Abstract
Background
There is a well-documented decline in fertility treatment success with increasing female age; however, there are few preconception cohort studies examining female age and natural fertility. In addition, data on male age and fertility is inconsistent. Given the increasing number of couples attempting conception at older ages, a more detailed characterization of age-related fecundability in the general population is of great clinical utility.
Objective
To examine the association between female and male age with fecundability.
Study Design
We conducted a web-based preconception cohort study of pregnancy planners from the United States and Canada. Participants enrolled between June 2013 and July 2017. Eligible participants were aged 21–45 years (females) or ≥21 years (males), and not using fertility treatments. Couples were followed until pregnancy or for up to 12 menstrual cycles. We analyzed data from 2,962 couples who had been trying to conceive for ≤3 cycles at study entry and reported no history of infertility. We used life-table methods to estimate the unadjusted cumulative pregnancy proportion at 6 and 12 cycles by female and male age. We used proportional probabilities regression models to estimate fecundability ratios, the per-cycle probability of conception for each age category relative to the referent (21–24 years), and 95% confidence intervals.
Results
Among females, the unadjusted cumulative pregnancy proportion at 6 cycles of attempt time ranged from 62.0% (age 28–30 years) to 27.6% (age 40–45 years); the cumulative pregnancy proportion at 12 cycles of attempt time ranged from 79.3% (age 25–27 years) to 55.5% (age 40–45 years). Similar patterns were observed among males, although differences between age groups were smaller. After adjusting for potential confounders, we observed a nearly monotonic decline in fecundability with increasing female age, with the exception of 28–33 years, where fecundability was relatively stable. Fecundability ratios were 0.91 (95% confidence interval: 0.74–1.11) for ages 25–27, 0.88 (95% confidence interval: 0.72–1.08) for ages 28–30, 0.87 (95% confidence interval: 0.70–1.08) for ages 31–33, 0.82 (95% confidence interval: 0.64–1.05) for ages 34–36, 0.60 (95% confidence interval: 0.44–0.81) for ages 37–39, and 0.40 (95% confidence interval: 0.22–0.73) for ages 40–45, compared with the reference group (ages 21–24 years). The association was stronger among nulligravid women. Male age was not appreciably associated with fecundability after adjustment for female age, although the number of men over age 45 years was small (n=37).
Conclusion
In this preconception cohort study of North American pregnancy planners, increasing female age was associated with an approximately linear decline in fecundability. While we found little association between male age and fecundability, the small number of men in our study over age 45 years limited our ability to draw conclusions on fecundability in older men.
Keywords: age, fecundability, fertility, preconception cohort, time-to-pregnancy
INTRODUCTION
Over the last several decades, couples in Western societies have been gradually postponing conception until older ages.1 There are several hypothesized reasons for delayed childbearing,2 including increased access to effective contraception;3 higher female educational attainment;4–6 increased female participation in the workforce;7 cultural shifts concerning the ideal number of children;8 improved gender equity;9–12 economic uncertainty,13, 14 and the absence of family-friendly workplace policies.15, 16 Given the increasing number of couples attempting conception at older ages, a more detailed characterization of age-related fecundability in the general population is of great clinical utility.
There is a well-documented decline in fertility treatment success with increasing female age.17, 18 In addition, data from non-contracepting natural fertility populations have shown that marital fertility rates decline with increasing female age, with peak fecundability in the early to mid-twenties and a steady decline at older ages; in some populations, a more rapid decline was observed after age 30 years.19–21
Studies examining the association between age and fecundability in infertile populations or populations of pregnant women are subject to selection bias22 and misclassification.23 Though limited in number, preconception cohort studies of women from the general population avoid these biases and support the hypothesis that a woman’s fecundability begins to decline during her early thirties. In a Danish preconception cohort study, fecundability peaked at around age 30 and then declined steadily at older ages. The age-related decline in fecundability was stronger among nulliparous women.24 In a preconception cohort study of American women age 30–44 years, fecundability began to decline around age 34 years; this association was more marked among women who had never conceived before.25
Studies also indicate that increasing male age, independent of female age, is associated with reduced fertility. Meta-analyses have shown age-related declines in semen quality, including volume, motility, morphology and DNA integrity.26, 27 However, prospective cohort studies examining male age and natural fertility24, 28 and success of assisted reproductive technologies29–32 report conflicting results. In particular, in a preconception cohort from seven European cities, among couples in which the female was age 35 years, the crude probability of conceiving within 12 cycles decreased from 82% if the male was 35 years old to 72% if the male was 40 years old.28 However, in a Danish preconception cohort study, the crude probability of conceiving within 12 cycles did not vary substantially by male age (86%, 81%, and 86% among men aged 30–34, 35–39, and ≥40 years, respectively), and men aged ≥40 years had 0.95 times the fecundability of men aged 21–24 years after adjusting for covariates.24
To better characterize the age-related decline in fecundability among couples attempting to conceive naturally, we examined the association between female and male age and fecundability in a preconception cohort study of pregnancy planners from North America.
