction.49 Furthermore, Kashef et al showed decreased expression of AnkB and its downstream targets in human ischemic and non-ischemic failing heart tissue, and calpain, a calcium-dependent protease, was identified as a regulator of AnkB in ischemic disease.50
Mechanisms of functional specificity
There is a striking amount of homology amongst the ankyrin proteins, yet they have completely unique, non-redundant functions. In 2002, Mohler et al explored the non-redundant functions of AnkB and AnkG through the use of ankyrin-B/G chimeras. They found that AnkG was unable to rescue aberrant expression of AnkB-binding partners in AnkB−/− cardiomyocytes and attributed the functional specificity of AnkB to its regulatory domain.20, 51 It is also fascinating that AnkB has numerous functions within cardiomyocytes. Alternative splicing of the ANK2 mRNA transcript is one mechanism that has been explored to explain this characteristic. Cunha et al were the first to identify alternative splicing in ANK2 and show a heterogeneous population of AnkB polypeptides in the heart, likely explaining the functional specificity observed in AnkB. Recently, two novel AnkB isoforms have been identified, AnkB-188 and AnkB-212 that also display unique functions. AnkB-188 functions in the localization and expression of NCX at the T-tubules, while AnkB-212 interacts with obscurin at the M-line.53, 54 The role of these variants in vivo is not yet known. Another mechanism of functional specificity is inter-domain interactions within AnkB. The C-terminal portion of the regulatory domain binds the membrane binding domain, and this binding is necessary for proper localization of IP3R.19 While studying the localization of AnkB in human bronchial epithelial (HBE) cells, He et al identified a linker peptide that interacts with the ANK repeat domains and the ZU52-UPA module and regulates the binding activity of AnkB, preventing membrane binding in HBE cells. This B-linker peptide is highly conserved in AnkB, and offers an additional mechanism by which AnkB and AnkG maintain divergent functions.55
Future investigations
Human disease-causing variants in ANK2 present with incomplete penetrance and variable phenotypes (Table 1), as is commonly observed in familial human genetics.56, 57 Phenotypic variability is likely due to the presence of genetic modifiers, a concept that dates back to 1941. Genetic modifiers may have a significant influence over the expressed phenotype that is a result of monogenic disease. Additional genetic variants as well as epigenetic modifications due to environmental influences may act as genetic modifiers. Chemical mutagenesis was recently used in zebrafish to identify genetic modifiers of cardiomyopathy, and a similar approach in mice was employed to identify genetic mechanisms of congenital heart disease.59, 60 Future efforts must be placed on identifying genes and elucidating mechanisms that contribute to the phenotypic variability associated with ANK2 variants.
Additional research to understand in the molecular roles of cardiac AnkB will also benefit several other areas of disease. To date, functional roles for AnkB have been implicated in neuronal disease, diabetes, and cancer.61–63 AnkB, along with many other cardiac ion channel-related proteins, is also expressed in neurons. AnkB−/− mice are born with severe neurological defects including brain and optical nerve malformation.64 Recently, AnkB and AnkG were shown to be decreased in human neurons with deletion of L1CAM, a gene that has been associated with hydrocephalus and intellectual disability.65 Furthermore, seizures have been shown to correlate with AnkB-associated cardiac channelopathies.38, 66, 67 Initially discovered in 1993 and 1995, the 440kDa AnkB (AnkB-440) and 480kDa AnkG (AnkG-480) isoforms, also known as giant ankyrins, are a result of the addition of a single large exon.68, 69 The role of giant ankyrins in patterning the axon initial segment was demonstrated in Drosophila and was shown to be conserved across Bilatera. These giant ankyrins have specific functions in neurobiology71–73, yet nothing is known about the role of giant ankyrins in the heart. The re-emergence of giant ankyrins in recent literature warrants investigation into potential localization and functions specific to these giant ankyrins in the heart.