MATERIAL AND METHODS
Study design and population
Pregnancy Study Online (PRESTO) is an ongoing prospective cohort study of North American couples attempting conception.33 Recruitment began in June 2013 using primarily web-based methods. We used banner ads on social networking sites (i.e., Facebook) that targeted women based on age, gender, and marital status. We also advertised on health-related websites, pregnancy-related websites, and parenting blogs. Eligible women were 21–45 years old, residents of the United States or Canada, in a stable relationship with a male partner, and attempting to conceive without use of fertility treatments. Female participants could invite their male partner to participate if the partner was ≥21 years of age (58% of participating women invited their male partners and 51% of males invited chose to participate). Participation for both partners involved a baseline questionnaire on demographics, lifestyle and behavioral factors, and medical and reproductive histories. Women completed shorter bimonthly follow-up questionnaires for up to 12 months to ascertain pregnancies and update exposure information.
The study protocol was approved by the institutional review board at Boston University Medical Center. All participants provided informed consent online before initiating the study.
Exclusions
Over 50 months of recruitment, 5,249 women completed the baseline questionnaire. We excluded couples in which the woman had implausible or missing LMP data (n=175), or was pregnant at study entry (n=46), and couples who had been attempting conception for longer than 3 cycles at study entry (n=1,856). We also excluded couples with a history of infertility (n=210) for a final analytic sample of 2,962 couples.
Definition of study variables
On the female baseline questionnaire, women reported their date of birth and their partner’s current age. On the male baseline questionnaire, men reported their date of birth. We calculated female and male ages at baseline from date of birth and date of female baseline questionnaire completion. When both partners participated in the study, we used information from the male questionnaire to measure male age. When only the female partner participated, we used information from the female questionnaire to measure male age. Agreement between female and male reports of age was high: among the 842 couples in which both partners contributed data, 810 (96.2%) reported male age identically, 30 (3.6%) reported ages discrepant by 1 year, 1 (0.1%) reported ages discrepant by 2 years, and 1 (0.1%) reported ages discrepant by 5 years.
We measured fecundability using data from the female baseline and follow-up questionnaires. We asked women with regular menstrual cycles about their typical cycle length. For women with irregular menstrual cycles, we estimated cycle length based on last menstrual period (LMP) dates at baseline and over follow-up. We estimated time-to-pregnancy in discrete menstrual cycles using the following formula: [(cycles of attempt at study entry)+[(LMP date from most recent follow-up questionnaire–date of baseline questionnaire)/cycle length]+1]. Only observed cycles at risk (those that occurred after study entry) were included in the analysis. Women who did not complete any follow-ups (n=304) were assigned one cycle of observation; their outcome information was imputed (see multiple imputation procedures below).
We obtained additional information on female and male demographics and behaviors from the female baseline questionnaire. Women reported their race/ethnicity, education level, household income, menstrual cycle characteristics, weight, height, physical activity, pregnancy history, smoking history, current alcohol and caffeine intake, intercourse frequency, use of methods to improve chances of conception (i.e., recording basal body temperature, monitoring cervical mucus, using an ovulation test kit, and other methods), and last method of contraception. Women also reported their male partner’s weight, height, education level, and smoking status. Body mass index (BMI) for females and males was calculated as weight (kg) divided by height (m2). Vigorous physical activity for females was calculating by summing the hours per week spent participating in each of the following activities: biking, jogging, swimming, racquetball, aerobics, and free weights.
Data analysis
All analyses were conducted using SAS version 9.4.34 We applied life-table methods to estimate the cumulative pregnancy proportion at 6 and 12 cycles, overall and by age group. We measured effects of factors affecting fecundability with the fecundability ratio (FR), which is the average per-cycle probability of conception in exposed compared with unexposed women; a FR <1.00 indicates that exposure has an adverse association with fecundability. We fit proportional probabilities regression models to compute FRs and 95% confidence intervals (CI) by age category. This model adjusts for changes in fecundability over time since beginning pregnancy attempts by including indicators of cycle attempt, and it accounts for left truncation by allowing for delayed entry into the risk set.35, 36 Crude fecundability declines rapidly with time since attempting pregnancy because there is steady depletion of the most fertile couples from the eligible pool. Follow-up was censored at reported pregnancy, initiation of fertility treatment, cessation of pregnancy attempt, loss to follow-up, or 12 cycles, whichever came first.
We adjusted for female education (≤high school, some college, college degree, graduate school), female smoking history (never, former, current occasional, current regular), female alcohol use (0, 1–6, 7–13, ≥14 drinks/week), female BMI (<25, 25–29, 30–34, ≥35 kg/m2), female vigorous physical activity (0, 1–3, 4–6, ≥7 hours/week), parity (nulliparous, parous), male BMI (<25, 25–29, 30–34, ≥35 kg/m2), male education (≤high school, some college, college degree, graduate school), male smoking (not current, current), annual household income (<$50,000, $50,000–$99,999, $100,000–$149,999, ≥$150,000), intercourse frequency (<1, 1, 2–3, ≥4 times/week), use of methods to improve chances of conception (recording basal body temperature, monitoring cervical mucus, using an ovulation predictor kit, and other; not mutually exclusive), and last method of contraception (hormonal methods, barrier methods, withdrawal/rhythm methods). Models were additionally adjusted for partner age (<25, 25–27, 28–30, 31–33, 34–36, 37–39, ≥40 years).
We fit restricted cubic splines to allow for non-linear relations between age and fecundability.37, 38 We stratified final models by gravidity to determine if the relation between age and fecundability differed between women with and without a prior pregnancy. Lastly, given that miscarriage is more common among older women,39 we ran separate models with viable pregnancy as the outcome, censoring pregnancy losses at their LMP date.
Data missingness ranged from 0% (age) to 3% (income). We used multiple imputation to account for missing data on covariates. Our imputation model contained 150 variables, and we created five imputation data sets using PROC MI. We used PROC MIANALYZE to combine betas and standard errors from each imputation data set.
RESULTS
Among women who completed the eligibility screening questionnaire, 89% were eligible for participation. Of those eligible, 65.8% completed the baseline questionnaire. We included 2,962 couples with no history of infertility who had been trying to conceive for ≤3 cycles at study entry in the present analysis; 62.4% of these couples conceived over follow-up, 6.0% started fertility treatment, 0.6% stopped trying to conceive, 19.8% were lost to follow-up before 12 cycles, and 11.2% were censored at 12 cycles. Women who completed the study (n=2,377) were more likely to have a college degree (82.3% vs. 71.5%), less likely to have a household income <$50,000/year (18.5% vs. 27.1%), and more likely to be white, non-Hispanic (80.8% vs. 77.2%) than women who were lost to follow-up. However, they were similar with respect to age (mean female age=29.9 years in both groups; mean male age=31.7 and 32.1 years, respectively).
The average ages of female and male PRESTO participants were 29.9 (standard deviation (SD)=4.0) and 31.8 (SD=5.1) years, respectively. Female age ranged from 21 to 44 years; male age ranged from 21 to 65 years. Female and male age were positively correlated (Pearson’s correlation coefficient (r)=0.62). Female age was positively associated with female education, household income, parity, gravidity, cycle regularity, female BMI, and doing something to improve chances of conception; it was inversely associated with cycle length, female current smoking and alcohol intake, and intercourse frequency (Table 1). Male age was positively associated with male education, household income, having ever impregnated a partner, and male alcohol intake; it was inversely associated with intercourse frequency.
Table 1.
Baseline characteristics of 2,962 North American female pregnancy planners and their male partners by age at baseline, PRESTO, 2013–2017.
| Characteristics | Female age (years)
|
Male age (years)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21–24 | 25–29 | 30–34 | 35–39 | 40–45 | 21–24 | 25–29 | 30–34 | 35–39 | ≥40 | |
| Number | 242 | 1185 | 1175 | 322 | 38 | 139 | 886 | 1197 | 517 | 223 |
| Partner’s age (years), mean | 26.3 | 29.9 | 33.0 | 37.3 | 40.1 | 24.4 | 27.5 | 30.3 | 32.9 | 34.0 |
| Cycles of attempt at study entry, mean | 1.4 | 1.3 | 1.3 | 1.4 | 1.8 | 1.6 | 1.3 | 1.3 | 1.4 | 1.3 |
| Non-white, %a | 17.8 | 13.3 | 15.7 | 12.4 | 39.5 | 7.3 | 11.7 | 14.2 | 9.7 | 25.5 |
| Regular cycles, % | 55.0 | 49.8 | 55.2 | 65.8 | 79.0 | -- | -- | -- | -- | -- |
| Cycle length (days), mean | 30.1 | 30.4 | 29.7 | 28.9 | 27.7 | -- | -- | -- | -- | -- |
| Cycle length <27 days, % | 9.5 | 10.4 | 12.4 | 12.1 | 23.7 | -- | -- | -- | -- | -- |
| Cycle length ≥32 days, % | 24.8 | 24.1 | 20.9 | 13.0 | 10.5 | -- | -- | -- | -- | -- |
| BMI (kg/m2), mean | 28.2 | 26.2 | 25.9 | 27.4 | 29.1 | 27.5 | 27.4 | 27.7 | 27.9 | 28.5 |
| Vigorous physical activity (hours/week), meana | 2.3 | 3.2 | 3.3 | 2.7 | 2.2 | 2.5 | 2.9 | 3.2 | 2.9 | 2.4 |
| <College degree, % | 54.1 | 18.5 | 13.2 | 12.1 | 18.4 | 68.4 | 36.6 | 32.0 | 30.2 | 35.9 |
| Annual household income <$50,000, % | 49.6 | 15.2 | 9.0 | 10.6 | 18.4 | 54.7 | 20.3 | 8.7 | 8.9 | 18.4 |
| Parous, % | 22.7 | 19.5 | 26.5 | 47.5 | 55.3 | -- | -- | -- | -- | -- |