Swayne et al recently reported the first account of a loss-of-function disease-causing ANK2 variant in the membrane-binding domain in families with Long QT syndrome. While this finding is significant in itself, this group also reported the presence of congenital malformations associated with the AnkB p.S646F variant.38 Interestingly, AnkB−/− mice suffer neonatal lethality21, which could be associated with a congenital heart defect. Additionally, these families displayed an incidence of cerebral aneurysms, suggesting a novel vascular role for AnkB. Further investigation and characterization of neonatal hearts and adult vasculature from AnkB deficient mice may provide insight into potentially novel roles for AnkB in cardiovascular development and disease.
Acknowledgments
This work was supported by the National Institutes of Health (grant numbers HL135754, HL134824, and HL114383), the Ohio State University JB Project, and the William D. and Jacquelyn L. Wells Fund for Cardiovascular Research.
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gallagher PG, Forget BG. An alternate promoter directs expression of a truncated, muscle-specific isoform of the human ankyrin 1 gene. J Biol Chem. 1998;273:1339–1348. doi: 10.1074/jbc.273.3.1339. [DOI] [PubMed] [Google Scholar]
- 2.Curran J, Mohler PJ. Coordinating electrical activity of the heart: Ankyrin polypeptides in human cardiac disease. Expert Opin Ther Targets. 2011;15:789–801. doi: 10.1517/14728222.2011.575363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, Bennett V. Nav1.5 e1053k mutation causing brugada syndrome blocks binding to ankyrin-g and expression of nav1. 5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004;101:17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe JS, Palygin O, Bhasin N, Hund TJ, Boyden PA, Shibata E, Anderson ME, Mohler PJ. Voltage-gated nav channel targeting in the heart requires an ankyrin-g dependent cellular pathway. J Cell Biol. 2008;180:173–186. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 6.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: Species-dependent differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994;74:1071–1096. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- 8.Bardou AL, Auger PM, Birkui PJ, Chasse JL. Modeling of cardiac electrophysiological mechanisms: From action potential genesis to its propagation in myocardium. Crit Rev Biomed Eng. 1996;24:141–221. doi: 10.1615/critrevbiomedeng.v24.i2-3.20. [DOI] [PubMed] [Google Scholar]
- 9.Cunha SR, Bhasin N, Mohler PJ. Targeting and stability of na/ca exchanger 1 in cardiomyocytes requires direct interaction with the membrane adaptor ankyrin-b. J Biol Chem. 2007;282:4875–4883. doi: 10.1074/jbc.M607096200. [DOI] [PubMed] [Google Scholar]
- 10.Li ZP, Burke EP, Frank JS, Bennett V, Philipson KD. The cardiac na+-ca2+ exchanger binds to the cytoskeletal protein ankyrin. J Biol Chem. 1993;268:11489–11491. [PubMed] [Google Scholar]
- 11.Li J, Kline CF, Hund TJ, Anderson ME, Mohler PJ. Ankyrin-b regulates kir6. 2 membrane expression and function in heart. J Biol Chem. 2010;285:28723–28730. doi: 10.1074/jbc.M110.147868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunha SR, Hund TJ, Hashemi S, et al. Defects in ankyrin-based membrane protein targeting pathways underlie atrial fibrillation. Circulation. 2011;124:1212–1222. doi: 10.1161/CIRCULATIONAHA.111.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kline CF, Scott J, Curran J, Hund TJ, Mohler PJ. Ankyrin-b regulates cav2.1 and cav2. 2 channel expression and targeting. J Biol Chem. 2014;289:5285–5295. doi: 10.1074/jbc.M113.523639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT, Bennett V. A cardiac arrhythmia syndrome caused by loss of ankyrin-b function. Proc Natl Acad Sci U S A. 2004;101:9137–9142. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kline CF, Cunha SR, Lowe JS, Hund TJ, Mohler PJ. Revisiting ankyrin-insp3 receptor interactions: Ankyrin-b associates with the cytoplasmic n-terminus of the insp3 receptor. J Cell Biochem. 2008;104:1244–1253. doi: 10.1002/jcb.21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohler PJ, Davis JQ, Davis LH, Hoffman JA, Michaely P, Bennett V. Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-b. J Biol Chem. 2004;279:12980–12987. doi: 10.1074/jbc.M313979200. [DOI] [PubMed] [Google Scholar]
- 17.Mohler PJ, Yoon W, Bennett V. Ankyrin-b targets beta2-spectrin to an intracellular compartment in neonatal cardiomyocytes. J Biol Chem. 2004;279:40185–40193. doi: 10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- 18.Bhasin N, Cunha SR, Mudannayake M, Gigena MS, Rogers TB, Mohler PJ. Molecular basis for pp2a regulatory subunit b56alpha targeting in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293:H109–119. doi: 10.1152/ajpheart.00059.2007. [DOI] [PubMed] [Google Scholar]
- 19.Abdi KM, Mohler PJ, Davis JQ, Bennett V. Isoform specificity of ankyrin-b: A site in the divergent c-terminal domain is required for intramolecular association. J Biol Chem. 2006;281:5741–5749. doi: 10.1074/jbc.M506697200. [DOI] [PubMed] [Google Scholar]
- 20.Mohler PJ, Gramolini AO, Bennett V. The ankyrin-b c-terminal domain determines activity of ankyrin-b/g chimeras in rescue of abnormal inositol 1,4,5-trisphosphate and ryanodine receptor distribution in ankyrin-b (−/−) neonatal cardiomyocytes. J Biol Chem. 2002;277:10599–10607. doi: 10.1074/jbc.M110958200. [DOI] [PubMed] [Google Scholar]
- 21.Tuvia S, Buhusi M, Davis L, Reedy M, Bennett V. Ankyrin-b is required for intracellular sorting of structurally diverse ca2+ homeostasis proteins. J Cell Biol. 1999;147:995–1008. doi: 10.1083/jcb.147.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohler PJ, Schott JJ, Gramolini AO, et al. Ankyrin-b mutation causes type 4 long-qt cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Spicarova Z, Rydholm S, Li J, Brismar H, Aperia A. Ankyrin b modulates the function of na, k-atpase/inositol 1,4,5-trisphosphate receptor signaling microdomain. J Biol Chem. 2008;283:11461–11468. doi: 10.1074/jbc.M706942200. [DOI] [PubMed] [Google Scholar]
- 24.Gudmundsson H, Hund TJ, Wright PJ, et al. Eh domain proteins regulate cardiac membrane protein targeting. Circ Res. 2010;107:84–95. doi: 10.1161/CIRCRESAHA.110.216713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curran J, Makara MA, Little SC, et al. Ehd3-dependent endosome pathway regulates cardiac membrane excitability and physiology. Circ Res. 2014;115:68–78. doi: 10.1161/CIRCRESAHA.115.304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camors E, Mohler PJ, Bers DM, Despa S. Ankyrin-b reduction enhances ca spark-mediated sr ca release promoting cardiac myocyte arrhythmic activity. J Mol Cell Cardiol. 2012;52:1240–1248. doi: 10.1016/j.yjmcc.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeGrande S, Nixon D, Koval O, et al. Camkii inhibition rescues proarrhythmic phenotypes in the model of human ankyrin-b syndrome. Heart Rhythm. 2012;9:2034–2041. doi: 10.1016/j.hrthm.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum ca2+ leak and increased na+-ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popescu I, Galice S, Mohler PJ, Despa S. Elevated local [ca2+] and camkii promote spontaneous ca2+ release in ankyrin-b-deficient hearts. Cardiovasc Res. 2016;111:287–294. doi: 10.1093/cvr/cvw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ Res. 2017;120:1969–1993. doi: 10.1161/CIRCRESAHA.117.310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf RM, Glynn P, Hashemi S, Zarei K, Mitchell CC, Anderson ME, Mohler PJ, Hund TJ. Atrial fibrillation and sinus node dysfunction in human ankyrin-b syndrome: A computational analysis. Am J Physiol Heart Circ Physiol. 