| Ever pregnant/ever impregnated partner, %a | 43.8 | 37.2 | 44.2 | 62.4 | 81.6 | 26.8 | 28.0 | 43.9 | 53.0 | 60.0 |
| Current regular smoker, % | 12.0 | 3.1 | 3.3 | 4.0 | 5.3 | 14.4 | 7.7 | 6.8 | 8.1 | 10.3 |
| Alcohol intake (drinks/week), meana | 1.8 | 3.3 | 3.9 | 3.9 | 2.5 | 4.2 | 5.9 | 7.0 | 6.2 | 8.2 |
| Intercourse frequency <1 time/week, % | 10.7 | 16.5 | 24.1 | 34.8 | 39.5 | 11.5 | 13.9 | 23.1 | 29.8 | 27.8 |
| Intercourse frequency ≥4 times/week, % | 31.8 | 16.2 | 10.6 | 13.4 | 7.9 | 30.2 | 18.7 | 12.0 | 11.0 | 13.9 |
| Doing something to improve chances of conception, % | 66.9 | 71.9 | 73.3 | 76.7 | 55.3 | 66.2 | 70.9 | 74.3 | 71.8 | 73.1 |
| Methods to improve chances, % | ||||||||||
| Basal body temperature | 18.2 | 23.4 | 19.6 | 16.5 | 13.2 | 17.3 | 21.1 | 21.6 | 19.7 | 17.0 |
| Cervical mucus | 33.1 | 39.5 | 37.2 | 37.0 | 21.1 | 34.5 | 38.5 | 37.8 | 38.3 | 32.3 |
| LH/ovulation test kit | 23.6 | 27.9 | 30.0 | 29.5 | 15.8 | 26.6 | 27.4 | 28.1 | 30.6 | 29.6 |
| Other | 21.9 | 21.3 | 22.2 | 25.2 | 21.1 | 18.7 | 22.7 | 22.9 | 19.0 | 25.1 |
| Hormonal last method of contraception, % | 40.5 | 39.7 | 36.0 | 34.2 | 39.5 | 41.0 | 39.2 | 38.1 | 35.0 | 33.6 |
Data on these variables were available for the 842 men who completed the baseline questionnaire.
The cumulative pregnancy proportions after 6 and 12 cycles of attempt time were 58.1% and 74.9%, respectively. Among couples attempting to conceive for 0 or 1 cycles at study entry, these proportions were 63.9% and 78.7%, respectively. Females in the 25–27 year age group had the highest crude probability of pregnancy after 12 cycles (79.3%), whereas women in the 40–45 year age group had the lowest probability (55.5%) (Table 2). Variation in the crude cumulative probability of pregnancy after 12 cycles was less substantial across male age groups; the probability was lowest among men aged 21–24 years (70.0%) and men aged ≥40 years (68.9%), and was relatively similar for men aged 25–39 years.
Table 2.
Cumulative pregnancy proportion within 6 and 12 cycles and fecundability by female and male age at baseline, PRESTO, 2013–2017.
| Pregnancies N | Participants N | Cycles N | Cumulative pregnancy proportiona
|
Unadjusted FR (95% CI) | Adjusted FRb (95% CI) | ||
|---|---|---|---|---|---|---|---|
| 6 cycles % (95% CI) | 12 cycles % (95% CI) | ||||||
| Female age (y) | |||||||
| 21–24 | 138 | 242 | 931 | 56.8 (49.9, 63.6) | 70.8 (62.9, 78.6) | 1.00 (Reference) | 1.00 (Reference) |
| 25–27 | 356 | 558 | 2184 | 59.0 (54.6, 63.5) | 79.3 (74.7, 83.9) | 1.06 (0.88, 1.28) | 0.91 (0.74, 1.11) |
| 28–30 | 606 | 946 | 3659 | 62.0 (58.6, 65.3) | 77.9 (74.4, 81.5) | 1.08 (0.91, 1.29) | 0.88 (0.72, 1.08) |
| 31–33 | 460 | 710 | 2858 | 60.7 (56.8, 64.5) | 76.6 (72.7, 80.5) | 1.06 (0.88, 1.26) | 0.87 (0.70, 1.08) |
| 34–36 | 201 | 327 | 1343 | 55.9 (50.0, 61.7) | 74.8 (68.8, 80.8) | 1.02 (0.83, 1.25) | 0.82 (0.64, 1.05) |
| 37–39 | 74 | 141 | 664 | 46.3 (37.3, 55.3) | 67.4 (57.7, 77.0) | 0.75 (0.58, 0.98) | 0.60 (0.44, 0.81) |
| 40–45 | 13 | 38 | 201 | 27.6 (12.1, 43.1) | 55.5 (30.7, 80.2) | 0.48 (0.27, 0.85) | 0.40 (0.22, 0.73) |
| Male age (y) | |||||||
| 21–24 | 76 | 139 | 600 | 50.2 (42.3, 59.0) | 70.0 (58.8, 81.2) | 1.00 (Reference) | 1.00 (Reference) |
| 25–27 | 248 | 400 | 1596 | 58.0 (52.7, 63.2) | 75.3 (70.0, 80.7) | 1.14 (0.90, 1.46) | 1.11 (0.87, 1.43) |
| 28–30 | 383 | 754 | 2848 | 62.7 (58.8, 66.5) | 78.2 (74.4, 82.0) | 1.24 (0.99, 1.55) | 1.14 (0.89, 1.46) |
| 31–33 | 479 | 741 | 2955 | 61.1 (57.3, 64.9) | 77.8 (73.8, 81.9) | 1.16 (0.92, 1.46) | 1.05 (0.82, 1.36) |
| 34–36 | 305 | 475 | 1923 | 58.1 (53.4, 62.8) | 75.2 (70.4, 79.9) | 1.15 (0.91, 1.45) | 1.04 (0.80, 1.36) |
| 37–39 | 140 | 230 | 950 | 58.5 (51.4, 65.5) | 78.6 (71.1, 86.1) | 1.09 (0.84, 1.41) | 1.13 (0.84, 1.51) |
| ≥40 | 116 | 223 | 968 | 47.8 (40.5, 55.0) | 68.9 (60.6, 77.2) | 0.91 (0.69, 1.19) | 1.02 (0.75, 1.38) |
Cumulative pregnancy proportions were calculated using life-table methods that account for censoring.
Adjusted for female education, female smoking, female alcohol use, female BMI, female vigorous physical activity, male education, male BMI, male smoking, household income, using methods to improve chances of conception, intercourse frequency, last method of contraception, and partner age.
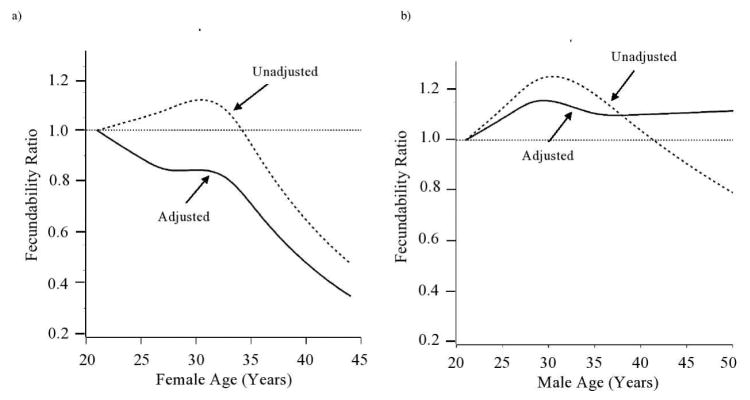
In unadjusted models, we observed an inverted-J shaped association between female age and fecundability (Table 2 and Figure 1). Compared with women aged 21–24 years, FRs for women aged 25–27, 28–30, 31–33, 34–36, 37–39, and 40–45 years were 1.06 (95% CI: 0.88, 1.28), 1.08 (95% CI: 0.91, 1.29), 1.06 (95% CI: 0.88, 1.26), 1.02 (95% CI: 0.83, 1.25), 0.75 (95% CI: 0.58, 0.98), and 0.48 (95% CI: 0.27, 0.85), respectively. However, after adjustment for female and male covariates, we observed a more linear decline, with FRs of 0.91 (95% CI: 0.74, 1.11), 0.88 (95% CI: 0.72, 1.08), 0.87 (95% CI: 0.70, 1.08), 0.82 (95% CI: 0.64, 1.05), 0.60 (95% CI: 0.44, 0.81), and 0.40 (95% CI: 0.22, 0.73), respectively.
Figure 1.
Association between female and male age and fecundability, fitted by restricted cubic splines, PRESTO, 2013–2017. The reference value was 21 years, the youngest age in the cohort. Both unadjusted (dotted line) and adjusted (solid line; adjusted for female education, female smoking, female alcohol use, female BMI, female vigorous physical activity, male education, male BMI, male smoking, household income, using methods to improve chances of conception, intercourse frequency, last method of contraception, and partner age) results are presented. Knots are located at the 10th, 25th, 75th, and 90th percentiles (25, 27, 33, and 36 years for females; 26, 28, 35, and 39 years for males). The spline for male age is trimmed at the 99th percentile (50 years).
The unadjusted association between male age and fecundability was roughly similar to that for female age, although not declining as steeply (Table 2 and Figure 1). FRs for men aged 25–27, 28–30, 31–33, 34–36, 37–39, and ≥40 years compared with men aged 21–24 years were 1.14 (95% CI: 0.90, 1.46), 1.24 (95% CI: 0.99, 1.55), 1.16 (95% CI: 0.92, 1.46), 1.15 (95% CI: 0.91, 1.45), 1.09 (95% CI: 0.84, 1.41), and 0.91 (95% CI: 0.69, 1.19), respectively. However, after adjustment for female age and other covariates, there was no substantial association between male age and fecundability.
The inverse association between older female age and fecundability was slightly stronger among nulligravid women (Table 3). FRs for women aged 34–36, 37–39, and 40–45 years compared with women aged 21–24 years were 0.68 (95% CI: 0.48, 0.96), 0.51 (95% CI: 0.30, 0.85), and 0.20 (95% CI: 0.03, 1.44), respectively, among nulligravid women and 0.96 (95% CI: 0.67, 1.40), 0.70 (95% CI: 0.46, 1.08), and 0.48 (95% CI: 0.24, 0.94), respectively, among gravid women.