2013;304:H1253–1266. doi: 10.1152/ajpheart.00734.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curran J, Musa H, Kline CF, Makara MA, Little SC, Higgins JD, Hund TJ, Band H, Mohler PJ. Eps15 homology domain-containing protein 3 regulates cardiac t-type ca2+ channel targeting and function in the atria. J Biol Chem. 2015;290:12210–12221. doi: 10.1074/jbc.M115.646893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Scouarnec S, Bhasin N, Vieyres C, et al. Dysfunction in ankyrin-b-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A. 2008;105:15617–15622. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glukhov AV, Fedorov VV, Anderson ME, Mohler PJ, Efimov IR. Functional anatomy of the murine sinus node: High-resolution optical mapping of ankyrin-b heterozygous mice. Am J Physiol Heart Circ Physiol. 2010;299:H482–491. doi: 10.1152/ajpheart.00756.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schott JJ, Charpentier F, Peltier S, Foley P, Drouin E, Bouhour JB, Donnelly P, Vergnaud G, Bachner L, Moisan JP, et al. Mapping of a gene for long qt syndrome to chromosome 4q25–27. Am J Hum Genet. 1995;57:1114–1122. [PMC free article] [PubMed] [Google Scholar]
- 37.Mohler PJ, Davis JQ, Bennett V. Ankyrin-b coordinates the na/k atpase, na/ca exchanger, and insp3 receptor in a cardiac t-tubule/sr microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swayne LA, Murphy NP, Asuri S, et al. Novel variant in the ank2 membrane-binding domain is associated with ankyrin-b syndrome and structural heart disease in a first nations population with a high rate of long qt syndrome. Circ Cardiovasc Genet. 2017:10. doi: 10.1161/CIRCGENETICS.116.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith SA, Sturm AC, Curran J, et al. Dysfunction in the betaii spectrin-dependent cytoskeleton underlies human arrhythmia. Circulation. 2015;131:695–708. doi: 10.1161/CIRCULATIONAHA.114.013708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohler PJ, Le Scouarnec S, Denjoy I, et al. Defining the cellular phenotype of “ankyrin-b syndrome” variants: Human ank2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation. 2007;115:432–441. doi: 10.1161/CIRCULATIONAHA.106.656512. [DOI] [PubMed] [Google Scholar]
- 41.Ichikawa M, Aiba T, Ohno S, et al. Phenotypic variability of ank2 mutations in patients with inherited primary arrhythmia syndromes. Circ J. 2016;80:2435–2442. doi: 10.1253/circj.CJ-16-0486. [DOI] [PubMed] [Google Scholar]
- 42.Sherman J, Tester DJ, Ackerman MJ. Targeted mutational analysis of ankyrin-b in 541 consecutive, unrelated patients referred for long qt syndrome genetic testing and 200 healthy subjects. Heart Rhythm. 2005;2:1218–1223. doi: 10.1016/j.hrthm.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 43.Ayalon G, Hostettler JD, Hoffman J, Kizhatil K, Davis JQ, Bennett V. Ankyrin-b interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury. J Biol Chem. 2011;286:7370–7378. doi: 10.1074/jbc.M110.187831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kizhatil K, Yoon W, Mohler PJ, Davis LH, Hoffman JA, Bennett V. Ankyrin-g and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J Biol Chem. 2007;282:2029–2037. doi: 10.1074/jbc.M608921200. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Yu C, Ye F, Wei Z, Zhang M. Structure of the zu5-zu5-upa-dd tandem of ankyrin-b reveals interaction surfaces necessary for ankyrin function. Proc Natl Acad Sci U S A. 2012;109:4822–4827. doi: 10.1073/pnas.1200613109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sedlacek K, Stark K, Cunha SR, Pfeufer A, Weber S, Berger I, Perz S, Kaab S, Wichmann HE, Mohler PJ, Hengstenberg C, Jeron A. Common genetic variants in ank2 modulate qt interval: Results from the kora study. Circ Cardiovasc Genet. 2008;1:93–99. doi: 10.1161/CIRCGENETICS.108.792192. [DOI] [PubMed] [Google Scholar]