Table 3.
Female age and fecundability, stratified by gravidity, PRESTO, 2013–2017.
| Exposure | Nulligravid (n=1,664)
|
Gravid (n=1,298)
|
||||
|---|---|---|---|---|---|---|
| Pregnancies N | Cycles N | Adjusted FRa (95% CI) | Pregnancies N | Cycles N | Adjusted FRa (95% CI) | |
| Female age (y) | ||||||
| 21–24 | 75 | 568 | 1.00 (Reference) | 63 | 363 | 1.00 (Reference) |
| 25–27 | 218 | 1462 | 0.88 (0.66, 1.16) | 138 | 722 | 0.92 (0.68, 1.24) |
| 28–30 | 352 | 2441 | 0.80 (0.59, 1.07) | 254 | 1218 | 0.95 (0.70, 1.30) |
| 31–33 | 243 | 1617 | 0.84 (0.62, 1.14) | 217 | 1241 | 0.88 (0.64, 1.22) |
| 34–36 | 87 | 736 | 0.68 (0.48, 0.96) | 114 | 607 | 0.96 (0.67, 1.40) |
| 37–39 | 21 | 263 | 0.51 (0.30, 0.85) | 53 | 401 | 0.70 (0.46, 1.08) |
| 40–45 | 1 | 49 | 0.20 (0.03, 1.44) | 12 | 152 | 0.48 (0.24, 0.94) |
Adjusted for female education, female smoking, female alcohol use, female BMI, female vigorous physical activity, male education, male BMI, male smoking, household income, using methods to improve chances of conception, intercourse frequency, last method of contraception, and partner age.
When we specified viable pregnancy as the outcome variable, our results were slightly stronger (FRs for women aged 25–27, 28–30, 31–33, 34–36, 37–39 and 40–45 years compared with 21–24 years were 0.91 (95% CI: 0.74, 1.13), 0.85 (95% CI: 0.68, 1.05), 0.84 (95% CI: 0.67, 1.05), 0.79 (95% CI: 0.61, 1.03), 0.61 (95% CI: 0.44, 0.84), and 0.38 (95% CI: 0.20, 0.73), respectively).
COMMENT
In this North American preconception cohort study of pregnancy planners, we observed an approximately linear decline in fecundability with increasing female age. Women aged 40–45 years were 60% less likely to conceive in any given cycle than women aged 21–24 years. The age-related decline in fecundability was more pronounced among women who had never conceived before. Results were stronger when considering viable pregnancies rather than all pregnancies as the outcome variable, indicating that older women are both less likely to conceive and less likely to carry a pregnancy term if they do conceive. We excluded women with a history of infertility from our analysis and adjusted for selected factors that may be related to both age and fecundability (e.g., intercourse frequency, partner age). After accounting for female age, male age was not substantially associated with fecundability.
Diminished ovarian reserve and periodic anovulation are thought to contribute to lower fecundability among older women.40, 41 Other preconception cohort studies have also reported a female age-related decline in fecundability. In a cohort study of older American women (age 30–44 years), a gradual association was observed between increasing female age and reduced fecundability, with the decline starting around age 34 years.25 Results were not adjusted for potential confounding factors. In a Danish preconception cohort study, adjusted fecundability increased slightly from ages 20 through 28, then decreased linearly at older ages.24 In the present study, we also found a slightly stronger association between age and fecundability among nulligravid women, as has been observed in other studies.24, 25 These findings may reflect prior unplanned pregnancies among the gravid women, as older women with no history of unplanned pregnancy may be inherently less fertile.
We found little association between male age and fecundability after accounting for female age. This finding generally agrees with the similarly-designed Danish preconception cohort study, where only slight reductions in fecundability were observed among men aged ≥40 years.24 However, our results conflict with those from a preconception cohort study comprising couples from seven European cities,28 and with two pregnancy-based studies that found stronger associations between advanced male age and fertility.42, 43 Despite evidence that male reproductive capacity exists well into the sixties, we had very few men in our study over age 45 (n=37); therefore we were unable to examine fecundability separately for the oldest age ranges of men.
Our study population comprised pregnancy planners recruited via the internet. While there are differences between users and non-users of the internet,44 these differences would not affect our comparisons unless the relationship between age and fecundability differs between internet users and non-users, which seems unlikely.45 Furthermore, our study46 and others47, 48 have demonstrated that even when participation in a cohort study is related to characteristics such as age and parity, bias due to self-selection is not necessarily present.
Our study population was restricted to couples planning a pregnancy. If unplanned pregnancies are more prevalent among fertile couples and younger women,49 exclusion of unplanned pregnancies could have introduced selection bias. Because younger, more fertile women may be underrepresented in our study population, the FRs for older women may be biased upwards.
Although 19.8% of our participants were lost to follow-up before completing the study, mean female and male ages were similar among those who did and did not complete the study. Therefore, we do not believe that differential loss to follow-up is a major source of bias in this analysis.
The present study improves upon prior preconception cohort studies by controlling for potential confounders and categorizing age more finely. Our results confirm the decline in fertility with increasing female age, but we found an approximately linear relationship throughout the reproductive years. The oldest women in our cohort (40–45 years) had 60% lower fecundability and approximately three-quarters the probability of conceiving within 12 cycles than women 21–24 years of age. In contrast, we found that male age is not strongly associated with fertility, although we were unable to examine the relationship between age and fecundability among men ≥45 years due to small numbers.
Acknowledgments
Source of funding: This study was funded by NICHD Grants R21 HD072326, R01 HD086742, R03 HD090315, and T32 HD052458. The funding sources had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
We thank Mr. Michael Bairos of Slone Epidemiology Center for his technical support with developing the web-based infrastructure of PRESTO (funding provided by the NICHD).
Footnotes
Paper presentation information: These findings were accepted as an abstract at the Society for Epidemiologic Research 50th Annual Meeting in Seattle, WA, June 20–23, 2017.
Conflicts of interest: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathews TJ, Hamilton BE. NCHS data brief. 21. Hyattsville, MD: National Center for Health Statistics; 2009. Delayed childbearing: More women are having their first child later in life. [PubMed] [Google Scholar]
- 2.Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17:848–60. doi: 10.1093/humupd/dmr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldin C, Katz LF. The power of the pill: oral contraceptives and women's career and marriage decisions. J Pol Econ. 2002;110:730–70. [Google Scholar]
- 4.Rindfuss RR, Morgan SP, Offutt K. Education and the changing age pattern of American fertility: 1963–1989. Demography. 1996;33:277–90. [PubMed] [Google Scholar]
- 5.Rindfuss RR, Morgan SP, Swicegood G. First Births in America. Changes in the Timing of Parenthood. Berkeley, CA: University of California Press; Number of pages. [Google Scholar]
- 6.Martin SP. Diverging fertility among U.S. women who delay childbearing past age 30. Demography. 2000;37:523–33. doi: 10.1353/dem.2000.0007. [DOI] [PubMed] [Google Scholar]
- 7.Voydanoff P. Work role characteristics, family structure demands and work-family conflict. J Mar Fam. 1988;50:749–62. [Google Scholar]
- 8.Goldstein J, Lutz W, Testa MR. The emergence of sub-replacement family size ideals in Europe. Popul Res Policy Rev. 2003;22:479–96. [Google Scholar]
- 9.Mason K, KO . Gender and demographic change: what do we know? In: Jones GW, Douglas RM, Caldwell JC, D'Souza RM, editors. The Continuing Demographic Transition. Oxford: Clarendon Press; 1997. [Google Scholar]
- 10.McDonald P. Gender equity in theories of fertility transition. Popul Dev Rev. 2000;26:427–39. [Google Scholar]
- 11.McDonald P. Gender equality, social institutions and the future of fertility. J Popul Res. 2000;17:1–16. [Google Scholar]
- 12.Neyer G. MPIDR Working Paper 2006–10. Max Planck Institute for Demographic Research; 2006. Family Policies and Fertility in Europe: Fertility Policies at the Intersection of Gender Policies, Employment Policies, and Care Policies. [Google Scholar]
- 13.Mills M, Blossfeld H-P, Klijzing E. Becoming an adult in uncertain times: a 14-country comparison of the losers of globalization. In: Blossfeld H-P, Klijzing E, Mills M, Kurz K, editors. Globalization, Uncertainty and Youth in Society. London: Routledge; 2005. [Google Scholar]
- 14.Adsera A. Changing fertility rates in developed countries. The impact of labor market institutions. J Popul Econ. 2004;17:17–43. [Google Scholar]
- 15.Neyer G, Andersson G. Consequences of family policies on childbearing behavior: effects or artifacts? Popul Dev Rev. 2008;34:699–724. [Google Scholar]
- 16.Letablier M-T, Luci A, Thevenon O. Directorate-General Employment SAaEO, editor. The Cost of Raising Children and the Effectiveness of Policies to Support Parenthood in European Countries: A Literature Review. Brussels: European Commission; 2009. [Google Scholar]
- 17.Centers for Disease Control and Prevention. Assisted Reproductive Technology (ART) Data. Vol. 2017 Atlanta, GA: Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 18.Schwartz D, Mayaux MJ. Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. Federation CECOS N Engl J Med. 1982;306:404–6. doi: 10.1056/NEJM198202183060706. [DOI] [PubMed] [Google Scholar]
- 19.Larsen U, Yan S. The age pattern of fecundability: an analysis of French Canadian and Hutterite birth histories. Soc Biol. 2000;47:34–50. doi: 10.1080/19485565.2000.9989008. [DOI] [PubMed] [Google Scholar]