- 47.Musa H, Murphy NP, Curran J, et al. Common human ank2 variant confers in vivo arrhythmia phenotypes. Heart Rhythm. 2016;13:1932–1940. doi: 10.1016/j.hrthm.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 48.Lopes LR, Syrris P, Guttmann OP, O’Mahony C, Tang HC, Dalageorgou C, Jenkins S, Hubank M, Monserrat L, McKenna WJ, Plagnol V, Elliott PM. Novel genotype-phenotype associations demonstrated by high-throughput sequencing in patients with hypertrophic cardiomyopathy. Heart. 2015;101:294–301. doi: 10.1136/heartjnl-2014-306387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hund TJ, Wright PJ, Dun W, Snyder JS, Boyden PA, Mohler PJ. Regulation of the ankyrin-b-based targeting pathway following myocardial infarction. Cardiovasc Res. 2009;81:742–749. doi: 10.1093/cvr/cvn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kashef F, Li J, Wright P, Snyder J, Suliman F, Kilic A, Higgins RS, Anderson ME, Binkley PF, Hund TJ, Mohler PJ. Ankyrin-b protein in heart failure: Identification of a new component of metazoan cardioprotection. J Biol Chem. 2012;287:30268–30281. doi: 10.1074/jbc.M112.368415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohler PJ, Hoffman JA, Davis JQ, Abdi KM, Kim CR, Jones SK, Davis LH, Roberts KF, Bennett V. Isoform specificity among ankyrins. An amphipathic alpha-helix in the divergent regulatory domain of ankyrin-b interacts with the molecular co-chaperone hdj1/hsp40. J Biol Chem. 2004;279:25798–25804. doi: 10.1074/jbc.M401296200. [DOI] [PubMed] [Google Scholar]
- 52.Cunha SR, Le Scouarnec S, Schott JJ, Mohler PJ. Exon organization and novel alternative splicing of the human ank2 gene: Implications for cardiac function and human cardiac disease. J Mol Cell Cardiol. 2008;45:724–734. doi: 10.1016/j.yjmcc.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu HC, Yamankurt G, Luo J, Subramaniam J, Hashmi SS, Hu H, Cunha SR. Identification and characterization of two ankyrin-b isoforms in mammalian heart. Cardiovasc Res. 2015;107:466–477. doi: 10.1093/cvr/cvv184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cunha SR, Mohler PJ. Obscurin targets ankyrin-b and protein phosphatase 2a to the cardiac m-line. J Biol Chem. 2008;283:31968–31980. doi: 10.1074/jbc.M806050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He M, Tseng WC, Bennett V. A single divergent exon inhibits ankyrin-b association with the plasma membrane. J Biol Chem. 2013;288:14769–14779. doi: 10.1074/jbc.M113.465328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper DN, Krawczak M, Polychronakos C, Tyler-Smith C, Kehrer-Sawatzki H. Where genotype is not predictive of phenotype: Towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum Genet. 2013;132:1077–1130. doi: 10.1007/s00439-013-1331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Genin E, Feingold J, Clerget-Darpoux F. Identifying modifier genes of monogenic disease: Strategies and difficulties. Hum Genet. 2008;124:357–368. doi: 10.1007/s00439-008-0560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haldane JBS. The relative importance of principal and modifying genes in determining some human diseases. Journal of Genetics. 1941;41:149–157. [Google Scholar]