- 20.Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233:1389–94. doi: 10.1126/science.3755843. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor KA, Holman DJ, Wood JW. Declining fecundity and ovarian ageing in natural fertility populations. Maturitas. 1998;30:127–36. doi: 10.1016/s0378-5122(98)00068-1. [DOI] [PubMed] [Google Scholar]
- 22.Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Selection bias in determining the age dependence of waiting time to pregnancy. Am J Epidemiol. 2000;152:565–72. doi: 10.1093/aje/152.6.565. [DOI] [PubMed] [Google Scholar]
- 23.Lum KJ, Sundaram R, Buck Louis GM. Women's lifestyle behaviors while trying to become pregnant: evidence supporting preconception guidance. Am J Obstet Gynecol. 2011;205:203, e1–7. doi: 10.1016/j.ajog.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman KJ, Wise LA, Sorensen HT, Riis AH, Mikkelsen EM, Hatch EE. Volitional determinants and age-related decline in fecundability: a general population prospective cohort study in Denmark. Fertil Steril. 2013;99:1958–64. doi: 10.1016/j.fertnstert.2013.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steiner AZ, Jukic AM. Impact of female age and nulligravidity on fecundity in an older reproductive age cohort. Fertil Steril. 2016;105:1584–88. e1. doi: 10.1016/j.fertnstert.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237–48. doi: 10.1016/s0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 27.Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. doi: 10.1016/j.arr.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol. 2004;103:51–6. doi: 10.1097/01.AOG.0000100153.24061.45. [DOI] [PubMed] [Google Scholar]
- 29.Dain L, Auslander R, Dirnfeld M. The effect of paternal age on assisted reproduction outcome. Fertil Steril. 2011;95:1–8. doi: 10.1016/j.fertnstert.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 30.Paulson RJ, Milligan RC, Sokol RZ. The lack of influence of age on male fertility. Am J Obstet Gynecol. 2001;184:818–22. doi: 10.1067/mob.2001.113852. discussion 22-4. [DOI] [PubMed] [Google Scholar]
- 31.Baird DT, Collins J, Egozcue J, et al. Fertility and ageing. Hum Reprod Update. 2005;11:261–76. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- 32.Sharma R, Agarwal A, Rohra VK, Assidi M, Abu-Elmagd M, Turki RF. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol. 2015;13:35. doi: 10.1186/s12958-015-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wise LA, Rothman KJ, Mikkelsen EM, et al. Design and Conduct of an Internet-Based Preconception Cohort Study in North America: Pregnancy Study Online. Paediatr Perinat Epidemiol. 2015;29:360–71. doi: 10.1111/ppe.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SAS. SAS Institute Inc. SAS/STAT® 9.4 User’s Guide. Cary, NC: SAS Institute; 2014. Number of pages. [Google Scholar]
- 35.Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol. 2013;27:491–502. doi: 10.1111/ppe.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howards PP, Hertz-Picciotto I, Poole C. Conditions for bias from differential left truncation. Am J Epidemiol. 2007;165:444–52. doi: 10.1093/aje/kwk027. [DOI] [PubMed] [Google Scholar]
- 37.Durrleman S, Simon R. Flexible regression models with cubic splines. Statist Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 38.Li R, Hertzmark E, Spiegelman D. The SAS GLMCURV9 Macro. Boston, MA: Channing Laboratory; 2008. [Google Scholar]
- 39.de la Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod. 2002;17:1649–56. doi: 10.1093/humrep/17.6.1649. [DOI] [PubMed] [Google Scholar]
- 40.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–54. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 41.Heffner LJ, Schust DJ. The Reproductive System at a Glance. 4. Oxford, UK: Wiley Blackwell; 2014. Menopause. [Google Scholar]
- 42.Ford WC, North K, Taylor H, Farrow A, Hull MG, Golding J. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. The ALSPAC Study Team (Avon Longitudinal Study of Pregnancy and Childhood) Hum Reprod. 2000;15:1703–8. doi: 10.1093/humrep/15.8.1703. [DOI] [PubMed] [Google Scholar]
- 43.Hassan MA, Killick SR. Effect of male age on fertility: evidence for the decline in male fertility with increasing age. Fertil Steril. 2003;79(Suppl 3):1520–7. doi: 10.1016/s0015-0282(03)00366-2. [DOI] [PubMed] [Google Scholar]
- 44.Keiding N, Slama R. Commentary: time-to-pregnancy in the Real World. Epidemiology. 2015;26:119–21. doi: 10.1097/EDE.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 45.Rothman KJ, Gallacher JE, Hatch EE. Why representativeness should be avoided. Int J Epidemiol. 2013;42:1012–4. doi: 10.1093/ije/dys223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatch EE, Hahn KA, Wise LA, et al. Evaluation of Selection Bias in an Internet-based Study of Pregnancy Planners. Epidemiology. 2016;27:98–104. doi: 10.1097/EDE.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology. 2006;17:413–8. doi: 10.1097/01.ede.0000220549.14177.60. [DOI] [PubMed] [Google Scholar]
- 48.Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 49.Mosher WD, Jones J, Abma JC. Intended and unintended births in the United States: 1982–2010. Natl Health Stat Report. 2012:1–28. [PubMed] [Google Scholar]