- 59.Ding Y, Long PA, Bos JM, et al. A modifier screen identifies dnajb6 as a cardiomyopathy susceptibility gene. JCI Insight. 2016:1. doi: 10.1172/jci.insight.88797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Klena NT, Gabriel GC, et al. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature. 2015;521:520–524. doi: 10.1038/nature14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kline CF, Kurata HT, Hund TJ, Cunha SR, Koval OM, Wright PJ, Christensen M, Anderson ME, Nichols CG, Mohler PJ. Dual role of k atp channel c-terminal motif in membrane targeting and metabolic regulation. Proc Natl Acad Sci U S A. 2009;106:16669–16674. doi: 10.1073/pnas.0907138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Healy JA, Nilsson KR, Hohmeier HE, Berglund J, Davis J, Hoffman J, Kohler M, Li LS, Berggren PO, Newgard CB, Bennett V. Cholinergic augmentation of insulin release requires ankyrin-b. Sci Signal. 2010;3:ra19. doi: 10.1126/scisignal.2000771. [DOI] [PubMed] [Google Scholar]
- 63.Hesson LB, Ng B, Zarzour P, et al. Integrated genetic, epigenetic, and transcriptional profiling identifies molecular pathways in the development of laterally spreading tumors. Mol Cancer Res. 2016;14:1217–1228. doi: 10.1158/1541-7786.MCR-16-0175. [DOI] [PubMed] [Google Scholar]
- 64.Scotland P, Zhou D, Benveniste H, Bennett V. Nervous system defects of ankyrinb (−/−) mice suggest functional overlap between the cell adhesion molecule l1 and 440-kd ankyrinb in premyelinated axons. J Cell Biol. 1998;143:1305–1315. doi: 10.1083/jcb.143.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patzke C, Acuna C, Giam LR, Wernig M, Sudhof TC. Conditional deletion of l1cam in human neurons impairs both axonal and dendritic arborization and action potential generation. J Exp Med. 2016;213:499–515. doi: 10.1084/jem.20150951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ratnapriya R, Satishchandra P, Dilip S, Gadre G, Anand A. Familial autosomal dominant reflex epilepsy triggered by hot water maps to 4q24–q28. Hum Genet. 2009;126:677–683. doi: 10.1007/s00439-009-0718-6. [DOI] [PubMed] [Google Scholar]
- 67.Hata Y, Yoshida K, Kinoshita K, Nishida N. Epilepsy-related sudden unexpected death: Targeted molecular analysis of inherited heart disease genes using next-generation DNA sequencing. Brain Pathol. 2016 doi: 10.1111/bpa.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kordeli E, Lambert S, Bennett V. Ankyring. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of ranvier. J Biol Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- 69.Chan W, Kordeli E, Bennett V. 440-kd ankyrinb: Structure of the major developmentally regulated domain and selective localization in unmyelinated axons. J Cell Biol. 1993;123:1463–1473. doi: 10.1083/jcb.123.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jegla T, Nguyen MM, Feng C, Goetschius DJ, Luna E, van Rossum DB, Kamel B, Pisupati A, Milner ES, Rolls MM. Bilaterian giant ankyrins have a common evolutionary origin and play a conserved role in patterning the axon initial segment. PLoS Genet. 2016;12:e1006457. doi: 10.1371/journal.pgen.1006457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koch I, Schwarz H, Beuchle D, Goellner B, Langegger M, Aberle H. Drosophila ankyrin 2 is required for synaptic stability. Neuron. 2008;58:210–222. doi: 10.1016/j.neuron.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 72.Hortsch M, Paisley KL, Tian MZ, Qian M, Bouley M, Chandler R. The axonal localization of large drosophila ankyrin2 protein isoforms is essential for neuronal functionality. Mol Cell Neurosci. 2002;20:43–55. doi: 10.1006/mcne.2002.1113. [DOI] [PubMed] [Google Scholar]
- 73.Tseng WC, Jenkins PM, Tanaka M, Mooney R, Bennett V. Giant ankyrin-g stabilizes somatodendritic gabaergic synapses through opposing endocytosis of gabaa receptors. Proc Natl Acad Sci U S A. 2015;112:1214–1219. doi: 10.1073/pnas.1417989112. [DOI] [PMC free article] [PubMed] [Google Scholar]